Summary
Arteriovenous malformation (AVM) is an abnormal connection between arteries and veins, bypassing the capillary system. In most cases, the disorder may be asymptomatic. The objective of endovascular AVM treatment is set individually for each case upon consultations with a neurosurgeon and a neurologist. The endpoint of the treatment should consist in prevention of AVM bleeding in a management procedure characterized by a significantly lower risk of complications as compared to the natural history of AVM. Endovascular interventions within AVM may include curative exclusion of AVM from circulation, embolization adjuvant to resection or radiation therapy, targeted closure of a previously identified bleeding site as well as palliative embolization. Onyx was first described in the 1990s. It is a non-adhesive and radiolucent compound. Onyx-based closure of the lumen of the targeted vessel is obtained by means of precipitation. The process is enhanced peripherally to the main flux of the injected mixture. This facilitates angiographic monitoring of embolization at any stage. The degree of lumen closure is associated with the location of the vessel. Supratentorial and cortical locations are most advantageous. Dense and plexiform structure of AVM nidus as well as a low number of supplying vessels and a single superficial drainage vein are usually advantageous for Onyx administration. Unfavorable factors include nidus drainage into multiple compartments as well as multiarterial supply of the AVM, particularly from meningeal arteries, en-passant arteries or perforating feeders. Onyx appears to be a safe and efficient material for embolization of cerebral AVMs, also in cases of intracranial bleeding associated with AVM. Curative embolization of small cerebral AVMs is an efficient and safe alternative to neurosurgical and radiosurgical methods. Careful angiographic assessment of individual arteriovenous malformations should be performed before each Onyx administration.
Keywords: Onyx, brain arteriovenous malformation, embolization
Background
The goal of this study is to present an overview of reports regarding the use of Onyx in embolization of cerebral arteriovenous malformations. The overview is foreworded by a brief presentation of epidemiological and clinical aspects of the disorder.
Arteriovenous malformation (AVM) is an abnormal connection between arteries and veins, bypassing the capillary system. The connections are most probably formed during embryonic or fetal development. The anatomical structure of arteriovenous malformations is known as nidus. Nidus area is formed by one or several supplying arterioles together with drainage veins, usually dilated and tangled.
The incidence of AVM is hard to estimate due to asymptomatic course of the disorder in most cases. Autopsy data that do not meet the criteria of population studies suggest that the incidence of AVM is in the range of 5 to 600 cases per 100,000 individuals [1]. Population studies allowing to achieve reliable significance factors would require a large number of patients to be recruited [2]. The available results fall within the range of 140–500 cases per 100,000 individuals, i.e. 0.14–0.5% of overall population [3]. The etiology of AVM is a subject of extensive dispute in the literature. Initially, AVMs were considered congenital defects. De novo cases of AVM have been described [4–6]. Currently, an increasing number of publications presents theories regarding the occurrence of congenital defects in vascular cells (7–9).
AVM may occur simultaneously with hereditary hemorrhagic telangiectasia, Ehlers-Danlos syndrome or Sturge-Weber syndrome. The disorder is also a part of the Bonnet-Dechaume-Blanc syndrome [10]. Malformations within the posterior cranial fossa are an element of the PHACE syndrome [11].
Clinical Relevance
Discrepant data are available regarding the incidence of AVM symptoms. In most cases, the disorder may be asymptomatic. Most common symptoms include bleeding [12], in some cases leading to focal neurological defects. Seizures and recurring headaches may occur as a consequence of local intracranial compression or as sequential aftermath of bleeding within the AVM. AVM rupture factors include the presence of aneurysms, size (diameter <3 cm) [13], deep/periventricular location, high pressure in supplying arteries, pregnancy. Anatomical conditions at particularly high risk of acute hemorrhage include blood being supplied from vertebrobasilar circulation or via perforating feeders, as well as drainage occurring via few or insufficient veins [14]. The mortality following rupture of non-treated AVM is 53.0–81.0% [15].
The necessity to assess the risk of treatment complications led to the development of various assessment scales. A common one is the Spetzler-Martin scale (Table 1) which, although initially proposed for surgical AVM treatment, is being used for endovascular treatment as well [16]. Other scales were established for endovascular procedures by Richling et al. [17] and Valavanis and Yasargil [18].
Table 1.
Spetzler-Martin scale. Source: Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations 1986. J Neurosurg, 2008; 108(1): 186–93.
| Criterion | Score | |
|---|---|---|
| Diameter | <3 cm | 1 |
| 3–6 cm | 2 | |
| >6 cm | 3 | |
| Location | Functionally relevant area | 0 |
| Functionally irrelevant area | 1 | |
| Drainage veins | Superficial | 0 |
| Deep | 1 |
Non=invasive assessment of AVM is based on magnetic resonance imaging. The anatomy of malformation is assessed by standard spin echo and gradient echo sequences – T1, T2, and T1 with contrast enhancement. The examination is recommended in non-hemorrhagic malformations, particularly when conservative treatment is anticipated. The available imaging options include the assessment of ischemic lesions (DWI), approximate imaging of lesion morphology (TOF, SLINKY) and mapping of eloquent cortex areas (fMRI).
Computed tomography is used to assess intracranial bleeding; however, the sensitivity of this technique in detecting AVM as the cause of the bleeding is low.. In rare cases, the diagnosis may be suggested by calcifications. Non-specific lesions, such as compression or dilation of the ventricular system may also be visible. Angio-CT scans provide information on the presence of an AVM nidus and visualize the venous drainage of the nidus. However, they do not provide information on local flow hemodynamics, thus not being superior to angiography when planning the AVM management.
Digital subtractive angiography is the gold standard in AVM assessment. It provides exact information on hemodynamic properties of the malformation, such as arterial supply, flow rate in individual components, and venous drainage, as well as visualizes the morphology of the AVM nidus. In addition, DSA visualizes venous drainage of normal cerebral tissue. Examination consists in visualization of cranial vessels – external carotid arteries, internal carotid arteries, vertebral arteries – in desired projections and 3D images. The examination is completed by superselective assessment of arterial blood supply of the AVM, which might constitute preparation for embolization.
The objective of endovascular AVM treatment is set individually for each case and the decision on the appropriate management strategy is made in a team including a neurosurgeon and a neurologist. The treatment endpoint should consist in prevention of AVM bleeding in a management procedure characterized by a significantly lower risk of complications as compared to the natural history of AVM. Thus, potential endovascular interventions within AVM may include curative (independent and complete) exclusion of AVM from circulation, embolization adjuvant to resection or radiation therapy, targeted closure of a previously identified bleeding site as well as palliative embolization (alleviation of symptoms other than bleeding).
The natural history of cranial AVMs has been presented in individual reports; however, these are very diverse. An multicenter, randomized ARUBA study has been ongoing since January 2006 to assess the five-year history of previously non-bleeding cerebral AVMs in 800 patients. The first arm of the study consists of patients who have been offered pharmaceutical treatment with possible procedural intervention in case of complications. The second arm consists of patients receiving full range of procedural treatment aimed at complete eradication of AVM. The inclusion and exclusion criteria, as well as the ethical principles of the study, are available on the Internet.
The procedure of administering Onyx to the desired location within the AVM is the same as that of superselective angiography of the nidus (Figure 1). Local anesthesia is required if the Wada test is to be used for prospective assessment of neurological defects. Thus, the choice of general anesthesia requires fMRI scan being performed first. General anesthesia increases the safety of the procedure and reduces patient’s stress due to the procedure. Also important is the maintenance of patient’s arterial pressure during and immediately after the procedure below 90 mmHg. Most common vascular access is achieved through the femoral artery. Using a system of coaxial catheters, a microcatheter is placed selectively in the selected AVM supplying vessel, constituting preparation for the embolization procedure (Figure 2). Onyx administration (Figure 3) should be preceded by the product vial being shaken for at least 20 minutes to obtain a homogeneous mixture. The dead space of the microcatheter should be filled with DMSO immediately before Onyx injection. The procedure is completed by follow-up administration of contrast agent to the vascular AVM supply system (Figure 4).
Figure 1.

Angiography of the internal carotid artery, late arterial phase. (A) – venous drainage of the AVM, (B) – main arterial supplying vessel, arrow – AVM nidus.
Figure 2.

Selective AVM angiography. Black arrow – microcatheter advanced through arterial supplying vessel, white arrow – venous drainage of the AVM, M – AVM nidus.
Figure 3.
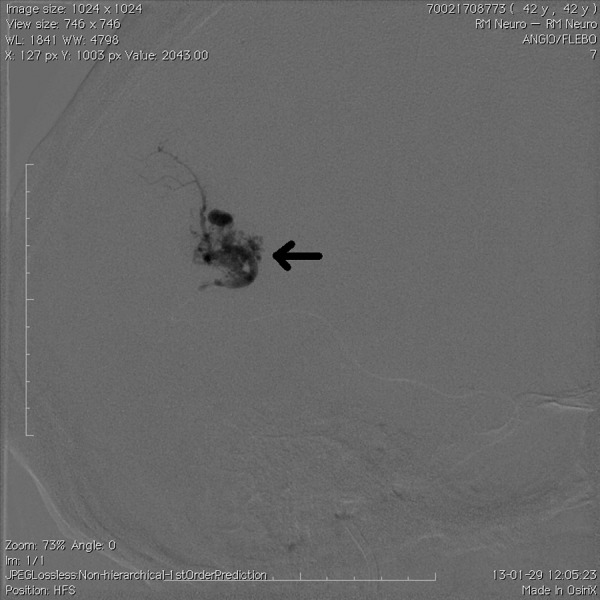
Follow-up X-ray image obtained immediately after injection of Onyx (arrow) through a microcatheter.
Figure 4.
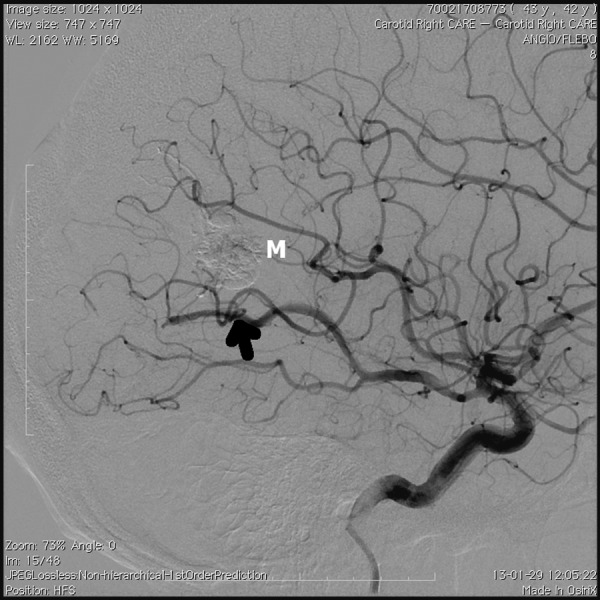
Angiography upon completion of embolization, arterial phase. Arrow – main arterial supplying vessel, M – contrasting embolization material mass.
Embolization Material – Onyx
Onyx (ev3 Neurovascular, Irvine, CA) was first described by Taki et al. [19] and Terada et al. [20] in the 1990s. From the chemical standpoint, it is an ethylene-vinyl alcohol copolymer (EVOH) dissolved in dimethyl sulfoxide (DMSO). It is a non-adhesive and radiolucent compound. Onyx-based closure of the lumen of the targeted vessel is obtained by means of precipitation, initiated after diffusion of DMSO in the presence of water. The process is enhanced peripherally to the main flux of the injected mixture. These properties facilitate angiographic monitoring of embolization at any stage. Fluoroscopic visualization of the injected Onyx mass is possible through the addition of tantalum filings. Currently, Onyx is available at different viscosity grades: 18, 20, 34 centipoise and HD 500. The variable factor is the concentration of EVOH, being 6, 6.5, 8, and 20%, respectively. First two of these concentrations are used in AVM embolization procedures. The rapid flow components of the arteriovenous malformation should be embolized with the product at 8% concentration, while the accompanying aneurysms should be closed with HD 500 grade [21]. The technical efficacy depends on finding appropriate proportion between product’s penetration capability within the nidus and retrograde reflux into the microcatheter.
Review of Current Reports
Weber et al. present their experience in a group of 93 patients with 94 AVMs [22]. The objective of AVM embolization was to prepare patients for neurosurgical or radiosurgical treatment, depending on anatomical availability of individual cases. The volume of malformations as measured by the Pasqualin method ranged from 1.0 to 5.8 mL (mean volume 9 mL). According to the Spetzler-Martin scale, 48 AVMs were grade I–II, 24 AVMs were grade III and 22 AVMs were grade IV–V malformations. Symptoms accompanying AVMs included seizures in 48% of patients, bleeding from the AVM nidus in 20% of patients, headaches in 17% of patients, and neurological defects in 4.3% of patients. Nine asymptomatic patients (9.6%) were also qualified for the treatment. Most common AVM locations included frontal location – 19 (20%), temporal – 15 (16%), occipital – 13 (14%), frontal-temporal – 9 (9,6%), parietal-occipital – 9 (9,6%), parietal – 8 (9%), cerebellar – 5 (5%). Onyx administration was started at the flow rate of 0.1 mL/min. The initial goal was to create a volume of Onyx around the tip of the microcatheter so as to prevent its reflux within the embolized vessel. After waiting 2 minutes for the plug to become stabilized, administration of Onyx was continued until optimum filling of the AVM from the particular vascular access. Administration of Onyx was discontinued when AVM drainage veins or meningeal branches were closed or when Onyx reflux was too high. Increased resistance upon Onyx injection also required procedure discontinuation due to the risk of damaging the microcatheter or local vessels. The release of the plug from the microcatheter was achieved by creating reduced pressure within the syringe barrel and alternating attempts of retracting and advancing the microcatheter tip until it was freely retracted.
Onyx 18 and Onyx 20 were mainly used. As the authors grew in their experience, they preferred to start the procedure using mixtures of higher viscosity (Onyx 20 or, in rare cases, Onyx 34), followed by viscosity reduction. The average volume of Onyx administered per single AVM was 3.3 mL (range of 0.1–14.8 mL). Closure of a single artery supplying blood to an AVM required administration of an average of 1.4 mL of Onyx (range of 0.1–7.0 mL). Embolization of a single AVM was obtained after closing an average of 2.3 supplying vessels. The average reduction in AVM volume following embolization was 79.5% (<50%, 7; 50– 69%, 11; 70–79%, 12; 80– 89%, 22; 90–99%, 23; 100%, 19). The comparison was valid for 61 available patients and was made between the follow-up angiography after last embolization and a follow up examination performed after 9.5 months.
The percentage of complete AVM embolizations within the study group was 20%, with 2% experiencing recurrence upon follow-up angiography performed 3 months after embolization. Embolization or embolization and surgical resection led to curative outcome in 71% and 83%, respectively.
Technical complications were encountered in 6% of cases: 4% – microcatheter entrapment, 2% – peripheral vessel perforations, immediately closed with Onyx without clinical consequences. Most of these complications were encountered in first ten patients. Embolization-related complications were encountered in 12% of cases. Acute intracranial hemorrhage was experienced by 2% of patients, resulting in a necessity of surgical decompression and AVM resection, completed without detectable neurological defects. Significant post-embolization defects were observed in 5% of patients. In addition, occipital location of AVM was associated with 4% of cases of quadrantopsia or hemianopsia.
A similar study was conducted in 2007 by W.J. van Rooij et al, who presented their own preliminary results and conclusions on the use of Onyx in AVM embolization procedure [23]. The study was conducted in a group of 44 patients at the age of 14–71 years, average of 44 years. The estimated AVM diameter was in the range of 2–7 cm, average of 3.9 cm. The incidence of symptoms was as follows: : seizures – 59%, AVM bleeding – 30%, subarachnoid bleeding from a concomitant aneurysm – 7%, disturbed vision – 2.3%. No AVM symptoms were observed in 1 patient (2.3% of the study group). The author performed a total of 52 embolization procedures of 138 supplying vessels as the only operator in the study group. Angiographic assessment of the efficacy of the procedure was performed 6–12 weeks after the procedure. The average AVM reduction was 75%. Total AVM closure was obtained in 7 patients (16%). Partial embolizations were completed with surgical (10 patients) or radiosurgical procedures (20 patients). Complications were observed in 6 patients, of whom 1 died and 2 experienced persistent paresis.
V. Katsaridis et al. resolved to assess the usefulness of Onyx in the curative treatment of AVM, including repeated embolization with Onyx in persistent management of AVM following previous attempts [24]. Upon publication, the total number of patients in the retrospective study was 101, and the total number of embolization procedures was 219. Eleven AVMs (10.9%) were located within the cerebellum, while 6 (5.9%) were located in deep cerebral structures: 2 within the geniculate body, 4 within the basal ganglia. The remaining 84 AVM locations within the study group were distributed as follows: 24 (28.6%) – frontal lobe, 18 (21.4%) – parietal lobe, 13 (15.5%) – occipital lobe, 10 (11.9%) – frontal-parietal location, 5 (6%) – temporal-occipital location, 4 (4.8%) – temporal location, 4 (4.%) – parietal-occipital location, 3 (3.6%) – temporal-parietal location, and 3 (3.6%) – frontal-temporal-parietal location. Arterial supply from the pachymaninx was observed in 57 (83.8%) AVMs, involving the branches of the middle cerebral artery, superficial temporal artery, occipital artery, ocular artery as well as extracranial musculodermal branches of the vertebral artery. The diameter of malformations ranged from 2 to 12 cm, with the average of 5.2 cm. In the Spetzler– Martin scale, 6.9% of cases were classified as grade I, 17.8 of cases were classified as grade II, 38.6% of cases were classified as grade III, 32.7% of cases were classified as grade IV and 4% of cases were classified as grade V. The group of patients who completed treatment consisted of 52 individuals. Of these, the intended complete closure of AVM was achieved in 28 patients. Death occurred in 3 cases (3%), while persistent neurological defects were described in 8 (8%) patients.
The final conclusion from the study was included a large rate of complete or nearly complete (>80% of volume) closure of AVM using Onyx, including non-simultaneous malformation closures. The mortality rate and the rate of persistent complications within the study group seem acceptable.
Curative embolization of AVM with Onyx was also the subject of a retrospective study conducted by van Rooij et al in 2012 (25). The principal difference with respect to the study mentioned above was the selection of superficial location of AVMs with diameters not larger than 3 cm (range 1–3 cm, median 2 cm). The group of 24 patients, including 7 women and 17 men at the average age of 41 years (range of 6–74 years) reported symptoms that were qualitatively and quantitatively consistent with those reported in the previous studies. AVM rupture was observed in the angiograms of 14 patients. AVMs were located as follows: 11 – frontal lobe, 6 – occipital lobe, 4 – parietal lobe and 3 – temporal lobe. Embolizations led to complete closure of AVMs in all 24 patients. No hemorrhagic complications, ischemic complications or defects were observed (0%; 95% CI, 0–16.3%). One patient from the group of patients with pre-procedural AVM rupture has died. Evacuation of a large hemorrhage from the frontal region was ineffective. In the remaining patients, follow-up angiography was performed after 3 months. In this group, a small residue of AVM was detected and closed using a radiosurgical procedure.
Administration of Onyx upon stopped blood flow within the supplying vessel, referred to as balloon assisted technique (BAT), was presented by Orozco et al. in 2012 [26]. Two cases of Onyx embolizations were reported for AVMs located in the geniculate body region following closure of catheterized supplying vessels using Scepter C balloons. Common features of the embolized AVMs included large size and a high number of supplying vessels branching off perigeniculate arteries. The first patient suffered of intraventricular bleeding and hydrocephaly, while the other reported with a long history of seizures and small intracranial bleeding. Administration of Onyx upon locally stopped blood flow in these patients was characterized by complete penetration of Onyx within the AVM nidus. Technical limitations of the method may involve the possibility of an important arterial vessel located proximally to the AVM catheterization sites being closed by the balloon, as well as poor availability of appropriate balloons.
Mounayer et al. suggested possible use of n-butyl cyanoacrylate (n-BCA) in arteriovenous fistulas (AVF) and in cases when AVM nidus catheterization is not feasible. In this observation, the flow of Onyx within the supplying vessel was too strong for its use. Some authors obtained good results of closures of AVFs associated with AVM flow when using Onyx 34 or Onyx 500. However, the proposal to embolize the high-flow compartments of the AVM nidus with an adhesive embolization agent seems to effectively complement this limitation in the applicability of Onyx [27].
Small and moderate-sized AVMs seem to be embolizable upon a single Onyx procedure. Möhlenbruch et al. obtained results confirming this hypothesis in the analysis of the history of treatment of 24 patients in whom AVMs were of an average diameter of 2.2 cm (median 2 cm; range 1–3 cm). Complete closure of malformation was achieved in all patients in a single procedure [28].
Bleeding as a complication of cerebral AVM is the most common cause of neurological defects and deaths associated with AVM. A precise angiographic assessment and efficient embolization of the bleeding site may attenuate the expected clinical outcomes by reducing the incidence of recurrent bleedings. The use of Onyx for embolization of bleedings associated with AVM, as assessed retrospectively by Rooij et al., allowed for complete exclusion of malformations in 57% of patients and partial exclusion in the remaining 43%. After a 21-month follow-up period, 1 patient died, while no bleeding symptoms were observed in the remaining patients [29].
Conclusions
The authors compare the technical advantages of Onyx against the cyanoacrylate glue that had been used hitherto. The possibility to divide the Onyx administration process into stages separated by control contrast administration offers a chance to better control the site of precipitation of the embolization material. Resuming Onyx administration after angiographic control leads to a change in the direction of its distribution within the nidus, leading to the desirable effect of filling other patent parts of the nidus. W.J. van Rooij et al confirmed the applicability of Onyx 34 in embolization of large arteriovenous fistulas within AVMs.
The higher possibility to control the filling of the AVM nidus presents a challenge to identify and eliminate the risk factors of complications resulting from unfavorable distribution of Onyx within the hemodynamic compartment being embolized. Onyx administration process should be monitored on an ongoing basis using biplanar fluoroscopy or uniplanar fluoroscopy with frequent changes in the imaging angle. Onyx reflux over a distance of 1.5–2 cm relative to the microcatheter is an indication to discontinue administration of the material and to retract the catheter for 1–2 minutes. This results in formation of a solidified plug slightly behind the microcatheter tip. The plug usually blocks the reflux of Onyx, also in case of the aforementioned change in the direction of distribution within the AVM nidus. Reflux encountered upon repeated attempt to administer Onyx indicates the need for administration being discontinued from the particular vascular access site. A separate case of retrograde Onyx flow is reflux into other arteries supplying the AVM via the nidus. This might be technically difficult to observe using single-plane or low image quality fluoroscopy. However, it is the cause of defect-type complications of background ischemia and is an absolute contraindication for Onyx administration. Possibility of changing the access to the embolized area as well as the concentration of the embolization mixture should be considered. Partial closure of AVM nidus supplying vessels while retaining open venous drainage vessels may lead to vein thrombosis and subsequent increase in blood pressure in insufficiently drained AVM with significant risk of AVM bleeding. The risk of relocation of an already administered portion of embolization material into the venous part of the nidus may be another cause of hemorrhagic complications of AVM administration. When using Onyx, the risk should be minimized by an earlier thorough analysis of hemodynamic conditions within the AVM, appropriate selection of concentration of the embolization mixture and the control of administration rate.
The degree of lumen closure is associated with the location of the vessel. Supratentorial and cortical locations are most advantageous. Dense and plexiform structure of AVM nidus as well as a low number of supplying vessels and a single superficial drainage vein are usually advantageous for Onyx administration. Prognostic factors for incomplete AVM embolization with <70% lumen reduction include nidus drainage into multiple compartments as well as multiarterial supply of the AVM, particularly from meningeal arteries, en-passant arteries or perforating feeders. It seems that complete or nearly complete Onyx-based embolization of nidus is feasible in most AVM cases. However, most of the cited authors recommend scheduling multisession Onyx treatments, if possible, accompanied by gradual, searching evaluation of embolization effects, particularly when closing large AVMs or AVMs with multiple hemodynamic compartments. This is to reduce the risk of complications including patient’s death, neurological defects of microcatheter entrapment. Each decision regarding the scope of embolization should be made by a team of experts with consideration to the AVM bleeding risk factors.
Increased resistance to the syringe plunger movement is a signal to discontinue administration of Onyx, perform a thorough fluoroscopic review of the embolized site and verify the patency of the microcatheter. The phenomenon may be due to complete embolization of the vascular bed; however, when ignored, it may cause pressure-related AVM damage or a technical failure of the procedure. The volume of the contracting space filled with Onyx may be larger than the space previously visualized upon superselective AVM angiography.
Onyx appears to be a relatively safe and efficient material for embolization of cerebral AVMs, also in cases of intracranial bleeding associated with AVM. Curative embolization of small cerebral AVMs is an efficient and safe alternative to neurosurgical and radiosurgical methods. Careful angiographic assessment of individual arteriovenous malformations should be performed before each Onyx administration.
References:
- 1.Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986;9:177–216. doi: 10.1007/BF01743136. [DOI] [PubMed] [Google Scholar]
- 2.Stapf C, Mohr JP, Pile-Spellman J, et al. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus. 2001;11(5):e1. doi: 10.3171/foc.2001.11.5.2. [DOI] [PubMed] [Google Scholar]
- 3.Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations: an analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467–90. doi: 10.3171/jns.1966.25.4.0467. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan A, Manchandia TC, Gould G, et al. De novo arteriovenous malformations: case report and review of the literature. Neurosurg Rev. 2010;33(1):115–19. doi: 10.1007/s10143-009-0227-z. [DOI] [PubMed] [Google Scholar]
- 5.McKinney JS, Steineke T, Nochlin D, et al. De novo formation of large arteriovenous shunting and a vascular nidus mimicking an arteriovenous malformation within an anaplastic oligodendroglioma: treatment with embolization and resection. J Neurosurg. 2008;109(6):1098–102. doi: 10.3171/JNS.2008.109.12.1098. [DOI] [PubMed] [Google Scholar]
- 6.Motegi H, Kuroda S, Ishii N, et al. De novo formation of cavernoma after radiosurgery for adult cerebral arteriovenous malformation – case report. Neurol Med Chir (Tokyo) 2008;48(9):397–400. doi: 10.2176/nmc.48.397. [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias PL. Brain and Spine AVMs, Vein of Galen Malformation. Treatments and Embryological Considerations. Interv Neuroradiol. 2003;9(3):263–72. doi: 10.1177/159101990300900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiex R, Mulliken JB, Revencu N, et al. A novel association between RASA1 mutations and spinal arteriovenous anomalies. Am J Neuroradiol. 2010;31(4):775–79. doi: 10.3174/ajnr.A1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863–73. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt D, Agostini H, Schumacher M. Twenty-seven years follow-up of a patient with congenital retinocephalofacial vascular malformation syndrome and additional congenital malformations (Bonnet-Dechaume-Blanc syndrome or Wyburn-Mason syndrome) Eur J Med Res. 2010;15(2):89–91. doi: 10.1186/2047-783X-15-2-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puttgen KB, Lin DD. Neurocutaneous vascular syndromes. Childs Nerv Syst. 2010;26(10):1407–15. doi: 10.1007/s00381-010-1201-3. [DOI] [PubMed] [Google Scholar]
- 12.Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–91. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 13.Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76(6):918–23. doi: 10.3171/jns.1992.76.6.0918. [DOI] [PubMed] [Google Scholar]
- 14.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350–55. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 15.Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331–37. doi: 10.3171/jns.1983.58.3.0331. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34(1):2–6. [PubMed] [Google Scholar]
- 17.Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(5 Suppl.3):S148–57. doi: 10.1227/01.NEU.0000237408.95785.64. discussion S3–13. [DOI] [PubMed] [Google Scholar]
- 18.Valavanis A, Pangalu A, Tanaka M. Endovascular treatment of cerebral arteriovenous malformations with emphasis on the curative role of embolisation. Interv Neuroradiol. 2005;11(Suppl.1):37–43. doi: 10.1177/15910199050110S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita K, Taki W, Iwata H, et al. Characteristics of ethylene vinyl alcohol copolymer (EVAL) mixtures. Am J Neuroradiol. 1994;15(6):1103–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer. Report of three cases. J Neurosurg. 1991;75(4):655–60. doi: 10.3171/jns.1991.75.4.0655. [DOI] [PubMed] [Google Scholar]
- 21.Ayad M, Eskioglu E, Mericle RA. Onyx: a unique neuroembolic agent. Expert Rev Med Devices. 2006;3(6):705–15. doi: 10.1586/17434440.3.6.705. [DOI] [PubMed] [Google Scholar]
- 22.Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61(2):244–52. doi: 10.1227/01.NEU.0000255473.60505.84. [DOI] [PubMed] [Google Scholar]
- 23.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28(1):172–77. [PMC free article] [PubMed] [Google Scholar]
- 24.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50(7):589–97. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 25.van Rooij WJ, Jacobs S, Sluzewski M, et al. Curative embolization of brain arteriovenous malformations with Onyx: patient selection, embolization technique, and results. Am J Neuroradiol. 2012;33(7):1299–304. doi: 10.3174/ajnr.A2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orozco LD, Luzardo GD, Buciuc RF. Transarterial balloon assisted Onyx embolization of pericallosal arteriovenous malformations. J Neurointerv Surg. 2013;5(4):e18. doi: 10.1136/neurintsurg-2012-010388. [DOI] [PubMed] [Google Scholar]
- 27.Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. Am J Neuroradiol. 2007;28(3):518–23. [PMC free article] [PubMed] [Google Scholar]
- 28.Möhlenbruch M, Bendszus M, Rohde S. Comment on: Curative Embolization of Brain Arteriovenous Malformations with Onyx: Patient Selection, Embolization Technique, and Results. Clin Neuroradiol. 2012;22(2):181–82. doi: 10.1007/s00062-012-0149-y. [DOI] [PubMed] [Google Scholar]
- 29.van Rooij WJ, Jacobs S, Sluzewski M, et al. Endovascular Treatment of Ruptured Brain AVMs in the Acute Phase of Hemorrhage. Am J Neuroradiol. 2012;33(6):1162–66. doi: 10.3174/ajnr.A2995. [DOI] [PMC free article] [PubMed] [Google Scholar]


