Abstract
Two porphyrins, CoTPPS and MnTMPyPCl5, were tested for their photodynamic activity and potential novel use in a therapy of human cancers. We investigated an effect of photodynamic reaction (PDR), electroporation (EP) and their combination (electro-photodynamic reaction [EP-PDR]) on human colon adenocarcinoma cell lines (LoVo and resistant to doxorubicin LoVoDX), human breast adenocarcinoma (wild type MCF-7/WT and resistant to doxorubicin MCF-7/DOX), and human melanoma (Me45). The efficiency of macromolecules transport was examined with cytofluorymetry by assessing the degree of propidium iodide (PI) penetration. Additionally, cellular ultrastructure after EP was evaluated. We determined cyto- and photo-cytotoxic effect on the cells viability (MTT assay) after standard PDR and PDR combined with EP. Intracellular distribution and mitochondrial colocalization of both porphyrins was also performed. The experiments proved that both complexes exhibit desirable photodynamic properties on LoVo LoVoDX cells, and EP effectively supports photodynamic method in this type of cancer. The application of EP provided shorter time of incubation (only 10 min) and enhanced effect of applied therapy. The porphyrins did not affect the MCF-7 and Me45 cell lines.
Keywords: Electroporation, Photodynamic reaction, Porphyrins, Colon adenocarcinoma
Introduction
Photodynamic therapy (PDT) is a technique of cancer treatment based on the effects of the light-sensitive agents. This method involves the combination of light, a photosensitizer and oxygen. It is based on the production of reactive oxygen species upon irradiation of the photosensitizer in the presence of oxygen. However, researchers are still developing new sensitizers with potential application in photodynamical treatment (Kulbacka et al. 2010; Saczko et al. 2008; Labanauskiene et al. 2007).
The therapeutic effect of porphyrins and their derivatives, in combination with visible light, have attracted much attention (Milgrom and O’Neill 1993; Grosseweiner 1994; Yslas et al. 2000). These compounds belong to a large class of fluorescent crystalline pigments, which are of natural or synthetic origin, having in common a substituted aromatic macrocyclic ring consisting of four pyrrole-type residues, linked together by four methine bridging groups. Porphyrins are verified as ideal photosensitizers because they are non-toxic, selectively retained in tumor tissue in high concentrations, water-soluble to a certain level, and cleared in a reasonable time from the body and rapidly from the skin, which prevents photosensitive reaction (Pushpan et al. 2002; Allison and Sibata 2010).
In some cases, PDT is not efficient enough and requires application of other enhancing methods (nanotechnology, electroporation [EP], etc.) (Allison et al. 2008, 2010). EP is a modern and versatile method that enables penetration of macromolecules from intercellular space into cells. Electropores generated with electromagnetic fields provide an additional way of a drug transport. Chemotherapy assisted by EP is known as electrochemotherapy (ECT) (Sersa et al. 2008; Escoffre and Rols 2012). Thus, ECT and PDT are low-invasive and targeted therapies. A combination of these therapies can allow a synergistic effect (Kotulska et al. 2013).
One of the most crucial elements of PDT is the ability of a photosensitizer to induce efficient transmembrane transport and the intracellular accumulation. Depending on a photosensitizer, its physicochemical properties, and uptake mechanisms, dyes can reach different intracellular concentrations and localize in different subcellular compartments, which may trigger various pathways of events (Teissie and Rols 1988; Chen et al. 2006). Understanding and controlling these pathways will allow for a better tailored therapy with a high specificity and increased efficiency.
The aim of our study was to assess the sensitivity to PDT, with two porphyrins never tested for their potential anticancer effect, on a few human cancer cells: human colon adenocarcinoma (LoVo and resistant to doxorubicin LoVoDX), human breast adenocarcinoma (MCF-7/WT and resistant to doxorubicin MCF-7/DOX), and human melanoma (Me45). We also tested the influence of EP on the therapeutic effects with these compounds.
Material and methods
Porphyrin dyes
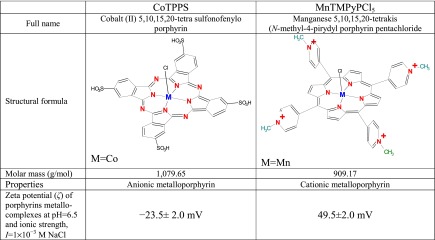
We used two porphyrins: an anionic metalloporphyrin CoTPPS and a cationic metalloporphyrin MnTMPyPCl5 (their structures and full names are presented in Table 1). Both porphyrins were purchased from Midcentury Chemicals.
Table 1.
Characteristics of the porphyrins selected for our study
Cell lines
The studies were performed on human adenocarcinoma cell lines: doxorubicin-sensitive (LoVo, MCF-7/WT) and doxorubicin-resistant (LoVoDX, MCF-7/DOX), and human melanoma (Me45). Colon adenocarcinoma cell lines were obtained from the Institute of Immunology and Experimental Therapy from Wroclaw. Human adenocarcinoma cells, MCF-7/WT and MCF-7/DOX, and melanoma cells Me45 were a kind gift from the Department of Tumor Biology, Comprehensive Cancer Center, Maria Sklodowska-Curie Memorial Institute (Gliwice, Poland). The colon adenocarcinoma cells were grown in Ham F-12 medium (Lonza) MCF-7 and Me45 cells were grown in Dulbecco's medium with addition of 10 % fetal bovine serum (Biowhittaker) and supplemented with antibiotics (penicillin/streptomycin; Sigma). For the experiments the cells were removed by trypsinization (Trypsin 0.025 % and EDTA 0.02 %; Sigma) and washed with PBS (Sigma). The cells were maintained in a humidified atmosphere at 37°C and 5 % CO2.
Spectroscopic assay
Solutions of porphyrins were dissolved in PBS and EtOH to a concentration of 6 μM. Spectra of absorbance were measured with EnSpire Multimode Reader (Perkin Elmer, Poland).
Zeta potential measurements
Electrophoretic mobility in Zetasizer nano ZS was measured using the Laser Doppler Velocimetry (LDV) technique (measurement range of 3 nm to 10 μm). In this technique, a voltage was applied across a pair of electrodes placed at both ends of a cell containing the particle dispersion. Charged particles were attracted to the oppositely charged electrode, and their velocity was measured and expressed per unit field strength as the electrophoretic mobility μe.
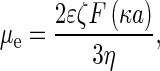 |
1 |
where ε is the dielectric constant of water and F(κa) is the function of dimensionless parameter κa, 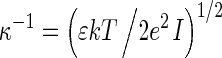 is the double-layer thickness, e is the elementary charge, k is the Boltzmann constant, T is the absolute temperature,
is the double-layer thickness, e is the elementary charge, k is the Boltzmann constant, T is the absolute temperature, 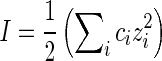 is the ionic strength, ci is the ion concentrations, and a is the characteristic dimension of the particle. Electrophoretic mobility was determined at fixed pH range and ionic strength, regulated by addition of NaCl. Then, the zeta potential was calculated using Henry’s equation
is the ionic strength, ci is the ion concentrations, and a is the characteristic dimension of the particle. Electrophoretic mobility was determined at fixed pH range and ionic strength, regulated by addition of NaCl. Then, the zeta potential was calculated using Henry’s equation
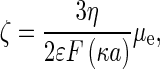 |
2 |
where ζ is the zeta potential of the porphyrin. Data are presented in Table 1.
Transmission electron microscope (TEM)
The ultrastructural analysis after EP was examined by TEM Zeiss EM 900. After EP, the cells were fixed for 30 min in 2.5 % (vol/vol) glutaraldehyde and 0.1 M phosphate buffer (pH 7.4). After postfixation in 1 % (wt/vol) osmium tetroxide, cells were dehydrated through a graded series of alcohol and propylene oxide and embedded in Epon. The Epon blocks were cut on the ultramicrotome (Ultracut E, Reihert, Germany). Ultrathin sections were contrasted with uranyl acetate and lead citrate according to the method described by Skolucka et al. (2011) and examined with a TEM Zeiss EM 900 (Carl Zeiss, Oberkochen, Germany).
Electroporation
EP was carried out with ECM 830 Square Wave Electroporation System (BTX, purchased from Syngen Biotech, Poland). The EP method was selected based on our previous experiments (Saczko et al. 2010; Kulbacka et al. 2011). We applied electrical pulses with magnitude 250, 1,250 and 2,500 V/cm, 50 μs long, in the series of five impulses. As electrodes we used two thin aluminum parallel plates, 4 mm apart. They were connected to the voltage generator and produced a uniform electric field in the cuvette (Cuvettes Plus 640, 800 μl). Cells in suspension were centrifuged for 3 min at 537 × g and resuspended in the EP buffer with low electrical conductivity (10 mM phosphate, 1 mM MgCl2, 250 mM sucrose, pH 7.4) (Saczko et al. 2010). After pulsation, cells were left for 10 min with addition of 1,800 μl culture medium, then washed and centrifuged twice with culture medium, and seeded into 96-well microculture plates for the MTT assay.
Electroporation efficiency — iodide propidium and porphyrins uptake (FACS)
Electropermeabilization of cells was quantified by the penetration of impermeant dye. Immediately before EP cells were put into propidium iodide (PI; P4170, Sigma) or porphyrins: CoTPPS or MnTMPyPCl5. The concentration of PI in cuvette in the EP buffer was 10 μmol/l, and the concentration of porphyrins was 6 μmol/l. After EP, cells were incubated for 15 min (PI) or 10 min (porphyrins) at 37 °C in a humidified atmosphere containing 5 % CO2. Then, cells were washed twice in PBS and resuspended in 1 ml PBS. Samples were analyzed immediately after electropermeabilization on FACS Calibur flow cytometer (Becton Dickinson). At least 50,000 viable cells were measured from each sample at a rate of up to 1000 cells/s. The samples were excited using the 488-nm line of an argon laser and red detection of fluorescence was performed at 650 nm. For porphyrins, the samples were excited using 530 nm line. Light-scatter and fluorescence measurements were used as an indication of object size and shape, allowing discrimination between cells, microspheres, and debris. Data were analyzed using CellQuest software (Becton Dickinson) and presented as histograms, as well as the geometric mean (GMean) fluorescent emission intensities of positive cells.
Photodynamic reaction (PDR)
The phototoxic effect of the two dyes was determined after 1 and 4 h of incubation with 6 and 12 μM concentration of applied compounds. Then the cultures were irradiated and culture medium was exchanged. Both porphyrins were irradiated using a lamp (OPTEL Fiber Illuminator, Opole, Poland) with polarized light (fluence at the level of the cell monolayer — 10 mW/cm2) and a two filters (λmax=435 and 530 nm). Twenty-four hours after irradiation, the MTT assay was performed.
Electroporation and photodynamic reaction (EP-PDR)
With standard PDR cells were incubated for 1 and 4 h before irradiation. The cells were irradiated with a lamp (OPTEL Fiber Illuminator, Opole, Poland) with polarized light (fluence at the level of the cell monolayer — 10 mW/cm2) and two filters (λmax=435 and 530 nm). Cells were incubated for 3 min in the presence of porphyrins in the dark. Then the EP with selected parameters (1,250 V/cm, 50 μs and five impulses) was applied and the cells were left for 10 min with addition of 1,800 μl culture medium. Next, the cells were irradiated for 10 min by the light of intensity 1.9 J/cm2. All irradiations were performed at room temperature. Finally, the cells were washed and centrifuged twice with a fresh culture medium and seeded into 96-well microculture plates for the MTT assay.
MTT assay
After standard PDR and PDR combined with EP we determined the cells viability with MTT assay. The MTT assay (Sigma) was used to test mitochondrial metabolic functioning. Cells were seeded into 96-well microculture plates at 1×104 cells/well and allowed to attach overnight. After incubation with various concentrations of the porphyrins, the assay was performed according to the manufacturer’s protocol. The absorbance was measured using a multiwell scanning spectrophotometer at 570 nm (Multiscan MS microplate reader). The result was expressed as the percentage of viable cells relative to untreated control cells.
Intracellular localization
Microcultures were trypsinized from the culture flasks and seeded on cover glasses (24x24 mm, Thermo Scientific) in 35 mm Petri dishes (Nunc). The cells were incubated with the compounds (6 μM) for 1 h. In case of EP-treated cells, the medium was removed and 1 ml of EP buffer with porphyrin (6 μM) was added; adherent cells were pulsed on the cover glasses, using Petri Pulser applicator (BTX Harvard Apparatus, purchased from Syngen Biotech, Poland) with ECM 830 generator. After 10 min of incubation in 37°C, the buffer was removed. Then the cells were stained with MitoTracker® Deep Red FM (1 μM, Molecular Probes®, Life Technologies) for 15 min. After mitochondrial staining the cells were washed in PBS, fixed in 4 % buffered formalin (Polysciences, Inc.) and washed in PBS. The cells were examined under a confocal scanning laser microscope (Carl Zeiss GmbH, Jena, Germany). The porphyrin dyes emission was observed using the 575–754 nm with the 475–565 nm excitation filter. The MitoTracker® Deep Red FM was excited with 644 nm and detected with emission filter: 665 nm.
Statistics
Data were analyzed statistically with Microsoft Office Excel 2007. Each outcome is a mean of results measured for at least three samples. The results of the MTT assays were reported as means ± SD. Statistical significance was determined by Pearson chi-square: p < 0.05 or p < 0.005 values (two-sided test) were assumed as statistically significant. The section of statistical analysis concerned samples after performed [electrochemical/photodynamical/both] reaction(s) in relation to untreated control cells. The main hypothesis of this analysis was whether or not two different populations (control and treated) are different enough in some aspect of their behavior.
Results
Spectroscopic studies of porphyrins
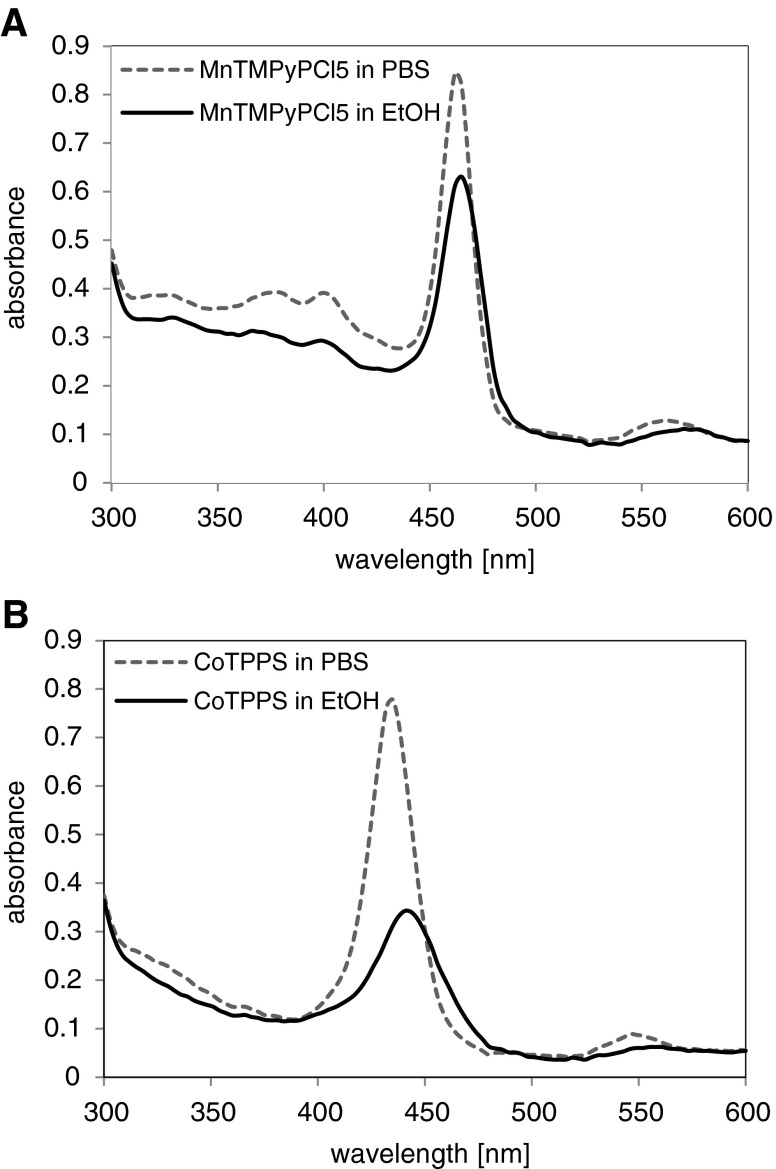
Two absorption bands can be observed in the absorption spectra of the two porphyrins (CoTPPS and MnTMPyPCl5) in EtOH and PBS solutions. The recorded spectra are presented in Fig. 1a and b. The absorption bands were located at shorter wavelengths of about 450 nm (CoTPPS: 440 nm in EtOH and 435 nm in PBS), and at longer wavelengths of about 550 nm (MnTMPyPCl5: 570 nm in EtOH and 561 nm in PBS). As shown in Fig. 1a and b, the absorbance peaks recorded for solutions in EtOH had “red shift” and revealed decreased values of absorbance. The porphyrins absorbance spectrum is sensitive to the environment of the porphyrins. Changes in acidity, hydrophobicity, ion content, etc., can reveal an increase or decrease of the absorbance intensity; thus, we suppose that the observed redshift and decreased absorbance are involved in this dependence, which can be related to acidity of the environment.
Fig. 1.
Absorbance spectrum of a MnTMPyPCl5 and b CoTPPS in PBS and EtOH solutions
Cytotoxicity and photodynamic reaction
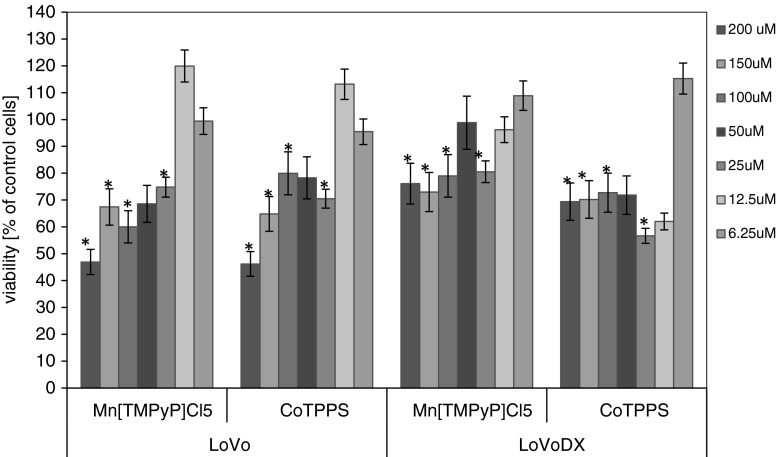
Among the five cell lines tested in our study, two colon adenocarcinoma lines showed sensitivity to the treatment with MnTMPyPCl5 and CoTPPS porphyrins. The breast adenocarcinoma cell line (MCF-7/WT and MCF-7/DOX) and melanoma cell line (Me45) were not affected by the porphyrins, neither in the standard PDR nor EP-PDR experiments (data not shown). Figure 2 shows the cytotoxicity of MnTMPyPCl5 and CoTPPS in LoVo and LoVoDX cell lines. The proliferation assay performed after 24 h revealed which dye concentration was not cytotoxic and could be included for further examinations. As we could also observe, LoVoDX cell line was more sensitive to the applied porphyrins.
Fig. 2.
The cytotoxicity assay performed in human colon adenocarcinoma cell lines after 24 h of incubation. Each bar represents the mean of at least four separate experiments; bars are the standard errors, *p ≤ 0.05
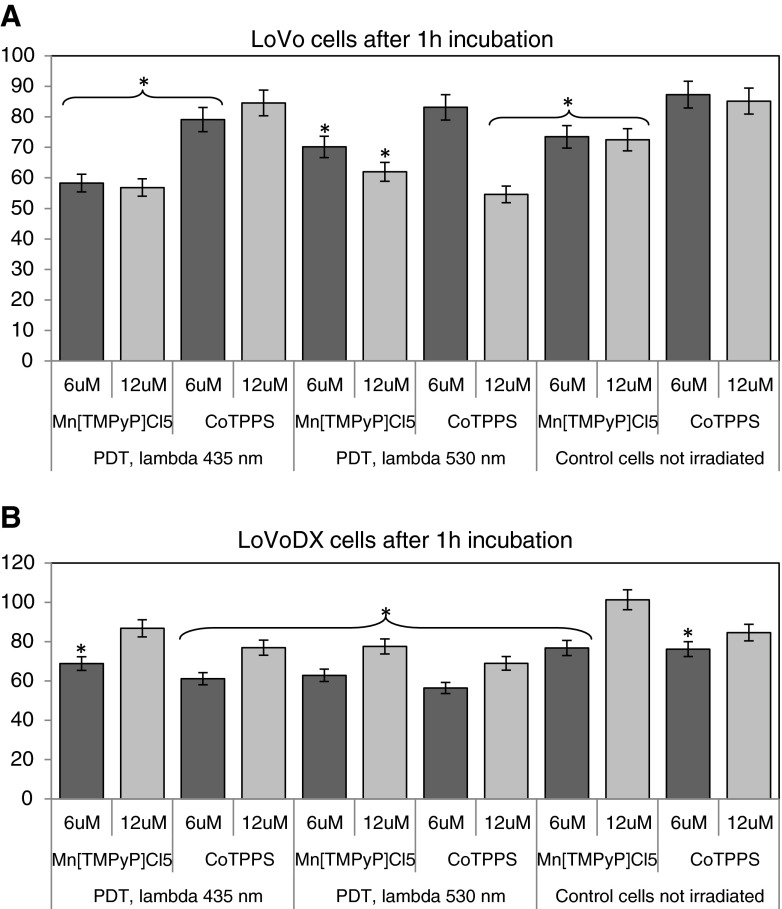
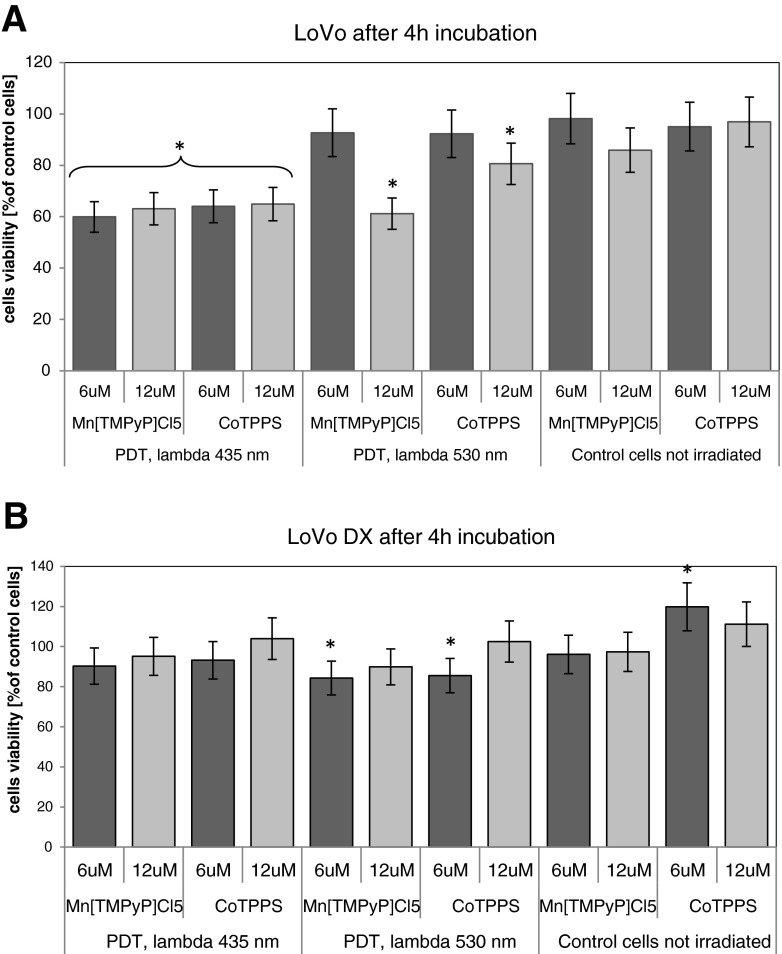
In the standard PDR experiment, cells were incubated for 1 or 4 h before irradiation. The results of the standard PDR are presented in Figs. 3 and 4a and b. After 1 h of incubation with a dye, the cells viability after irradiation with 430 nm reached about 60 % for LoVo cells and 60–80 % in case of LoVoDX cells, for both dyes. The results obtained for LoVo cells present over 20 % decrease (relative to control untreated cells) after irradiation of MnTMPyPCl5 at λ=435 nm. In case of irradiation with 530 nm, the photodynamic effect was not significant. After irradiation after 1 h with 530–550 nm, for LoVo/DX and CoTPPS, the cells reached 44.2 % of control cells viability. The porphyrin CoTPPS induced the decrease of cells proliferation only at the concentration of 12 μM and λ=530 nm.
Fig. 3.
The photocytotoxicity assay performed in a LoVo and b LoVoDX cells. Cells were incubated with porphyrins during 1 h and then irradiated with two wavelengths (435 and 530 nm), then 24 h of incubation without the compound was followed. Each bar represents the mean of at least three separate experiments; bars are the standard errors, *p ≤ 0.005
Fig. 4.
The photocytotoxicity assay performed in a LoVo and b LoVoDX cells. Cells were incubated with porphyrins during 4 h and then irradiated with two wavelengths (435 and 530 nm) then 24 h of incubation without the compound followed. Each bar represents the mean of at least four separate experiments; bars are the standard errors, *p ≤ 0.005
Interestingly, in the case of resistant LoVoDX cells, after 4 h of incubation the PDR effect was minor (only 10–15 % decrease in comparison with dark control cells). It could indicate that the pump out effect of the drug, often occurring in the cells with drug resistance, came to the fore after a prolonged of exposure to the drug.
Electropermeabilization (EP) of colon adenocarcinoma cells
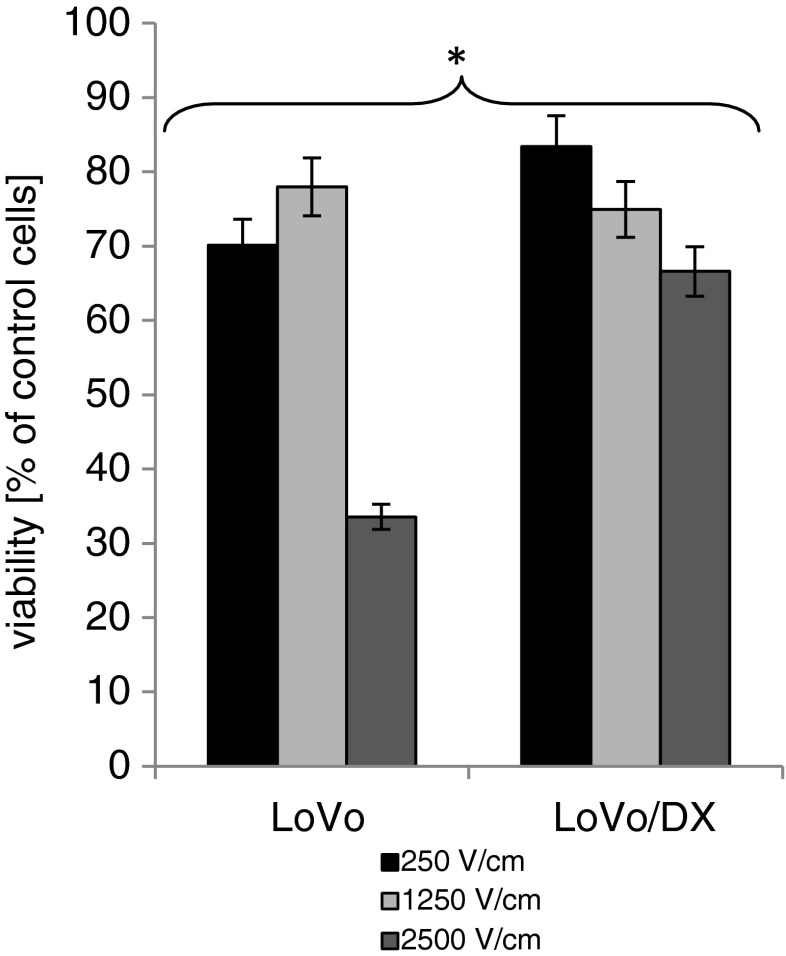
Figure 5 shows the influence of EP parameters for LoVo and LoVoDX cells. EP at 1,250 V/cm (50 μs and five impulses) turned out as relatively non-toxic: 78 % for LoVo and 75 % for LoVoDX, and was selected for further experiments. EP at 2,500 V/cm reduced cells proliferation to 33 % in LoVo cells and to 66 % in resistant cell line. The cell line sensitive to doxorubicin was usually more sensitive to external electric field.
Fig. 5.
The influence of electroporation parameters on human colon cancer cell lines performed by MTT assay. Electroporation parameters: 250, 1,250 and 2,500 V/cm, 50 μs, five impulses; cells were electroporated and 24 h of incubation followed. Each bar represents the mean of at least four separate experiments; bars are the standard errors, *p ≤ 0.05
Intracellular distribution of MnTMPyPCl5 and CoTPPS — CLSM study
Intracellular distribution of MnTMPyPCl5 and CoTPPS is presented in Fig. 6. We detected stronger fluorescence signal in the case of MnTMPyPCl5 dye for both cell lines. This porphyrin was dispersed throughout cytoplasm and in cellular compartments. As we could observe, the fluorescence intensity of Mn-porphyrin increased proportionally to the electric field parameters. There was a noticeable colocalization with cellular mitochondria in LoVo cells. A much weaker intensity was demonstrated with CoTPPS in both cell lines; however, in resistant cells the fluorescent signal was stronger and dispread in cytoplasm.
Fig. 6.
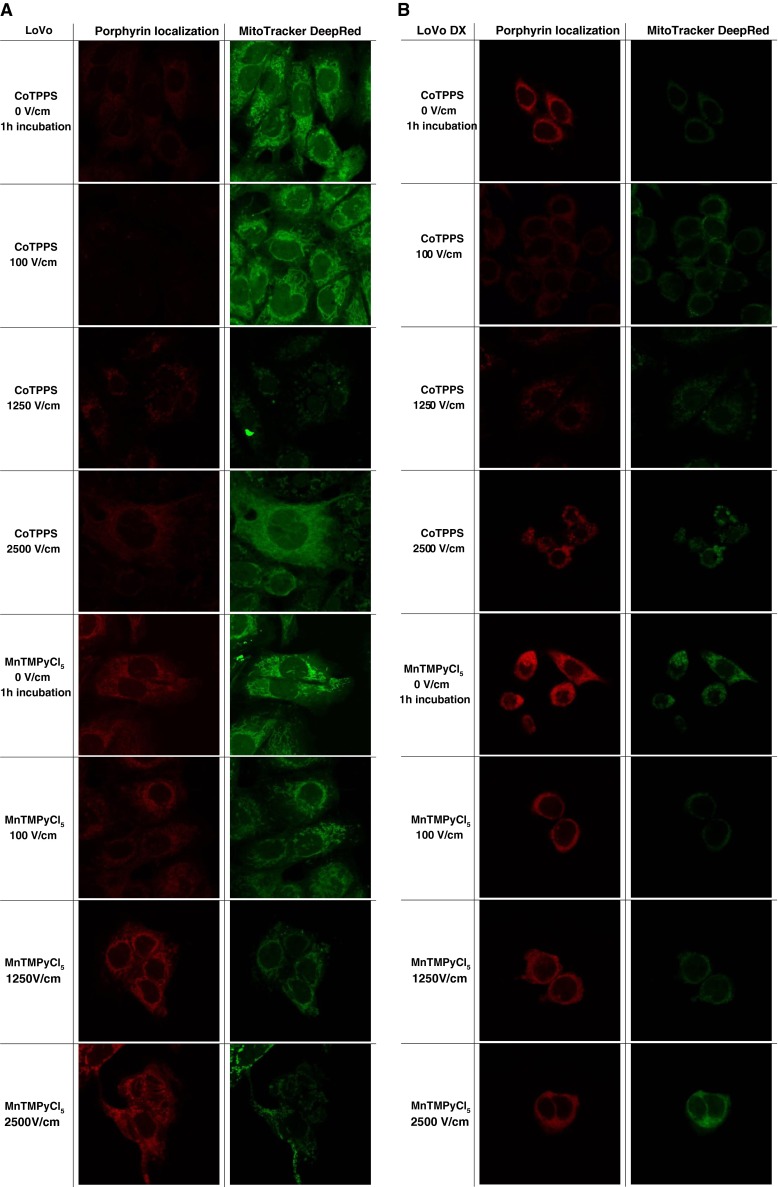
Intracellular distribution of porphyrins: MnTMPyCl5 and CoTPPS in a LoVo and b LoVoDX cells without electroporation after 1 h incubation, and with EP after 10 min incubation with dye
FACS analysis — IP and porphyrins uptake
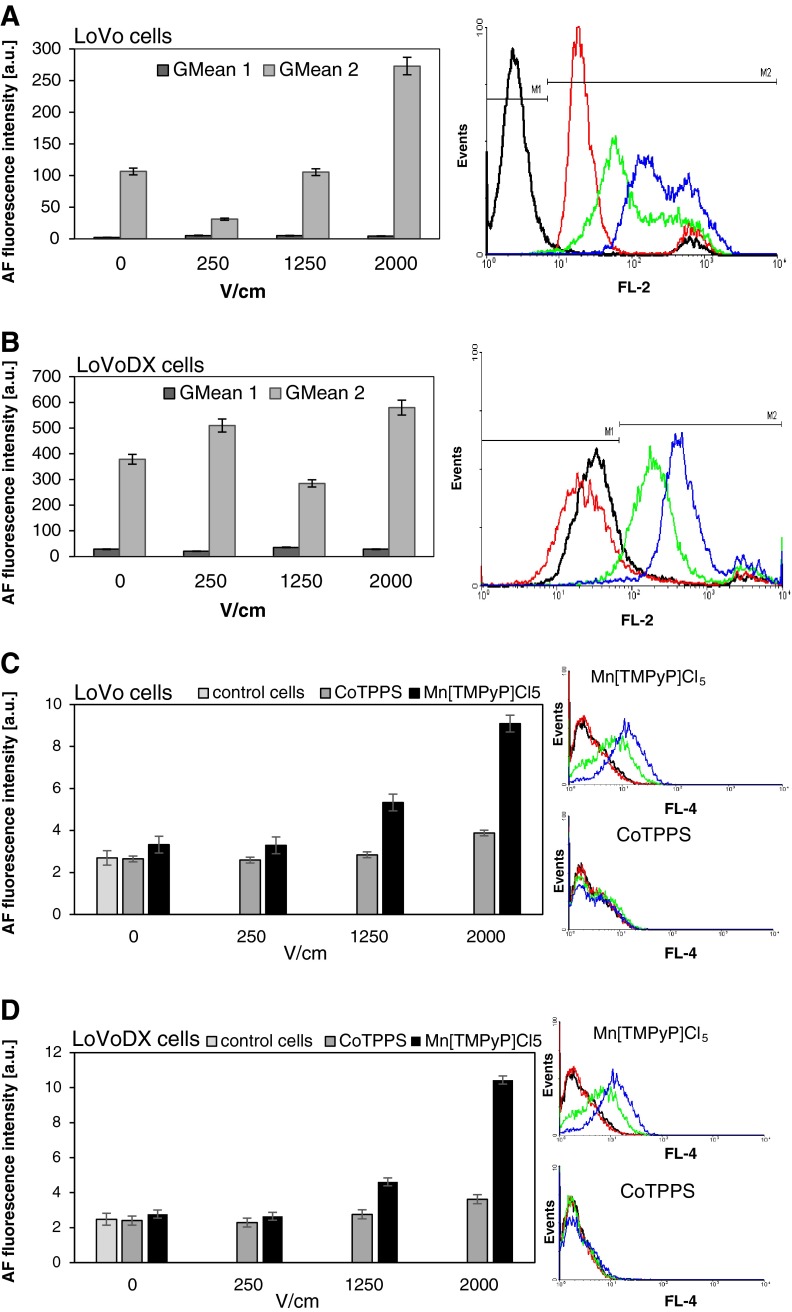
FACS analysis, presented in Fig. 7a–d, showed the efficiency of applied EP parameters in human colon adenocarcinoma cell lines. As observed, in LoVo cells the higher electric field the greater intensity of PI signal. This proves better PI transport into cells. In the case of LoVoDX cells, we observed a similar but weaker effect (Fig. 7a). The uptake of porphyrins revealed a better efficiency in the case of MnTMPyPCl5. FACS analysis demonstrated the increased dye uptake with increasing electrical field parameters. In the case of CoTPPS, no significant effect was observed.
Fig. 7.
Flow cytometry analysis of human colon adenocarcinoma cells treated with iodide propidium (PI) after electroporation: a LoVo and b LoVoDX; treated with both porphyrins (CoTPPS and Mc[TMPyP]Cl5) after electroporation: c LoVo and d LoVoDX. Diagram represents the GMean (MFI) intensity signal of PI/porphyrins control samples and delivered to the cells after electroporation at different voltages 0, 250, 1,250, and 2,000 V/cm (black, red, green, blue and purple line, respectively), * p ≤ 0.05
TEM — ultrastructural analysis after EP
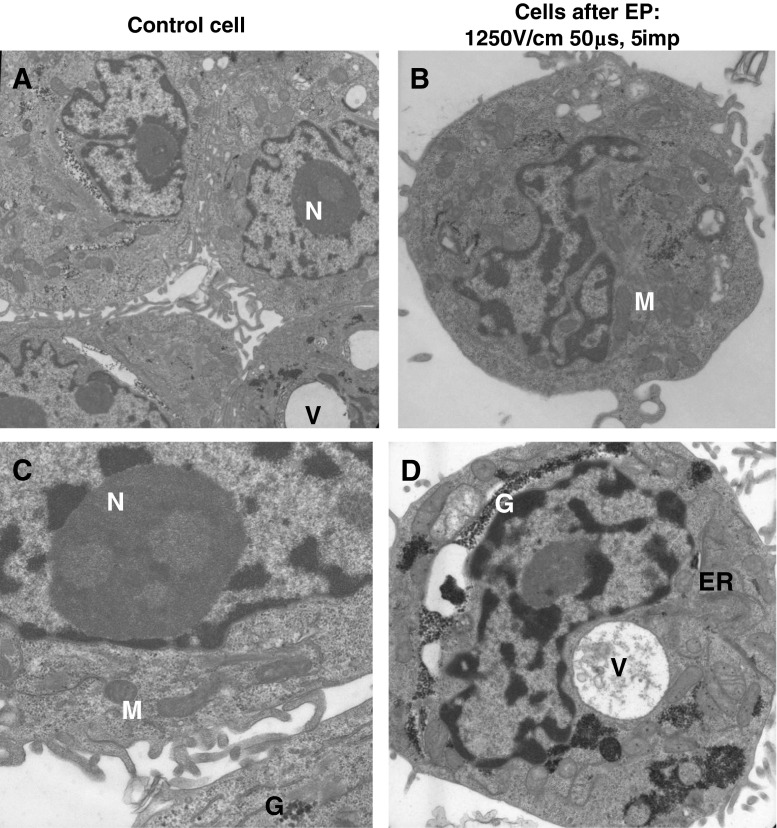
With selected parameters, we conducted an ultrastructural examination of both human colon adenocarcinoma cell lines. Electrograms presented in Fig. 8a–d were performed for control cells and cells treated with external field (1,250 V/cm). Electrograms for control cells of both cell lines were normal without any major alterations. In LoVo cells we observed non-significant changes after application of 1,250 V/cm. We could see a lot of heterochromatin; myelin bodies (MB), several vacuoles and normal mitochondria. In the case of LoVoDX cells, translucent mitochondrial matrix appeared after electropermeabilization, the cristae were swollen and lysed. Some vacuoles containing electron dense material (EDM) also occurred. In the resistant cell line, we could also observe many deposits of glycogen.
Fig. 8.
Ultrastructure of human colon adenocarcinoma a LoVo control cells, magnification ×7,000; b LoVo cells after EP, magnification ×12,600; c LoVoDX control cells, magnification ×20,600; d LoVoDX cells after EP, magnified ×12,600. N nucleus, M mitochondria, ER endoplasmic reticulum, V vacuoles, MB myelin body, G glycogen
PDR-EP study in human cancer cells
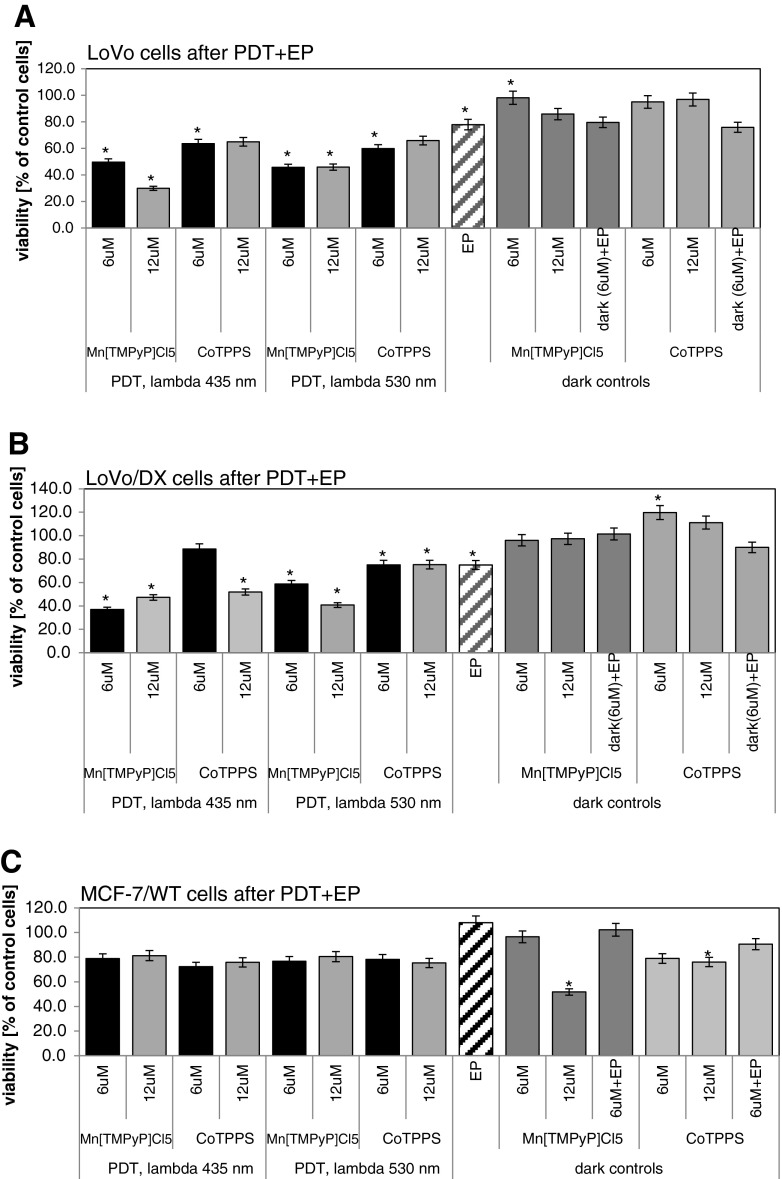
Figure 9a–e present the results obtained from the combined “therapy”: EP with PDR. The results show that the effect of porphyrins in dark condition on electroporated cells is usually smaller (in some cases significantly) than that in irradiated experiment, which proves the therapeutic potential of the method. In the case of LoVo cells (Fig. 9a), at 6 μM of MnTMPyPCl5, the dark EP control cells showed 79.7 % viability, while in the full irradiated experiment only 45.8 % (530 nm) and 49.6 % (435 nm) survived. For LoVoDX cells (Fig. 9b), the effect was even more significant — at 6 μM of MnTMPyPCl5, the dark EP control cells showed 101.5 % of viability while in the full irradiated experiment only 58.8 % (530 nm) and 37.0 % (435 nm) survived. In the case of 12 μM of MnTMPyPCl5 irradiated with 435 nm, irradiation proliferation was reduced to 30 % of LoVo cells, and over 60 % for LoVoDX cells (Fig. 9).
Fig. 9.
The photodynamic reaction in combination with electroporation performed on a LoVo and b LoVoDX cell lines. After electroporation with parameters: 1,250 V/cm, 50 μs, five impulses; cells were incubated with porphyrins during 20 min then irradiated with two wavelengths (435 and 530 nm) then 24 h of incubation without the compound followed. Each bar represents the mean of at least four separate experiments; bars are the standard errors, *p ≤ 0.05
CoTPPS revealed a weaker effect — at 6 μM, the dark EP control cells showed 75.9 % of viability while in the full irradiated experiment only 59.9 % (530 nm) and 63.7 % (435 nm) LoVo cells survived. For LoVoDX cells no significant effect was observed — at 6 μM of CoTPPS, the dark EP control cells showed 90 % of viability while in the full irradiated experiment only 75.2 % (530 nm) and 88.6 % (435 nm) survived. After EP application to LoVo cells with CoTPPS at 12 μM and irradiation with 435 nm, cell viability decreased to about 65 % of control cells (Fig. 9a) and for LoVoDX to 52 % (Fig. 9b).
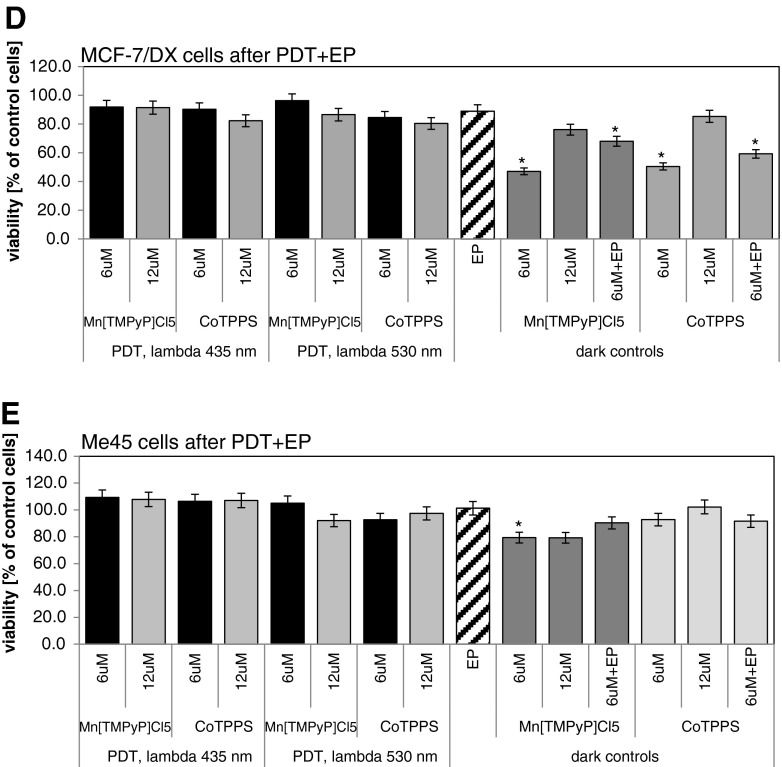
In the case of other treated cell lines, no significant effect of combination EP with PDR was observed. For breast adenocarcinoma cells, wild type (MCF-7/WT), after therapy 80 % of control was achieved (Fig. 9c); for resistant cells (MCF-7/DX,) 90 % of control cells (Fig. 9d). Human melanoma cells (Me45) were extremely resistant for applied EP+PDR reaction and reached over 100 % of control cells (Fig. 9e).
Discussion
Photodynamic treatment is a commonly applied therapeutic method. However, researchers are still seeking new photosensitizing drugs or techniques for their intracellular delivery. In this study, we suggest EP as an effective method for a photosensitizer transport into cancer cells. EP involves the application of short high-voltage pulses to permeabilize the cell membrane. By applying an external electric field, which surpasses the capacitance of the cell membrane, transient and reversible breakdown of the membrane can be induced (Gehl 2003; Breton and Mir 2012). Enhancing PDT of cancer by electropremeabilization increases the therapeutic efficiency of PDT. What is more, this combined therapy is low invasive and selective. In our previous study the effective action of EP with PDR with hematoporphyrin derivative (HpD) was proven (Skołucka et al. 2010; Saczko et al. 2010; Kulbacka et al. 2011). The electro-photodynamic therapy applied to lung cancer cells (A549) revealed a significant increase of the cytotoxic effect on cells when compared to HpD-PDT alone, even at drug doses significantly lower than typical levels. The results also demonstrated that the drug was delivered within minutes, instead of hours as in standard PDT (Skolucka et al. 2011). Labanauskiene et al. (2009) examined Chinese hamster lung fibroblast cell line (DC-3F) (2007) and murine hepatoma MH22A cells. Cells were affected by photosensitizers chlorin e(6) and aluminium phthalocyanine tetrasulfonate (AlPcS4).These data showed that the cytotoxicity of PDT in combination with EP increased the efficacy 4-fold on the average (Labanauskiene et al. 2007, 2009). For the review of combined application of PDR and EP, see Kotulska et al. (2013).
Some authors examined the usefulness of ECT against colorectal cancer (CRC) using a mouse model. Kuriyama et al. (2000) applied bleomycin (BLM), 5-fluorouracil (5-FU) and cisplatin in combination with EP. For this type of cancer, they obtained a positive treatment effect only for BLM (Kuriyama et al. 2000). Edhemovic et al. (2011) described a case of a successful treatment of a solitary metastasis of colorectal cancer in the liver. The procedure was performed intraoperatively by inserting long needle electrodes, two in the center of the tumor and four around the tumor into the normal tissue. The authors obtained good antitumor effectiveness with complete tumor destruction, which was confirmed with a histological analysis (Edhemovic et al. 2011).
In our work, we examined five human cancer cell lines (colon and breast adenocarcinoma, and melanoma) for treatment with compounds from a group of porphyrins: CoTPPS and MnTMPyPCl5, which have not been tested in PDR so far. The cell lines of colon adenocarcinoma (LoVo and LoVoDX) showed therapeutic sensitivity to the porphyrins combined with EP. The application of EP provided shorter time of incubation (only 10 min) and enhanced the effects of applied therapy. The increase of the electric field intensity induced higher permeability of cell membranes without any significant changes in the cellular ultrastructure, which was proved with TEM analysis.
In case of LoVo and LoVoDX cells, the synergistic effect of EP and photodynamic treatment was reported. The porphyrin MnTMPyPCl5 with EP gave the best results. In particular, the doxorubicin resistant cell line irradiated with 435 nm showed 3-fold decrease of the cell viability, as compared to its dark electroporated control.
The method with new porphyrins proposed in our work could become an option for reducing the surgery area or necessity for protecting the organism from the severe side effects of systemic chemotherapy, which also affects healthy cells of the organism. The therapy could provide an option for a low invasive and targeted treatment of high efficiency. The in vitro studies on internal organ cancer cells show potential applicability of this therapeutic approach. These results demonstrate the problem validity of the effective delivery of anticancer substances into the cancer cells, especially those with multidrug resistance (MDR). Some cancers (particularly from the gastrointestinal tract) are characterized by acquired resistance, and part of the tumor develops resistance to drugs due to prolonged chemotherapy. The introduction of a new and low invasive treatment of tumors of this type is extremely important for the success of cancer treatment and patient comfort.
Acknowledgments
This work was supported by National Science Center; grant No. 2011/01/D/NZ4/01255 (J. Kulbacka).
Abbreviations
- PDR
Photodynamic reaction
- PDT
Photodynamic therapy
- EP
Electroporation
- ECT
Electrochemotherapy
- PI
Propidium iodide
- MB
Myelin bodies
- EDM
Electron dense material
References
- Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis Photodyn Ther. 2010;7(2):61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Allison RR, Mota HC, Bagnato VS, Sibata CH. Bio-nanotechnology and photodynamic therapy—state of the art review. Photodiagnosis Photodyn Ther. 2008;5(1):19–28. doi: 10.1016/j.pdpdt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Allison RR, Bagnato VS, Sibata CH. Future of oncologic photodynamic therapy. Future Oncol. 2010;6(6):929–940. doi: 10.2217/fon.10.51. [DOI] [PubMed] [Google Scholar]
- Breton M, Mir LM. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnet. 2012;33:106–123. doi: 10.1002/bem.20692. [DOI] [PubMed] [Google Scholar]
- Chen C, Smye SW, Robinson MP, Evans JA. Membrane electroporation theories: a review. Med Biol Eng Comput. 2006;44:5–14. doi: 10.1007/s11517-005-0020-2. [DOI] [PubMed] [Google Scholar]
- Edhemovic I, Gadzijev EM, Brecelj E, Miklavcic D, Kos B, Zupanic A, Mali B, Jarm T, Pavliha D, Marcan M, Gasljevic G, Gorjup V, Music M, Vavpotic TP, Cemazar M, Snoj M, Sersa G. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10(5):475–485. doi: 10.7785/tcrt.2012.500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffre JM, Rols MP. Electrochemotherapy: progress and prospects. Curr Pharm Des. 2012;18(23):3406–3415. doi: 10.2174/138161212801227087. [DOI] [PubMed] [Google Scholar]
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Grosseweiner LI (1994) The science of phototherapy. In: Photodynamic therapy. CRC Press, London, pp 139–155
- Kotulska M, Kulbacka J, Saczko J (2013) Advances in photodynamic therapy assisted by electroporation. Curr Drug Metab 14(No. 3), in press [DOI] [PubMed]
- Kulbacka J, Chwilkowska A, Bar J, Pola A, Banas T, Gamian A, Saczko J. Oxidative alterations induced in vitro by the photodynamic reaction in doxorubicin-sensitive (LoVo) and -resistant (LoVoDX) colon adenocarcinoma cells. Exp Biol Med (Maywood) 2010;235(1):98–110. doi: 10.1258/ebm.2009.009162. [DOI] [PubMed] [Google Scholar]
- Kulbacka J, Nowak M, Skołucka N, Saczko J, Kotulska M. The influence of electroporation on in vitro photodynamic therapy of human breast carcinoma cells. Folia Biol (Praha) 2011;57(3):112–118. doi: 10.14712/fb2011057030112. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Matsumoto M, Mitoro A, Tsujinoue H, Nakatani T, Fukui H, Tsujii T. Electrochemotherapy for colorectal cancer with commonly used chemotherapeutic agents in a mouse model. Dig Dis Sci. 2000;45(8):1568–1577. doi: 10.1023/A:1005565027969. [DOI] [PubMed] [Google Scholar]
- Labanauskiene J, Gehl J, Didziapetriene J. Evaluation of cytotoxic effect of photodynamic therapy in combination with electroporation in vitro. Bioelectrochem. 2007;70(1):78–82. doi: 10.1016/j.bioelechem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Labanauskiene J, Satkauskas S, Kirveliene V, Venslauskas M, Atkocius V, Didziapetriene J. Enhancement of photodynamic tumor therapy effectiveness by electroporation in vitro. Med (Kaunas) 2009;45(5):372–377. [PubMed] [Google Scholar]
- Milgrom LR, O’Neill F. The chemistry of natural products. In: Thomson RH, editor. Porphyrins. London: Blackie; 1993. pp. 329–376. [Google Scholar]
- Pushpan SK, Venkatraman S, Anand VG, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar TK. Porphyrins in photodynamic therapy — a search for ideal photosensitizers. Curr Med Chem Anticancer Agents. 2002;2(2):187–207. doi: 10.2174/1568011023354137. [DOI] [PubMed] [Google Scholar]
- Saczko J, Chwiłkowska A, Kulbacka J, Berdowska I, Zieliński B, Drąg-Zalesińska M, Wysocka T, Ługowski M, Banaś T. Photooxidative action in cancer and normal cells induced by the use of Photofrin® in photodynamic therapy. Folia Biol (Praha) 2008;54:24–29. doi: 10.14712/fb2008054010024. [DOI] [PubMed] [Google Scholar]
- Saczko J, Nowak M, Skołucka N, Kulbacka J, Kotulska M. The effects of the electro-photodynamic in vitro treatment on human lung adenocarcinoma cells. Bioelectrochem. 2010;79:90–94. doi: 10.1016/j.bioelechem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34(2):232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Skolucka N, Daczewska M, Saczko J, Chwilkowska A, Choromanska A, Kotulska M, Kaminska I, Kulbacka J. ETM study of electroporation influence on cell morphology in human malignant melanoma (Me-45) and human primary gingival fibroblast (HGFs) cells. Asian Pacific J Trop Biomed. 2011;2:1–5. doi: 10.1016/S2221-1691(11)60003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skołucka N, Saczko J, Kotulska M, Kulbacka J. Elektroporacja i jej zastosowanie, (Electroporation and its application) Pol Merk Lek. 2010;XXVIII(168):501–504. [PubMed] [Google Scholar]
- Teissie J, Rols MP. Electropermeabilization and electrofusion of cells. In: Latruffe N, Gaudemer Y, Vignais P, Azzi A, editors. Dynamic of membrane proteins and cellular energetics. Berlin: Springer Verlag; 1988. pp. 249–268. [Google Scholar]
- Yslas I, Alvarez MG, Marty C, Mori G, Durantini EN, Rivarola V. Expression of Fas antigen and apoptosis caused by 5,10,15,20-tetra(4-methoxyphenyl)porphyrin (TMP) on carcinoma cells: implication for photodynamic therapy. Toxicology. 2000;149:69–74. doi: 10.1016/S0300-483X(00)00221-3. [DOI] [PubMed] [Google Scholar]