Abstract
Cigarette smoke extracts (CSE) induce oxidative stress, an important feature in chronic obstructive pulmonary disease (COPD), and oxidative stress contributes to the poor clinical efficacy of corticosteroids in COPD patients. Carbocysteine, an antioxidant and mucolytic agent, is effective in reducing the severity and the rate of exacerbations in COPD patients. The effects of carbocysteine on CSE-induced oxidative stress in bronchial epithelial cells as well as the comparison of these antioxidant effects of carbocysteine with those of fluticasone propionate are unknown. The present study was aimed to assess the effects of carbocysteine (10−4 M) in cell survival and intracellular reactive oxygen species (ROS) production (by flow cytometry) as well as total glutathione (GSH), heme oxygenase-1 (HO-1), nuclear-related factor 2 (Nrf2) expression and histone deacetylase 2 (HDAC-2) expression/activation in CSE-stimulated bronchial epithelial cells (16-HBE) and to compare these effects with those of fluticasone propionate (10−8 M). CSE, carbocysteine or fluticasone propionate did not induce cell necrosis (propidium positive cells) or cell apoptosis (annexin V-positive/propidium-negative cells) in 16-HBE. CSE increased ROS production, nuclear Nrf2 and HO-1 in 16-HBE. Fluticasone propionate did not modify intracellular ROS production, GSH and HDCA-2 but reduced Nrf2 and HO-1 in CSE-stimulated 16-HBE. Carbocysteine reduced ROS production and increased GSH, HO-1, Nrf2 and HDAC-2 nuclear expression/activity in CSE-stimulated cells and was more effective than fluticasone propionate in modulating the CSE-mediated effects. In conclusion, the present study provides compelling evidences that the use of carbocysteine may be considered a promising strategy in diseases associated with corticosteroid resistance.
Keywords: Cigarette smoke, Airway epithelial cells, Reactive oxygen species
Introduction
The airway epithelium is emerging as a regulator of innate immune responses to a variety of insults including cigarette smoke. In this regard, it has been demonstrated that upon cigarette smoke extracts (CSE) stimulation, the airway epithelium is able to release increased concentrations of IL-8, but reduced IFN-gamma-inducible protein 10 (Pace et al. 2008a), thus amplifying the inflammatory and immune responses.
Chronic obstructive pulmonary disease (COPD) is mainly caused by cigarette smoke exposure, and oxidative stress induced by chronic smoke exposure is considered to be a crucial event in the COPD pathogenesis (Brusselle et al. 2011). The inflammatory events present in COPD are largely resistant to corticosteroids (Adcock et al. 2010). Oxidative stress contributes to the low response to corticosteroids through the downregulation of histone deacetylase (HDAC) activity, a crucial event in the trans-repression activities of corticosteroids (Barnes and Adcock 2009; Malhotra et al. 2011; Barnes 2011). HDACs, deacetylating histones which bind to inflammatory gene promoters, limit the access of the transcriptional machinery to these genes, thus repressing their transcription (Barnes 2011).
Carbocysteine (CARB), an antioxidant and mucolytic agent, is effective in reducing the severity and the rate of exacerbations in COPD patients (Zheng et al. 2008). The clinical efficacy of carbocysteine seems to be more related to its antioxidant and anti-inflammatory effects than to its mucolytic activity (Rahman and Macnee 2012). Limited information is available on the comparative effects between carbocysteine and a potent corticosteroid, fluticasone, in cellular models with elevated oxidative stress due to cigarette smoke exposure.
The present work was aimed to understand whether, in bronchial epithelial cells stimulated with CSE, CARB was similar or superior to fluticasone propionate (FP) in controlling oxidative stress and antioxidant responses and whether it was able to modulate histone deacetylase 2 (HDAC-2) expression and activity.
Materials and methods
Preparation of cigarette smoke extracts
Commercial cigarettes (Marlboro) were used in this study. Cigarette smoke solution was prepared as described previously (Su et al. 1998). Each cigarette was smoked for 5 min, and two cigarettes were used per 20 ml of PBS to generate a CSE-PBS solution. The CSE solution was filtered through a 0.22-μm pore filter to remove bacteria and large particles. The smoke solution was then adjusted to pH 7.4 and used within 30 min of preparation. This solution was considered to be 100 % CSE and diluted to obtain the desired concentration in each experiment. The concentration of CSE was calculated spectrophotometrically, measuring the OD as previously described (Luppi et al. 2005) at the wavelength of 320 nm. The pattern of absorbance, among different batches, showed very little differences, and the mean OD of the different batches was 1.37 ± 0.16. The presence of contaminating LPS on undiluted CSE was assessed by a commercially available kit (Cambrex Corporation, East Rutherfort, NJ) and was below the detection limit of 0.1 EU/ml.
Stimulation of epithelial cell lines
16-HBE, an immortalised normal bronchial epithelial cell line (Cozens et al. 1992), RPMI 2650a, a nasal epithelial cell line (Pace et al. 2012a), and a mucin-producing human NCI-H292 airway epithelial cell line (Nogawa et al. 2009) were used in this study.
16-HBE was maintained in MEM (Gibco, BRL, Germany) supplemented with 10 % foetal calf serum (Gibco) and 0.5 % gentamicin (Gibco). Cell cultures were maintained in a humidified atmosphere of 5 % CO2 in air at 37 °C. Cell lines were cultured as previously reported in the presence of CSE (5 and 10 %; Pace et al. 2008a) and in the presence and absence of FP (10−4, 10−7, 10−8 and 10−9 M; Sigma-Aldrich, St. Louis, MO) and CARB (10−3, 10−4, 10−5 and 10−8 M; Dompè, Italy; Garavaglia et al. 2008). Three time points were initially tested: 2, 4 and 18 h. CARB was added 1 h before CSE cell stimulation. RPMI 2650 and H292 were cultured for 18 h in the presence of CSE (10 %) and in the presence and absence of FP (10−8 M; Sigma-Aldrich) and of CARB (10−4 M), respectively. At the end of stimulation, cells were collected for further evaluations. Three replicates were performed for each experiment.
Cell necrosis and cell apoptosis
Cell necrosis and cell apoptosis were evaluated, as previously described (Pace et al. 2007), by staining with annexin V–fluorescein isothiocyanate and propidium iodide (PI) using a commercial kit (Bender MedSystem, Vienna, Austria) following the manufacturer’s directions. Cells were analysed using a FACS Calibur (Becton Dickinson, Mountain View, CA) analyser equipped with an argon ion laser (Innova 70 Coherent) and Consort 32 computer support. The PI-negative and annexin V-negative cells (i.e. viable cells) were present in the lower left quadrant, the PI cells (i.e. necrotic cells) were present in the upper left quadrant, the PI and annexin V double-positive cells (i.e. late apoptotic cells) were present in the upper right quadrant, and the single annexin V-positive cells (i.e. early apoptotic cells) were present in the lower right quadrant.
Analysis of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) were measured by the conversion of the non-fluorescent dichlorofluorescein diacetate (DCFH-DA; Sigma) in a highly fluorescent compound, DCF, by monitoring the cellular esterase activity in the presence of peroxides, as previously described (Bruno et al. 2011). ROS generation was assessed by the uptake of 1 μM DCFH-DA, incubation for 10 min at room temperature in the dark, followed by flow cytometric analysis.
Measurement of cellular glutathione content
Intracellular total glutathione (GSH) content was assessed in cell extracts as previously reported (Rahman et al. 2006). Briefly, cell extracts were prepared in 0.1 M potassium phosphate extraction buffer containing 0.6 % (w/v) sulfosalicylic acid, 0.1 % (v/v) Triton X-100 and 5 mM EDTA. After harvesting and resuspension in the extraction buffer, cells were sonicated in ice-cold water and underwent two cycles of freezing and thawing. Supernatants/extracts were collected by centrifugation and used for the following colorimetric assay: 10 μl of the extracts was incubated in the presence of 60 μl 0.6 mg/ml 5,5′-dithiobis(2-nitrobenzoic acid) and of 60 μl of 250 U/ml glutathione reductase for 30 s at RT; 50 μl of 0.6 mg/ml β-NADPH was added and 2-nitro-5-thiobenzoic acid formation was immediately evaluated by measuring the absorbance at 412 nm in a microplate reader. The concentration of GSH in the cell extracts was calculated using a standard curve, normalised for the total protein content and expressed as nanomoles per milligram protein.
Western blot
The expression of heme oxygenase-1 (HO-1), nuclear-related factor 2 (Nrf2) and HDAC-2 was evaluated by western blot analysis as previously described (Pace et al. 2012b). The concentration of proteins in cell lysates was measured using the Bradford assay. The following primary antibodies were used: rabbit polyclonal anti-human HO-1 (Assay Design, SPA-896, Anna Arbor, MI), rabbit polyclonal anti-human Nrf2 and mouse anti-human HDAC-2 (sc-81599, Santa Cruz) and rabbit anti-ß-actin (Sigma). To study Nrf2 and HDAC-2 nuclear translocation, the protein extracts were treated to separate the cytoplasmic and nuclear protein fractions as previously described (Pace et al. 2008b) by using a commercial kit following the manufacturer’s directions (Pierce, Rockford, IL). Secondary anti-rabbit and anti-mouse antibodies were purchased from Sigma. Revelation was performed with an enhanced chemiluminescence system (GE Healthcare, Chalfont St. Giles, UK) followed by autoradiography. Beta-actin (Sigma) was used as the housekeeping protein to normalise differences in protein loading.
Immunoprecipitation and HDAC-2 activity
HDAC-2 activity was assessed as previously described (Malhotra et al. 2011). HDAC2 was immunoprecipitated from cell lysates (100 μg) by overnight incubation with anti-HDAC-2 at 4 °C and then incubated with protein G-agarose beads.
Beads were extensively washed with lysis buffer and resuspended in reducing sample buffer for western blot analysis. HDAC-2 activity was measured using a commercial kit (Enzo Lifesciences, Farmingdale, NY, USA). An acetylated lysine substrate is incubated with samples with HDAC activity. Deacetylation sensitises the substrate such that treatment with the HDAC developer in the second step releases a fluorescent product. The assay was performed exactly as recommended by the manufacturer. Fluorescence was measured in a microplate reader using Ex 355 and Em 460.
Statistics
Data are expressed as the mean counts ± standard deviation. Comparison between different experimental conditions was evaluated using paired t test. A value of P <0.05 was accepted as statistically significant.
Results
Effects of CSE on ROS production in bronchial epithelial cells
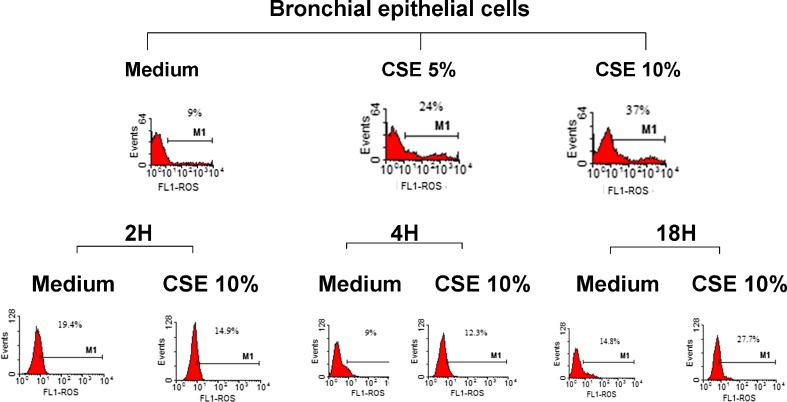
The sources of the increased oxidative stress in COPD patients derive from the increased burden of inhaled oxidants such as cigarette smoke and from the increase in ROS generated by several inflammatory, immune and structural airways cells (Faux et al. 2009). We tested the effect of CSE on ROS production in bronchial epithelial cells at three different time points (2, 4 and 18 h) to test whether a short- or a long-term incubation was suitable for evaluating ROS formation. The highest ROS production was observed with CSE 10 % and at 18 h (Fig. 1). We then selected CSE 10 % and 18 h of incubation for assessing ROS in the presence of CARB and FP.
Fig. 1.
Effects of CSE in ROS production in bronchial epithelial cells. 16-HBE cells were cultured in the presence and absence of CSE (5 and 10 %) for 2, 4 and 18 h and then were used for assessing ROS production using flow cytometry (see “Materials and methods” for details). Representative histogram plots from two experiments are shown
Effects of FP and CARB on ROS formation in bronchial epithelial cells
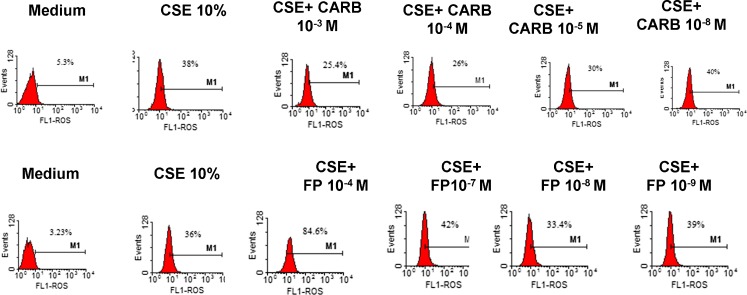
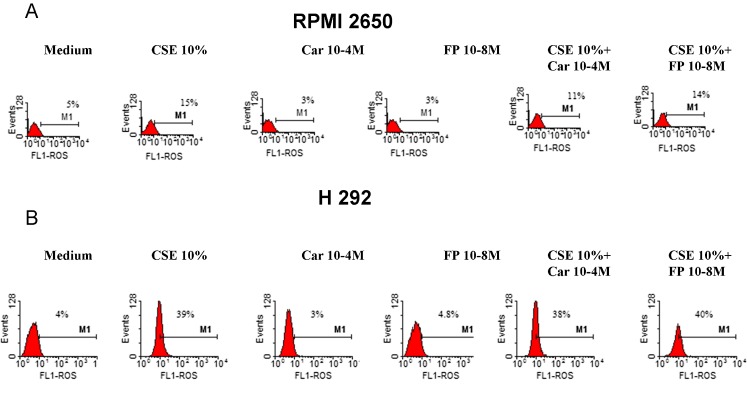
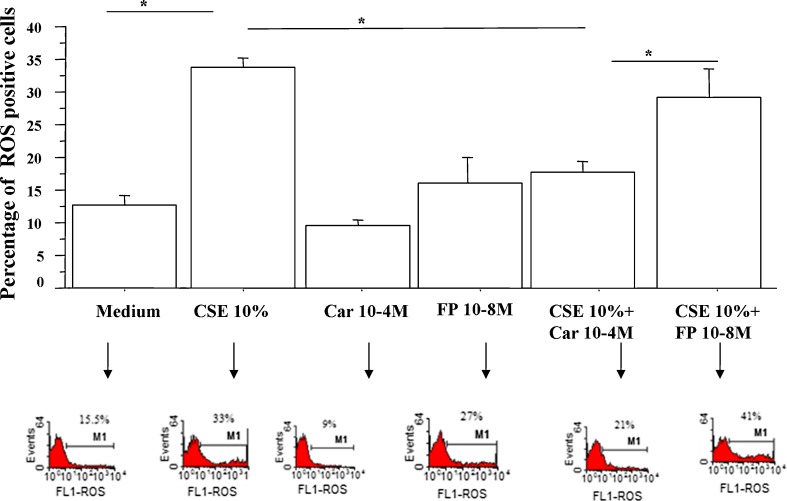
We next tested the effects of FP and carbocysteine on ROS formation in bronchial epithelial cells. In a preliminary dose–response experiment, three different concentrations of CARB (10−3, 10−4, 10−5 and 10−8 M) and of FP (10−4, 10−7, 10−8 and 10−9 M) were tested. Since the obtained results did not show any relevant difference between CARB concentrations of 10−3 and 10−4 M (Fig. 2), the lower concentration of CARB of 10−4 M was selected. At all the tested concentrations, FP was unable to limit ROS production. On the contrary, the concentration of FP of 10−4 M induced relevant ROS production. The concentration of FP of 10−8 M was selected because it was associated with the lowest ROS production. The effect of CARB and FP on ROS formation in a nasal epithelial cell line (RPMI 2650) and in another airway epithelial cell line (H292) was also tested. The effect of CARB (10−4 M, for 18 h) on ROS formation in RPMI 2650 and in H292 was modest (Fig. 3a, b), and we decided to continue the study on the bronchial epithelial cell line because it was more sensitive to CARB effects. In bronchial epithelial cells, CARB significantly reduced CSE-induced ROS production, and this effect was significantly higher than the effect exerted by FP (Fig. 4).
Fig. 2.
Dose–response experiments for CARB and FP. 16-HBE cells were cultured in the presence and absence of CSE (10 %), CARB (10−3, 10−4, 10−5 and 10−8 M) and FP (10−4, 10−7, 10−8 and 10−9 M) for 18 h and then were used for assessing ROS production using flow cytometry (see “Materials and methods” for details). Representative histogram plots from two experiments are shown
Fig. 3.
Effects of CARB and FP on ROS production in nasal and airway epithelial cells. Nasal epithelial cells (RPMI 2650) (a) and airway epithelial cells (H292, n = 2) (b) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h and then were used for assessing ROS production using flow cytometry (see “Materials and methods” for details). Representative histogram plots are shown
Fig. 4.
Effects of CARB and FP on ROS production in bronchial epithelial cells. 16-HBE (n = 6) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h and then were used for assessing ROS production using flow cytometry (see “Materials and methods” for details). Data are expressed as the percentage of ROS-positive cells ± SD. Representative histogram plots are also shown
Effects of CSE, FP and CARB on necrosis and apoptosis of bronchial epithelial cells
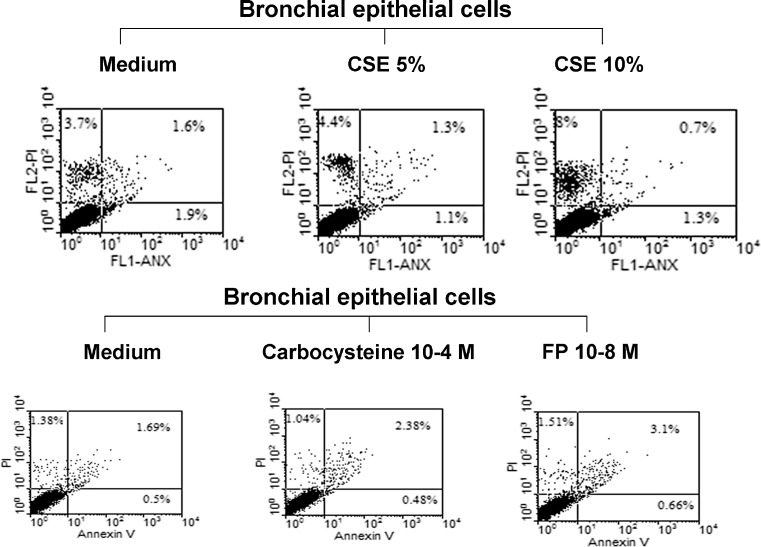
We tested whether, at the used concentrations, CSE (5 and 10 %), FP (10−8 M) and CARB (10−4 M) induced cell apoptosis or necrosis in bronchial epithelial cells using the PI/annexin V binding method. Neither CSE (5 or 10 %) nor FP (10−8 M) or CARB (10−4 M) induced relevant numbers of necrotic (PI-positive) or apoptotic (annexin V-positive) cells (Fig. 5).
Fig. 5.
Effects of CSE, CARB and FP on the necrosis and apoptosis of bronchial epithelial cells (16-HBE). 16-HBE cells were cultured in the presence and absence of CSE (5 and 10 %), CARB (10−4 M) and FP (10−8 M) for 18 h and then were used for assessing cell necrosis and cell apoptosis by the annexin V/PI method using flow cytometry (see “Materials and methods” for details). Representative dot plots showing the percentage of annexin V, PI and annexin V/PI-positive 16-HBE at baseline and following the exposure of CSE (5 and 10 %), CARB (10−4 M) and FP (10−8 M) are shown
Effects of FP and CARB on GSH expression in bronchial epithelial cells
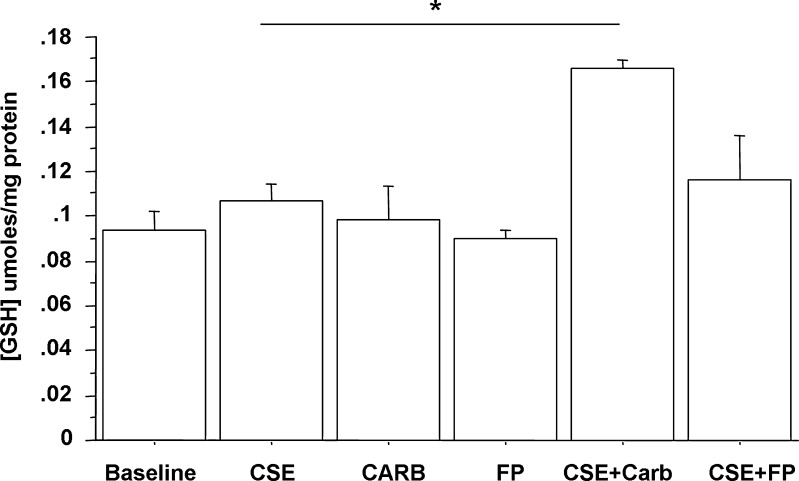
GSH is one of the most important defensive mechanisms against oxidative stress (Biswas and Rahman 2009; Ghezzi 2011). The effect of CARB and FP on GSH expression in CSE-stimulated bronchial epithelial cells was explored. CSE, CARB and FP alone did not significantly increase GSH expression in bronchial epithelial cells. CARB, but not FP, significantly increased GSH expression in CSE-stimulated bronchial epithelial cells (Fig. 6).
Fig. 6.
Effects of CARB and FP on GSH expression in bronchial epithelial cells. 16-HBE (n = 6) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h and then were used for assessing GSH content (see “Materials and methods” for details). Data are expressed as micromoles GSH per milligram proteins ± SD
Effects of FP and CARB on HO-1 expression in bronchial epithelial cells
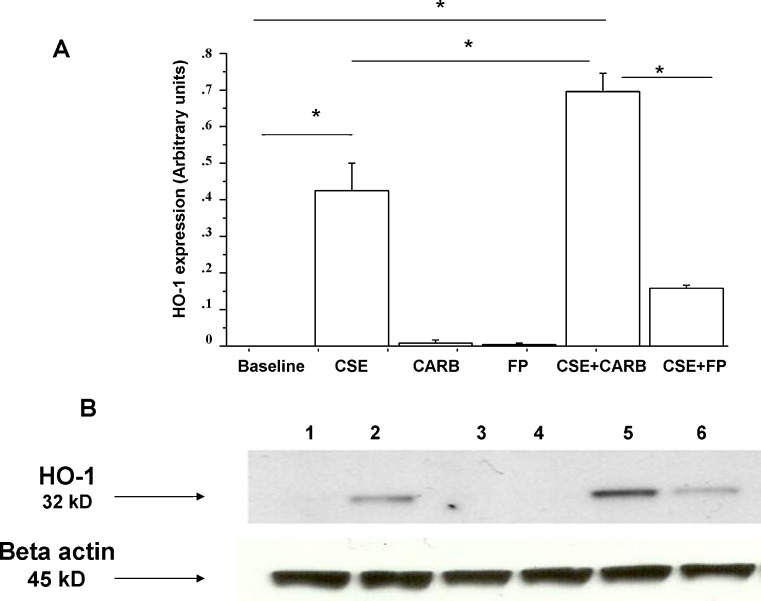
The inducible stress protein HO-1 has been implicated in cytoprotection against the toxic action of CSE (Dolinay et al. 2012; Raval and Lee 2010). The effect of CARB and FP on HO-1 expression in CSE-stimulated bronchial epithelial cells was explored. CSE increased HO-1 expression in bronchial epithelial cells. CARB and FP alone did not significantly induce HO-1 expression. FP significantly reduced HO-1 expression in CSE-stimulated bronchial epithelial cells. CARB significantly increased HO-1 expression in CSE-stimulated bronchial epithelial cells, and its effect was opposite to the effect exerted by FP (Fig. 7a, b).
Fig. 7.
Effects of CARB and FP on HO-1 expression in bronchial epithelial cells. 16-HBE (n = 5) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h. Total proteins were extracted and analysed for HO-1 expression using western blot analysis. Membranes were then stripped and incubated with rabbit polyclonal anti-ß-actin. a Densitometric analysis of HO-1 expression. Signals corresponding to HO-1 on the various western blots were semi-quantified by densitometric scanning, normalised and expressed after correction with the density of the band obtained for beta-actin. Data are expressed as arbitrary units ± SD. b Representative western blot performed on nuclear extracts. Lane 1, baseline; lane 2, CSE 10 %; lane 3, CARB (10−4 M); lane 4, FP (10−8 M); lane 5, CSE 10 % + CARB (10−4 M); lane 6, CSE 10 % + FP (10−8 M)
Effects of FP and CARB on Nrf2 and HDAC-2 nuclear expression in bronchial epithelial cells
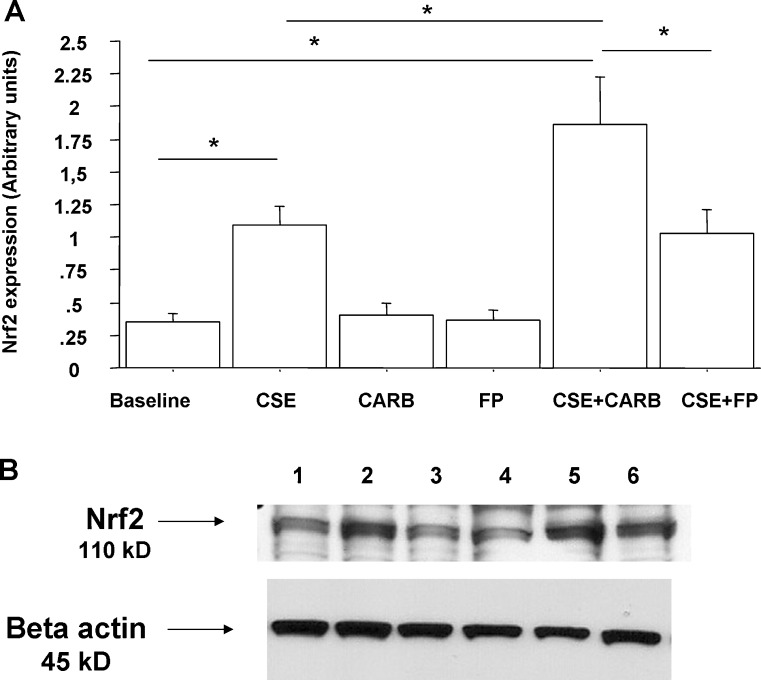
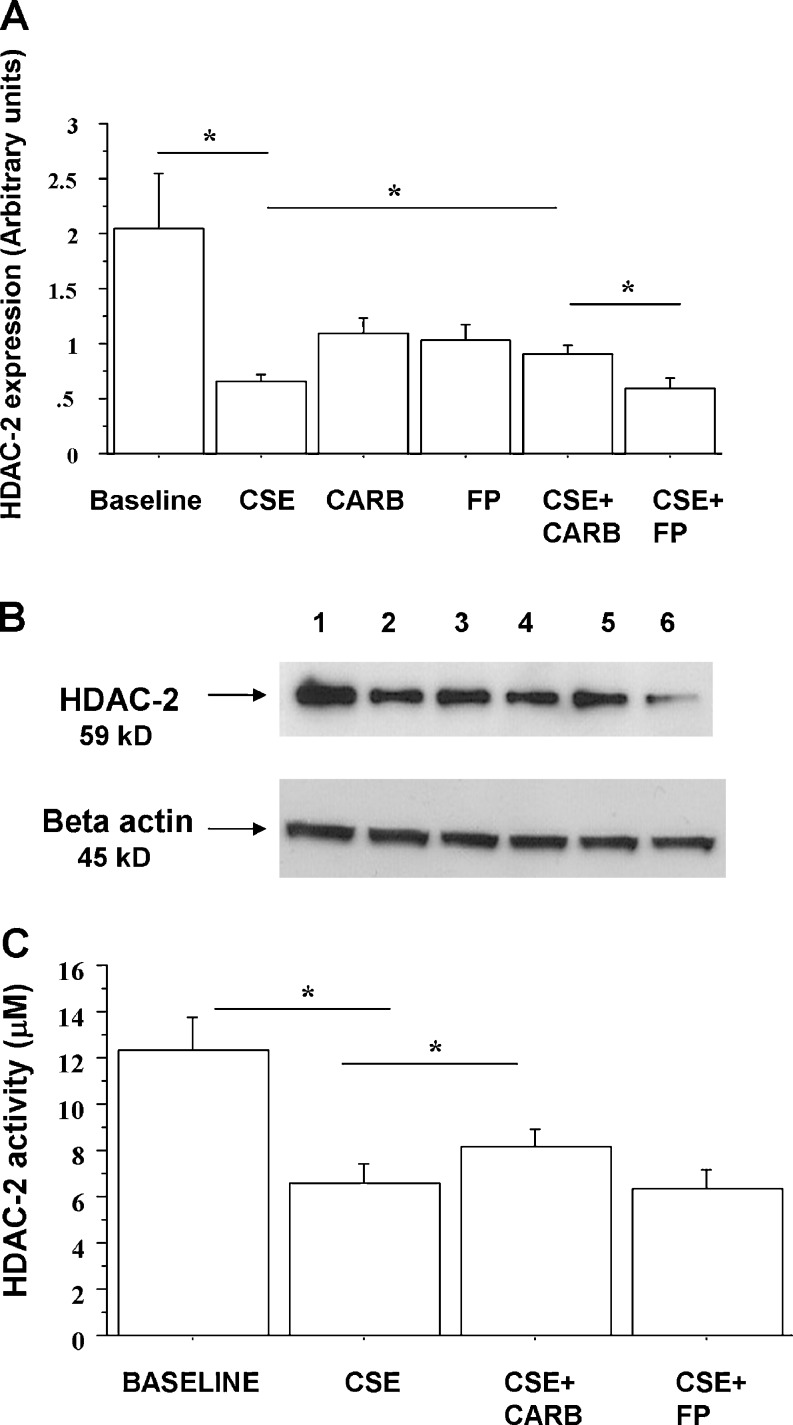
Since CSE affects Nrf2 nuclear translocation and reduces the expression of HDAC-2 (Malhotra et al. 2011), the effects of carbocysteine and FP on nuclear Nrf2 and HDAC-2 expression in CSE-stimulated bronchial epithelial cells were explored. CSE increased nuclear Nrf2 expression in bronchial epithelial cells. CARB significantly increased Nrf2 nuclear expression in CSE-stimulated bronchial epithelial cells. Nuclear Nrf2 was significantly higher in cells treated with both CARB and CSE than in cells treated with FP and CSE (Fig. 8a, b). With regard to HDAC-2 nuclear expression, CSE reduced the nuclear expression of HDAC-2. CARB and FP alone did not affect nuclear HDAC-2 expression. CARB, but not FP, slightly, but significantly, increased HDAC-2 nuclear expression in CSE-stimulated bronchial epithelial cells (Fig. 9a, b).
Fig. 8.
Effects of CARB and FP on Nrf2 nuclear expression in bronchial epithelial cells. 16-HBE (n = 5) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h. Total proteins were extracted and nuclear proteins were collected and analysed for Nrf2 expression by western blot analysis. Membranes were then stripped and incubated with rabbit polyclonal anti-ß-actin. a Densitometric analysis of Nrf2 expression. Signals corresponding to Nrf2 on the various western blots were semi-quantified by densitometric scanning, normalised and expressed after correction with the density of the band obtained for β-actin. Data are expressed as arbitrary units ± SD. b Representative western blot performed on nuclear extracts. Lane 1, baseline; lane 2, CSE 10 %; lane 3, CARB (10−4 M); lane 4, FP (10−8 M); lane 5, CSE 10 % + CARB (10−4 M); lane 6, CSE 10 % + FP (10−8 M)
Fig. 9.
Effects of carbocysteine and FP on HDAC-2 nuclear expression in bronchial epithelial cells. 16-HBE (n = 6) cells were cultured in the presence and absence of CSE (10 %), CARB (10−4 M) and FP (10−8 M) for 18 h. Total proteins were extracted and nuclear proteins were collected and analysed for HDAC-2 expression by western blot analysis. Membranes were then stripped and incubated with rabbit polyclonal anti-ß-actin. a Densitometric analysis of HDAC-2 expression. Signals corresponding to HDAC-2 on the various western blots were semi-quantified by densitometric scanning, normalised and expressed after correction with the density of the band obtained for beta-actin. Data are expressed as arbitrary units ± SD. b Representative western blot performed on nuclear extracts. Lane 1, baseline; lane 2, CSE 10 %; lane 3, CARB (10−4 M); lane 4, FP (10−8 M); lane 5, CSE 10 % + CARB (10−4 M); lane 6, CSE 10 % + FP (10−8 M). c In some experiments (n = 3), nuclear proteins were immunoprecipitated and then assessed for HDAC activity. Data are expressed as micromolar ± SD using as reference a curve of the Fluor-de-Lys deacetylated standard (provided with the kit)
Effects of FP and carbocysteine on HDAC-2 activity in bronchial epithelial cells
Cigarette smoke extracts induce posttranslational modifications (nytrosilation) of HDAC-2, leading to inactivity (Yang et al. 2006). The effects of carbocysteine and FP on HDAC-2 activity in CSE-stimulated bronchial epithelial cells were evaluated. CSE reduced HDAC-2 activity. Carbocysteine, but not FP, was able to partially counteract the effects of CSE on HDAC-2 activity in bronchial epithelial cells (Fig. 9c).
Discussion
The bronchial epithelium has a central role in coordinating many of the responses that are observed in COPD (Puchelle et al. 2006). Cigarette smoke is the major risk factor for airflow limitation in COPD. Cigarette smoke, promoting oxidative stress- and nitrosative stress-induced posttranslational modifications, leads to HDAC2 inactivity and to corticosteroid resistance, phenomena both frequently observed in COPD patients. The main aims and the novelty of this work were to provide data to support the use of CARB to control oxidative stress and to improve the antioxidant (GSH) as well as the protective (Nrf2 and HO-1) mechanisms in CSE-exposed airway epithelial cells. The present study demonstrates that, in bronchial epithelial cells, CARB counteracts the oxidative stress induced by CSE exposure, by increasing GSH, HO-1, Nrf2 and HDAC-2 nuclear expressions and activity, and is more potent in these effects than fluticasone propionate. Furthermore, the finding that CARB increases nuclear translocation and activity of HDAC-2 in cigarette smoke-stimulated bronchial epithelial cells supports a new potential role in improving corticosteroid activity, suggesting the combined use of FP and carbocysteine to better control inflammation and oxidative stress, both important features of COPD pathogenesis.
Increased oxidative burden and antioxidant imbalance generated by inhaled oxidants or by endogenous sources are involved in cellular and tissue damage related to the pathogenesis of many acute and chronic respiratory diseases, including COPD. For this purpose, strategies aimed at reducing oxidative burden or increasing antioxidants are central in the therapy of COPD.
Antioxidant agents, such as carbocysteine, scavenge free radicals and oxidants (Zheng et al. 2008). COPD patients treated with carbocysteine experienced fewer numbers of exacerbations per year (Zheng et al. 2008). This effect may be linked to the reduced adherence of bacterial pathogens to airway epithelial cells (Rahman and Macnee 2012) and to the downregulation of ICAM-1, a receptor for the major group of rhinovirus (Yasuda et al. 2006). Moreover, carbocysteine inhibiting cell apoptosis and promoting the reversal of the imbalance of proteolytic and anti-proteolytic enzyme activities protects against the development of CSE-induced emphysema in rats (Hanaoka et al. 2011). To counteract oxidative stress, cells enlist a battery of antioxidant systems, and carbocysteine in a lung “epithelial-like” cell line (WI-26VA4) (Garavaglia et al. 2008) induces an increase in the chloride and GSH anionic fluxes, along with an increase in GSH permeability. Here, it has been demonstrated that, in bronchial epithelial cells stimulated with CSE (10 %), ROS production started to increase after 4 h and reached higher levels after 18 h of incubation, and for this reason, this last time point was selected for all subsequent experiments.
Carbocysteine, but not FP, was able to counteract the oxidant activities of CSE, reducing ROS production. However, CARB, at the selected concentration (10−4 M), resulted less potent in controlling ROS formation in a nasal epithelial cell line and in another airway epithelial cell line (Nogawa et al. 2009). Furthermore, in bronchial epithelial cells, carbocysteine, but not FP, was able to counteract the oxidant activities of CSE, also increasing GSH, HO-1 and Nrf2. HO-1 is an enzyme that catalyses the degradation of the heme group into iron, biliverdin, and carbon monoxide and acts as a stress response protein (Morse and Choi 2002). This enzyme is highly inducible in response to oxidants and inflammatory molecules and might serve as a protective mechanism against oxidative stress and inflammation-induced lung injury. In this regard, it has been demonstrated that acrolein, an aldehyde present also in cigarette smoke, induces HO-1 in bronchial epithelial cells (Zhang and Forman 2008). An imbalance between HO-1 and inducible nitric oxide synthase may be associated with the development of severe impairment in chronic obstructive pulmonary disease patients (Maestrelli et al. 2003). In addition, it is noteworthy that n-aceylcysteine is able to induce HO-1 also in the presence of CSE in alveolar “epithelial-like” cells (Kosmider et al. 2011). Herein, we show that carbocysteine is able to further increase the expression of HO-1 in CSE-stimulated bronchial epithelial cells while FP has an opposite effect. The increased expression of HO-1 is normally linked to the inactivation of the Kelch-like ECH-associated protein 1 (Keap1), resulting in nuclear accumulation of the transcriptional regulator Nrf2. CSE oxidises Keap1 thiol groups, resulting in the dissociation of Nrf2 from Keap1 and the translocation of Nrf2 into the nucleus leading to increased HO-1 mRNA and protein in human macrophages (Goven et al. 2009). It has also been previously demonstrated that during keratinocyte differentiation, HO-1 may be modulated via a Nrf2-independent pathway (Ballatori et al. 2009).
The data herein reported confirm the effect of CSE in increasing the nuclear translocation of Nrf2 and show a significant increase of Nrf2 nuclear expression in CSE-stimulated cells treated with CARB compared to FP. The observed reduced nuclear expression of Nrf2 in CSE-stimulated cells treated with FP confirms previous observations concerning a suppressive role of glucocorticoids in Nrf2-dependent antioxidant response (Kratschmar et al. 2012). Furthermore, in bronchial epithelial cells stimulated with CSE, CARB was able to further increase Nrf2 nuclear expression, suggesting that the further increased expression of HO-1 in this condition was dependent on the modulation of the Nrf2 pathway. In the present study, carbocysteine improves the cytoprotective events against oxidative stress, increasing Nrf2 and HO-1 expression and GSH content. Interestingly, the increased GSH content of cells treated with both CSE and carbocysteine further supports the cytoprotective role of carbocysteine. GSH is a major low molecular weight antioxidant thiol and its levels decline in both ageing and COPD (Drost et al. 2005). GSH levels can control both inflammation and oxidative stress, two major factors contributing to COPD.
The increase of GSH induced by carbocysteine in bronchial epithelial cells stimulated with CSE may also promote, as previously reported, the denitrosylation of proteins by means of a trans-nitrosylation reaction (Benhar et al. 2009). Exogenous GSH treatment of macrophages from individuals with COPD restores HDAC-2 activity and induces a concomitant decrease in S-nitrosylation of HDAC2 (Malhotra et al. 2011).
HDAC plays a critical role in the regulation of inflammatory responses (Shakespear et al. 2011). Class I HDACs (HDAC-1, HADC-2, HDAC-3 and HDAC-8) mediate the deacetylation of histones proteins. Upon the deacetylation of histones, chromatin condensation and transcriptional repression occur, while the deacetylation of transcription factors alters protein–protein interactions and DNA binding, thus regulating the transcriptional programme (Shakespear et al. 2011). The decrease in HDAC-2 activity in alveolar macrophages and in lung epithelial cells, which occurs concomitantly with COPD progression, is responsible for the amplified inflammation and glucocorticorticoid resistance observed in these patients (Cosio et al. 2004). The anti-inflammatory response induced by glucocorticoids is linked to the trans-repression of NF-κB-dependent cytokine gene expression (Ito et al. 2006). HDAC-2 is a critical component for the trans-repression of NF-κB transcriptional activity by deacetylating histones in the pro-inflammatory gene promoters and deacetylating the glucocorticoid receptor (Ito et al. 2006). Moreover, HDAC-dependent mechanisms contribute to the maintenance of the adult lung structure since in animal models HDAC inhibition leads to emphysema (Mizuno et al. 2011). Pretreatment of A549, an adenocarcinomic alveolar basal epithelial cell line, with N-acetyl-l-cysteine attenuated the oxidant-mediated reduction in HDAC activity (Moodie et al. 2004). According to these data, we now demonstrate that carbocysteine is able, differently from FP, to increase HDAC-2 nuclear translocation and to improve HDAC-2 activity in CSE-stimulated bronchial epithelial cells. Additional experiments will be required in the future to better clarify whether the increased HDAC-2 activity caused by carbocysteine could improve the response to corticosteroids in models with elevated oxidative stress.
In conclusion, the present study demonstrates that, in bronchial epithelial cells stimulated with CSE, carbocysteine reduces ROS production, increases cytoprotective events, including GSH and HO-1 expression, and counteracts the effects of CSE in reducing HDAC-2 activity, thus providing new mechanisms that are not shared with steroids, whose activity is impaired in models with high oxidative stress.
The main aims and the novelty of this work were to provide data to support the use of CARB to control, as well known, the oxidative stress and to improve the antioxidant (GSH) and the protective (Nrf2 and HO-1) events in CSE-exposed airway epithelial cells. Furthermore, the finding that CARB increases, in cigarette smoke-stimulated bronchial epithelial cells, the nuclear translocation and activity of HDAC-2 supports a new potential role in improving corticosteroid activity. Taken together, these data support a combined use of FP and carbocysteine to better control inflammation and oxidative stress, both important features of COPD pathogenesis.
Acknowledgments
This work was mainly supported by the Italian National Research Council and by Dompè, Italy. Elisabetta Pace declares that she has had access to and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- CSE
Cigarette smoke extracts
- CARB
Carbocysteine
- FP
Fluticasone propionate
- ROS
Reactive oxygen species
- GSH
Glutathione
- HO-1
Heme oxygenase-1
- Nrf2
Nuclear-related factor 2
- HDAC-2
Histone deacetylase 2
References
- Adcock IM, Marwick J, Casolari P, Contoli M, Chung KF, Kirkham P, Papi A, Caramori G. Mechanisms of corticosteroid resistance in severe asthma and chronic obstructive pulmonary disease (COPD) Curr Pharm Des. 2010;16:3554–3573. doi: 10.2174/138161210793797889. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A, et al. Apigenin affects leptin/leptin receptor pathway and induces cell apoptosis in lung adenocarcinoma cell line. Eur J Cancer. 2011;47:2042–2051. doi: 10.1016/j.ejca.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200:689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Yamaya M, et al. A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell Dev Biol. 1992;28A:735–744. doi: 10.1007/BF02631062. [DOI] [PubMed] [Google Scholar]
- Dolinay T, Choi AM, Ryter SW. Heme oxygenase-1/CO as protective mediators in cigarette smoke-induced lung cell injury and chronic obstructive pulmonary disease. Curr Pharm Biotechnol. 2012;13:769–776. doi: 10.2174/138920112800399338. [DOI] [PubMed] [Google Scholar]
- Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14:90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- Garavaglia ML, Bononi E, Dossena S, Mondini A, Bazzini C, Lanata L, Balsamo R, Bagnasco M, Conese M, Bottà G, Paulmichl M, Meyer G. S-CMC-Lys protective effects on human respiratory cells during oxidative stress. Cell Physiol Biochem. 2008;22:455–464. doi: 10.1159/000185494. [DOI] [PubMed] [Google Scholar]
- Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med. 2011;4:105–113. doi: 10.2147/IJGM.S15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goven D, Boutten A, Leçon-Malas V, Boczkowski J, Bonay M. Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters NRF2 and Bach1 expression in human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS Lett. 2009;583:3508–3518. doi: 10.1016/j.febslet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Hanaoka M, Droma Y, Chen Y, Agatsuma T, Kitaguchi Y, Voelkel NF, Kubo K. Carbocisteine protects against emphysema induced by cigarette smoke extract in rats. Chest. 2011;139:1101–1108. doi: 10.1378/chest.10-0920. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratschmar DV, Calabrese D, Walsh J, Lister A, Birk J, Appenzeller-Herzog C, Moulin P, Goldring CE, Odermatt A. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cells. PLoS One. 2012;7:e36774. doi: 10.1371/journal.pone.0036774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi F, Aarbiou J, van Wetering S, Rahman I, de Boer WI, Rabe KF, Hiemstra PS. Effects of cigarette smoke condensate on proliferation and wound closure of bronchial epithelial cells in vitro: role of glutathione. Respir Res. 2005;6:140. doi: 10.1186/1465-9921-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrelli P, Páska C, Saetta M, Turato G, Nowicki Y, Monti S, Formichi B, Miniati M, Fabbri LM. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. Eur Respir J. 2003;21:971–976. doi: 10.1183/09031936.03.00098203. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, Barnes P, Biswal S. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest. 2011;121:4289–4302. doi: 10.1172/JCI45144. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mizuno S, Yasuo M, Bogaard HJ, Kraskauskas D, Natarajan R, Voelkel NF. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol. 2011;300:L402–L413. doi: 10.1152/ajplung.00207.2010. [DOI] [PubMed] [Google Scholar]
- Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- Morse D, Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- Nogawa H, et al. Carbocisteine can scavenge reactive oxygen species in vitro. Respirology. 2009;14:53–59. doi: 10.1111/j.1440-1843.2008.01424.x. [DOI] [PubMed] [Google Scholar]
- Pace E, Bruno TF, Berenger B, Mody CH, Melis M, Ferraro M, Tipa A, Bruno A, Profita M, Bonsignore G, Gjomarkaj M. Elevated expression of prostaglandin receptor and increased release of prostaglandin E2 maintain the survival of CD45RO+ T cells in the inflamed human pleural space. Immunology. 2007;121:427–436. doi: 10.1111/j.1365-2567.2007.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace E, Ferraro M, Siena L, Melis M, Montalbano A, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M. Cigarette smoke increases TLR4 and modifies LPS mediated responses in airway epithelial cells. Immunology. 2008;124:401–411. doi: 10.1111/j.1365-2567.2007.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace E, Ferraro M, Mody CH, Melis M, Bonanno A, Profita M, Giarratano A, Gjomarkaj M. Pleural mesothelial cells express both BLT2 and PPARa and mount an integrated response to pleural LTB4. J Immunol. 2008;181:7292–7299. doi: 10.4049/jimmunol.181.10.7292. [DOI] [PubMed] [Google Scholar]
- Pace E, Scafidi V, Di Bona D, Siena L, Chiappara G, Ferraro M, La Grutta S, Gallina S, Speciale R, Ballacchino A, Bachert C, Bousquet J, Gjomarkaj M. Increased expression of IL-19 in the epithelium of patients with chronic rhinosinusitis and nasal polyps. Allergy. 2012a;67:878–886. doi: 10.1111/j.1398-9995.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- Pace E, Ferraro M, Minervini MI, Vitulo P, Pipitone L, Chiappara G, Siena L, Montalbano AM, Johnson M, Gjomarkaj M. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One. 2012b;7:e33601. doi: 10.1371/journal.pone.0033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- Rahman I, Macnee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol. 2012;12:256–265. doi: 10.1016/j.coph.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Raval CM, Lee PJ. Heme oxygenase-1 in lung disease. Curr Drug Targets. 2010;11:1532–1540. doi: 10.2174/1389450111009011532. [DOI] [PubMed] [Google Scholar]
- Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1998;19:819–825. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- Yang SR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Yamada M, Asada M, Yoshida M, Suzuki T, Nishimura H, Sasaki H. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. Eur Respir J. 2006;28:51–58. doi: 10.1183/09031936.06.00058505. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, Shi Y, Liu CT, Chen P, Li Q, Wang ZS, Huang YJ, Luo ZY, Chen FP, Yuan JZ, Yuan BT, Qian HP, Zhi RC, Zhong NS. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): a randomised placebo-controlled study. Lancet. 2008;371:2013–2018. doi: 10.1016/S0140-6736(08)60869-7. [DOI] [PubMed] [Google Scholar]