Abstract
Clusterin, a protein chaperone found at high levels in physiological fluids, is expressed in nervous tissue and upregulated in several neurological diseases. To assess relevance to amyotrophic lateral sclerosis (ALS) and other motor neuron disorders, clusterin expression was evaluated using long-term dissociated cultures of murine spinal cord and SOD1G93A transgenic mice, a model of familial ALS. Motor neurons and astrocytes constitutively expressed nuclear and cytoplasmic forms of clusterin, and secreted clusterin accumulated in culture media. Although clusterin can be stress inducible, heat shock failed to increase levels in these neural cell compartments despite robust upregulation of stress-inducible Hsp70 (HspA1) in non-neuronal cells. In common with HSPs, clusterin was upregulated by treatment with the Hsp90 inhibitor, geldanamycin, and thus could contribute to the neuroprotection previously identified for such compounds in disease models. Clusterin expression was not altered in cultured motor neurons expressing SOD1G93A by gene transfer or in presymptomatic SOD1G93A transgenic mice; however, clusterin immunolabeling was weakly increased in lumbar spinal cord of overtly symptomatic mice. More striking, mutant SOD1 inclusions, a pathological hallmark, were strongly labeled by anti-clusterin. Since secreted, as well as intracellular, mutant SOD1 contributes to toxicity, the extracellular chaperoning property of clusterin could be important for folding and clearance of SOD1 and other misfolded proteins in the extracellular space. Evaluation of chaperone-based therapies should include evaluation of clusterin as well as HSPs, using experimental models that replicate the control mechanisms operant in the cells and tissue of interest.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0427-x) contains supplementary material, which is available to authorized users.
Keywords: Clusterin, Amyotrophic lateral sclerosis, Hsp90 inhibitor, Motor neurons, Astrocytes, Heat shock protein
Introduction
Clusterin, also called apolipoprotein J, sulfated glycoprotein-2, secreted glycoprotein gp80, complement lysis inhibitor, testosterone-repressed prostate message 2 (TRPM-2), or complement-associated protein SP40-40, is found at high level in physiological fluids (seminal fluid, blood plasma, and cerebral spinal fluid) and is expressed constitutively at variable levels in a wide variety of tissues (Rizzi et al. 2009). At least two transcripts are derived from use of distinct promoters in the clusterin gene, but multiple protein isoforms have been identified (Rizzi et al. 2009). The mature secreted form of the protein is a glycosylated, 75–80-kDa disulfide-linked heterodimer of α and β subunits (produced by internal cleavage) and shares homology with the small heat shock protein family of molecular chaperones (Wilson and Easterbrook-Smith 2000). Clusterin has been compared to heat shock proteins (HSP) due to its chaperoning activity and increased expression following cellular stress (Nizard et al. 2007; Poon et al. 2000; Wilson and Easterbrook-Smith 2000). In fact, the heat shock transcription factors, Hsf1 and Hsf2, can activate transcription of the clusterin gene by binding to the 14-bp CLE element in the clusterin promoter, which differs by only one base from the conserved heat shock element (HSE) in the promoter of heat shock genes (Wilson and Easterbrook-Smith 2000).
Clusterin also exists as a nuclear, unglycosylated 60-kDa protein (Nizard et al. 2007). Nuclear clusterin is thought to promote cell death by inhibiting DNA repair through binding to Ku70 (Leskov et al. 2003; Yang et al. 2000). On the other hand, accumulation of clusterin in the cytosol is associated with cell survival. Stress-induced translocation of clusterin from the endoplasmic reticulum to the cytosol has been demonstrated (Nizard et al. 2007) and could prevent apoptosis by antagonizing Bax, preventing mitochondrial release of cytochrome c and consequent caspase activation (Zhang et al. 2005). In fact, downregulation of clusterin by siRNA induced apoptosis in cancer cells (Trougakos and Gonos 2006). On the other hand, ectopic expression of high levels of clusterin damaged mitochondria (Debure et al. 2003). Thus, the regulation and biological effects of clusterin are complex and circumstance dependent.
In the nervous system, clusterin is widely expressed in neurons, being prominent in spinal cord motor neurons and pontobulbar neurons, and in astrocytes (Charnay et al. 2008; Pasinetti et al. 1994; Van Beek et al. 2000; Wiggins et al. 2003). In keeping with a role in stress response in the nervous system, clusterin is upregulated in experimental models of traumatic brain injury (May et al. 1992), nerve crush (Bonnard et al. 1997; Ohlsson et al. 2003), and status epilepticus (Dragunow et al. 1995; Schreiber et al. 1993), as well as in a variety of neurological conditions including multiple sclerosis (Rithidech et al. 2009) and Alzheimer’s disease (Ghiso et al. 1993; Giannakopoulos et al. 1998; Lidstrom et al. 1998; May et al. 1989; Polihronis et al. 1993). A recent report identifies polymorphism in CLU as a risk factor for Alzheimer’s disease.
Amyotrophic lateral sclerosis (ALS) is a fatal, adult-onset, neurodegenerative disorder characterized by gradual loss of muscle function due to death of motor neurons in the cortex, brain stem, and spinal cord. Mutations in several genes have been linked to familial forms, including fALS1 due to dominant mutations in SOD1 (Rosen et al. 1993). Evidence points to misfolding and altered solubility of the mutant protein as the underlying gain of toxic function leading to disease, with multiple downstream effects including mitochondrial abnormalities, calcium dysregulation, and formation of cytosolic protein aggregates (Boillee et al. 2006). Inducing expression of HSPs [stress-inducible Hsp70 (HspA1) and Hsp40 (DNAJ)] is protective in cell culture models, including a primary culture model developed in our laboratory by expressing wild-type or mutant SOD1 in primary motor neurons of dissociated spinal cord–dorsal root ganglion (DRG) cultures (Batulan et al. 2006). In addition to accumulating in the cytoplasm, mutant SOD1 is secreted from cells and thus could exert toxicity through extracellular mechanisms (Turner et al. 2005; Urushitani et al. 2006). We therefore asked what effect expression of mutant SOD1 or treatments known to induce HSPs would have on the expression and secretion of clusterin from spinal cord cells, given that clusterin is a stress protein, cytosolic clusterin is protective, and secreted clusterin can prevent the aggregation of misfolded proteins in the extracellular milieu (Poon et al. 2000).
In this study, the distribution of clusterin was examined in motor neurons and astrocytes of long-term (3–6 weeks) spinal cord–DRG cultures using antibodies recognizing either nuclear clusterin or cytoplasmic/secreted clusterin. Each cell type expressed both nuclear and cytoplasmic clusterin, the strong constitutive expression of nuclear clusterin arguing against an apoptotic role. In addition, clusterin was secreted into the culture medium. Our previous studies using this model showed that motor neurons have a high threshold for stress-induced upregulation of HSPs, but astrocytes mount a robust stress response (Batulan et al. 2003), replicating the properties of these cells in vivo (Manzerra and Brown 1996). However, thermal stress sufficient to induce expression of Hsp70 in astrocytes failed to increase expression of clusterin in either astrocytes or motor neurons. In contrast to heat shock, treatment of spinal cord–DRG cultures with the Hsp90 inhibitor, geldanamycin, did induce expression of nuclear and cytoplasmic clusterin in both motor neurons and astrocytes, as previously shown for Hsp70 and Hsp40 (Batulan et al. 2003, 2006). Thus, clusterin could contribute to the neuroprotective properties of Hsp90 inhibitors. Expression of mutant SOD1 in cultured motor neurons or astrocytes did not alter clusterin expression. In lumbar spinal cord of overtly symptomatic SOD1G93A mice, the major finding was strong immunolabeling of mutant SOD1 inclusions, a hallmark of disease, in common with other proteins associated with protein quality control. The data indicate both commonalities and differences in the regulation of clusterin and other stress-inducible genes, depending on the cell type, conditions, and type of stress, in keeping with the complex regulation of these genes.
Materials and methods
Dissociated spinal cord–DRG cultures
Primary dissociated spinal cord–DRG cultures were prepared from embryonic day 13 CD1 mice (Charles River Laboratories, Saint Constant Canada) as previously described (Durham et al. 1997). Briefly, spinal cords with attached DRGs were removed, dissociated in trypsin (Life Technologies, Burlington Canada) and plated at a density of 350,000–400,000 cells on 18-mm glass coverslips coated with poly-d-lysine (Sigma-Aldrich, St. Louis, MO) and Matrigel (BD Biosciences, Mississauga, Canada). Cultures were maintained in a modified N3 medium supplemented with 2.5 % horse serum (Life Technologies). At 90 % confluency (about 4–6 days from initial plating), cultures were treated with 1.4 μg/mL cytosine-β-d-arabinoside (EMD Millipore, Billerica, MA) for 4–5 days in order to inhibit proliferation of non-neuronal cell types. Motor neurons were identified in 3–6-week-old cultures by their large size and morphology as previously described (Roy et al. 1998).
Immunocytochemistry (cultures)
Cells were fixed in 3 % paraformaldehyde in phosphate-buffered saline (PBS), blocked in 5 % horse serum in PBS (30 min at room temperature (RT) or 24 h at 4 °C), and incubated with primary antibody for 45 min at RT. Samples were washed (3 × 4 min) with PBS and incubated with secondary antibody for 30 min. After washing, coverslips were mounted onto glass slides using Immunomount (Fisher Scientific, Ottawa, Canada) and stored at 4 °C.
Semi-quantitative analysis of protein expression in situ
Following labeling of the protein of interest by immunocytochemistry, intensity of epifluorescence in individual cells was measured using Universal Imaging MetaFluor® Software (Molecular Devices, Sunnyvale, CA). Images were captured using a Hamamatsu cooled CCD camera (Hamamatsu Photonics K.K., Japan), and labeling was quantified by tracing individual cells and measuring average pixel density using Metafluor. Fluorescent images were captured within the linear range of pixel density. Approximately 30 neurons per coverslip were measured.
SDS-PAGE and Western blotting
For detection of intracellular proteins, cultures were rinsed with cold PBS, harvested in Tris–EDTA (TE) buffer, and sonicated for 3-s pulses at power 50. Samples were centrifuged at 18,000×g for 10 min. For detection of secreted proteins, cultures were placed in serum-free medium (serum contains high levels of clusterin). The medium was harvested after a minimum of 24 h and clarified by centrifugation at 21,000×g for 5 min. The supernatant was concentrated using 10-kDa-cut-off Nanosep tubes (Pall Corporation, Mississauga, Canada) by centrifugation at 14,000×g for 10 min. The retained volume was adjusted to 100 μL using TE buffer. Cells from the same cultures were harvested concomitantly. Protein concentrations for both cell lysates and culture media were determined using the DC Protein Assay Kit I (Bio-Rad, Mississauga, Canada). Cell lysate (15 μg/mL) and culture medium (25 μg/mL) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10 % acrylamide gels.
To detect clusterin using the SC-8354 primary antibody, samples were run under reducing conditions (3 % β-mercaptoethanol), but non-reducing conditions were used for analysis using the SC-6419 primary antibody (under reducing conditions, a protein smear was obtained). Gels were transferred to nitrocellulose membranes and subjected to Western analysis, using 5 % skim milk to block nonspecific binding (1 h at RT). Primary antibodies were applied overnight at 4 °C, followed by washing 3 × 10 min and incubation with HRP-conjugated secondary antibody for 1 h at R.T. Loading controls were bovine serum albumin (BSA) for secretion experiments or actin or Hsc70 for culture lysates. After washing, blots were developed using the ECL detection system (Perkin Elmer, Waltham, MA). Band intensities were quantified using NIH Image J software and pixel density for proteins of interest normalized to the corresponding loading control.
Antibodies
Primary antibodies used for immunocytochemistry (IC) and Western blotting (WB) were goat anti-clusterin antibody (Santa Cruz Biotechnology, Dallas TX, SC-6419; 1:50 for IC and 1:500 for WB), rabbit anti-clusterin antibody (Santa Cruz, SC-8354; 1:50 for IC, 1:500 for WB), rabbit anti-glial fibrillary acidic protein (GFAP) antibody (DAKO, Burlington Canada, Z0334; 1:400), mouse anti-GFAP (Sigma-Aldrich, G3893; 1:400), mouse anti-SOD1 antibody (Sigma Aldrich, SD-G6; 1:400), mouse anti-Hsp70 (BioLynx Inc, Brockville Canada, SPA 810; 1:1,000), and mouse anti-bovine serum albumin (Sigma-Aldrich, B2901; 1:400). Secondary antibodies for IC were donkey anti-goat Cy3 (Jackson ImmunoResearch, Westgrove PA; 1:400) and donkey anti-rabbit Cy3 and Cy2 (Jackson ImmunoResearch; 1:400). Secondary antibodies for WB were donkey anti-goat conjugated to horseradish peroxidase (HRP; Jackson ImmunoResearch; 1:5,000), donkey anti-rabbit HRP (Jackson ImmunoResearch; 1:5,000), and goat anti-mouse HRP (Jackson ImmunoResearch; 1:5,000).
Specificity of clusterin antibodies was determined by standard preabsorption assay. Each antibody (diluted 1:500) was incubated with 500 ng/mL recombinant clusterin protein (R&D Systems-Cedarlane, Burlington, Canada, 2747-HS). The antibody–antigen complex was incubated for 24 h at 4 °C, followed by centrifugation. The supernatant was collected and used as primary antibody for Western blotting (data not shown).
Induction of cellular stress by heat shock
Briefly, coverslips were transferred to 35-mm dishes containing 2 mL of incubation medium (Delbecco’s minimum essential medium enriched with 5 g/L, pH 7.4). Dishes covered and sealed with Parafilm were floated in a 43 °C water bath for 30 min followed by a recovery period in regular culture medium at 37 °C, in a 4 % CO2 humidified incubator. Clusterin expression was assayed by immunocytochemistry after fixation at 1, 6, 24, and 48 h post-heat shock. Cell viability post-heat shock was assessed by Trypan Blue exclusion.
Induction of cellular stress by expression of mutant SOD1
To express human wild-type or mutant SOD1 in astrocytes of spinal cord–DRG cultures, cultures were transfected with pcDNA-SOD1WT or pcDNA-SOD1G93A using LipofectamineTM 2000 Transfection Reagent (Life Technologies) according to manufacturer’s instructions (2 μL lipofectamine per each 50 μL of Opti-MEM medium; 2 μg plasmid/coverslip). Gene transfer into motor neurons was accomplished by intranuclear microinjection of 30 μg/mL plasmid along with a 70-kDa dextran-FITC marker (30 μg/mL; Life Technologies), as previously described (Durham et al. 1997) as they are not amenable to liposome-mediated transfection. Expression of human SOD1 was verified by immunocytochemistry using the SD-G6 anti-SOD1 antibody (Sigma-Aldrich), which does not cross-react with the endogenous murine SOD1.
SOD1G93A transgenic mice
The B6SJL-TgN(SOD1G93A)1Gur mouse line, transgenic for human SOD1 with the ALS-associated mutation, G93A, was maintained in the animal facility at the Montreal Neurological Institute. Mice hemizygous for the transgene were obtained by breeding hemizygous males with nontransgenic B6SJL females, genotyping conducted according to JAX’s protocol. All experiments were approved by the McGill University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care. For this study, approximately three transgenic and three nontransgenic littermates from the same litter were sampled at each of postnatal day (P) 45 (early presymptomatic), P80 (presymptomatic before significant neuronal loss), and at symptomatic stage (>P140). SOD1G93A transgenic mice were designated “symptomatic” by positive hindlimb extensor reflex (Gurney 1994).
For immunohistochemistry, mice were euthanized by CO2 followed by cervical dislocation, frozen in isopentane cooled to −35 to −40 °C and stored at −80 °C. Tissue blocks were prepared containing the lumbar spinal cord with the spinal ganglia, roots and rootlets, nerves, vertebrae, and muscles. For simultaneous processing of tissues from each experimental group, 10-μm-thick coronal sections were mounted on microslides (Fisherbrand Superfrost Plus, cat. 12-550-15) in an array with three sections from each of four mice on each slide (nontransgenic and different aged SOD1G93A transgenic). Antibodies and parameters for fixation and immunohistochemistry are presented in Table 1.
Table 1.
Antibodies and conditions for immunohistochemistry on mouse spinal cord sections
| Primary antibody | Fixation and labeling pattern |
|---|---|
| Goat anti-clusterin-α (Santa Cruz SC-6420 1:50) | Acetone fixation. This antibody labeled aggregates/inclusions in degenerating motor neurons scattered in the ventral spinal cord of symptomatic TgMSOD1, but not in control and non-symptomatic mice. |
| Goat anti-clusterin-α (Santa Cruz, SC-6419 1:50) | No results obtained with this antibody |
| Rabbit anti-αB-crystallin (StressGen, SPA-223 1:300) | 4% paraformaldehyde fixation 60 min: cell bodies with perinuclear immunolabeling and dystrophic dendrites |
| Rabbit anti-SOD1 (assay design cat# 500 100 1:300–1:500) | 4% paraformaldehyde fixation: cell body immunolabeling |
| Acetone fixation: degenerating neuronal somata and proximal dendrites immunolabeled | |
| Mouse monoclonal anti-SOD1 (clone SD-G6 Sigma-Aldrich 1:10; 1:50 1:100) | No results obtained with this antibody |
| Rabbit anti-Iba1 (Waco, 019–197411:300 to 1:600) | 4 % formaldehyde fixation: detectable in microglial cells |
| Rabbit anti-GFAP (Chemicon AB5804 1:1,000) | 4 % formaldehyde fixation: detectable in astrocytes |
Primary antibodies were incubated at 4 °C, overnight. Biotinylated secondary antibodies (1:100) were used for single labels. For colocalization studies, secondary antibodies were donkey anti-goat Alexa Fluor 488 (Life Technologies) diluted 1:150 and donkey anti-rabbit Alexa Fluor 555 (Life Technologies) diluted 1:100
Statistical analysis
Statistical significance was assessed using a two tailed t test, one-way ANOVA, or two-way factorial ANOVA for independent samples. Results were considered significant at p < 0.05. Culture experiments were completed in triplicate and results verified in one to two additional culture batches.
Results
Clusterin expression in motor neurons and astrocytes
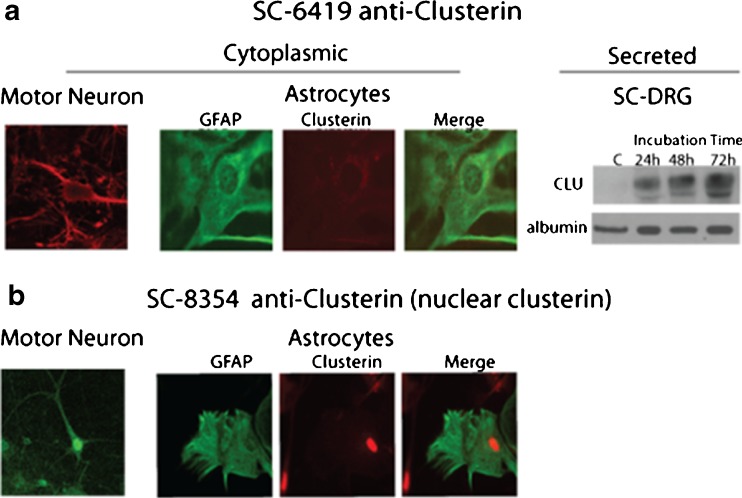
Intracellular distribution and secretion of clusterin were evaluated in dissociated mouse spinal cord–DRG cultures using two commercially available antibodies (Santa Cruz SC-6419 and Santa Cruz SC-8354). Clusterin immunolabeling in situ varied depending on the antibody used, SC-6419 detecting cytoplasmic clusterin (Fig. 1a) and SC-8354 detecting nuclear clusterin (Fig. 1b). Astrocytes were identified by double labeling with antibody to GFAP, a classic astrocytic marker (Fig. 1a, b). Motor neurons were identified based on size and morphology, as previously validated by neuronal markers (Roy et al. 1998). Expression of nuclear clusterin in astrocytes and motor neurons was constitutive in apparently healthy cells, contrary to previous reports that nuclear clusterin was associated with a loss of cellular integrity or cell death (Leskov et al. 2003; Yang et al. 2000). Specificity of labeling was demonstrated by preadsorbing antibodies with recombinant clusterin (data not shown).
Fig. 1.
Detection of cytoplasmic, secreted, and nuclear clusterin in spinal cord–DRG cultures maintained 3–6 weeks in vitro. a Antibody SC-6419 (produced in goat) detected cytoplasmic clusterin in motor neurons and astrocytes by indirect immunocytochemistry, as well as clusterin secreted into serum-free culture medium by Western analysis. Secondary antibody was cy3-conjugated anti-goat IgG. Motor neurons were identified by their characteristic morphology and astrocytes by double label with rabbit anti-GFAP visualized by cy2-conjugated anti-rabbit IgG. Medium was collected at 24, 48, and 72 h after exchange, clarified by centrifugation, and concentrated using 10-kDa-cut-off Nanosep tubes prior to SDS-PAGE/Western analysis. Albumin, a constituent of the culture medium, was used as loading control. b Antibody SC-8354 (produced in rabbit) detected only nuclear clusterin. Secondary antibody was cy3-anti-rabbit IgG. Specificity of both antibodies was validated by standard preabsorption with recombinant protein (not shown)
Clusterin is a secreted protein and serves as an extracellular chaperone. Western blot analysis showed that antibody SC-6419 also detected clusterin secreted from spinal cord–DRG cells, accumulating in the culture medium collected at 24, 48, and 72 h of incubation in fresh, serum-free medium (Fig. 1a). Serum-free medium was used because serum contains substantial amounts of clusterin (data not shown).
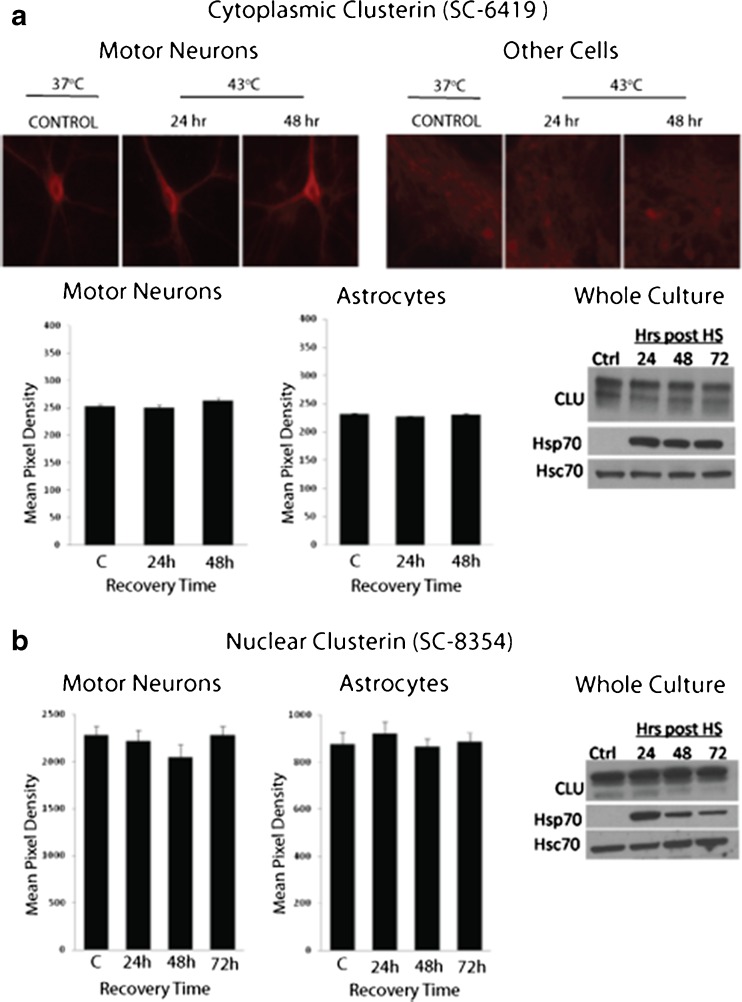
Clusterin expression was not induced by heat shock
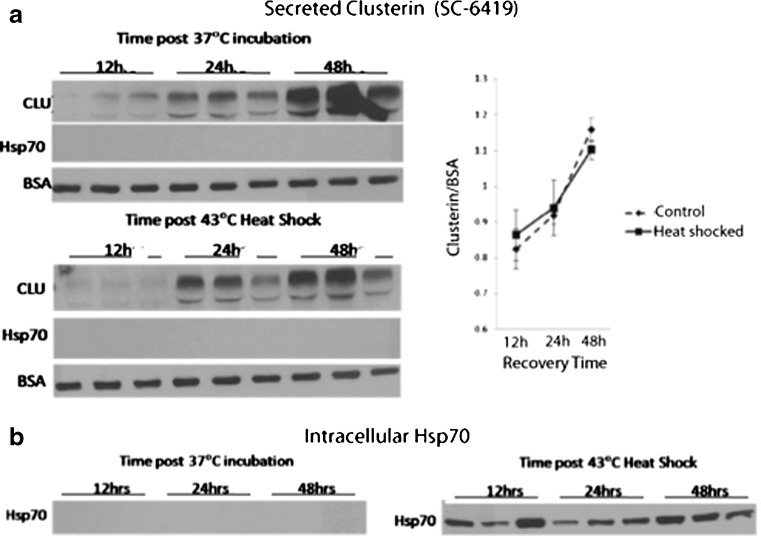
Heat shock induces expression of HSPs (Hsp70 and Hsp40) in astrocytes, but not in motor neurons, of dissociated spinal cord cells (Batulan et al. 2003). To determine the effect of heat shock on clusterin expression, mouse spinal cord–DRG cultures were heat shocked (shown are results at 43 °C for 30 min) and assessed at recovery times up to 72 h. Clusterin levels specifically in motor neurons and non-neuronal cells were measured in cultures immunolabeled with anti-clusterin (SC-6419 or SC-8354). Expression was quantified as the mean pixel density/unit area of epifluorescence of antibody labeling in defined regions of interest, as described in “Materials and methods.” Heat shock failed to induce expression of clusterin in either cell type (Fig. 2). This finding was validated on Western blots of total culture lysates, showing increase in expression of stress-inducible Hsp70 (positive control) but not clusterin or constitutively expressed Hsc70 (commonly used as a negative loading control for shock experiments (Didelot et al. 2008)). Thus, clusterin protein levels in mouse spinal cord–DRG cultures were not increased following induction of cellular stress by heat shock nor did heat shock increase clusterin secretion (Fig. 3). The amount of clusterin in the medium harvested at 24, 48, or 72 h post-heat shock (30 min at 43 °C), normalized to BSA (a component of the culture medium), increased with the duration of incubation to a similar extent as in cultures maintained at 37 °C (p > 1, two-way factorial ANOVA for independent samples). Although secretion of Hsp70 has been demonstrated in several cell lines (Broquet et al. 2003; Guzhova et al. 2001; Hightower and Guidon 1989), no Hsp70 was detected in medium from spinal cord–DRG cultures either constitutively or after heat shock, assessed by Western analysis of concentrated medium. This was despite robust heat shock-induced expression within cells (p = 1, two-way factorial ANOVA for independent samples) (Fig. 3b). These results were confirmed using the dot blot technique, with sensitivity to readily detect recombinant protein above background at 0.011 μg/mL (Online resource 1).
Fig. 2.
Sublethal heat shock does not increase clusterin levels in motor neurons or astrocytes, or generally in spinal cord–DRG cultures. a Immunolabeling of cytoplasmic clusterin in motor neurons and astrocytes using antibody SC-6419. Cultures were maintained at 37 °C for 24 or 48 h after heat shock at 43 °C for 30 min. Graphs show quantitation of clusterin levels in each cell type as mean pixel density of fluorescence of cy3-anti-goat IgG. Shown are means ± SEM, three cultures per condition and minimum of 20 cells per culture. Levels of clusterin in whole cultures were measured by SDS-PAGE/Western analysis, using stress inducible Hsp70 (HspA1) as a positive control for heat shock and Hsc70 (HspA8) as a negative/loading control. b Experiments conducted using antibody to nuclear clusterin (SC-8354) showing no change in expression with heat shock (p > 0.05, one-way ANOVA)
Fig. 3.
a Sublethal heat shock does not increase secretion of clusterin or Hsp70 from spinal cord–DRG cultures. Cultures were heat shocked at 43 °C for 30 min, while control cultures were maintained at 37 °C. Cultures were incubated in serum-free medium, which was collected after 12, 24, or 48 h of recovery at 37 °C. Media were clarified by centrifugation, concentrated using 10-kDa-cut-off Nanosep tubes and subjected to SDS-PAGE/Western analysis using antibody SC-6419, which recognizes secreted clusterin. Graphed is mean pixel density ± SEM of clusterin bands relative to BSA (a component of the culture medium as loading control). Although Hsp70 (HspA1) was assessed potentially as a positive control, no Hsp70 was detected in media from heat-shocked cultures, despite robust intracellular expression in cells of the same heat-shocked cultures, as shown in b. (p = 1; two-way factorial ANOVA for independent samples). Note, samples of cultures maintained at 37 °C and heat shocked at 43 °C shown in b were processed on the same blots
These results confirm that clusterin is constitutively secreted from spinal cord–DRG cultures and demonstrate that heat shock-induced cellular stress does not increase levels of cell-associated or secreted clusterin.
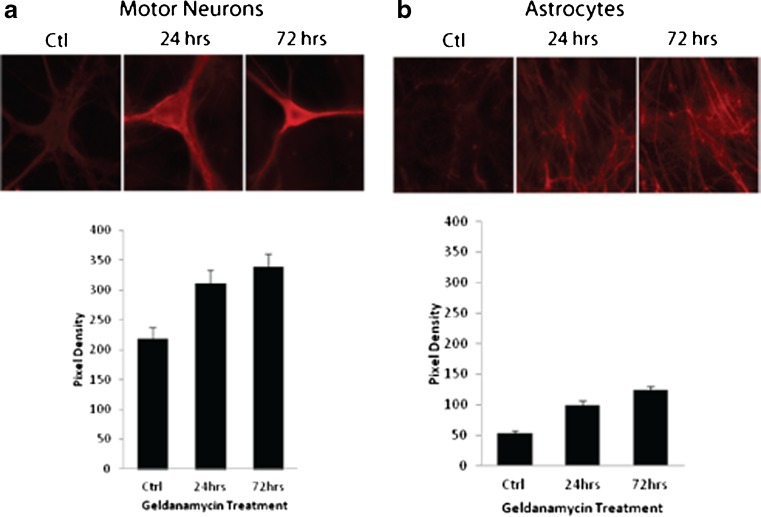
Clusterin expression is induced by the Hsp90 inhibitor, geldanamycin, in motor neurons and non-neuronal cells in spinal cord–DRG cultures
Geldanamycin is an Hsp90 inhibitor thought to induce HSPs by releasing the heat shock transcription factor 1 (Hsf1) from Hsp90 complexes in the cytosol, thereby facilitating translocation to the nucleus (Voellmy 2004). In addition to the classical heat shock element (HSE), Hsf1 also binds to the CLE promoter element of the clusterin gene because of its close homology to HSE (Loison et al. 2006; Wilson and Easterbrook-Smith 2000). Previous studies by our lab showed that treating spinal cord–DRG cultures with 0.01 μM geldanamycin induces expression of Hsp70 and Hsp40 in all cell types, including motor neurons (Batulan et al. 2003, 2006). To determine if this HSP inducer would increase clusterin expression, cultures were treated with 0.01 μM geldanamycin for 24, 48, or 72 h. Cultures were then immunolabeled with the SC-6419 anti-clusterin antibody, and protein levels were estimated by quantifying pixel density of secondary antibody fluorescence in motor neurons and astrocytes. Cultures were immunolabeled for Hsp70 (HspA1) expression as a positive control. Both clusterin and Hsp70 were upregulated by geldanamycin treatment (Fig. 4). Clusterin levels were roughly 1.5-fold higher in motor neurons (Fig. 4a) and twofold higher in astrocytes (Fig. 4b) in geldanamycin-treated cultures relative to untreated cultures (p < 0.0005; one-way ANOVA). These experiments point to different mechanisms of control on expression of clusterin and Hsp70 according to the stressful stimulus and cell type.
Fig. 4.
The Hsp90 inhibitor, geldanamycin, increases expression of clusterin in a motor neurons and b astrocytes of spinal cord–DRG cultures. Cultures were treated with 0.01 μM geldanamycin or vehicle for 24–72 h and then fixed and immunolabeled with antibody SC-6419 against cytoplasmic clusterin, using Cy3 conjugated anti-goat IgG as secondary antibody. Graphs show mean ± SEM of pixel density of fluorescence in three cultures (at least 20 neurons per culture) (p < 0.0005, one-way ANOVA)
Clusterin was not increased in cultured motor neurons or astrocytes expressing the ALS-associated mutant protein, SOD1G93A
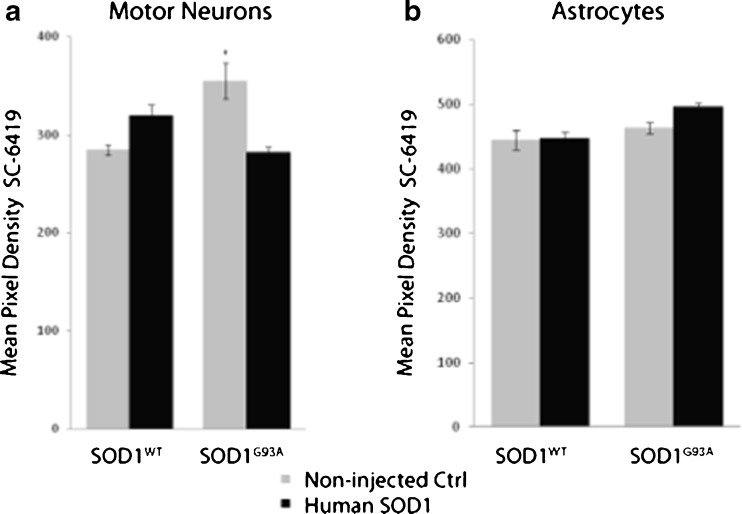
Our particular interest in understanding stress-induced upregulation of proteins with chaperoning activity relates to the potential therapy of neurodegenerative disorders involving protein misfolding. Thus, we investigated the effect of an ALS-associated mutant protein on clusterin expression, specifically dominantly inherited mutations in SOD1 (Rosen et al. 1993). Human SOD1wt or SOD1G93A was expressed in motor neurons by intranuclear microinjection of plasmid expression vector. Three days later, cultures were immunolabeled with anti-clusterin and anti-human SOD1 primary antibodies. Expression of cytoplasmic clusterin was measured 3 days after plasmid microinjection. Injected cells were identified by the presence of a co-injected 70-kDa dextran-FITC marker, and expression of the transgene was verified by immunolabeling with antibody recognizing human but not mouse SOD1 (SD-G6). Semi-quantitation of clusterin levels was accomplished by measuring the mean pixel density of epifluorescence in motor neuron cell bodies as described in “Materials and methods.” Mean clusterin levels in SOD1WT- or SOD1G93A-expressing cells was not increased, but decreased, relative to non-injected cells on the same coverslip (Fig. 5a). Thus, neither SOD1WT nor SOD1G93A increased clusterin expression in cultured motor neurons.
Fig. 5.
Expression of mutant SOD1 associated with familial ALS does not upregulate clusterin in motor neurons or astrocytes of spinal cord-DRG cultures. a Human SOD1G93A, or SOD1WT as control, was expressed in motor neurons by intranuclear microinjection of plasmid expression vector. b Astrocytes were transfected with the same vectors using lipofectamine (which does not transfect motor neurons). Expression of mutant protein was verified by double labeling with antibody that recognizes human, but not mouse, SOD1 (not shown). Clusterin expression was assessed by double-label with antibody SC-6419 using Cy3-conjugated secondary antibody and quantified as pixel density of fluorescence. Graphed is mean pixel density of clusterin signals ± SEM from neurons in three cultures per condition (minimum 20 neurons per culture). Fluorescence in non-injected neurons on the same culture was also quantified as control
Clusterin is widely expressed in the central nervous system including in glial cells (Murakami et al. 1988; Pasinetti et al. 1994). Previous reports indicate that clusterin is upregulated in astrocytes in conditions of cellular stress and in Alzheimer’s disease (Danik et al. 1993; Giannakopoulos et al. 1998). To assess whether expression of mutant SOD1 would increase clusterin expression in astrocytes, mouse spinal cord–DRG cultures were transfected with plasmid encoding mutant human SOD1G93A or SOD1WT. As expected, motor neurons were not transfected, but expression of human SOD1 was detected in a number of astrocytes, identified by double labeling with anti-GFAP. Protein levels were estimated by mean pixel density of epifluorescence following indirect immunocytochemistry with SC-6419 anti-clusterin on day 3 post-transfection. No significant difference in fluorescence intensity between SOD1WT or SOD1G93A transfected and non-transfected astrocytes on the same coverslips was observed (Fig. 5b). These results indicate that expression of mutant SOD1G93A per se had no effect on clusterin protein levels in astrocytes of long-term dissociated spinal cord–DRG cultures.
Clusterin is a constituent of mutant SOD1 inclusions in symptomatic SOD1G93A transgenic mice
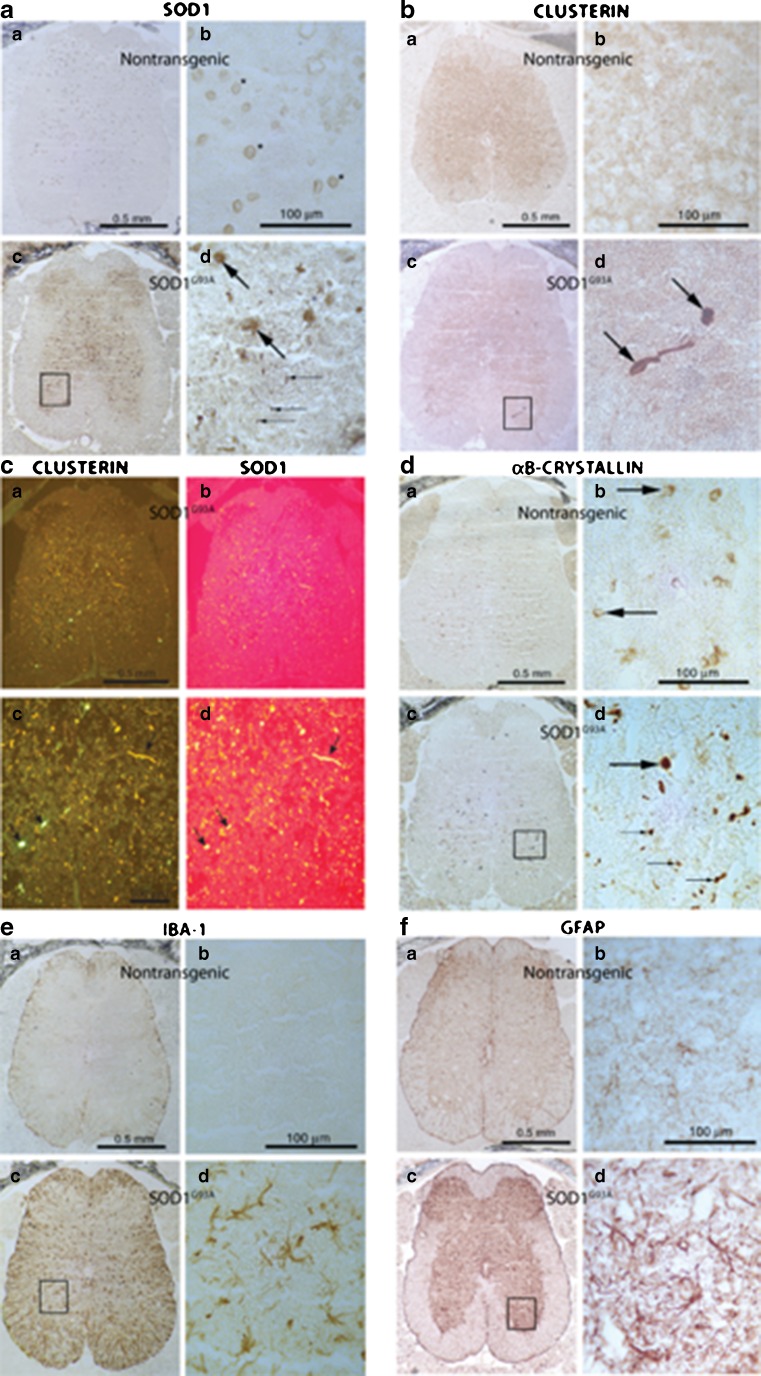
Transgenic mice hemizygous for the SOD1G93A transgene were used to investigate the effect of mutant SOD1 on clusterin expression in vivo. Lumbar spinal cords from transgenic mice and nontransgenic littermates were examined at ages corresponding to different stages of pathogenesis (Fig. 6, Table 2): early presymptomatic (P45), late presymptomatic, just prior to motor neuron loss (P75–80), and overtly symptomatic, as determined by abnormal hind limb extension reflex (>P140) (Gurney et al. 1994). Expression of cytoplasmic clusterin was assessed by immunohistochemistry with SC-6419 antibody and compared to expression and distribution of the following proteins: SOD1 (focusing on presence in inclusions in motor neurons, a pathological hallmark of toxicity), αB-crystallin (a small HSP upregulated by stress), Iba1 (a marker of microglial activation), and GFAP (a marker of astrocyte activation). Iba1 and GFAP are markers of disease progression in ALS and other neurodegenerative disorders. Table 2 summarizes the relative intensity of immunolabeling of these proteins in lumbar spinal cord sections according to age, symptoms, and genotype. Immunoreactivity of clusterin was weakly increased in sections from overtly symptomatic SOD1G93A mice (>P140), predominantly in dystrophic structures scattered over gray matter, similar to αB-crystallin. GFAP and Iba1 labeling were strongly increased as typically found in these mice.
Fig. 6.
Expression of clusterin in lumbar spinal cord of symptomatic mice transgenic for mutant (G93A) SOD1 in comparison to other markers of disease progression. Mice were assessed symptomatic by abnormal hindlimb splay reflex. WT SOD1 transgenic mice were analyzed as a control for overexpression of SOD1. Shown are both low magnification images and higher magnification of specific regions. 10 μm coronal cryostat sections from symptomatic aged mice (>140 days postnatal) were immunolabeled with antibodies to a and c b and d SOD1, b and c a and c clusterin (SC- 6420), d the small heat shock protein, αB-crystallin, e the microglial marker, Iba1, and f the astroglial marker, GFAP. Conditions for fixation and immunolabeling were optimized for each antibody (see Table 1). In a, b, d, e, and f, biotinylated secondary antibodies were used and developed using the peroxidase method. In c, fluorescent secondary antibodies were used. Arrows in a d point to inclusion bodies in sections from SOD1G93A mice immunolabeled by anti-SOD1, a pathological hallmark. b d Clusterin antibody strongly labeled inclusions in sections of lumbar spinal cord from these mice; c d labeling of sections with both antibodies revealed coexistence of clusterin and SOD1 in the same inclusions. d Inclusions positive for αB-crystallin were also present (arrows in d d). Antibodies against Iba1 (e) and GFAP (f) revealed activation of microglia and astrocytes, respectively, in SOD1G93A sections, a known pathological feature of disease in these mice. Characterization of abnormalities observed at different stages of disease are presented in Table 2
Table 2.
Intensity of immunolabeling of lumbar spinal cord gray matter in sections from SOD1G93A transgenic mice (+) and nontransgenic littermates (−)
| Mouse number | Age (days) | Sex | B.W. (g) | G93A mutant | Symptoms | Clusterin | αB-crystallin | GFAP | Iba1 |
|---|---|---|---|---|---|---|---|---|---|
| Early presymptomatic age | |||||||||
| 274/6 | 45 | M | 24 | − | − | − | − | ++ | + |
| 274/7 | 45 | M | 22 | − | − | − | − | ++ | − |
| 274/5 | 45 | M | 22 | + | − | − | − | ++ | +++ |
| 274/4 | 45 | M | 20 | + | − | − | − | ++ | + |
| 274/8 | 45 | M | 24 | + | − | − | − | ++ | + |
| Late presymptomatic age | |||||||||
| 273/3 | 79 | F | 18 | − | − | − | − | ++ | + |
| 273/4 | 79 | M | 31 | − | − | − | − | ++ | + |
| 273/5 | 79 | M | 30 | − | − | − | − | ++ | − |
| 273/1 | 79 | F | 20 | + | − | − | − | +++ | ++ |
| 273/2 | 79 | F | 22 | + | − | − | − | ++++ | + |
| 273/6 | 79 | M | 26 | + | − | − | − | +++ | + |
| Overtly symptomatic age | |||||||||
| 267/3 | 141 | M | 32 | − | − | − | − | ++ | + |
| 256/3 | 191 | F | 19 | − | − | − | − | ++ | − |
| 262/5 | 179 | M | 44 | − | − | − | − | +++ | − |
| 260/4 | 164 | M | 32 | − | − | − | − | ++ | − |
| 256/4 | 191 | F | 22 | + | − | − | + | ++++ | +++ |
| 267/4 | 141 | M | 23 | + | y | ++ | ++ | +++++ | ++++ |
| 260/3 | 164 | F | 21 | + | y | ++ | +++ | +++++ | +++++ |
| 262/4 | 179 | M | 34 | + | y | ++ | ++ | +++++ | ++ |
B.W. body weight, + very weak, ++ weak, +++ medium, ++++ strong, +++++ very strong immunoreaction
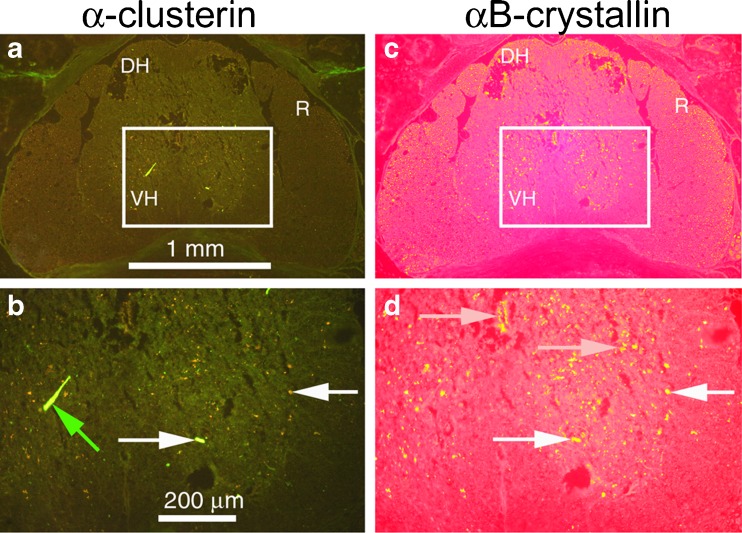
The most notable clusterin abnormality was strong labeling of inclusions in lumbar spinal cord of symptomatic SOD1G93A transgenic mice (Fig. 6b). Clusterin-containing inclusions were co-labeled by antibody to SOD1 (Fig. 6c), but this labeling was distinct from the distribution of Iba1 (Fig. 6e) and GFAP (Fig. 6f). Clusterin and αB-crystallin did colocalize in some but not all inclusions (Fig. 7).
Fig. 7.
Clusterin colocalizes with αB-crystallin in some but not all inclusions in lumbar spinal cord of symptomatic SOD1G93A transgenic mice; 10 μm coronal cryostat sections through the lumbar spinal cord were labeled with both goat anti-clusterin (SC-6420, green) and rabbit anti-αB-crystallin (red). b and d are higher magnifications of boxed regions of images in a and c
In summary, clusterin level was slightly increased in lumbar spinal cord of SOD1G93A transgenic mice, but only during the period of neuronal death, reactive gliosis, and overt symptomatology, and clusterin was a constituent of mutant SOD1 inclusions.
Discussion
Protein chaperones are relevant to diseases associated with protein misfolding and aggregation, prompting the search for chemical compounds to upregulate these networks therapeutically. Our particular interest is in disorders of motor neurons and peripheral nerve. Cell types, including spinal motor neurons and astrocytes, have different sensitivities to stress-induced upregulation of HSPs (Batulan et al. 2003; Kalmar et al. 2002; Manzerra and Brown 1992, 1996; Robinson et al. 2005). Clusterin is a secreted chaperone that can also be regulated by the heat shock transcription factor, Hsf1. This study evaluated clusterin expression in long-term cultures of spinal cord–DRG cultures. Both nuclear and cytoplasmic/secreted forms of clusterin were constitutively expressed in motor neurons and astrocytes, being differentially recognized by two commercial antibodies (SC-6419 and SC-8354, validated by preadsorption with recombinant clusterin) (Fig. 1). Both similarities and differences were identified in constitutive and stress-induced expression of clusterin in these cell types in long-term primary culture and in comparison to findings in proliferating cell lines.
Previous studies in cell lines have implicated nuclear clusterin in cell death (Leskov et al. 2003; Yang et al. 2000); however, this was not the case in primary cultured spinal cord cells, which constitutively express clusterin in the nucleus (this study), or in rat brain following excitotoxic, deafferenting, or ischemic lesions (May et al. 1992). Furthermore, sublethal heat shock had no effect on clusterin levels in (Fig. 2) or secretion from (Fig. 3) cells in spinal cord–DRG cultures, despite it being considered a stress-inducible protein (Fig. 3). Heat shock has induced clusterin in other systems including A431, MCF1, and rat sertoli cells (Clark and Griswold 1997), but with variable speed and sensitivity. Clusterin levels in PNT1A or PC-3 cells were sensitive to imminent cell death but did not correlate with heat shock per se (Caccamo et al. 2006). In the Caccamo study, clusterin accumulated intracellularly following nonlethal heat shock due to inhibition, rather than increase, of secretion; however, in spinal cord–DRG cultures, neither intracellular nor secreted clusterin levels increased following thermal stress. Cells in dissociated spinal cord–DRG cultures have several properties that could influence clusterin response to heat shock. They are primary cells and a mix of cell types; they are not dividing, being either postmitotic or contact-inhibited for a prolonged period. Thus, not only cell type but also mitotic status or cell–cell contact could influence clusterin regulation.
Expression of clusterin in heat-shocked motor neurons and astrocytes did not perfectly mirror stress-inducible Hsp70 (HspA1), despite both being subject to Hsf1 regulation. In motor neurons, heat shock failed to upregulate either clusterin (this study) or Hsp70 (Batulan et al. 2003). Although astrocytes in spinal cord–DRG cultures do show a robust response to heat shock by upregulating Hsp70 (Batulan et al. 2003), increase in nuclear, cytoplasmic, or secreted clusterin levels was not found, despite strong expression of Hsp70 (Fig. 3).
Secretion of Hsp70 has been described in experiments using a wide variety of cell types (Broquet et al. 2003; Evdokimovskaya et al. 2010; Guzhova et al. 2001; Hightower and Guidon 1989; Mambula and Calderwood 2006) including cultured spinal astrocytes and skeletal muscle (Robinson et al. 2005). Surprisingly, Hsp70 could not be detected in the medium of heat-shocked spinal cord–DRG cultures, despite strong intracellular expression and sensitivity of detection comparable to other studies. Although it is possible that secreted Hsp70 is bound to a more extensive extracellular matrix in these cultures, secreted clusterin was readily detected in culture medium. As discussed for clusterin above, factors such as mitotic status and cell contacts could influence secretion of Hsp70.
Previous work by our lab has shown that Hsp90 inhibitors increase expression of Hsp70 and Hsp40 in motor neurons, although heat shock does not (Batulan et al. 2006). The same exposures to the Hsp90 inhibitor, geldanamycin, induced upregulation of clusterin expression in both motor neurons and astrocytes, in fact generally in cultured spinal cord–DRG cells in parallel with Hsp70 (Fig. 4). Hsp90 inhibitors are thought to free Hsf1 from Hsp90 complexes in the cytoplasm, resulting in its translocation to the nucleus and binding to heat shock elements (HSE) (Voellmy 2004). The CLU element in the clusterin gene promoter has sequence homology with HSE (Wilson and Easterbrook-Smith 2000), but whether geldanamycin upregulates clusterin through Hsf1 is not known. The clusterin promoter also contains an activator protein-1 domain which could be involved (Trougakos and Gonos 2006). Clearly, regulation of HSP and clusterin expression is complex and not completely understood. Even the mechanism by which Hsp90 inhibitors result in Hsf1 activation and HSP expression is unclear (Batulan et al. 2006; Taylor et al. 2007a; Taylor et al. 2007b). Regardless of the mechanism, clusterin upregulation could contribute to the neuroprotective properties of this class of drug. Previous studies from our lab and others have demonstrated effectiveness of Hsp90 inhibitors in models of neurodegenerative disorders including ALS due to mutations in SOD1 (Batulan et al. 2006).
Mutant SOD1 proteins associated with familial ALS are secreted from cells, an important factor in their toxicity (Turner et al. 2005; Urushitani et al. 2006). Clusterin secreted from neurons or astrocytes could be protective by chaperoning misfolded SOD1 in the extracellular space. In addition, clusterin suppresses inflammatory responses through several mechanisms (Falgarone and Chiocchia 2009) and could attenuate neuroinflammation, which is an important process in progression of ALS and other neurodegenerative disorders (Villoslada et al. 2008). Thus, we investigated the effect of mutant SOD1 on clusterin expression in established culture (Fig. 5) and transgenic mouse models of this form of ALS (Figs. 6 and 7). Expression of the mutant protein per se was not sufficient to induce clusterin expression in either model. However, in symptomatic SOD1G93A transgenic mice, weak increase in clusterin immunolabeling was detected in sections of lumbar spinal cord and antibody against cytoplasmic/secreted clusterin strongly labeled mutant SOD1 inclusions, a pathological hallmark of the disease. Increased clusterin mRNA levels have been detected in the motor cortex and spinal cord from cases of sporadic ALS (Grewal et al. 1999) and increase in clusterin has been found in other neurodegenerative disorders as well, including Alzheimer’s disease (Ghiso et al. 1993; Giannakopoulos et al. 1998; Lidstrom et al. 1998; May et al. 1989; Polihronis et al. 1993). Our data in SOD1G93A transgenic mice suggest that increase in clusterin is a late injury response in this model associated with both neuronal degeneration and gliosis.
The identification of clusterin in neuronal inclusions in these mice is consistent with other constituents associated with protein quality control including Hsc70, ubiquitin, and proteasome subunits (Watanabe et al. 2001).
In conclusion, as with other chaperones, regulation of clusterin is complex and variable with cell type and conditions. Its particular role as an extracellular chaperone could be highly relevant to neurological disease, and it should be considered along with HSPs when chaperone-based therapies are being evaluated. When screening for compounds to upregulate these proteins, it is important that the experimental model replicate the control mechanisms operant in the cells and tissue of interest.
Electronic supplementary material
(PDF 120 kb)
Acknowledgments
This work was supported by the Canadian Institutes for Health Research (MOP77743). Immunohistochemistry on mouse spinal cord sections and characterization were conducted by Cytochem, Inc., Montreal, Canada (Dr. Martin Marcinkiewicz, President and CSO) through a fee for service contract. The data obtained in culture models were included in the M.Sc. thesis of S.Z.
References
- Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J, Durham HD. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis. 2006;24:213–225. doi: 10.1016/j.nbd.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande VC, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bonnard AS, Chan P, Fontaine M. Expression of clusterin and C4 mRNA during rat peripheral nerve regeneration. Immunopharmacology. 1997;38:81–86. doi: 10.1016/S0162-3109(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Caccamo AE, Desenzani S, Belloni L, Borghetti AF, Bettuzzi S. Nuclear clusterin accumulation during heat shock response: implications for cell survival and thermo-tolerance induction in immortalized and prostate cancer cells. J Cell Physiol. 2006;207:208–219. doi: 10.1002/jcp.20561. [DOI] [PubMed] [Google Scholar]
- Charnay Y, Imhof A, Vallet PG, Hakkoum D, Lathuiliere A, Poku N, Aronow B, Kovari E, Bouras C, Giannakopoulos P. Clusterin expression during fetal and postnatal CNS development in mouse. Neuroscience. 2008;155:714–724. doi: 10.1016/j.neuroscience.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Clark AM, Griswold MD. Expression of clusterin/sulfated glycoprotein-2 under conditions of heat stress in rat Sertoli cells and a mouse Sertoli cell line. J Androl. 1997;18:257–263. [PubMed] [Google Scholar]
- Danik M, Chabot JG, Hassan-Gonzalez D, Suh M, Quirion R. Localization of sulfated glycoprotein-2/clusterin mRNA in the rat brain by in situ hybridization. J Comp Neurol. 1993;334:209–227. doi: 10.1002/cne.903340205. [DOI] [PubMed] [Google Scholar]
- Debure L, Vayssiere JL, Rincheval V, Loison F, Le DY, Michel D. Intracellular clusterin causes juxtanuclear aggregate formation and mitochondrial alteration. J Cell Sci. 2003;116:3109–3121. doi: 10.1242/jcs.00619. [DOI] [PubMed] [Google Scholar]
- Didelot C, Lanneau D, Brunet M, Bouchot A, Cartier J, Jacquel A, Ducoroy P, Cathelin S, Decologne N, Chiosis G, Dubrez-Daloz L, Solary E, Garrido C. Interaction of heat-shock protein 90 beta isoform (HSP90 beta) with cellular inhibitor of apoptosis 1 (c-IAP1) is required for cell differentiation. Cell Death Differ. 2008;15:859–866. doi: 10.1038/cdd.2008.5. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Preston K, Dodd J, Young D, Lawlor P, Christie D. Clusterin accumulates in dying neurons following status epilepticus. Brain Res Mol Brain Res. 1995;32:279–290. doi: 10.1016/0169-328X(95)00088-A. [DOI] [PubMed] [Google Scholar]
- Durham HD, Roy J, Dong L, Figlewicz DA. Aggregation of mutant Cu/Zn superoxide dismutase proteins in a culture model of ALS. J Neuropathol Exp Neurol. 1997;56:523–530. doi: 10.1097/00005072-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Evdokimovskaya Y, Skarga Y, Vrublevskaya V, Morenkov O. Secretion of the heat shock proteins HSP70 and HSC70 by baby hamster kidney (BHK-21) cells. Cell Biol Int. 2010;34:985–990. doi: 10.1042/CBI20100147. [DOI] [PubMed] [Google Scholar]
- Falgarone G, Chiocchia G. Chapter 8: Clusterin: a multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res. 2009;104:139–170. doi: 10.1016/S0065-230X(09)04008-1. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski T, Frangione B. The cerebrospinal-fluid soluble form of Alzheimer's amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993;293(Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Kovari E, French LE, Viard I, Hof PR, Bouras C. Possible neuroprotective role of clusterin in Alzheimer's disease: a quantitative immunocytochemical study. Acta Neuropathol. 1998;95:387–394. doi: 10.1007/s004010050815. [DOI] [PubMed] [Google Scholar]
- Grewal RP, Morgan TE, Finch CE. C1qB and clusterin mRNA increase in association with neurodegeneration in sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1999;271:65–67. doi: 10.1016/S0304-3940(99)00496-6. [DOI] [PubMed] [Google Scholar]
- Gurney ME. Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med. 1994;331:1721–1722. doi: 10.1056/NEJM199412223312516. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng H-X, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/S0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Burnstock G, Vrbova G, Greensmith L. The effect of neonatal nerve injury on the expression of heat shock proteins in developing rat motoneurones. J Neurotrauma. 2002;19:667–679. doi: 10.1089/089771502753754127. [DOI] [PubMed] [Google Scholar]
- Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278:11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- Lidstrom AM, Bogdanovic N, Hesse C, Volkman I, Davidsson P, Blennow K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer's disease. Exp Neurol. 1998;154:511–521. doi: 10.1006/exnr.1998.6892. [DOI] [PubMed] [Google Scholar]
- Loison F, Debure L, Nizard P, Le GP, Michel D, Le DY. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1-HSF2 heterocomplexes. Biochem J. 2006;395:223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Brown IR. Expression of heat shock genes (hsp70) in the rabbit spinal cord: Localization of constitutive and hyperthermia-inducible mRNA species. J Neurosci Res. 1992;31:606–615. doi: 10.1002/jnr.490310404. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Brown IR. The neuronal stress response: nuclear translocation of heat shock proteins as an indicator of hyperthermic stress. Exp Cell Res. 1996;229:35–47. doi: 10.1006/excr.1996.0341. [DOI] [PubMed] [Google Scholar]
- May PC, Johnson SA, Poirier J, Lampert-Etchells M, Finch CE. Altered gene expression in Alzheimer's disease brain tissue. Can J Neurol Sci. 1989;16:473–476. doi: 10.1017/s0317167100029796. [DOI] [PubMed] [Google Scholar]
- May PC, Robison P, Fuson K, Smalstig B, Stephenson D, Clemens JA. Sulfated glycoprotein-2 expression increases in rodent brain after transient global ischemia. Brain Res Mol Brain Res. 1992;15:33–39. doi: 10.1016/0169-328X(92)90148-5. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ushio Y, Morino Y, Ohta T, Matsukado Y. Immunohistochemical localization of apolipoprotein E in human glial neoplasms. J Clin Invest. 1988;82:177–188. doi: 10.1172/JCI113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizard P, Tetley S, Le DY, Watrin T, Le GP, Wilson MR, Michel D. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8:554–565. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Bellander BM, Langmoen IA, Svensson M. Complement activation following optic nerve crush in the adult rat. J Neurotrauma. 2003;20:895–904. doi: 10.1089/089771503322385827. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Johnson SA, Oda T, Rozovsky I, Finch CE. Clusterin (SGP-2): a multifunctional glycoprotein with regional expression in astrocytes and neurons of the adult rat brain. J Comp Neurol. 1994;339:387–400. doi: 10.1002/cne.903390307. [DOI] [PubMed] [Google Scholar]
- Polihronis M, Paizis K, Carter G, Sedal L, Murphy B. Elevation of human cerebrospinal fluid clusterin concentration is associated with acute neuropathology. J Neurol Sci. 1993;115:230–233. doi: 10.1016/0022-510X(93)90230-V. [DOI] [PubMed] [Google Scholar]
- Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000;39:15953–15960. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]
- Rithidech KN, Honikel L, Milazzo M, Madigan D, Troxell R, Krupp LB. Protein expression profiles in pediatric multiple sclerosis: potential biomarkers. Mult Scler. 2009;15:455–464. doi: 10.1177/1352458508100047. [DOI] [PubMed] [Google Scholar]
- Rizzi F, Coletta M, Bettuzzi S. Chapter 2: Clusterin (CLU): from one gene and two transcripts to many proteins. Adv Cancer Res. 2009;104:9–23. doi: 10.1016/S0065-230X(09)04002-0. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Roy J, Minotti S, Dong L, Figlewicz DA, Durham HD. Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J Neurosci. 1998;18:9673–9684. doi: 10.1523/JNEUROSCI.18-23-09673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SS, Tocco G, Najm I, Baudry M. Seizure activity causes a rapid increase in sulfated glycoprotein-2 messenger RNA in the adult but not the neonatal rat brain. Neurosci Lett. 1993;153:17–20. doi: 10.1016/0304-3940(93)90066-T. [DOI] [PubMed] [Google Scholar]
- Taylor DM, De KP, Minotti S, Durham HD. Manipulation of protein kinases reveals different mechanisms for upregulation of heat shock proteins in motor neurons and non-neuronal cells. Mol Cell Neurosci. 2007;34:20–33. doi: 10.1016/j.mcn.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Tradewell ML, Minotti S, Durham HD. Characterizing the role of Hsp90 in production of heat shock proteins in motor neurons reveals a suppressive effect of wild-type Hsf1. Cell Stress Chaperones. 2007;12:151–162. doi: 10.1379/CSC-254R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40:1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Atkin JD, Farg MA, Zang dW, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–117. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- Van Beek J, Chan P, Bernaudin M, Petit E, MacKenzie ET, Fontaine M. Glial responses, clusterin, and complement in permanent focal cerebral ischemia in the mouse. Glia. 2000;31:39–50. doi: 10.1002/(SICI)1098-1136(200007)31:1<39::AID-GLIA40>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Moreno B, Melero I, Pablos JL, Martino G, Uccelli A, Montalban X, Avila J, Rivest S, Acarin L, Appel S, Khoury SJ, McGeer P, Ferrer I, Delgado M, Obeso J, Schwartz M. Immunotherapy for neurological diseases. Clin Immunol. 2008;128:294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- Wiggins AK, Shen PJ, Gundlach AL. Delayed, but prolonged increases in astrocytic clusterin (ApoJ) mRNA expression following acute cortical spreading depression in the rat: evidence for a role of clusterin in ischemic tolerance. Brain Res Mol Brain Res. 2003;114:20–30. doi: 10.1016/S0169-328X(03)00124-4. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci. 2000;25:95–98. doi: 10.1016/S0968-0004(99)01534-0. [DOI] [PubMed] [Google Scholar]
- Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, Boothman DA. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci U S A. 2000;97:5907–5912. doi: 10.1073/pnas.97.11.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 120 kb)