Summary
Background:
The purpose of this study was to determine the specific and detailed anatomic sites and morphologic characteristics of mediastinal lymph nodes on spiral computed tomography for the purpose of differentiation between sarcoidosis and Hodgkin’s lymphoma.
Material/Methods:
Anatomical distribution of mediastinal lymph nodes on spiral CT was reviewed in 39 patients with sarcoidosis and 37 patients with Hodgkin’s lymphoma using the International Association for the Study of Lung Cancer (IASLC) lymph node map. Other morphologic features such as lymph node calcification or coalescence of adjacent lymph nodes were also compared.
Results:
Zone 10 was involved more often in sarcoidosis than in Hodgkin’s lymphoma. On the other hand, there was a higher tendency for presence of zone 1 and 3 as well as retrocrural and internal mammary lymphadenopathy in Hodgkin’s lymphoma than in sarcoidosis. Sarcoidosis presented with intranodal calcifications more often than Hodgkin’s lymphoma. Coalescence, pressure effect on adjacent structures and central cavitations were significantly more common in Hodgkin’s lymphoma.
Conclusions:
Findings of the present study indicate that specific anatomical distribution and morphological patterns of mediastinal lymph nodes, as demonstrated on spiral CT, can be useful in differentiating sarcoidosis from Hodgkin’s lymphoma.
Keywords: sarcoidosis, Hodgkin’s lymphoma, lymphadenopathy, IASLC lymph node map, spiral CT scan
Background
Sarcoidosis is a systemic disorder of unknown etiology with a wide variety of clinical and radiological manifestations. Pathologically, the disease is characterized by presence of widespread non-caseating granulomas. These granulomas, however, are not unique to sarcoidosis and can be seen in other conditions including tuberculosis, fungal disease, berylliosis, and in association with carcinoma and lymphoma [1].
Clinical manifestations of sarcoidosis are widespread, but the lung and intrathoracic lymph nodes are almost universally affected. Among patients with sarcoidosis, 75–90% will present with mediastinal and hilar lymphadenopathy. In fact, the most frequent radiological abnormality involves enlarged bilateral hilar and right paratracheal lymph nodes [2].
Computed tomography (CT) is superior to chest radiography for delineating the full extent of lymphadenopathy in the mediastinum [3,4].
Although sarcoidosis classically involves the right paratracheal and hilar lymph nodes, CT scan often demonstrates enlargement of lymph nodes in previously unsuspected locations including the anterior mediastinum, axillary, internal mammary chain and retrocrural and infradiaphragmatic locations [3–5].
On the other hand, lymphoma is a systemic disease where the mediastinal lymph nodes are often involved, and it is easily confused with sarcoidosis. Parenchymal presentations of lymphoma and sarcoidosis are similar and indistinguishable as well.
It is extremely important for clinicians and radiologists to be able to distinguish between these two entities in a clinical setting. With a CT scan, anatomical sites and characteristics of enlarged lymph nodes can be accurately determined. To the best of authors’ knowledge, a detailed comparison between sarcoidosis and lymphoma regarding anatomical sites of mediastinal lymph nodes on CT scan has not yet been reported.
In the present study, spiral CT scan features were retrospectively reviewed in a group of 39 patients with sarcoidosis and 37 cases of mediastinal Hodgkin’s lymphoma for the purpose of assessing the differences in anatomical distribution and imaging characteristics of mediastinal lymphadenopathy between the two entities.
Material and Methods
Thirty-nine consecutive patients with untreated sarcoidosis and 37 consecutive patients with untreated mediastinal Hodgkin’s lymphoma who had undergone a chest CT scan were enrolled at the Department of Radiology, Masih Daneshvari Hospital, a tertiary referral center for pulmonary diseases, from January 2010 to April 2012. The criteria to establish the diagnosis of sarcoidosis included 1) histological evidence of non-caseating granuloma in the samples obtained by bronchoscopic biopsy, surgical biopsy or transthoracic needle aspiration biopsy, 2) compatible clinical and imaging evidence and 3) negative bacterial and fungal studies for sputum, bronchoalveolar lavage or biopsied tissues. Patients with a history of pervious or concurrent tuberculosis were excluded from the study. Subjects who met all of these criteria were recruited. Chest CT scan had been performed in these patients due to several reasons including minimal or atypical chest radiography presentations, ruling out malignancy and pre-bronchoscopic planning.
The diagnosis of mediastinal lymphoma was made by biopsy of the enlarged lymph nodes.
The criterion for significant lymph node enlargement of mediastinal lymph nodes was considered to be a short axis dimension ≥10 mm for all zones except for subcarinal lymph nodes (zone 7) were a measurement ≥12 mm was used. For axillary, internal mammary, peridiaphragmatic and retrocrural lymph nodes, short axis cut off points equal to or more than 10 mm, 5 mm, 5 mm and 6 mm were used respectively.
All scans were obtained on a spiral CT scanner (Siemens SOMATOM Emotional, Munich, Germany). Scans were taken from the supraclavicular fossa down to the level of adrenal glands. The following parameters were used: 130 kV, 90 mAs, Gantry rotation time of 0.8 s, pitch of 1.5, and collimation of 5 mm. Intravenous contrast was used in 32 patients from the sarcoidosis group and in 35 patients from the lymphoma group. A 100-mL bolus of contrast material (Omnipaque, 240 mg iodine/ml; GE healthcare Inc, Cork, Ireland) was injected at a rate of 2.5 mL per second; The delay time was 35 seconds. In the remaining patients (7 patients from the sarcoidosis group and 2 patients from the lymphoma group), there were contraindications to contrast administration, but fortunately there was sufficient amount of mediastinal fat to allow adequate discrimination of different compartments.
Two experienced thoracic radiologists who were unaware of final diagnoses reviewed all CT scan images independently. If patients had multiple CT scans throughout the course of their disease, the earliest scan was reviewed.
For each patient, characteristics of the enlarged lymph nodes were recorded, including anatomical site, margin, presence or absence of calcifications, and enhancement patterns on axial and coronal multiplanar (MPR) images using a soft-tissue reconstruction algorithm. Discrepancies in interpretation between observers were resolved by consensus.
Association for the Study of Lung Cancer (IASLC) proposed a lymph node map that reconciles the discrepancies between two previous maps (American Thoracic Society lymph node map and Japan Lung cancer society lymph node map) and other published proposals, and provides detailed nomenclature for the anatomical boundaries of lymph node locations. This map is now the recommended method of describing regional lymph node involvement in lung cancers [6].
According to this map, mediastinal lymph nodes were divided into 10 zones. The aforementioned zones were as follows: 1 (low cervical, supraclavicular and sternal notch), 2R (right upper paratracheal), 2L (left upper paratracheal), 3 (prevascular and retrotracheal), 4R (right lower paratracheal), 4L (left lower paratracheal), 5 (subaortic), 6 (paraaortic), 7 (subcarinal), 8 (paraesophageal), 9 (pulmonary ligament), 10R (right hilar), and 10L (left hilar).
Coalescence of lymph nodes was defined as conglomerate of adjacent lymph nodes without discernable borders. Lymph node caviation was presumed when low density area was present in its center.
Data analysis was carried out using the commercially available SPSS program (Version 17.0; Chicago, IL, USA). The differences in anatomical distribution and morphological patterns of mediastinal lymph nodes between sarcoidosis and lymphoma were examined with a chi-square test. P<0.05 was considered statistically significant.
Results
The mean age of patients with sarcoidosis (49.9 years, range 28–75 years) was significantly higher than patients with Hodgkin’s lymphoma (39.1 years, range 11–60 years) (p<0.001). 18 patients (46.1%) with sarcoidosis were men and 21 patients (53.9%) were women. 17 patients (45.9%) with Hodgkin’s lymphoma were men and 20 patients were women (54.1%). 20 patients (51.3%) with sarcoidosis had history of smoking, while 13 (35.1%) patients with Hodgkin’s lymphoma had history of smoking (p=0.156).
Sarcoidosis predominantly affected the lymph nodes in the following zones: 4 (lower paratracheal; 97.4%), 10 (hilar; 78%), 7 (subcarinal; 76.9%) and 5 (subaortic; 69.2%). In patients with Hodgkin’s disease, enlarged lymph nodes were distributed mainly in the zones: 4 (lower paratracheal; 89.2%), 3 (prevascular; 81.1%), 7 (subcarinal; 73.0%) and 2 (upper paratracheal; 70.2%). Zone 9 (pulmonary ligament) and peridiaphragmatic zones were rarely affected in either Hodgkin’s lymphoma or sarcoidosis cases. Sarcoidosis involved the 10 (hilar) zone significantly more often than Hodgkin’s lymphoma but the 1 (low cervical, supraclavicular and sternal notch), 3 (prevascular), internal mammary and retrocrural zones were less likely to be involved in sarcoidosis compared to Hodgkin’s lymphoma.
Coalescence of enlarged lymph nodes was seen significantly more often in Hodgkin’s lymphoma (94.6%) compared to sarcoidosis (5.3%, p<0.001). Calcification within the nodes was demonstrated in one patient (2.7%) with Hodgkin’s lymphoma and in 12 patients (30.8%) with sarcoidosis. None of the sarcoidosis cases exhibited pressure effect or displacement of adjacent structures by the enlarged lymph nodes, while it was seen in 59.5% of Hodgkin’s lymphoma cases (p<0.001). Two patients (5.4%) with Hodgkin’s lymphoma presented with lymph node cavitation, while it was not present in patients with sarcoidosis.
Table 1 shows the distribution of affected lymph nodes in sarcoidosis and Hodgkin’s lymphoma.
Table 1.
Anatomic distribution of lymph nodes in 76 patients with sarcoidosis or Hodgkin’s lymphoma.
| Site of involvement* | Sarcoidosis (n=39) | Hodgkin’s Lymphoma (n=37) | p-value |
|---|---|---|---|
| 1 | 9 (23.1%) | 17 (45.9%) | 0.036 |
| 2 | 22 (56.4%) | 26 (70.3%) | 0.211 |
| 3 | 10 (25.6%) | 30 (81.1%) | 0.000 |
| 4 | 38 (97.4%) | 33 (89.2%) | 0.147 |
| 5 | 27 (69.2%) | 24 (64.9%) | 0.686 |
| 6 | 21 (53.8%) | 21 (56.8%) | 0.799 |
| 7 | 30 (76.9%) | 27 (73.0%) | 0.691 |
| 8 | 12 (30.8%) | 15 (40.5%) | 0.374 |
| 9 | 7 (17.9%) | 4 (10.8%) | 0.377 |
| 10** 10R 10L 10B |
35 (89.7%) 2 (5.1%) 1 (2.5%) 32 (82.0%) |
23 (62.2%) 7 (18.9%) 7 (18.9%) 9 (24.3%) |
0.005 0.082 0.027 0.000 |
| Axillary | 5 (12.8%) | 8 (21.6%) | 0.370 |
| Peridiaphragmatic | 1 (2.5%) | 2 (5.4%) | 0.610 |
| Internal mammary | 0 (0.0%) | 11 (29.7%) | 0.000 |
| Retrocrural | 0 (0.0%) | 5 (13.5%) | 0.024 |
10R (Right unilateral hilar), 10L (Left unilateral hilar), 10B (Bilateral hilar);
according to the International Association for the Study of Lung Cancer (IASLC) lymph node map [6].
Discussion
Sarcoidosis is one of the most important pulmonary diseases mostly presenting with enlarged mediastinal lymph nodes with typical pattern of involvement, which includes right paratracheal and bilateral hilar zones [2]. However, this pattern of involvement is not specific and can be seen in a wide variety of diseases such as Hodgkin’s lymphoma and tuberculosis [2]. On the other hand, atypical pattern of involvement of lymphadenopathy in sarcoidosis is not rare, which makes differentiation of sarcoidosis from other mediastinal diseases, especially Hodgkin’s lymphoma, more difficult [3]. Biopsy is considered the gold standard for diagnosis of diseased mediastinal lymph nodes, but it is time-consuming and invasive. As CT imaging evolved, multi-planar or three-dimensional reconstructed images became available. This was an important development for the detection of enlarged lymph nodes in the mediastinum [7].
In sarcoidosis, distribution of thoracic lymphadenopathy, according to the American Thoracic Society lymph node map, was evaluated in two studies [8,9]. The most commonly involved nodal locations, in decreasing order of frequency, were 4R (right lower paratracheal), 10R (right hilar), 7 (subcarinal), 5 (subaortic, i.e., aorto-pulmonic window), which is consistent with our findings. However, in our study prevalence of lymphadenopathy in different zones is generally higher compared to previous studies. Considering similar method and criteria, it is unclear whether the difference is due to patient population or higher detection rate by newer, higher-resolution CT images.
Several reports [3,4,10] show that in sarcoidosis, hilar lymphadenopathy is frequently associated with mediastinal lymph node enlargement as depicted on CT scans, especially including the right paratracheal and subaortic nodes. However, mediastinal lymphadenopathy without hilar involvement is rare and is more suggestive of lymphoma. Our findings are in line with these reports.
In concordance with previous studies [4,11], we found that unilateral hilar lymphadenopathy is seen in less than 8% of cases of sarcoidosis, but is not uncommon in Hodgkin’s lymphoma where it is present in 37.8% of cases.
In sarcoidosis, unilateral hilar lymphadenopathy (if present) is approximately twice as common on the right side compared to the left side, but it is equal in both sides in Hodgkin’s lymphoma. These results are in line with findings of previous studies as well [6,12].
Suwatanapongched et al. [10] found that unilateral hilar disease or mediastinal lymphadenopathy without hilar disease is rarely seen with sarcoidosis and is more suggestive of lymphoma. Our findings support this report.
In addition, in our study, retrocrural and internal mammary involvement is seen exclusively in Hodgkin’s lymphoma. Nunes et al. [7] also found that although occasionally present in sarcoidosis, enlargement of internal mammary and pericardial lymph nodes requires exclusion of lymphoma. Hamper et al. [3] found that enlarged lymph nodes in unusual locations such as axillary, peridiaphragmatic and internal mammary region, were much more frequently appreciated on CT images than previously believed. They showed that when sarcoidosis presented with generalized peripheral and thoracic lymphadenopathy, the differential diagnosis from lymphoma on the basis of CT scan was a challenge. (Figure 1).
Figure 1.
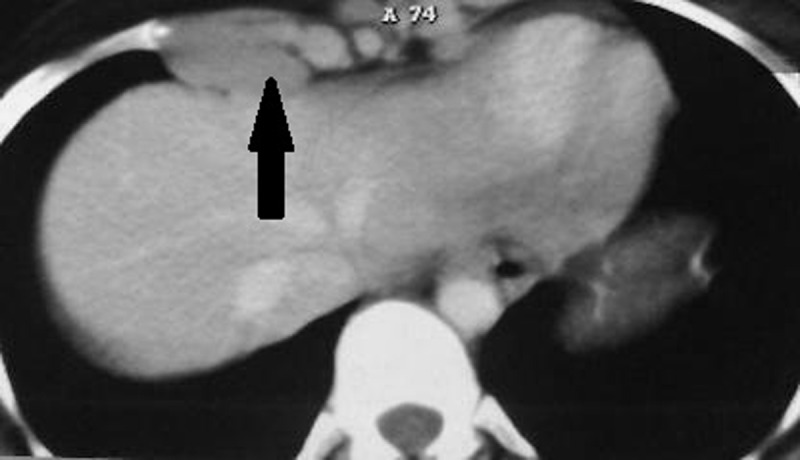
A 23-year-old man with Hodgkin’s lymphoma presented with enlarged lymph node (black arrow) in internal mammary zone. This pattern is found to be highly in favor of Hodgkin’s lymphoma rather than sarcoidosis.
To the best of our knowledge there is no published study comparing the distribution of lymphadenopathy in Hodgkin’s lymphoma and sarcoidosis using IASLC classification method. We found that zone 1 and 3 are more commonly involved in Hodgkin’s lymphoma and zone 10 is more commonly involved in sarcoidosis and these differences are significant.
It was found that in none of our sarcoidosis cases lymph nodes were compressed, which is supported by several previous studies [7,13]. Nevertheless, extrinsic compression by enlarged lymph nodes in thoracic sarcoidosis is reported in some other studies [14–16].
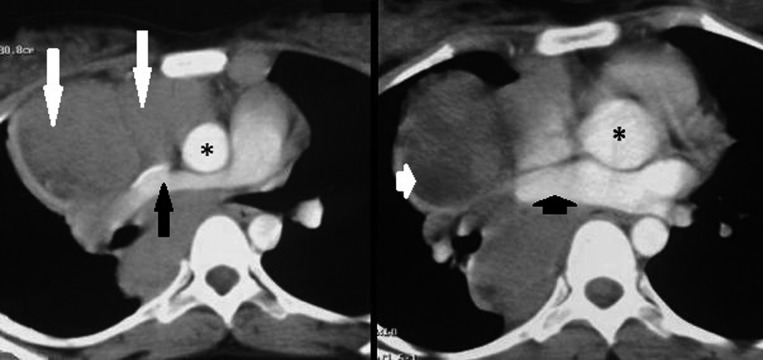
In contrast, lymph nodes in Hodgkin’s lymphoma show pressure effect on adjacent structures in more than half of cases in our study and this can be proposed as a helpful criterion, not characteristic of course, to differentiate lymphoma from sarcoidosis (Figure 2).
Figure 2.
Axial CT scans in a 21-year-old female with Hodgkin’s lymphoma show enlarged lymph nodes (long white arrow) compressing right pulmonary artery (long black arrow), SVC and right pulmonary vein (small black arrow). This compression effect is not seen in sarcoidosis. Asterisk represents ascending aorta. Also, note the hypodensity (small white arrow) in the central part of lymphadenopathy suggestive of cavitation.
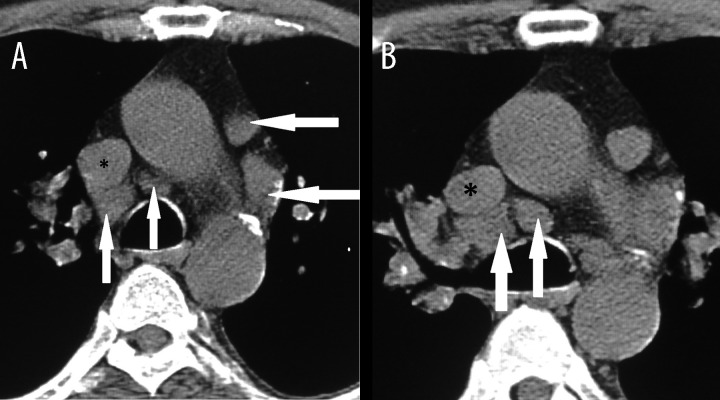
Granulomas in sarcoidosis gradually resolve or undergo healing by fibrosis. Nodular fibrous lesions representing healed granulomas are seen in lymph nodes. Fibrosis usually begins at the periphery and may extend centrally until the entire granuloma is replaced by fibrous tissue [17]. This fibrotic content may explain our findings regarding adjacent lymph nodes in sarcoidosis, as they preserve their sharp and distinct borders and do not coalesce; while lymph nodes in lymphoma contain highly proliferative lymphocyte precursors, which coalesce when their borders touch (Figure 3).
Figure 3.
Axial CT scans in a 38-year-old woman with sarcoidosis demonstrate adjacent enlarged lymph nodes (long white arrow), which do not coalesce despite adhering to each other. In contrast to Hodgkin lymphoma, no pressure effect on superior vena cava (asterisk) is notable.
Calcification may accompany extensive fibrosis and this fact may explain the presence of calcified lymph nodes in sarcoidosis but not in untreated Hodgkin’s lymphoma, as demonstrated in our study. In concordance with our findings, lymph node calcifications in sarcoidosis were reported in 5 to 50% of cases [12]. Miller et al. [18] found that the occurrence of lymph node calcification in sarcoidosis, as in other chronic granulomatous diseases, is closely related to the duration of disease. In a study of sarcoidosis patients known to have disease for up to 32 years, nodal calcification was noted in 53%, with eggshell calcification seen in 9%. In another report, calcification was identified in 20% of patients at presentation, increasing to 44% over a period of 4 years [19].
A potential limitation for the present study is that in sarcoidosis group only patients who had had chest CT scan before commencement of therapy were enrolled in the study. Considering the fact that some of the cases may be diagnosed based on clinical and pathological findings beside typical chest x-ray presentations and without evaluation by chest CT scan, our sarcoidosis group might have not been an exact representative of the entire patients population. Therefore, prospective studies performing chest CT scan in all cases may be required to overcome this limitation.
Conclusions
In summary, the findings of this study indicate that zone 1 and 3 (according to IASLC mapping) lymph nodes were involved in Hodgkin’s lymphoma more often than in sarcoidosis and internal mammary and retrocrural groups involvement was only seen in Hodgkin’s lymphoma. Hence, contrast-enhanced CT scan may be helpful in differentiating intrathoracic sarcoidosis from Hodgkin’s lymphoma based on the anatomical distribution of enlarged lymph nodes. Furthermore, presence of lymph node calcification, lack of pressure effect on adjacent structures and noncoalescent behavior were more consistent with sarcoidosis. Bilateral hilar involvement was more common in sarcoidosis than in Hodgkin’s lymphoma.
Footnotes
Conflict-of-interest disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References:
- 1.Vagal SA, Shipley R, Meyer CA. Radiological manifestations of sarcoidosis. Clin Dermatol. 2007;25:312–25. doi: 10.1016/j.clindermatol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Hansell DM, Armstrong P, Lynch DA. Imaging of diseases of the chest. 4th ed. Philadelphia, PA: Elsevier Mosby; 2005. pp. 631–52. [Google Scholar]
- 3.Hamper UM, Fishman EK, Khouri NF, et al. Typical and atypical CT manifestations of pulmonary sarcoidosis. J Comput Assist Tomogr. 1986;10:928–36. doi: 10.1097/00004728-198611000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Rockoff SD, Rohatgi PK. Unusual manifestations of thoracic sarcoidosis. Am J Roentgenol. 1985;144:513–28. doi: 10.2214/ajr.144.3.513. [DOI] [PubMed] [Google Scholar]
- 5.Saksouk FA, Haddad MC. Detection of mesenteric involvement in sarcoidosis using computed tomography. BrJ Radiol. 1987;60:1135–36. doi: 10.1259/0007-1285-60-719-1135. [DOI] [PubMed] [Google Scholar]
- 6.Byrd DR, Compton CC, Fritz AG, et al. AJCC Cancer Staging Manual. 7th ed. Chicago: Springer; 2010. Thorax; pp. 251–70. [Google Scholar]
- 7.Nunes H, Brillet PY, Valeyre D, et al. Imaging in sarcoidosis. Seminars in Respiratory and Critical Care Medicine. 2007;28(1):102–20. doi: 10.1055/s-2007-970336. [DOI] [PubMed] [Google Scholar]
- 8.Patil SN, Levin DL. Distribution of thoracic lymphadenopathy in sarcoidosis using computed tomography. J Thorac Imaging. 1999;14:114–17. doi: 10.1097/00005382-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Niimi H, Kang EY, Kwong JS, et al. CT of chronic infiltrative lung disease: prevalence of mediastinal lymphadenopathy. J Comput Assist Tomogr. 1996;20:305–8. doi: 10.1097/00004728-199603000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Suwatanapongched T, Gierada DS. CT of thoracic lymph nodes. Part II: diseases and pitfalls. Br J Radiol. 2006;79:999–1006. doi: 10.1259/bjr/82484604. [DOI] [PubMed] [Google Scholar]
- 11.Fraser RG, Pare JAP, Pare PD, et al. of diseases of the chest. 3rd ed. Philadelphia, PA: Saunders; 1991. pp. 2604–47. [Google Scholar]
- 12.Park HJ, Jung JI, Chung MH, et al. Typical and Atypical Manifestations of Intrathoracic Sarcoidosis. Korean J Radiol. 2009;10:623–31. doi: 10.3348/kjr.2009.10.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale ME, Karlinsky JB. Computed tomography of the chest: A teaching file. Chicago: Year Book Medical; 1988. [Google Scholar]
- 14.Aye M, Campbell AP, Greenstone MA. An unusual case of lobar collapse. Chest. 2002;122:1465–66. doi: 10.1378/chest.122.4.1465. [DOI] [PubMed] [Google Scholar]
- 15.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayan D, Brown L, Thayer JO. Surgical management of superior vena caval syndrome in sarcoidosis. Ann Thorac Surg. 1998;66:946–48. doi: 10.1016/s0003-4975(98)00564-5. [DOI] [PubMed] [Google Scholar]
- 17.Rosen Y. Pathology of Sarcoidosis. Semin Respir Critic Care Med. 2007;28(1):36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 18.Miller BH, Rosado-de-Christenson ML, McAdams HP, et al. Thoracic sarcoidosis: radiologic-pathologic correlation. Radiographics. 1995;15(2):421–37. doi: 10.1148/radiographics.15.2.7761646. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch J, Muller NL. Pulmonary sarcoidosis: changes on follow-up CT examination. Am J Roentgenol. 1992;159:473–77. doi: 10.2214/ajr.159.3.1503008. [DOI] [PubMed] [Google Scholar]