Abstract
The aim of this study was to investigate the effects of cold stress on the expression levels of heat shock proteins (Hsps90, 70, 60, 40, and 27) and inflammatory factors (iNOS, COX-2, NF-κB, TNF-α, and PTGEs) and oxidative indexes in hearts of chickens. Two hundred forty 15-day-old male chickens were randomly divided into 12 groups and kept at the temperature of 12 ± 1 °C for acute and chronic cold stress. There were one control group and five treatment groups for acute cold stress, three control groups, and three treatment groups for chronic cold stress. After cold stress, malondialdehyde level increased in chicken heart; the activity of superoxide dismutase and glutathione peroxidase in the heart first increased and then decreased. The inflammatory factors mRNA levels were increased in cold stress groups relative to control groups. The histopathological analysis showed that heart tissues were seriously injured in the cold stress group. Additionally, the mRNA levels of Hsps (70, 60, 40, and 27) increased significantly (P < 0.05) in the cold stress groups relative to the corresponding control group. Meanwhile, the mRNA level and protein expression of Hsp90 decreased significantly (P < 0.05) in the stress group, and showed a gradually decreasing tendency. These results suggested that the levels of inflammatory factors and Hsps expression levels in heart tissues can be influenced by cold stress. Hsps commonly played an important role in the protection of the heart after cold stress.
Keywords: Cold stress, Heat shock proteins, Inflammatory injury, Chicken heart
Introduction
Animals face a variety of environmental stressors, such as cold stress, that commonly exists in cold regions. When many organ systems of chickens are still developing, appropriate stress may have a long-lasting effect and can improve their ability to cope with stressors later in life (Zulkifli et al. 1995). Researchers have demonstrated that cold stress can influence the health and welfare of animals in the cold region (Tsutsayeva and Sevryukova 2001). When living organisms are exposed to various stress conditions, the synthesis of most proteins is retarded, but a group of highly conserved proteins known as heat shock proteins (Hsps) is rapidly synthesized (Al-Aqil and Zulkifli 2009). Hsps are major molecular chaperones that perform important functions in the folding/unfolding and translocation of proteins as well as in the assembly/disassembly of protein complexes (Bernabo et al. 2011). Meanwhile, Hsps are also expressed constitutively in poultry species (Givisiez et al. 2001). Among HSP families, Hsp70 is the most extensively studied because of its prominent response to diverse stressors, and increased synthesis of these inducible proteins is involved in the protection of stressed cells and organisms (Gabriel et al. 2002). The geese heart Hsp70 expression increased under heat stress suggesting that HSP70 had a protective function in cells (Wang et al. 2008). Overexpression of Hsp70 is associated with myocardial protection (Plumier et al. 1995; Jayakumar et al. 2001; Liu et al. 2007). Hao et al. (2010) reported the effect of transport stress on variation in the expression of Hsp27, Hsp70, and Hsp90, and the results suggested that Hsps could protect cardiac muscle cells from transport stress. Additionally, Hsp60, Hsp70, and Hsp90 expression levels in the hearts of chickens exposed to high temperatures have been reported, and the results suggested that Hsps had protective function against cardiac injury (Yu et al. 2008).
Hsp60 and Hsp27 have been extensively studied in heart disease because Hsps play an important role in the protection and repair of cells and tissues. For example, a Hsp60 expression increase was observed in heart failure (Lin et al. 2007; Wang et al. 2010). It is known that Hsp27 is usually expressed in heart muscle tissues under normal conditions where its function can be modified by different cell mechanisms operating either on their expression or their structure depending on cellular localization (Michaud et al. 1997; Anastasiya et al. 2005). However, several studies have indicated that overexpression of Hsp27 protects cardiac myocytes against ischemic injury (Yamboliev et al. 2000; Vander Heide 2002). Thus, numerous investigations have demonstrated the viability of Hsps in hearts of animals and humans; however, under cold stress conditions, chicken studies have not been reported, especially in the heart; therefore, we investigated the levels of Hsps in hearts of chickens exposed to a low-temperature environment in this study.
Exposure to low-temperature provokes physiological responses related to oxygen consumption and induces changes in energy production and subsequent reactive oxygen species (ROS) production (Blagojevic 2007). Several studies have indicated that exposure to low temperatures resulted in compensatory changes in the antioxidant defense system (Bottje et al. 1998; Lin et al. 2004; Mujahid 2010). In low-temperature exposed chicks, brain and heart malondialdehyde (MDA) levels were 2.1- and 1.2-fold higher, respectively, than that of control chicks (Mujahid and Furuse 2009). It was reported that cold stress causes the increase of MDA content, and the superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities of the chicken lung (Jia et al. 2009). These studies show that there is a relationship between cold exposure and the antioxidant defense system in animals.
Inflammation is an important indicator of animal tissue damage due to cold stress. Probably the most pivotal enzymes involved in maintaining inflammation are the inducible enzymes: inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), which are responsible for the catalysis of nitric oxide (NO) and prostaglandin E2 (PGE2), respectively (Surh et al. 2001). Nuclear factor-kappa B (NF-κB) is an inducible transcription factor in lymphocytes. Therefore, in this paper, we investigated inflammation factors in hearts of chickens exposed to a low-temperature environment.
Herein, we first investigated the antioxidant enzyme responses (SOD and GSH-Px) and MDA content in the hearts of chickens after cold treatment; second, we investigated expression of inflammation factors in the heart tissue under cold stress; and third, we investigated Hsps (Hsp90, 70, 60, 40, and 27) expression in the heart tissue under cold stress. Additionally, we investigated the protective role of Hsps in oxidative stress and inflammation induced by cold stress in hearts of chickens.
Materials and methods
Chickens and tissue collection
All procedures used in the present experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (Harbin, China). The chickens model of cold stress was developed as described in our previous studies (Wang and Xu 2008; Wang et al. 2009; Zhang et al. 2011). Briefly, 240 15-day-old male chickens were purchased from Weiwei Co. Ltd. (Harbin, China) and randomly allocated to 12 groups (6 groups for the acute cold stress experiment and 6 groups for the chronic cold stress experiment; n = 20/group). The chickens were maintained in our animal facility, kept under a 16 L:8D cycle and a temperature of 30 ± 2 °C, and given free access to standard food and water. During their second week of age, five groups were transferred to a cold environment (12 ± 1 °C) and kept for 1, 3, 6, 12, and 24 h, respectively, for acute cold stress, and one group was maintained at 25 °C as control (0-h group). Three groups were transferred to the cold environment (12 ± 1 °C) and kept for 5, 10, and 20 days, respectively, for chronic cold stress, and three groups were maintained at 25 °C for 5, 10, and 20 days as controls. The chickens were euthanized by sodium pentobarbital after stress termination. The heart tissues from each chicken were collected, immediately frozen on dry ice and then stored at −80 °C for RNA isolation and protein extract. Animal care and treatment complied with the standards described in the guidelines for the care and use of laboratory animals of the Northeast Agriculture University.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from the tissue samples (50 mg tissue; n = 6/group) using Trizol reagent according to the manufacturer's instructions (Invitrogen, China). The dried RNA pellets were re-suspended in 50 μl of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm (Gene Quant 1300/100, USA). First-strand cDNA was synthesized from 5 μg of total RNA using oligo dT18 primers and Superscript II reverse transcriptase according to the manufacturer's instructions (Invitrogen, China). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use.
To design primers, Primer Premier Software (PREMIER Biosoft International, USA) was used to design specific primers for Hsp90, Hsp70, Hsp60, Hsp40, Hsp27, COX-2, iNOS, PTGEs, TNF-α, NF-κB, and β-actin based on known chicken sequences (Table 1). General PCRs were first performed to confirm the specificity of the primers.
Table 1.
Gene special primers used in the real-time quantitative reverse transcription PCR
| Gene | Serial number | Primer sequence | Primer length (bp) | Size of the products (bp) |
|---|---|---|---|---|
| Hsp90 | NM_001109785.1 | Forward 5′-TCCTGTCCTGGCTTTAGTTT-3′ | 20 | 143 |
| Reverse 5′-AGGTGGCATCTCCTCGGT-3′ | 18 | |||
| Hsp70 | NM_001006685.1 | Forward 5′-CGGGCAAGTTTGACCTAA-3′ | 18 | 250 |
| Reverse 5′-TTGGCTCCCACCCTATCTCT-3′ | 20 | |||
| Hsp60 | NM_001012916.1 | Forward 5′-AGCCAAAGGGCAGAAATG-3′ | 18 | 208 |
| Reverse 5′-TACAGCAACAACCTGAAGACC-3′ | 21 | |||
| Hsp40 | NM_001199325.1 | Forward 5′-GGGCATTCAACAGCATAGA-3′ | 19 | 151 |
| Reverse 5′-TTCACATCCCCAAGTTTAGG-3′ | 20 | |||
| Hsp27 | NM_205290.1 | Forward 5′-ACACGAGGAGAAACAGGATGAG-3′ | 22 | 158 |
| Reverse 5′-ACTGGATGGCTGGCTTGG-3′ | 18 | |||
| COX-2 | NM_001167718 | Forward 5′-TGTCCTTTCACTGCTTTCCAT-3′ | 21 | 84 |
| Reverse 5′-TTCCATTGCTGTGTTTGAGGT-3′ | 21 | |||
| iNOS | NM_204961 | Forward 5′-CCTGGAGGTCCTGGAAGAGT-3′ | 20 | 82 |
| Reverse 5′-CCTGGGTTTCAGAAGTGGC-3′ | 19 | |||
| PTGEs | NM_001194983 | Forward 5′-GTTCCTGTCATTCGCCTTCTAC-3′ | 22 | 115 |
| Reverse 5′-CGCATCCTCTGGGTTAGCA-3′ | 19 | |||
| TNF-α | NM_204267 | Forward 5′-GCCCTTCCTGTAACCAGATG-3′ | 20 | 82 |
| Reverse 5′-ACACGACAGCCAAGTCAACG-3′ | 20 | |||
| NF-κB | NM_205134 | Forward 5′-TCAACGCAGGACCTAAAGACAT-3′ | 22 | 162 |
| Reverse 5′-GCAGATAGCCAAGTTCAGGATG-3′ | 22 | |||
| β-actin | L08165 | Forward 5′-CCGCTCTATGAAGGCTACGC-3′ | 20 | 128 |
| Reverse 5′-CTCTCGGCTGTGGTGGTGAA-3′ | 20 |
The qPCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems, USA). Reactions were performed in a 20-μl reaction mixture containing 10 μl of 2× SYBR Green PCR Master Mix (Roche, Switzerland), 2 μl of diluted cDNA, 0.6 μl of each primer (10 μM), and 6.8 μl of PCR-grade water. The PCR procedure for Hsp90, Hsp70, Hsp60, and β-actin consisted of heating the reaction mixture to 52 °C for 2 min and 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 20 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. A dissociation curve was run for each plate to confirm the production of a single product. The amplification efficiency for each gene was determined by using the DART-PCR program (Peirson et al. 2003). The mRNA relative abundance was calculated according to the method of Pfaffl (2001), accounting for gene-specific efficiencies and was normalized to the mean expression of β-actin.
Western blot analysis
Protein extracts were subjected to SDS-polyacrylamide gel electrophoresis under reducing conditions on 12 % gels. Separated proteins were then transferred to nitrocellulose membranes using a tank transfer for 2 h at 200 mA in Tris-glycine buffer containing 20 % methanol. Membranes were blocked with 5 % skim milk for 16–24 h and incubated overnight with diluted primary chicken antibody Hsp90 (1:500), Hsp70 (1:500), and Hsp60 (1:1,400; Hsp90, Hsp70, and Hsp60 production of polyclonal antibody by our lab) followed by a horseradish peroxidase (HRP)-conjugated secondary antibody against rabbit IgG (1:1,500, Santa Cruz, CA, USA). To verify equal loading of samples, the membrane was incubated with monoclonal β-actin antibody (1:1,000, Santa Cruz, CA, USA), followed by a HRP-conjugated goat anti-mouse IgG (1:1,000). The signal was detected by X-ray films (Trans Gen Biotech Co., China). The optical density (OD) of each band was determined by Image VCD gel imaging system, and the Hsp90, Hsp70, and Hsp60 expression were detected as the ratio of OD of Hsp90, Hsp70, Hsp60, and OD of β-actin, respectively.
Determination of antioxidant enzyme activities
Chicken hearts were homogenized on ice in physiological saline, centrifuged at 700 × g, and supernatants were collected. Here, we detected free radical scavenging enzymes such as SOD, metabolizing enzymes such as GSH-Px, and MDA as an index of oxidative damage. Commercial assay kits for SOD, GSH-Px, and MDA were provided by Jian cheng Biotechnology Research Institute (Nanjing, China). Measurements were performed according the protocol provided by the manufacturer in the laboratory of the Science and Technology Experiment Centre, Shanghai University of Traditional Chinese Medicine.
SOD activity in the homogenate was assayed by the inhibition at 25 °C of pyrogallol autoxidation by SOD (with and without sample) and was followed kinetically at 550 nm (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). One unit of SOD is defined as the amount of enzyme that causes 50 % inhibition of pyrogallol autoxidation. GSH-Px activity was measured by using H2O2 as a substrate by applying the method of Rotruck (Rotruck et al. 1973). MDA level was determined by 2-thiobarbituric acid reactive substance (TBARS) chromometry (Zhang et al. 2011).
Histopathologic examination
After necropsy, tissue specimens of heart were fixed in 4 % buffered formaldehyde and routinely processed in paraffin. Thin sections (5 μm) of each tissue were sliced from each block and mounted on glass. Slides were stained with hematoxylin and eosin (H&E). Histological slides were examined under an Olympus light microscope.
Statistical analysis
Statistical analysis of all data was performed by using SPSS for Windows (version 13, SPSS Inc., Chicago, IL, USA). When a significant value (P < 0.05) was obtained by one-way ANOVA, further analysis was carried out. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey's honestly significant difference test for post hoc multiple comparisons. Data are expressed as the mean ± SD.
Results
Changes in antioxidant enzyme activities
To examine whether cold stress exposure could cause oxidative stress, we detected GSH-Px and SOD activities, and measured MDA content in the hearts of chickens. Antioxidant enzymes activity and MDA content in the heart of chickens are shown in Tables 2 and 3. The SOD activities of heart tissue were significantly increased (P < 0.05) at the beginning of acute stress compared to the control group; however, at the end of acute cold stress, SOD activities of heart tissue were significantly decreased (P < 0.05). SOD activities of heart tissue were significantly decreased (P < 0.05) in the chronic stress groups compare to the corresponding control group.
Table 2.
Effects of acute cold stress on the SOD, GSH-Px activity, and MDA content in heart tissue
| 0 h | 1 h | 3 h | 6 h | 12 h | 24 h | |
|---|---|---|---|---|---|---|
| SOD (NU/mg.Pr) | 37.854 ± 4.135a | 42.439 ± 2.176b | 43.042 ± 2.714b | 29.498 ± 1.732c | 25.449 ± 1.007d | 22.764 ± 1.117e |
| GSH-Px (U/mg.Pr) | 1.23 ± 0.107a | 2.712 ± 0.204b | 3.849 ± 0.211c | 1.307 ± 0.113a | 0.986 ± 0.032a | 0.641 ± 0.025d |
| MDA (nmol/mg.Pr) | 2.432 ± 0.104a | 2.527 ± 0.223a | 2.986 ± 0.147b | 3.076 ± 0.245b | 3.647 ± 0.118c | 3.749 ± 0.194c |
Different lowercase letters indicate significant differences (P < 0.05) between any two means
Table 3.
Effects of chronic cold stress on the SOD, GSH-Px activity, and MDA content in heart tissue
| Group | 5 days | 10 days | 20 days | |
|---|---|---|---|---|
| SOD (NU/mg.Pr) | Control group | 34.967 ± 2.432 | 35.146 ± 3.047 | 33.982 ± 2.965 |
| Stress group | 24.492 ± 2.074* | 27.086 ± 1.443* | 29.796 ± 2.472 | |
| GSH-Px (U/mg.Pr) | Control group | 1.364 ± 0.117 | 1.276 ± 0.087 | 1.331 ± 0.065 |
| Stress group | 0.642 ± 0.044* | 1.027 ± 0.033 | 1.196 ± 0.041 | |
| MDA (nmol/mg.Pr) | Control group | 2.396 ± 0.087 | 2.407 ± 0.107 | 2.246 ± 0.102 |
| Stress group | 3.541 ± 0.117* | 3.209 ± 0.114* | 2.976 ± 0.087* |
*Significant differences are expressed as P < 0.05 between stress group and corresponding control group at the same point
The effects of cold stress on the GSH-Px activity in heart tissue are shown in Tables 2 and 3. GSH-Px activity in the acute stress group was first increased (P < 0.05) then decreased (P < 0.05) compared to that of the control group. In the chronic cold stress group, GSH-Px activity at 5-day stress group was decreased in heart tissue compared to that of each corresponding control group. However, GSH-Px activity was not significantly changed (P > 0.05) in the 10- and 20-day stress groups.
The effects of cold stress on the MDA content in heart tissue are shown in Tables 2 and 3. The MDA content in the examined tissues of the stress group chickens was significantly higher than that of the control group (P < 0.05). This significant difference was noted in both 12- and 24-h stress groups. The difference between the two groups was more pronounced at day 5.
Histological analysis
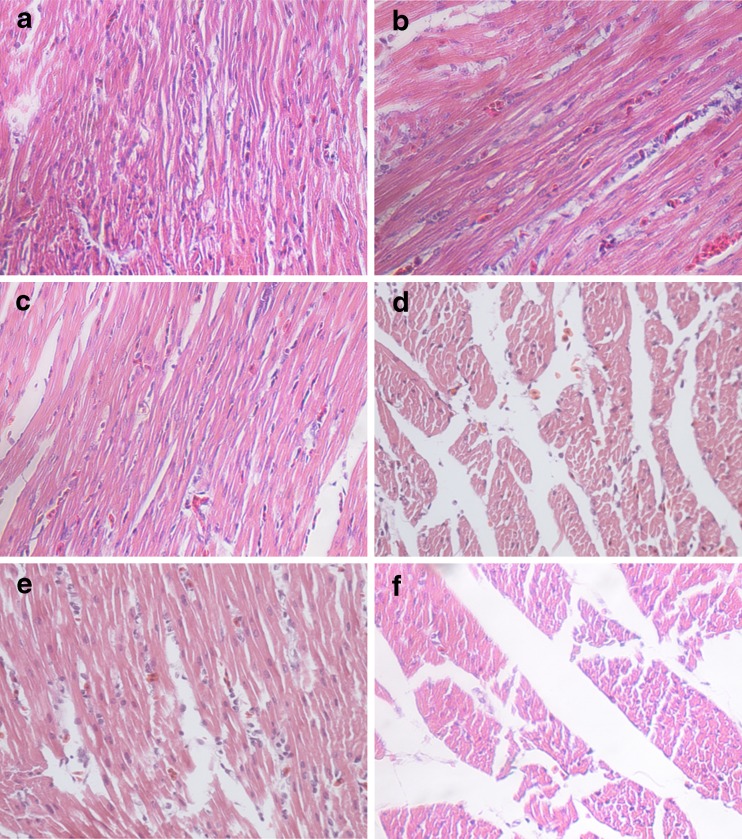
Heart tissues from the control group showed normal histological structures with regular cardiac muscle fibers (Fig. 1a). Heart tissues of animals treated with cold stress showed heart lesions with disordered or ruptured cardiac muscle fibers, cardiocyte hypertrophy, vacuolar degeneration, mesenchymal rarefaction, and infiltration of inflammatory cells (Fig. 1b–f).
Fig. 1.
H&E stained heart tissue sections of chicken: (a) heart of chicken from control group (H&E, ×400), (b) heart of chicken from 1-h stress group (H&E, ×400), (c) heart of chicken from 3-h group exposed to cold stress (H&E, ×400), (d) heart of chicken from 24-h group exposed to cold stress (H&E, ×400), (e) heart of chicken from 5-day group exposed to cold stress (H&E, ×400), and (f) heart of chicken from 20-day group exposed to cold stress (H&E, ×400)
Effects of cold stress on the mRNA levels of iNOS, COX-2, NF-κB, PTGEs, and TNF-α in hearts of chickens
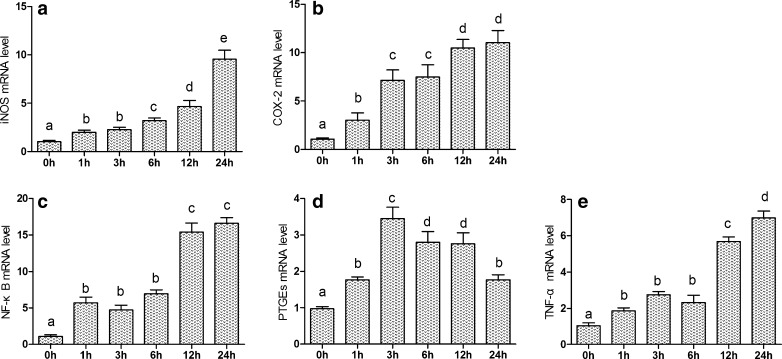
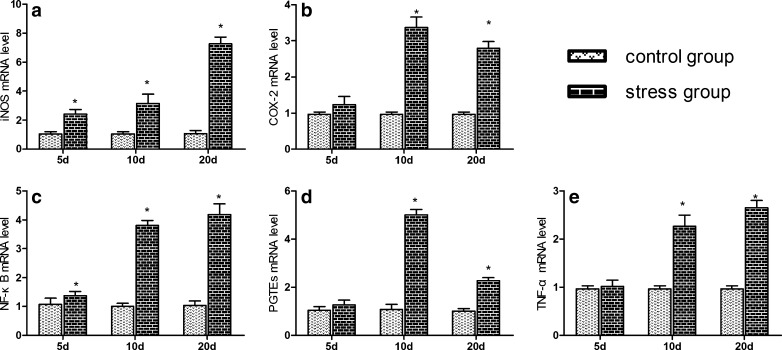
The effects of acute and chronic cold stress on the mRNA levels of the corresponding inflammation genes in heart are shown in Figs. 2 and 3. The iNOS mRNA levels of heart tissue were significantly increased (P < 0.05) in acute stress groups compared to control groups from 1 to 24 h of stress. In the chronic cold stress, the iNOS mRNA levels of heart tissue were significantly increased (P < 0.05) in stress groups compared to control group from 5 to 20 days of stress. The NF-κB and TNF-α mRNA levels of heart tissue were significantly increased (P < 0.05) in stress groups compared to control group. Moreover, the mRNA expression levels of NF-κB and TNF-α were similar. Compared with the control group, COX-2 and PTGEs mRNA expressions were significantly increased (P < 0.05) in stress groups.
Fig. 2.
Effects of acute cold stress on the expression of iNOS, COX-2, NF-κB, TNF-α, and PTGEs genes in hearts of chickens. a–e The mRNA expression of iNOS, COX-2, NF-κB, TNF-α, and PTGEs genes in acute cold stress, respectively. Relative mRNA levels of the inflammatory genes were detected by qPCR, and the different letters over the bars in a–e indicate that there are significant differences (P < 0.05) between groups
Fig. 3.
Effects of chronic cold stress on the mRNA expression of iNOS, COX-2, NF-κB, TNF-α, and PTGEs genes in heart of chickens. a–e The mRNA expression of iNOS, COX-2, NF-κB, TNF-α, and PTGEs genes in chronic cold stress, respectively. Relative mRNA levels of the iNOS, COX-2, NF-κB, TNF-α, and PTGEs genes were detected by qPCR. Asterisk, significant differences (P < 0.05) between the control group and the stress group at the same time point; each value represented the mean ± SD of six individuals
Effects of cold stress on the mRNA levels of hsp90, hsp70, and hsp60 in hearts of chickens
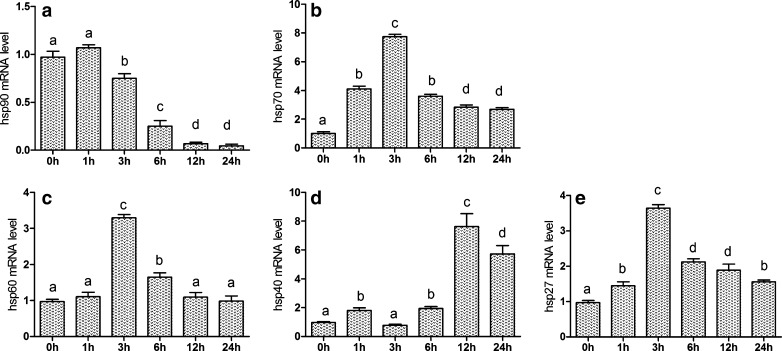
In order to determine the effects of cold stress on the expression of Hsp90, Hsp70, and Hsp60 genes in hearts of chickens during the different degrees of cold stress, the mRNA expression level of hsp90, hsp70, and hsp60 were examined by qPCR. From Figs. 4a and 5a, qPCR results revealed that the presence of hsp90 mRNA in hearts of chickens and the level of mRNA gradually decrease (P < 0.05). However, the level of hsp90 mRNA in the heart increased slightly at 1 h of cold stress (P > 0.05). Compared with the corresponding control groups, chronic cold stress in 5-, 10-, and 20-day treatment groups all had significantly decreased (P < 0.05) mRNA levels of the hsp90 gene in heart. Simultaneously, western blot of Hsp90 results were consistent with hsp90 mRNA response to cold stress (Fig. 6).
Fig. 4.
Effects of acute cold stress on the hsp90, hsp70, hsp60, hsp40, and hsp27 genes mRNA expression in hearts of chickens. a–e The mRNA expression of hsp90, hsp70, hsp60, hsp40, and hsp27 genes, respectively. Relative mRNA levels of the hsps genes were detected by qPCR. In the acute cold stress experiment, the relative mRNA levels from the 0-h control group were used as the reference values in a–e. The different letters over the bars in a–e indicate that there are significant differences (P < 0.05) between groups. Each value represented the mean ± SD of six individuals
Fig. 5.
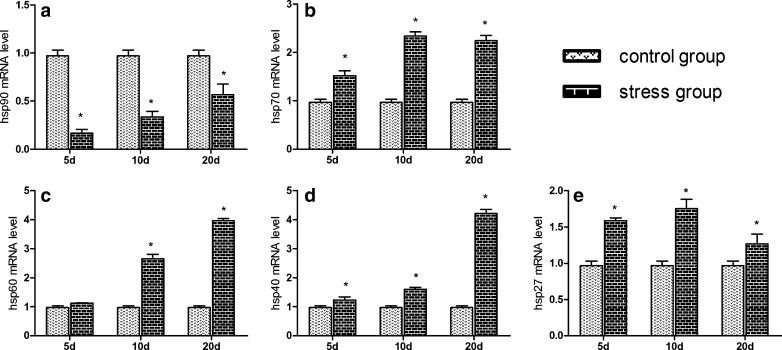
Effects of chronic cold stress on the hsp90, hsp70, hsp60, hsp40, and hsp27 genes mRNA expression in hearts of chickens. a–e The mRNA expression of hsp90, hsp70, hsp60, hsp40, and hsp27 genes, respectively. In the chronic cold stress experiment, relative mRNA levels of the hsps genes were detected by qPCR; the relative mRNA levels from the 5-, 10-, and 20-day control group were used as the reference values in a–e. Each value represented the mean ± SD of six individuals. Asterisk, significant differences (P < 0.05) between the control group and the stress group at the same time point
Fig. 6.
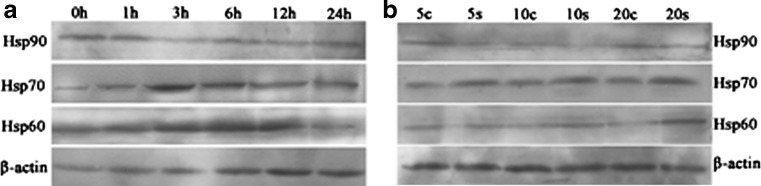
Effects of acute and chronic cold stress on the Hsp90, Hsp70, and Hsp60 protein expression in heart of chickens. a, b Acute and chronic cold stress groups, respectively. Acute cold stress groups (1, 3, 6, 12, and 24 h), 0 h = acute cold stress control group, chronic cold stress groups (5s, 10s, and 20s), and chronic corresponding control groups (5c, 10c, and 20c)
From Figs. 4b and 5b, qPCR results revealed the presence of hsp70 mRNA in hearts of chickens. Compared with the 0-h control group, acute cold stress treatment groups all significantly increased (P < 0.05) the mRNA levels of the hsp70 gene in heart, especially in the 3-h group. Compared with the corresponding control groups, chronic cold stress in treatment groups all significantly increased (P < 0.05) the mRNA levels of the hsp70 gene in heart. Simultaneously, western blot of Hsp70 results were consistent with hsp70 mRNA response to cold stress (Fig. 6).
Compared with the 0-h control group, acute cold stress in 3- and 6-h treatment groups significantly increased (P < 0.05) the mRNA levels of the Hsp60 gene in heart; however, 1-, 12-, and 24-h treatment groups had no significance (P > 0.05). Compared with the corresponding control groups, chronic cold stress in 10- and 20-day treatment groups all significantly increased (P < 0.05) the mRNA levels of the hsp60 gene in heart, except in the 5-day treatment groups (Figs. 4c and 5c). What is more, western blot of Hsp60 results was consistent with hsp60 mRNA response to cold stress (Fig. 6).
Effects of cold stress on the mRNA levels of hsp40 and hsp27 in hearts of chickens
The effects of acute and chronic cold stress on the mRNA levels of the hsp40 and hsp27 genes in hearts of chickens are shown in Figs. 4d, e and 5d, e. In acute cold stress, compared with the 0-h control group, all treatment groups significantly decreased (P < 0.05) the mRNA levels of the hsp40 gene in heart, except in the 3-h treatment group which had significant change (P > 0.05). Acute cold stress in all treatment groups significantly increased (P < 0.05) the mRNA levels of the hsp27 gene in heart. In chronic cold stress, compared with the corresponding control groups, 5-, 10-, and 20-day treatment groups all significantly increased (P < 0.05) the mRNA levels of the hsp40 and hsp27 genes in heart.
Western bolt analysis of Hsps levels
The protein expression levels of Hsp90, Hsp70, and Hsp60 were examined by western blots. The results (Fig. 6) revealed that the Hsp90 protein expression was gradually decreased in hearts of chickens compared with control group in the acute cold stress group; however, the Hsp90 protein expression at 1 h of cold stress was increased slightly. Compared with the corresponding control groups, chronic cold stress in 5-, 10-, and 20-day treatment groups all had significantly decreased protein expression of Hsp90 in heart. Compared with control group, Hsp70 protein expression in hearts of chickens was increased in acute and chronic cold stress, especially in the 3-h stress group. Compared with the 0-h control group, acute cold stress in 3-, 6-, and 12-h treatment groups significantly increased the protein levels of the Hsp60 gene in hearts of chickens; however, there was no significance in 1- and 24-h stress groups. Compared with the corresponding control groups, chronic cold stress in 10- and 20-day treatment groups significantly increased the protein levels of the Hsp60 in heart, except in the 5-day treatment groups.
Discussion
Cold stress can disrupt the balance of the oxidant/antioxidant system and cause oxidative damage to several tissues by altering the enzymatic and non-enzymatic antioxidant status, protein oxidation, and lipid peroxidation (Sahin and Gumuslu 2004). GSH-Px is considered to be the first line of cellular defense against oxidative damage (Ferreccio et al. 1998). The MDA levels serve as an index of oxidative damage in chickens exposed to low temperatures. Prior study indicated that cold stress induced the destruction of the oxidation–antioxidant balance in lung tissue of chickens, and caused oxidation damage of DNA (Jia et al. 2009). Additionally, in our previous study, we found that acute and chronic cold exposure induced oxidative damage in chicken intestine (Zhang et al. 2011). In the present study, MDA contents of heart increased due to cold stress, which implied the deposit of lipid peroxidation products (LPO) in the heart tissues. Meanwhile, GSH-Px and SOD activity decreased, which demonstrated that the ability to scavenge free radicals and antioxidation were impaired. Furthermore, LPO and free radicals accumulated in heart tissues. The status of heart tissues treated with cold stress showed heart lesions with disordered or ruptured cardiac muscle fibers, cardiocyte hypertrophy, mesenchymal rarefaction, and infiltration of inflammatory cells leading to histological changes, which indicated that cold stress caused oxidative damage to hearts of chickens.
In order to further detect cold stress-induced tissues damage and host defenses such as inflammatory reactions, the mRNA expression levels of NF-κB, COX-2, iNOS, TNF-α, and PTGEs were examined by qPCR. In the nucleus, NF-κB induces the transcription of a large variety of target genes by binding to the cis-acting κB element. The target genes are those that normally encode cytokines (Kiemer et al. 2003), cell adhesion molecules, and inflammatory enzymes including COX-2 (Abate et al. 1998) and iNOS (Lee et al. 2000). COX-2 inhibitors reduce mucosal damage, which further indicates the role of prostaglandins in tissue damage and inflammation reactions (Zheng et al. 2012). PTGES catalyzes the isomerization of PGH2 to PGE2, and the latter plays an important role in inflammation (Liu et al. 2012a), cell growth, and transformation (Sasaki et al. 2012). In the current study, we found that the NF-κB, iNOS, and TNF-α mRNA levels of heart tissue were significantly increased (P < 0.05) in stress groups. Similar to COX-2, PTGES expression was increased by cold exposure, suggesting that it may play a role in inducing inflammation in cold stress. In addition, iNOS and COX-2 are generally not expressed under normal conditions and can be induced by various cytokines or lipopolysaccharides. Although the induction of iNOS expression may be a vital means of establishing a host defense, increases in iNOS and COX-2 expression have been implicated in various pathophysiological processes of septic shock and chronic inflammatory diseases, with iNOS proposed to play a proinflammatory role. Prior research (Teshfam et al. 2006) indicated that cold exposure could significantly increase eNOS and iNOS gene expression in the lungs of broiler chickens. Acute immobilization and cold water-immersion stress increased TNF-α level at different time points (0, 30, 60, and 120 min) after stress in rats (Liu et al. 2012b). Macrophages from cold-stressed mice persistently produced high levels of TNF-alpha (Aviles and Monroy 2001). So, in our study, the increased inflammation factors suggested that the myocardial inflammatory response was induced by cold stress, which may lead to myocardial injury.
As molecular chaperones, Hsps are highly conserved cellular stress proteins present in every organism from bacteria to man. The cytoprotective role of Hsps has been observed in a wide variety of animals and in humans. Organisms are protected from a number of stress conditions including ischemia, metabolic disorders, inflammation and infection, heat stress, ischemic stress, and other stresses (Sreedhar and Csermely 2004). Hsps help to maintain the metabolic and structural integrity of the cell and act as a protective response to external stresses. For example, associations of high serum Hsp70 levels with disease severity were observed in patients with severe heart failure (Gombos et al. 2008; Locke et al. 1996). Several studies have shown that elevation of Hsp70 in heart provided protection against stress-induced myocardial injury. Additionally, Hsp90 and Hsp60 might play an important role in protecting small intestinal epithelial and mucosal cells from hydrogen peroxide-induced or indomethacin-induced cell injury (Takada et al. 2010; Tamaki et al. 2011). The variations of Hsp27 expression are associated with strengthening of the cytoskeleton and an increased tolerance of the cell to injury induced by stress (Guay et al. 1997). However, the effects of cold stress on the expression levels of Hsps (Hsps90, 70, 60, 40, and 27) in heart is unknown, especially in chicken; therefore, we studied the variation of these Hsps.
It has been demonstrated that the level of Hsp90 mRNA in the heart decreased significantly at 3 and 5 h of heat stress in broilers, respectively (Yu et al. 2008). Bao et al. (2008) also reported that Hsp90 dramatically decreased after 6 h of transportation in pigs. Consistent with these prior studies, our results suggested that with duration of the cold stress, Hsp90 expression was decreased (Fig. 4). It was suggested that Hsp90 expression may be inhibited by other Hsps or cold stress, which implied the heart had been damaged under cold stress. Hsp90 may act as important markers and protective proteins in response to the adverse environmental conditions. It is concluded from our results that the highest levels of Hsp70 (especially Hsp70), Hsp60, and Hsp27 mRNA occur in the heart at 3 h of cold stress, and the encoded proteins may act as important markers and protective proteins in response to the adverse environmental conditions. Hsp90, Hsp70, and Hsp60 also play a very important role in pig transport stress (Bao et al. 2008). In recent years, Hsp90, Hsp70, and Hsp60 as targets in the immune response of rats have been reported (Martinez et al. 2001). The expression of Hsp70, Hsp60, and Hsp27 showed a similar trend in our results (Fig. 4); synergies among Hsps were inferred. Our results showed that Hsp70 first expressed and then Hsp40 expression (Fig. 4). Hsp40 co-operates with Hsp70 to facilitate protein folding as reported previously (Li et al. 2009), so we speculated that Hsp40 expression could raise the Hsp70 expression to the highest level. In addition, one of the main roles of Hsp40 proteins is the regulation of the ATPase activity of Hsp70 (Cintron and Toft 2006). Synergistic relationships may occur between Hsp40 and Hsp70. In the present study, the results showed that different Hsps had different patterns of expression, and suggested that Hsps could have different responses to cold stress.
In conclusion, the present paper suggested that the antioxidant defense system could be damaged in hearts of chickens by cold stress. The increase in mRNA expression of inflammation factors further suggested that cold stress caused damage to heart tissue of the chicken. The increased Hsps (70, 60, 40, and 27) and decreased Hsp90 mRNA expression levels revealed that different Hsps may have the ability to protect chicken heart tissue from cold stress. However, so far, the specific cold stress-activated induction and regulation mechanisms among Hsps are unclear, and further studies are needed.
Acknowledgments
This work was supported by technological innovation projects special funds of Harbin, China (no. 2010RFXXN041) and science and technology innovation outstanding youth funds of Harbin (no. RC2013QN002065). The authors thank the members in the veterinary internal medicine laboratory, especially the members of the cold stress group, at College of Veterinary Medicine, Northeast Agricultural University (Harbin, China) for help in feeding the chicks and analyzing the data.
Footnotes
All authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the journal.
Contributor Information
Shu Li, Email: lishu@neau.edu.cn.
Shi-Wen Xu, Phone: +86-451-55190407, Email: shiwenxu@neau.edu.cn.
References
- Abate A, Oberle S, Schroder H. Lipopolysaccharide-induced expression of cyclooxygenase-2 in mouse macrophages is inhibited by chloromethylketones and a direct inhibitor of NF-kappa B translocation. Prostag Other Lipid Mediat. 1998;56:277–290. doi: 10.1016/S0090-6980(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Al-Aqil A, Zulkifli I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult Sci. 2009;88:1358–1364. doi: 10.3382/ps.2008-00554. [DOI] [PubMed] [Google Scholar]
- Anastasiya VP, Valeria VM, Ivan SC, Natalia AC, Dmitrii IL, Nikolai BG (2005) Effects of small heat shock proteins on the thermal denaturation and aggregation of F-actin. Bioche Biophy Res Commu 4:1548–1553 [DOI] [PubMed]
- Aviles H, Monroy FP. Immunomodulatory effects of cold stress on mice infected intraperitoneally with a 50% lethal dose of Toxoplasma gondii. Neuroimmunomodulation. 2001;9:6–12. doi: 10.1159/000049002. [DOI] [PubMed] [Google Scholar]
- Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008;13:459–466. doi: 10.1007/s12192-008-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabo P, Rebecchi L, Jousson O, Martinez-Guitarte JL, Lencioni V. Thermotolerance and hsp70 heat shock response in the cold-stenothermal chironomid Pseudodiamesa branickii (NE Italy) Cell Stress Chaperones. 2011;16:403–410. doi: 10.1007/s12192-010-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagojevic DP. Antioxidant systems in supporting environmental and programmed adaptations to low temperatures. Cryo-Letters. 2007;28:137–150. [PubMed] [Google Scholar]
- Bottje WG, Wang S, Beers KW, Cawthon D. Lung lining fluid antioxidants in male broilers: age-related changes under thermoneutral and cold temperature conditions. Poult Sci. 1998;77:1905–1912. doi: 10.1093/ps/77.12.1905. [DOI] [PubMed] [Google Scholar]
- Cintron NS, Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem. 2006;281:26235–26244. doi: 10.1074/jbc.M605417200. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Gonzalez Psych C, Milosavjlevic Stat V, Marshall Gredis G, Sancha AM. Lung cancer and arsenic exposure in drinking water: a case–control study in northern Chile. Cad Saude Publica. 1998;14(Suppl 3):193–198. doi: 10.1590/S0102-311X1998000700021. [DOI] [PubMed] [Google Scholar]
- Gabriel JE, da Mota AF, Boleli IC, Macari M, Coutinho LL. Effect of moderate and severe heat stress on avian embryonic hsp70 gene expression. Growth Dev Aging. 2002;66:27–33. [PubMed] [Google Scholar]
- Givisiez PEN, da Silva MM, Mazzi CM, Ferro MIT, Ferro JA, Gonzales E, Macari M. Heat or cold chronic stress affects organ weights and Hsp70 levels in chicken embryos. Can J Anim Sci. 2001;81:83–87. doi: 10.4141/A00-049. [DOI] [Google Scholar]
- Gombos T, Förhécz Z, Pozsonyi Z, Jánoskuti L, Prohászka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperon. 2008;13:199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Hao Q, Bao E, Zhang M, Yue Z, Hartung J. Variation in the expression of Hsp27, Hsp70, Hsp90 and their corresponding mRNA transcripts in the hearts of pigs during different transportation durations. Livest Sci. 2010;129:88–94. doi: 10.1016/j.livsci.2010.01.008. [DOI] [Google Scholar]
- Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH (2001) Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation 104:I303–I307 [DOI] [PubMed]
- Jia HY, Li JM, Yu Q, Wang JJ, Li S. The effect of cold stress on DNA oxidative damage of lung in chicken. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2009;25:373–376. [PubMed] [Google Scholar]
- Kiemer AK, Hartung T, Huber C, Vollmar AM. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway. J Hepatol. 2003;38:289–297. doi: 10.1016/S0168-8278(02)00417-8. [DOI] [PubMed] [Google Scholar]
- Lee YW, Han SH, Lee M, Yang KH, Kim HM, Jeon YJ. 2-Amino-3-methylimidazo [4,5-f] quinoline inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells by blocking p38 kinase activation. Cancer Lett. 2000;156:133–139. doi: 10.1016/S0304-3835(00)00452-3. [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Sha B. Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett. 2009;16:606–612. doi: 10.2174/092986609788490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Decuypere E, Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) Comp Biochem Physiol B Biochem Mol Biol . 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–H2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- Liu JC, He M, Wan L, Cheng XS (2007) Heat shock protein 70 gene transfection protects rat myocardium cell against anoxia-reoxygeneration injury. Chin Med J (Engl) 120:578–583 [PubMed]
- Liu T, Laidlaw TM, Feng C, Xing W, Shen S, Milne GL, Boyce JA. Prostaglandin E2 deficiency uncovers a dominant role for thromboxane A2 in house dust mite-induced allergic pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:12692–12697. doi: 10.1073/pnas.1207816109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Bi H, Fan R, Li YH, Wang YM, Chen YM, Chen JY, Chi SM, Pei JM. Effect of compound nutrients on acute immobilization and cold water-immersion stress-induced changes of Th1/Th2 cytokines. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012;28:601–603. [PubMed] [Google Scholar]
- Locke M, Tanguay RM, Ianuzzo CD. Constitutive expression of HSP 72 in swine heart. J Mol Cell Cardiol. 1996;28:467–474. doi: 10.1006/jmcc.1996.0043. [DOI] [PubMed] [Google Scholar]
- Martinez J, Perez-Serrano J, Bernadina WE, Rodriguez-Caabeiro F. HSP60, HSP70 and HSP90 from Trichinella spiralis as targets of humoral immune response in rats. Parasitol Res. 2001;87:453–458. doi: 10.1007/s004360000315. [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay RM (1997) Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci 53:104–113 [DOI] [PMC free article] [PubMed]
- Mujahid A. Acute cold-induced thermogenesis in neonatal chicks (Gallus gallus) Comp Biochem Physiol Mol Integr Physiol. 2010;156:34–41. doi: 10.1016/j.cbpa.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Mujahid A, Furuse M. Oxidative damage in different tissues of neonatal chicks exposed to low environmental temperature. Comp Biochem Physiol Mol Integr Physiol. 2009;152:604–608. doi: 10.1016/j.cbpa.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN (1995) Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest 95:1854–1860 [DOI] [PMC free article] [PubMed]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sahin E, Gumuslu S. Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol. 2004;48:165–171. doi: 10.1007/s00484-004-0205-7. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Kamei D, Ishikawa Y, Ishii T, Uematsu S, Akira S, Murakami M, Hara S. Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene. 2012;31:2943–2952. doi: 10.1038/onc.2011.472. [DOI] [PubMed] [Google Scholar]
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Takada M, Otaka M, Takahashi T, Izumi Y, Tamaki K, Shibuya T, Sakamoto N, Osada T, Yamamoto S, Ishida R, Odashima M, Itoh H, Watanabe S. Overexpression of a 60-kDa heat shock protein enhances cytoprotective function of small intestinal epithelial cells. Life Sci. 2010;86:499–504. doi: 10.1016/j.lfs.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Otaka M, Takada M, Yamamoto S, Odashima M, Itoh H, Watanabe S. Evidence for enhanced cytoprotective function of HSP90-overexpressing small intestinal epithelial cells. Dig Dis Sci. 2011;56:1954–1961. doi: 10.1007/s10620-010-1558-x. [DOI] [PubMed] [Google Scholar]
- Teshfam M, Brujeni GN, Hassanpour H. Evaluation of endothelial and inducible nitric oxide synthase mRNA expression in the lung of broiler chickens with developmental pulmonary hypertension due to cold stress. Br Poult Sci. 2006;47:223–229. doi: 10.1080/00071660600611169. [DOI] [PubMed] [Google Scholar]
- Tsutsayeva AA, Sevryukova LG. Effect of cold exposure on survival and stress protein expression of Drosophila melanogaster at different development stages. Cryo-Letters. 2001;22:145–150. [PubMed] [Google Scholar]
- Vander Heide RS (2002) Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 282:H935–H941 [DOI] [PubMed]
- Wang BW, Wu XP, Zhang XH, Jia XH, Zhang MA, Long FY, Yang ZG, Wang L. Expression and purification of goose HSP70 and compound formation with virus polypeptide. Agr Sci China. 2008;7:239–247. doi: 10.1016/S1671-2927(08)60045-0. [DOI] [Google Scholar]
- Wang JT, Li S, Li JL, Zhang JW, Xu SW. Effects of cold stress on the messenger ribonucleic acid levels of peroxisome proliferator-activated receptor-{gamma} in spleen, thymus, and bursa of Fabricius of chickens. Poult Sci. 2009;88:2549–2554. doi: 10.3382/ps.2009-00404. [DOI] [PubMed] [Google Scholar]
- Wang JW, Xu SW. Effects of cold stress on the messenger ribonucleic acid levels of corticotrophin-releasing hormone and thyrotropin-releasing hormone in hypothalami of broilers. Poult Sci. 2008;87:973–978. doi: 10.3382/ps.2007-00281. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen L, Hagiwara N, Knowlton AA. Regulation of heat shock protein 60 and 72 expression in the failing heart. J Mol Cell Cardiol. 2010;48:360–366. doi: 10.1016/j.yjmcc.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamboliev IA, Hedges JC, Mutnick JL, Adam LP, Gerthoffer WT (2000) Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP27 pathway. Am J Physiol Heart Circ Physiol 278:H1899–H1907 [DOI] [PubMed]
- Yu J, Bao E, Yan J, Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperon. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Lv ZH, Li JL, Li S, Xu SW, Wang XL. Effects of cold stress on nitric oxide in duodenum of chicks. Poult Sci. 2011;90:1555–1561. doi: 10.3382/ps.2010-01333. [DOI] [PubMed] [Google Scholar]
- Zheng M, Kang YM, Liu W, Zang WJ, Bao CY, Qin DN. Inhibition of cyclooxygenase-2 reduces hypothalamic excitation in rats with adriamycin-induced heart failure. PLoS One. 2012;7:e48771. doi: 10.1371/journal.pone.0048771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I, Siegel HS, Mashaly MM, Dunnington EA, Siegel PB. Inhibition of adrenal steroidogenesis, neonatal feed restriction, and pituitary-adrenal axis response to subsequent fasting in chickens. Gen Comp Endocrinol. 1995;97:49–56. doi: 10.1006/gcen.1995.1005. [DOI] [PubMed] [Google Scholar]