Abstract
Predicting the survival of a patient with heart failure (HF) is a complex problem in clinical practice. Our previous study reported that extracellular HSP70 (HSPA1A) correlates with markers of heart function and disease severity in HF, but the predictive value of HSP70 is unclear. The goal of this study was to analyze extracellular HSP70 as predictive marker of mortality in HF. One hundred ninety-five patients with systolic heart failure were enrolled and followed up for 60 months. By the end of follow-up, 85 patients were alive (survivors) and 110 died (nonsurvivors). HSP70 (measured by ELISA in the serum) was elevated in nonsurvivors, compared with survivors (0.39 [0.27–0.59] vs. 0.30 [0.24–0.43] ng/ml, respectively, p = 0.0101). In Kaplan–Meier survival analysis higher HSP70 levels above median were associated with a significantly increased mortality. In multivariable survival models, we show that HSP70 level above the median is an age-, sex-, body mass index-, creatinine-, and NT-proBNP-independent predictor of 5-year mortality in HF. Extracellular HSP70 could prove useful for estimating survival in patients with HF.
Keywords: HSP70, HSPA1A, Heart failure, Inflammation, Prognostic marker, Five-year survival
Introduction
Heart failure (HF) is a major public health problem associated with increased morbidity and mortality. As in the USA, life expectancy increases and the population ages, the overall prevalence of HF will continue to rise (Deedwania and Carbajal 2012). The prevalence of heart failure can be estimated at 1–2 % in the Western world and its incidence approaches 5–10 per 1,000 persons per year. Its prevalence is 10 % or higher among individuals aged 85 years or older (Mosterd and Hoes 2007). Heart failure has traditionally been considered a disease of the myocardium, with symptoms arising from altered hemodynamics. However, it is now recognized that, in addition to the marked neuroendocrine disturbance, there is a perturbation of cytokine expression in patients with heart failure. The resulting inflammatory imbalance plays a central role in the underlying pathophysiological processes of heart failure, and leads to disease progression, as well as to a poorer prognosis (Pugh et al. 2002).
Within the heat shock protein (HSP) family, HSP70 (HSPA1A) has been structurally and functionally conserved during evolution. It is ubiquitous in all organisms, from archaebacteria through plants to humans (Daugaard et al. 2007). HSP70 plays a multiple role in cellular homeostasis. Its level increases rapidly in response to various types of severe stress, as a protection against a subsequent, near-lethal, ischemic, or hypoxic event (Hecker and McGarvey 2011). Besides, it is involved in the folding of proteins, in their transport between cellular compartments, and in the breakdown of the irreversibly damaged ones. HSP70 has been detected also in the circulation, in the extracellular space, and its presence has been demonstrated in the serum of healthy individuals (Pockley et al. 1998). HSP70 could be passively released from necrotic cells, and actively excreted by a nonclassical secretory pathway (Asea 2007). HSP70 has a number of extracellular functions, some of which involve immunoregulation, and others that are more focused on cellular homeostasis and protection (Henderson and Pockley 2010).
Elevated HSP70 level in the serum or tissues appears to be a nonspecific indicator of organ ischemia or dysfunction (Hecker and McGarvey 2011). A large body of evidence from the literature shows that extracellular HSP70 is important and useful as a biomarker in different clinical situations, including heart failure. Genth-Zotz and colleagues showed first that serum HSP70 is elevated in HF patients as compared to healthy controls, and increased serum HSP70 is related to disease severity but not to survival (Genth-Zotz et al. 2004). Our group demonstrated previously that high serum HSP70 level is associated with the severity of heart failure. Indicating a complex stress response, HSP70 levels were associated with left ventricular ejection fraction and NT-proBNP (Gombos et al. 2008). Circulating HSP70 levels were elevated in patients with idiopathic left ventricular dysfunction (Giannessi et al. 2007). Serum HSP70 was also significantly higher in heart failure patients with different types of cardiomyopathy (Wei et al. 2009). Moreover, HSP70 plays an important role in acute myocardial ischemia since it is rapidly released into the circulation after acute myocardial infarction, has been suggested as a marker of myocardial damage (Dybdahl et al. 2005; Satoh et al. 2006). Increased levels of HSP70 were significantly associated with the risk and severity of the acute coronary syndrome (Zhang et al. 2010). Additionally, the role of HSP70 in the progression of vascular calcification was also demonstrated: its levels correlated with the severity of atherosclerosis in patients with carotid artery disease and chronic lower limb ischemia (Krepuska et al. 2011).
As an integrative marker of stress, and based on its relationship to disease severity, we hypothesized that HSP70 could be an independent predictor of mortality in HF. Accordingly, the present study aimed to analyze the association of HSP70 level with overall 5-year survival.
Results and discussion
One hundred ninety-five patients with systolic heart failure were enrolled. Our work group reported previously the baseline clinical characteristics of the study group (Gombos et al. 2008; Forhecz et al. 2009). The demographic data, clinical characteristics, and comparison of the survivors and nonsurvivors followed up for 5 years are presented in Table 1. All patients were followed-up for 60 months (contacted between 56 and 60 months; median, 60 months) after study entry. The primary outcome measure was the death of the patient from any cause. Eighty-five patients (43.6 %) were alive (survivors) at the end of the 5-year follow-up; 110 (56.4 %) died (nonsurvivors). First, we analyzed whether increased HSP70 levels are associated with all-cause mortality and found a significant (p = 0.0101) association (see Table 1). Advanced age, elevated NT-proBNP and creatinine levels, as well as decreased body mass index (BMI), and left ventricular ejection fraction (EF) were similarly associated with mortality (Table 1).
Table 1.
Demographic data and baseline clinical characteristics of the 85 survivors and 110 nonsurvivors (who died before the end of the 5-year-long follow-up period)
| Parameters | Survivors (N = 85) | Nonsurvivors (N = 110) | p valuea |
|---|---|---|---|
| Sex (males) | 61 (71.8 %)b | 84 (76.4 %) | 0.4658 |
| Age (years) | 67.07 (58.71–75.85)c | 71.50 (61.29–78.51) | 0.0253 |
| BMI (kg/m2) | 28.4 (25.2–31.5) | 25.7 (23.8–30.4) | 0.0112 |
| Heart rate (1/min) | 80 (68–93) | 78 (70–88) | 0.7131 |
| Systolic blood pressure (mmHg) | 125 (120–140) | 120 (110–133) | 0.0725 |
| Diastolic blood pressure (mmHg) | 80 (70–80) | 70 (65–80) | 0.0115 |
| NYHA class (I) | 31 (36.5 %) | 7 (6.4 %) | <0.0001 |
| NYHA class (II) | 31 (36.5 %) | 34 (30.9 %) | |
| NYHA class (III) | 21 (24.7 %) | 48 (43.6 %) | |
| NYHA class (IV) | 2 (2.4 %) | 21 (19.1 %) | |
| Left ventricular ejection fraction (%) | 37 (30–41) | 32 (24–40) | 0.0032 |
| NT-proBNP (fmol/ml) | 423.4 (268.6–869.3) | 1,133.5 (571.1–2,153.3) | <0.0001 |
| Etiology (ischemic heart disease) | 43 (50.6 %) | 70 (63.6 %) | 0.0672 |
| Duration of HF (years) | 2.6 (0.3–6.4) | 2.8 (1.0–6.1) | 0.7734 |
| Serum sodium (mmol/l) | 141 (139–143) | 140 (136–142) | 0.0056 |
| Serum creatinine (μmol/l) | 90 (75–106) | 107 (87–156) | 0.0002 |
| HSP70 (ng/ml) | 0.30 (0.24–0.43) | 0.39 (0.27–0.59) | 0.0101 |
The detailed description of the patient group and its management, as well as data collection, blood sampling, and laboratory measurements were reported previously (Gombos et al. 2008; Forhecz et al. 2009). Serum HSP70 levels (R and D Systems, Minneapolis, MN, USA) were measured by ELISA, according to the manufacturers’ instructions. We use the term “HSP70” as a synonym for HSPA1A under the new nomenclature (Kampinga et al. 2009)
BMI body mass index, NYHA New York Heart Association, NT-proBNP N-terminal prohormone of brain natriuretic peptide, HF heart failure
aMann–Whitney test (continuous variables) or Pearson’s chi-square test (categorical variable), or chi-square test for trend (multiple ordered categorical variables) between survivors and nonsurvivors. The statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA), and SPSS v13.0 (SPSS Inc., Chicago, IL, USA)
bNumber of cases with percentages
cMedian values with interquartile ranges
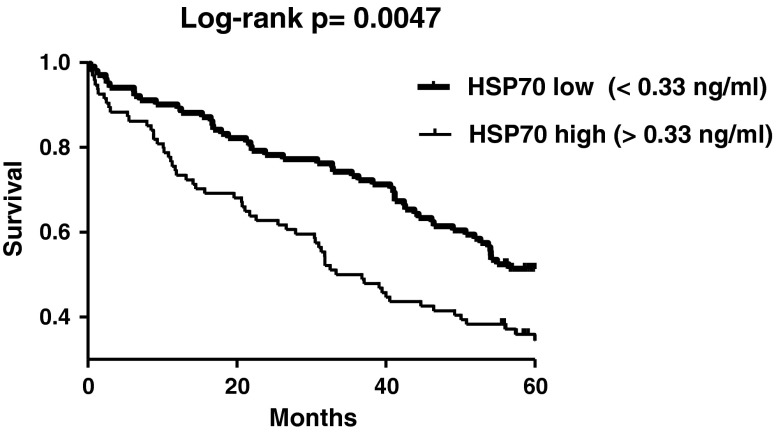
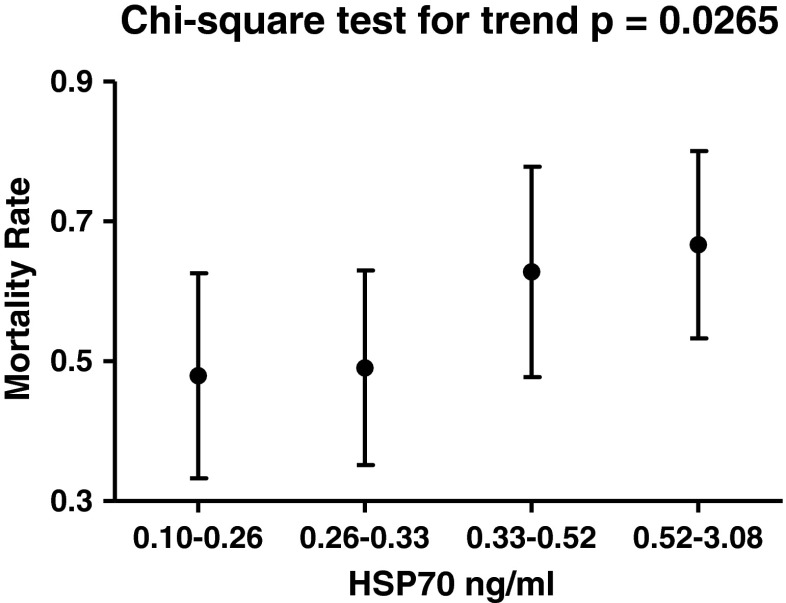
Next, we analyzed survival. The 195 patients were stratified, according to the median value of HSP70 (0.33 ng/ml), into low- or high-HSP70 groups. Kaplan–Meier survival analysis was done to describe mortality over time in both groups (Fig. 1). In our study, higher HSP70 levels were associated with an increased mortality (log-rank test, p = 0.0047, Fig. 1) during the 5-year-long follow-up period. Additionally, we observed a trend of increasing 5-year mortality rate in the groups with higher HSP70 levels. As presented in Fig. 2, mortality rates rose gradually in the subsets with elevated HSP70 levels (four subsets were created according to the 25th, median, and 75th percentiles of HSP70; chi-square test for trend, p = 0.0265, Fig. 2).
Fig. 1.
Kaplan–Meier survival plots in the two subgroups of HF patients stratified according to median HSP70 (HSPA1A) levels. The p value of the log-rank test is indicated. Survival times were measured from inclusion until the end of follow-up. Survival was plotted according to the Kaplan–Meier method, and differences in survival between the groups were compared with the log-rank test. The power of the log-rank test was sufficiently high (p = 0.9, alpha = 0.05) to support the above conclusion
Fig. 2.
Mortality rates (95 % CI) in the four subsets of HF patients stratified according to HSP70 (HSPA1A) levels (25th percentile, median, and 75th percentile). The p value obtained by the chi-square test for trend is indicated (chi-square = 4.92, degrees of freedom = 1). The patients were contacted between the 56th and 60th months (median, 60 months) after study entrance. The primary outcome measure was the death of the patient from any cause. We ascertained the death of the patients reported by their family members or the medical staff by checking the data of the national health registry. To compare mortality rates, we calculated person-time incidence rates in the four subsets with different ranges of HSP70 level: 0.10–0.26 ng/ml (9.58 deaths per 100 person-years), 0.26–0.33 ng/ml (9.81 deaths per 100 person-years), 0.33–0.52 ng/ml (12.55 deaths per 100 person-years), and 0.52–3.08 ng/ml (13.33 deaths per 100 person-years)
Therefore, HSP70 was evaluated as a potential predictor of outcome in univariable and multivariable Cox regression models. In the univariable Cox proportional hazards risk model, HSP70 (as a categorical variable, stratified according to the median level) was a significant predictor of 5-year mortality (hazard ratio, 1.712; 95 % confidence interval, 1.174–2.496 when comparing patients with high versus low HSP70 level, Wald chi-square = 7.877, p = 0.005, Table 2). In addition to HSP70 levels, the univariate survival analysis showed that age, BMI, and left ventricular EF, as well as NT-proBNP, and serum creatinine levels were also associated with 5-year mortality (data not shown). Therefore, multivariable Cox regression analysis was performed to test the independence of HSP70 from other covariates. In the multivariable Cox analysis adjusted for age, sex, BMI, serum creatinine, and NT-proBNP, HSP70 remained a significant predictor of 5-year mortality (hazard ratio, 1.556; 95 % confidence interval, 1.042–2.325 for comparison of patients with high or low HSP70 level, Wald chi-square = 4.654, p = 0.031, Table 2). Furthermore, the 5-year mortality predicted by HSP70 was adjusted in a subsequent model for age, sex, BMI, serum creatinine, and left ventricular EF (hazard ratio, 1.590; 95 % confidence interval, 1.046–2.417 for comparison of patient with high or low HSP70 level, Wald chi-square = 4.720, p = 0.030, Table 2). In summary, high (above median) HSP70 concentration predicts 5-year mortality in HF independent of age, sex, BMI, creatinine, and disease severity (reflected by NT-proBNP or left ventricular EF).
Table 2.
Prediction of 5-year mortality by HSP70, Cox regression analysis
| Models | HRa | 95 % CI | Chi-square | p value |
|---|---|---|---|---|
| Univariable model of HSP70 (high vs. low) | 1.712 | 1.174–2.496 | 7.877 | 0.005 |
| Multivariable model of HSP70 (high vs. low) adjusted for age, sex, BMI, serum creatinine and NT-proBNP | 1.556 | 1.042–2.325 | 4.654 | 0.031 |
| Multivariable model of HSP70 (high vs. low) adjusted for age, sex, BMI, serum creatinine and left ventricular EF | 1.590 | 1.046–2.417 | 4.720 | 0.030 |
HR hazard ratio
aHSP70 as a categorical variable, stratified according to the median level (HR for comparison of patients with high vs. low HSP70 level) with 95 % CI; Wald chi-square and p values of likelihood ratio tests are presented
A very large number of variables have been shown to correlate with the outcome in HF (McMurray et al. 2012). The assessment of prognosis is particularly important when counseling patients about devices and surgery (e.g., transplantation), as well as in planning end-of-life care (McMurray et al. 2012). However, predicting the survival of HF patients remains a complex and incomplete task in clinical practice and therefore, new prognostic markers are needed. As mentioned above, several studies corroborate that circulating HSP70 is detectable in the serum in response to various forms of stress (Dybdahl et al. 2005; Satoh et al. 2006; Wei et al. 2009). This marker might gain wide acceptance in predicting the prognosis of, or determining the patients’ risk status in cardiovascular diseases (Zhang et al. 2010). However, Genth-Zotz et al. reported conflicting data showing that serum HSP70 was elevated in HF patients but did not predict survival (Genth-Zotz et al. 2004). Their study was limited by the small number of subjects and by the poor detection limit of the assay used (HSP70 was detectable in only 41 % of HF patients). Our work group demonstrated previously that serum HSP70 levels are associated with disease severity in heart failure (Gombos et al. 2008), and using a sensitive immunoassay, HSP70 was a detectable in all of our patients. In the present study, we have shown for the first time that extracellular HSP70 is a predictor of all-cause, 5-year mortality in patients with heart failure. We found higher HSP70 levels in nonsurvivors than in survivors (Table 1).
Elevated HSP70 levels could be linked to cellular stress caused by mild hypoxia from heart failure. Activation of the neurohormonal system with increased catecholamine levels is known to occur in HF, with potential induction of heat-shock (stress) response reflected by elevated HSP70 levels (Moalic et al. 1989; Meng et al. 1996). In HF, hypoxia may also be persistently present especially during acute cardiac decompensation episodes. Hypoxia may further initiate oxidative stress, inflammation, and heat shock response as well as remodeling. As an indicator of the above complex stress response, HSP70 level correlated with left ventricular ejection fraction and NT-proBNP, as demonstrated previously (Gombos et al. 2008). According to previous data (Genth-Zotz et al. 2004) and to our current results, HSP70 seems to be a clinically useful biomarker for prediction of mortality in HF; however, further studies are necessary to identify if it is independent from other risk factors and to determine the best cutoff value.
HSP70 levels, age, BMI, left ventricular EF, creatinine, and NT-proBNP were also associated with 5-year mortality in our patients. Considering the contribution of these covariates, we performed Cox regression analysis to investigate their potential influence.
In conclusion, we established that the high level of extracellular HSP70 is an independent prognostic marker in HF and thus, HSP70 could prove suitable for predicting long-term survival in patients with heart failure.
Acknowledgments
This work was supported by the National Research Fund (NF72689), National Development Agency TÁMOP 4.2.2-08/01/KMR-2008-0004, and the Ministry of Welfare of Hungary (ETT229/2006). No extramural funding other than that listed above was used. The authors are solely responsible for the design and conduct of this study, for all study analyses, as well as for the drafting and editing of the paper and its final contents.
References
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32(3):579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Carbajal E. Evidence-based therapy for heart failure. Med Clin N Am. 2012;96(5):915–931. doi: 10.1016/j.mcna.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Genth-Zotz S, Bolger AP, Kalra PR, von Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol. 2004;96(3):397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D. Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones. 2007;12(3):265–274. doi: 10.1379/CSC-272.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos T, Forhecz Z, Pozsonyi Z, Janoskuti L, Prohaszka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperones. 2008;13(2):199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker JG, McGarvey M. Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones. 2011;16(2):119–131. doi: 10.1007/s12192-010-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Pockley AG. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol. 2010;88(3):445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepuska M, Szeberin Z, Sotonyi P, Sarkadi H, Fehervari M, Apor A, Rimely E, Prohaszka Z, Acsady G. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16(3):257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- Meng X, Brown JM, Ao L, Banerjee A, Harken AH. Norepinephrine induces cardiac heat shock protein 70 and delayed cardioprotection in the rat through alpha 1 adrenoceptors. Cardiovasc Res. 1996;32(2):374–383. doi: 10.1016/0008-6363(96)00078-8. [DOI] [PubMed] [Google Scholar]
- Moalic JM, Bauters C, Himbert D, Bercovici J, Mouas C, Guicheney P, Baudoin-Legros M, Rappaport L, Emanoil-Ravier R, Mezger V, et al. Phenylephrine, vasopressin and angiotensin II as determinants of proto-oncogene and heat-shock protein gene expression in adult rat heart and aorta. J Hypertens. 1989;7(3):195–201. doi: 10.1097/00004872-198903000-00005. [DOI] [PubMed] [Google Scholar]
- Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig. 1998;27(6):367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pugh PJ, Jones RD, Jones TH, Channer KS. Heart failure as an inflammatory condition: potential role for androgens as immune modulators. Eur J Heart Fail. 2002;4(6):673–680. doi: 10.1016/S1388-9842(02)00162-9. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8(8):810–815. doi: 10.1016/j.ejheart.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wei YJ, Huang YX, Shen Y, Cui CJ, Zhang XL, Zhang H, Hu SS. Proteomic analysis reveals significant elevation of heat shock protein 70 in patients with chronic heart failure due to arrhythmogenic right ventricular cardiomyopathy. Mol Cell Biochem. 2009;332(1–2):103–111. doi: 10.1007/s11010-009-0179-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, Hu FB, Tanguay RM, Wu T. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15(5):675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]