Summary
Background:
Implantable intrathecal drug delivery systems (IDDS) are basic tool enabling chronic intrathecal pharmacotherapy. Lack of expected clinical results of IDDS therapy necessitates search for the cause with the help of diagnostic imaging methods among other things. Beside radiological techniques, it is also possible to visually assess IDDS systems by nuclear medicine methods. In this study we assess utility of radioisotopic methods in differential diagnosis of failure of therapy with IDDS systems.
Material/Methods:
Scintigraphic studies were performed in selected patients with neurological diseases associated with spasticity, who had IDDS system implanted and were unable to maintain satisfying clinical effect of inrathecally infused baclofen. After emptying the IDDS system of the drug, radiotracer (99mTc-DTPA) solution was injected into the pump reservoir. Subsequently, a series of scintigraphic images was registered, demonstrating passage and distribution of the infused radiotracer.
Results:
In all investigated cases, scintigraphic study resulted in acquiring relevant additional diagnostic information. Normal or disrupted distribution of radiotracer in spinal canal allowed for a diagnosis drug resistance or demonstrated presence of arachnoid adhesions respectively. Early appearance of radiotracer in blood was considered a proof of leak. Our examinations had decisive influence on further patient treatment, allowing for diagnosis of drug resistance in one patient or complication related to IDDS system in three other cases including breakage of a catheter, pump malfunction and arachnoid adhesions.
Conclusions:
Scintigraphic methods carry significant amount of information facilitating final diagnosis of the cause of IDDS therapy failure. They should become an important element complementing the diagnostic strategy in patients with suspected failure of intrathecal drug administration systems. Interpretation of radioisotopic studies, since they are purely functional, must be performed in strict relation to clinical data and radiological examinations as they carry indispensable, basic information regarding morphology.
Keywords: intrathecal drug delivery system, intrathecal baclofen, intrathecal, baclofen, scintigraphy, 99mTc-DTPA
Background
Systems for chronic intrathecal drug delivery are important elements in management strategy of spasticity and pain resistant to non-invasive conventional treatment [1–8]. Intrathecal drug delivery allows for achieving high cerebrospinal fluid drug concentration and direct action on nervous system structures. Intrathecal drug delivery allows for treating patients resistant to conventional noninvasive treatment thanks to high drug concentrations in cerebrospinal fluid and avoiding some side effects [9]. Systems for intrathecal drug delivery (IDDS) typically consist of a pumping element with fillable drug reservoir and a set of catheters delivering the drug to subarachnoid space of vertebral canal. The main pumping element is most frequently implanted subcutaneously in the abdominal region. In the absence of expected therapeutic effect of pump implantation the following reasons should be considered: resistance to infused drug, pump mechanism or catheter system failure (kinking, blocking by fibrin clot, disconnection, breakage), inappropriate implantation of catheters and complication of catheter implantation in the subarachnoid space – dislodgement, granulation tissue or formation of adhesions near catheter tip [10–16]. Implantation of a catheter in subarachnoid space is a technically challenging procedure subject to the risk of improper placement. Before definitive diagnosis of drug resistance one should exclude with high confidence the possibility of drug delivery system failure. The purpose of the article is to demonstrate on selected cases the value of radioisotope studies in the diagnosis of therapy failure among patients treated with IDDS.
Material and Methods
The study includes four patients receiving intrathecal baclofen therapy (ITB). Our scintigraphic studies are based on proven methodology used by other authors with modification enabling usage 99mTc-DTPA (our radiotracer) instead of frequently used 111In-DTPA [9,17–22]. A day before the radioisotope study pump reservoir was emptied of the drug and physiologic saline solution was instilled to gradually replace the drug in pump and catheters. On the day of the study pump reservoir was emptied of saline and a 99mTc-DTPA radiotracer (OBRI Polatom, Otwock, Poland) with activity of 2 mCi was instilled. Infusion speed was increased to 1ml/hour. Directly after filling the pump reservoir with a radiotracer an early image of the pump was obtained to confirm presence of radiotracer in the reservoir. After 30 minutes we registered a scintigram of the pump to exclude leak in a pump region. Catheter scan was registered within the next 30–60 minutes scan and distribution of the radiotracer in the spinal canal was observed after 30–60 minutes. The exact timing of imaging was adjusted dynamically basing on observed passage of radiotracer on control display of the gamma camera Siemens MS2 (Siemens, Erlangen, Germany). We used 256×256 matrix, LEHR collimators, no zooming, “spot” technique. Images were acquired until registering between 200 to 500 kilocounts.
Results
Patient 1
Woman, 40 years old with IDDS implanted 7 years earlier (Synchromed I 40ml – Medtronic, Minneapolis, USA) for treatment of multiple sclerosis. Gradual disappearance of therapeutic effect of intrathecal baclofen (ITB) was observed for a year. Scintigraphy demonstrated proper distribution of radiotracer. Taking into consideration small volume of residual drug in reservoir during refill and normal x-ray image, resistance to intrathecal baclofen was finally diagnosed (Figures 1–3).
Figure 1.

Presence of radiotracer in pump reservoir.
Figure 3.

Proper distribution of radiotracer in cerebrospinal fluid (lateral projection).
Patient 2
A 28-year-old man treated for spastic paraparesis of unknown etiology had an ITB system implanted 5 years earlier (Synchromed I 20 ml – Medtronic, Minneapolis, USA). Gradual reduction of therapeutic effect of intrathecal baclofen was observed for 2 years. Due to high sensitivity of scintigraphy, appearance of considerable radiotracer amount in blood, soft tissues and urinary bladder is characteristic for a leak (no need to see pooling of radiotracer near leakage place). Catheter system in this patient was later repaired intraoperatively (Figures 4 and 5).
Figure 4.

Late posterior projection depicting absence of radiotracer distribution in the vertebral canal.
Figure 5.

High radiotracer activity in the circulation and body fluids – result of absorption of extravasated radiotracer.
Patient 3
A 29-year-old woman treated for Huntington’s chorea, with an ITB system implanted two years before had been complaining of weakening of therapeutic effect for a year. Scintigraphy reveals proper distribution of radiotracer in catheters and CSF. Malfunction of the pumping element was finally diagnosed, taking into consideration the increased volume of residual drug during periodic refills. Disruption of pump function was confirmed by manufacturer, resulting in replacement (Figures 6–8).
Figure 6.

Anterior projection demonstrating normal distribution of radiotracer in a pump and catheters.
Figure 8.

Proper distribution of radiotracer in cerebrospinal fluid (posterior projection).
Patient 4
A 10-year-old girl treated for spastic hemidystonia, 3 years after implantation of ITB (Synchromed II 20 ml – Medtronic, Minneapolis, USA), had been complaining of deterioration of therapeutic effect for a year. Scintigraphy demonstrates normal distribution of radiotracer in a catheter system, no signs of leakage or obstruction, distinct pooling of radiotracer at the site of intrathecal catheter orifice in the vertebral canal as well as free distribution of radiotracer in cerebro-spinal fluid. Pooling of radiotracer near catheter orifice suggests local outflow restriction. Finally, arachnoid adhesions in the catheter tip region was diagnosed and confirmed intraoperatively (Figures 9 and 10; Table 1).
Figure 9.
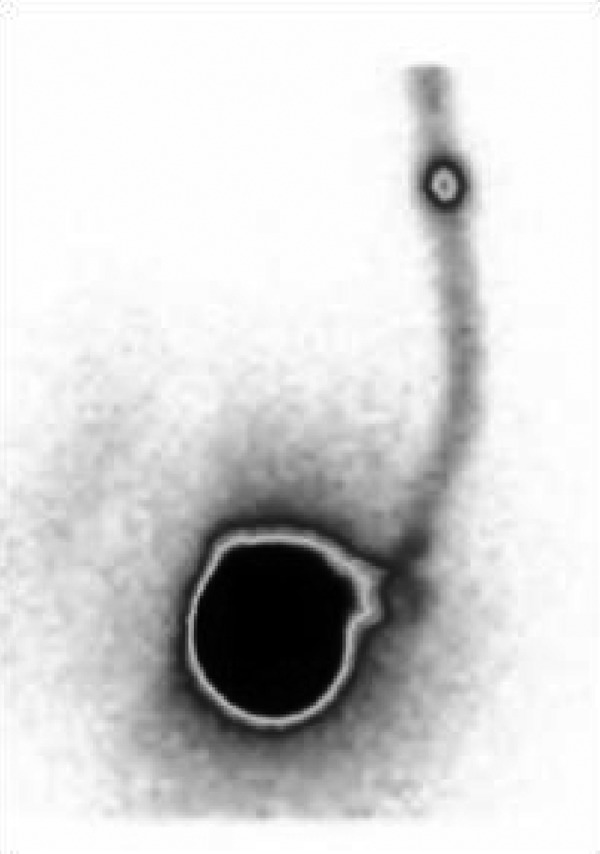
Anterior projection – in the upper part of the image there is focal pooling of radiotracer in a region of catheter tip in spinal canal.
Figure 10.

Lateral projection – distribution of radiotracer in cerebrospinal fluid.
Table 1.
Summary of the most significant clinical and diagnostic information.
| Patient | Sex | Age (years) | Disease treated | Implantation to malfunction interval (years) | Scintigraphic findings | Clinical findings | Conclusion |
|---|---|---|---|---|---|---|---|
| Case 1 | F | 40 | Sclerosis multiplex | 6 | Normal distribution | Since a year deterioration of clinical effect, small residual volume during refill | Resistance to drug |
| Case 2 | M | 28 | Spastic paraparesis of unknown etiology | 3 | High activity of tracer in body fluids, no tracer in spinal canal | Since two years deterioration of clinical effect | Disconnection or damage to catheters confirmed operatively |
| Case 3 | F | 29 | Huntington’s chorea | 1 | Normal distribution | Since a year deterioration of clinical effect, additionally Increased residual volume during pump refill | Malfunction of pumping mechanism, pump replacement solved the problem |
| Case 4 | F | 10 | hemidystonia | 2 | Pooling of radiotracer at the catheter tip | Since a year deterioration of clinical effect | Arachnoid adhesions confirmed operatively |
Discussion
IDDSs are clinically utilized since early 80s. Initially they were reserved for opioid analgesics. Their main advantage is direct delivery of drug into subarachnoid space enabling high drug concentrations around target organ (central nervous system). They minimize side effects associated with presence of high concentration of orally or intravenously administered drugs in blood and other body fluids and tissues.
Absence of expected clinical effect or weakening of clinical effect in relatively short period of time prompt a search for the cause of IDDS therapy failure. Possible reasons include: drug resistance, failure of pump system or catheters and restriction of free distribution of a drug in cerebrospinal fluid. First line diagnostic tool is typically plain radiography performed in several projections [19]. It allows for detection of mechanical pump failure (in rotor position test), kinking, cutoff or disconnection of catheter system. Some major dislodgments of catheter in vertebral canal can be also visualized. When plain films are inconclusive, the next diagnostic step is x-ray fluoroscopic imaging [19]. Performed without contrast media, it allows for dynamic assessment of possible position-related kinking or dislodgment. A dynamic x-ray can be improved by injection of small volume of contrast through an accessory port in the pump [19]. Drug reservoir cannot be used for contrast-enhanced radiological studies because of low pumping rate producing only low concentration of contrast in CSF or extravasation (accessory port allows for direct manual injection of contrast and reaching higher concentrations) [9]. Small diameter of catheters precludes observation of contrast migration by x-ray modalities. Addition of contrast allows for detection of contrast extravasation in case of disconnection or breakage and flow restriction by adhesions of subarachnoid space. CT imaging soon after contrast administration enables precise three-dimensional localization of catheter tip or detection of smaller contrast extravasations missed on planar imaging [19]. Granulation/inflammatory tissue around catheter tip can be another cause of improper intrathecal drug/contrast distribution and can be detected with contrast-enhanced MRI or computed tomography (contrast injected into CSF) [19]. Nuclear medicine scintigraphic imaging is typically reserved for cases unresolved with radiological modalities. Most relevant advantage of nuclear medicine imaging over x-ray methods is high sensitivity, as even very small amount of radiotracer may be identified by gamma camera detectors. It allows for use of a drug reservoir and pumping of the radiotracer at slow speed used in therapeutics, together with the drug or instead of drug. Sequentially registered scintigrams follow all stages of tracer passage from reservoir, through catheters, to subarachnoid space and subsequently its distribution in cerebrospinal fluid. Scintigraphy enables diagnosis of mechanical pump failure, catheter occlusion, catheter leak or restriction of free distribution in the CSF. Disadvantage of scintigraphic imaging is related to relatively poor spatial resolution and lack of anatomical landmarks in the image – precise location of occlusion, leak, or catheter position is difficult. Paucity of spatial resolution in scintigraphy can be overcome by image registration using dual-mode SPECT-CT scanners (scintigraphic and x-ray tomography registered and then displayed simultaneously), although metal artifacts sometimes disrupt the CT image near metallic elements. Diagnostic problems encountered in scintigraphy include differentiation between pump failure and high-grade catheter occlusion as well as differentiation between various causes of occlusion [9]. In lower-grade occlusion radiotracer typically can be seen in proximal catheter, while in high-grade occlusion and total pump failure activity is only present in the reservoir [9]. Scintigraphic imaging can be performed using two different radiotracers. The most frequently used radiotracer is 111In-DTPA – the possibility to admix it with drug in a reservoir and observe passage at unchanged, “normal” rate is a great advantage of this tracer, while the disadvantage is such that patients sometimes have to be examined for as long as 72 hours [9]. We used another radiotracer in our department – 99mTc-DTPA. Advantages of 99mTc-DTPA include its low cost, shortening of study duration to a maximum of few hours, good image quality and low radiation dose; the disadvantage is the need to reprogram the pump so that it would inject with high rate and, as consequence, necessity to remove the drug (from reservoir and catheter) before examination to avoid drug overdose [22]. Radiotracers utilized in IDDS scintigraphy (111In-DTPA or 99mTc-DTPA) are chemically different from radiological iodine contrast agents and are safe for the patient (they are apyrogenic, non-toxic, without potential for allergization, produce no risk of anaphylactic shock or thyroid function disruption, they do not alter CSF circulation and do not interact with drugs injected by IDDS). In all presented cases scintigraphic evaluation gave important information for diagnosis of IDDS therapy failure. Normal, fast distribution of radiotracer in IDDS and cerebrospinal fluid together with unchanged x-ray image and clinical information (small residual volume in drug reservoir) allowed for excluding catheter dislodgement or breakage, while restriction of distribution in the spinal canal enabled final diagnosis of drug resistance (Patient 1). Rapid diffusion of radiotracer 99m Tc-DTPA into body fluids enables unequivocal diagnosis of a leak even with normal accompanying imaging results (Patient 2). Normal distribution of radiotracer in cerebrospinal fluid in combination with increased residual drug volume during pump refill as well as normal radiological image of IDDS and spinal canal strongly suggests pump mechanism failure, which was confirmed by the manufacturer (Patient 3). Pooling of radiotracer in the region of catheter tip in spinal canal is pathognomonic for changes in spinal canal restricting free flow, for example arachnoid adhesions (Patient 4). Unfortunately due to relatively small number of patients in the study, the diagnostic accuracy, sensitivity and specificity of radionuclide imaging were not defined, but presented initial results seem promising.
Conclusions
Radiologic assessment of IDDS system is essential. It facilitates final diagnosis in many cases, is broadly available and carries basic morphologic information needed for interpretation of functional radioisotopic studies or later surgical intervention.
Scintigraphic imaging seems to be a valuable functional adjunct to first-line morphological studies enabling final diagnosis in many patients with potential malfunction of IDDS systems. Further studies encompassing higher number of patients are necessary to define the exact diagnostic accuracy.
Figure 2.

Proper distribution of radiotracer in a pump and catheter (anterior projection).
Figure 7.

Posterior projection demonstrating normal distribution of radiotracer in a pump and catheters.
References:
- 1.Dash A, Cudworth G. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty P, Staats P. Intrathecal drug therapy for chronic pain: from basic science to clinical practice. Anesthesiology. 1999;91:1891–918. doi: 10.1097/00000542-199912000-00044. [DOI] [PubMed] [Google Scholar]
- 3.Gilmartin R, Bruce D, Storrs BB, et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15:71–77. doi: 10.1177/088307380001500201. [DOI] [PubMed] [Google Scholar]
- 4.Guillaume D, Van Havenbergh A, Vloeberghs M, et al. A clinical study of intrathecal baclofen using a programmable pump for intractable spasticity. Arch Phys Med Rehabil. 2005;86:2165–71. doi: 10.1016/j.apmr.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Maron J, Loeser J. Spinal opioid infusions in the treatment of chronic pain of nonmalignant origin. Clin J Pain. 1996;12:174–79. doi: 10.1097/00002508-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Penn RD. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg. 1992;77:236–40. doi: 10.3171/jns.1992.77.2.0236. [DOI] [PubMed] [Google Scholar]
- 7.Stempien L, Tsai T. Intrathecal baclofen pump use for spasticity: a clinical survey. Am J Phys Med Rehabil. 2000;79:536–41. doi: 10.1097/00002060-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Van Schaeybroeck P, Nuttin B, Lagae L, et al. Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo-controlled, double-blind study. Neurosurgery. 2000;46:603–9. doi: 10.1097/00006123-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Pak S, Jallo GI, Biser A, et al. Indium-111 diethylene-triaminepentaacetic acid scintigraphy in the evaluation of function and patency of baclofen intrathecal infusion systems. Neurosurg Focus. 2007;23:E17. doi: 10.3171/foc.2007.23.1.17. [DOI] [PubMed] [Google Scholar]
- 10.Dawes WJ, Drake JM, Fehlings D. Microfracture of a baclofen pump catheter with intermittent under- and overdose. Pediatr Neurosurg. 2003;39:144–48. doi: 10.1159/000071652. [DOI] [PubMed] [Google Scholar]
- 11.Deer TR, Raso LJ, Garten TG. Inflammatory mass of an intrathecal catheter in patients receiving baclofen as a sole agent: a report of two cases and a review of the identification and treatment of the complication. Pain Med. 2007;8:259–62. doi: 10.1111/j.1526-4637.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 12.Flückiger B, Knecht H, Grossmann S, et al. Device-related complications of long-term intrathecal drug therapy via implanted pumps. Spinal Cord. 2008;46:639–43. doi: 10.1038/sc.2008.24. [DOI] [PubMed] [Google Scholar]
- 13.Gooch JL, Oberg WA, Grams B, et al. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1–6. doi: 10.1159/000070870. [DOI] [PubMed] [Google Scholar]
- 14.Li TC, Chen MH, Huang JS, et al. Catheter migration after implantation of an intrathecal baclofen infusion pump for severe spasticity: a case report. Kaohsiung J Med Sci. 2008;24:492–97. doi: 10.1016/S1607-551X(09)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107:32–35. doi: 10.3171/PED-07/07/032. [DOI] [PubMed] [Google Scholar]
- 16.Pasquier Y, Cahana A, Schnider A. Subdural catheter migration may lead to baclofen pump dysfunction. Spinal Cord. 2003;41:700–2. doi: 10.1038/sj.sc.3101536. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak EM, McGuire JR, Nelson ME. Incidence and identification of intrathecal baclofen catheter malfunction. PM R. 2010;2:751–56. doi: 10.1016/j.pmrj.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Le Breton F, Daviet JC, Monteil J, et al. Radioisotopic control for baclofen pump catheter failure. Spinal Cord. 2001;39:283–85. doi: 10.1038/sj.sc.3101142. [DOI] [PubMed] [Google Scholar]
- 19.Miracle AC, Fox MA, Ayyangar RN, et al. Imaging evaluation of intrathecal baclofen pump-catheter systems. Am J Neuroradiol. 2011;32:1158–64. doi: 10.3174/ajnr.A2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell M, Wong TZ, Forkheim KE, et al. Comparison of Tc99m-DTPA and indium-111 DTPA studies of baclofen pump function. Clin Nucl Med. 2004;29:578–80. doi: 10.1097/01.rlu.0000134995.55131.30. [DOI] [PubMed] [Google Scholar]
- 21.Rosenson AS, Ali A, Fordham EW, et al. Indium-111 DTPA flow study to evaluate surgically implanted drug pump delivery system. Clin Nucl Med. 1990;15:154–56. doi: 10.1097/00003072-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Stinchon JF, Shah NP, Ordia J, et al. Scintigraphic evaluation of intrathecal infusion systems: selection of patients for surgical or medical management. Clin Nucl Med. 2006;31:1–4. doi: 10.1097/01.rlu.0000190890.97713.34. [DOI] [PubMed] [Google Scholar]


