Summary
Background:
The aim of this study was to determine age, gender, and hemispheric differences in the volume of the human neostriatum (striatum) nucleus in healthy humans.
Material/Methods:
This study was performed on 120 normal human subjects (60 males, 60 females, right-handed) 15–65 years old, divided into two groups: young (<40 yrs) and old (=≥40 yrs). Sectional brain images were obtained via magnetic resonance imaging (MRI), analyzed and processed using the Image-J software, and the striatum volume was calculated using the Cavalieri’s principle, retrospectively.
Results:
The analyses revealed bilateral age-related shrinkage of the putamen in both genders and the putamen and caudate nucleus were significantly smaller in older than in younger subjects (P-value <0.001). The age-related shrinkage of the caudate and putamen nucleus in men and women was about 5%, 5% and 4%, 4%, respectively, and there were statistically significant volume differences between males and females (P-value <0.05). In both genders, a significant rightward asymmetry was observed in the caudate and putamen nucleus (3.89%, 4.21% in men and 4.51%, 3.32% in women).
Conclusions:
Bilateral age-related shrinkage and rightward asymmetry of the striate nucleus was found in healthy adults and there were significant volume differences between men and women. Obtained results provide useful baseline data on age and gender-related changes of the volume of the striatum.
Keywords: striate nucleus, age, gender, MRI, morphometry
Background
The neostriatum – caudate and putamen nucleus- plays an important role in controlling motor function, planning and execution [1,2]. The anatomy, function and metabolism of the human brain tissue undergo gradual changes over the life cycle [3]. Age-related changes are major risk factors in the most prevalent neurodegenerative diseases, including Parkinson’s and Huntington’s disease [4,5]. Attempts have been made to standardize the striatum volume variation related to normal aging and gender. Therefore, morphometric and volumetric studies of striate nuclei in normal persons are essential to identify changes related to neuro-degenerative diseases. Measurement of age-related changes in striate nuclei is a challenge and includes two approaches: postmortem studies and in vivo imaging studies. The results of the first method are not reliable due to fixation artifacts, bias in sample selection and continuous reduction in volume and weight of brain tissue which are not present in the latter method. Among different imaging modalities, Magnetic Resonance Imaging (MRI) provides excellent tissue contrast, absence of bone artifacts and is an accurate non-invasive method of in vivo measurement which increases the accuracy of morphometric measurements [6–8]. Besides, combination of MRI and current software techniques could potentially provide useful information in this area. MRI studies have shown that gray matter of the cerebral cortex and sub-cortical regions reveal a negative correlation with age [6,8–11] and their reduction patterns in different regions of brain tissue are different, e.g. rapid reduction in the frontal lobe compared to other lobes [12], age-related atrophy in volume of brain tissue [8] and age-related decrease in volume of thalamus, putamen and caudate nucleus [9]. Gender is another important factor in morphological changes of brain tissue. MRI studies have demonstrated effects of gender on age-related changes in brain structure [11–15] such as more of age-related changes of brain tissue volume in men compared to women [11], faster age-related atrophy of gray and white matter in men [16] and gender differences in the gray matter in middle-aged healthy men and women [17]. A few imaging studies have examined the effects of age on striate nucleus, and yet some fundamental questions on differences in gender- and age-related changes in striate nucleus remain unanswered. It is well known that functional and morphological asymmetries exist in human brain [11,15,18,19]. The majority of reports indicate intrahemispheric differences in brain structures such as volume of brain lobes and thalamus [5,7,16,18–20] There are fewer reports on the striate nucleus. Due to the fact that a disease can cause different age-related changes in the striatum, testing healthy persons was the most appropriate method for assessing age-related changes. This study was specifically designed using MRI morphometry of healthy male and female volunteers to find out whether there are any age-related changes in human striate nucleus and whether they are gender-dependent or not. Moreover, the study aimed to establish any differences in the volume of the striatum between two hemispheres. The obtained data may be used as reference data in comparative studies on neurodegenerative diseases such as Parkinson’s.
Material and Methods
Subjects
One hundred and twenty right-handed healthy volunteers (sixty men, sixty women) aged between 15 and 65 years recruited from the Babak imaging center were randomly selected. Each subject had a complete neuro-psychiatric and physical examination which excluded any risk factors of stroke, such as hypertension, diabetes or cardiovascular disease. The subjects were divided into two groups: young (<40 yrs) and old (≥40 yrs). There were 30 men (27.10±7.25 yrs) and 30 women (27.23±8.03 yrs) in the young group and 30 men (50.27±6.35 yrs) and 30 women (52.40±8.10 yrs) in the old group. There was no significant difference in age between men and women. The study was approved by the Ethics Committee of the Iran University of Medical Sciences and after full information on the procedure and outcome of the study was provided, a written consent was obtained from all subjects.
MRI acquisition
Brain images were obtained at the Babak imaging center using the Intera system (1.5 Tesla, Philips Gyroscan). Patients’ heads were aligned along the midline or the canthomeatal line at 0° using head support (standard imaging protocol). At first, a series of sagittal scout images (8–10 slices, 5 mm thick with a 1-mm interslice gap) was acquired to verify head position and quality of images. All the scans used for the volumetric analysis were (axial and coronal) T2-weighted spin-echo images with the following parameters: TE=100 msec, TR=2800 msec, slice thickness =2.5 mm, inter-slice gap =0.3 mm, repetition =1, acquisition angle =90°, FOV=220 mm, acquisition matrix =352×512.
The axial and coronal T2-weighted images are highly sensitive to local pathologies and provide a good demarcation of the CSF-containing spaces which results in high-spatial-resolution images with excellent contrast, to be used for measurement by means of manual tracing of striate nucleus structure.
MRI processing
The brain images were transferred to a PC workstation (21-inch video monitor screen with standard brightness and contrast) using the Image-J software (Version 1.34, National Institutes of Health) that provides reliable morphometry and manual tracing. After image acquisition, the axial slices were realigned parallel to the anterior commissure-posterior commissure (AC-PC) line and the images were filtered to reduce noise. After subtraction of brain tissue from cranial and extracranial structures in the obtained images, the images were divided into: images of white matter, gray matter and CSF. One of the basic considerations in the selection of a correct ROI (region of interest) is the exact delineation of structures for which several parameters such as size, contrast and brightness were changed to find a sharp border of the selected structure. The region of interest in this study includes the caudate and putamen nucleus, the volumes of which were calculated using the Cavalieri’s theorem of systemic sampling. For that purpose, a summation over the product of slice area and slice thickness was calculated and presented in cubic centimeters (cm3).
Delineation of ROIs
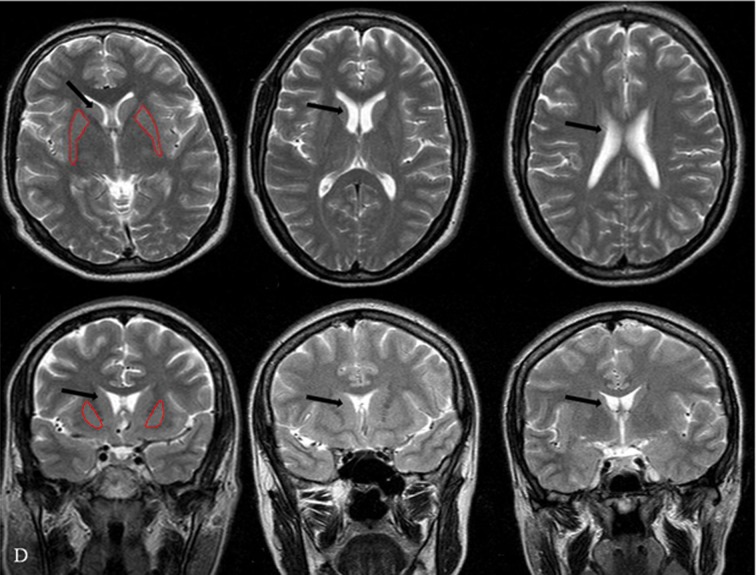
The caudate and putamen were manually outlined on the axial and coronal slices according to standard anatomical brain atlases (Figure 1). To reduce measurement variability, all structures were segmented and measured by one investigator who was blind to subject identity. Reliability of measurement was evaluated by a second investigator (double-blind study).
Figure 1.
Segmentation method: example for caudate nucleus on a T2-weighted MRI scan. (A–C) in the axial section and (D–F) in the coronal section; the border of the putamen nucleus is traced on every slice and saved as an object mark (in red). The volume of the segmented object map is calculated in cubic centimeters for the right and left side. Caudate nucleus in cross-sectional image (arrow).
Caudate nucleus
The volume of the head and body of the caudate nucleus was estimated on the basis of 20 coronal and axial slices. The caudate nuclei first appeared in the rostral section where the stria terminalis and terminal vein constituted the ventral boundary. The caudate was traced on each section until it was no longer visible. The lateral ventricle and the anterior limb of the internal capsule represented the medial and lateral boundaries, respectively.
The dorsal boundary of the caudate was surrounded by white matter. On more anterior sections of the caudate nucleus, the accumbens nucleus featured as the ventral border and the accumbens nucleus was included in the total caudate volume.
Putamen nucleus
The volume of the putamen was estimated from 25 slices and situated laterally to the caudate nucleus heads and thalami. Tracing began on the most rostral section, when the putamen was visible in the ventro-lateral regions of the caudate, and continued to the most caudal section on which it disappeared. The external and internal capsules formed the lateral and medial borders, respectively. In the slice where the anterior commissure was reached, the globus pallidus became the medial border of the putamen. The temporal stem, optic radiations, amygdala, temporal horn, and anterior commissure served as the ventral boundary of the putamen.
Intracranial volume (ICV)
Traced volumes of each ROI (Caudate and Putamen nuclei) were expressed as a percent of the traced ICV that was defined as cerebral hemispheres and measured in every slice between the vertex and the superior border of the mid-brain. ICV was estimated from the coronal sections (approximately 11–12 sections). In addition, the normalized volume of the ROI was defined using the following formula: (non-normalized ROI volume/ICV) ×100.
Statistical analysis
Statistical analysis was performed by SPSS 15 (SPSS/PC Inc., Chicago, IL). Data were presented as mean (SD). The relationship of putamen nucleus, caudate nucleus and hemispheric differences of caudate and putamen nucleus with age were tested using linear regression in each gender group. To assess the interaction and main effects of age and gender, regression analysis was used; in that model, the variables were entered as indicators. To assess the differences of age by gender groups (M <40 yrs, M ≥40 yrs, F<40 yrs and F ≥40 yrs) in the right or left side of putamen nucleus, caudate nucleus and hemispheric asymmetry, the analysis of variances (ANOVA) or Welch robust test was used. The latter was used in case of heterogeneous variances. The ANOVA or Welch robust test was followed by Tukey or Games-Howell test, respectively. Differences with P-value <0.05 were considered as significant.
Results
Effect of age on striatum volume
The results of the statistical analysis on age-related changes for both sides of the caudate and putamen volumes, corrected for intracranial volume, are presented in Table 1.
Table 1.
Age distribution of the subjects, intracranial volume (ICV) and mean volumes, index asymmetry of caudate and putamen nucleus in men and women.
| Male (mean ±SD) | Female (mean ±SD) | ||||
|---|---|---|---|---|---|
| Young | Old | Young | Old | ||
| Age | 27.10±7.25 | 50.27±6.35 | 27.23±8.03 | 52.40±8.10 | |
| ICV | 1026.09 | *909.49 | 995.02 | *907.09 | |
| Non-normalized | Right | 4.55±1.01 | *3.63±0.52 | 4.34±0.78 | *3.47±0.53 |
| Left | 4.37±0.92 | *3.49±0.47 | 4.08±0.78 | *3.32±0.44 | |
| Total | 8.92±1.05 | *7.12±0.85 | 8.42±1.40 | *6.79±0.87 | |
| Caudate | |||||
| Normalized | Right | 0.44±0.06 | *0.40±0.04 | 0.43±0.05 | *0.38±0.04 |
| Left | 0.42±0.06 | *0.38±0.03 | 0.41±0.06 | *0.36±0.04 | |
| Total | 0.86±0.12 | *0.78±0.07 | 0.84±0.11 | *0.74±0.08 | |
| Asymmetry | 3.93% | 3.85% | 4.58% | 4.45% | |
| Non-normalized | Right | 5.63±0.92 | *4.53±0.62 | 5.35±0.71 | *4.46±0.36 |
| Left | 5.46±0.88 | *4.29±0.59 | 5.19±0.68 | *4.31±0.47 | |
| Total | 11.09±1.60 | *8.82±1.01 | 10.54±1.38 | *8.77±0.83 | |
| Putamen | |||||
| Non-normalized | Right | 0.55±0.04 | *0.50±0.04 | 0.53±0.03 | *0.49±0.02 |
| Left | 0.53±0.04 | *0.47±0.03 | 0.52±0.04 | *0.47±0.03 | |
| Total | 1.08±0.08 | *0.96±0.07 | 1.05±0.07 | *0.96±0.05 | |
| Asymmetry | 3.01% | **5.40% | 3.04% | 3.60% | |
The mean for groups of the old was significantly different than the mean for the groups of the young (P-value <0.001);
The index of asymmetry was significantly higher than in the groups of the young (P-value <0.05).
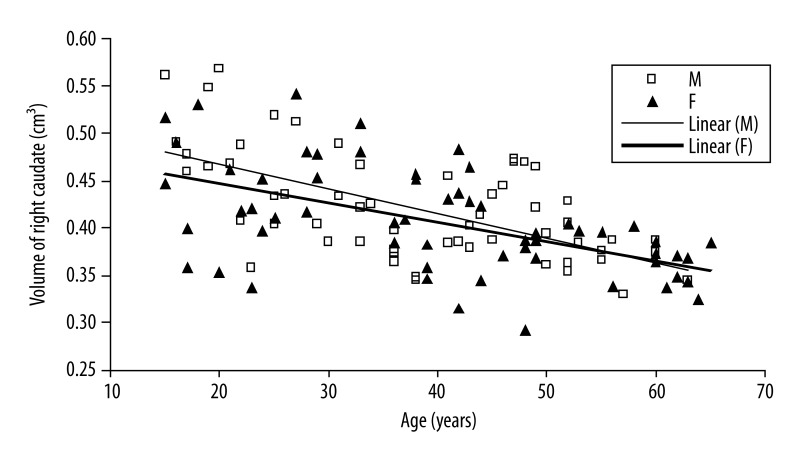
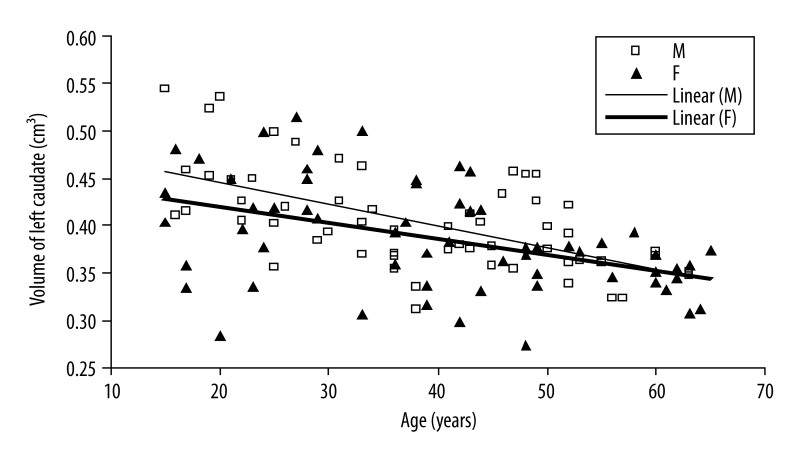
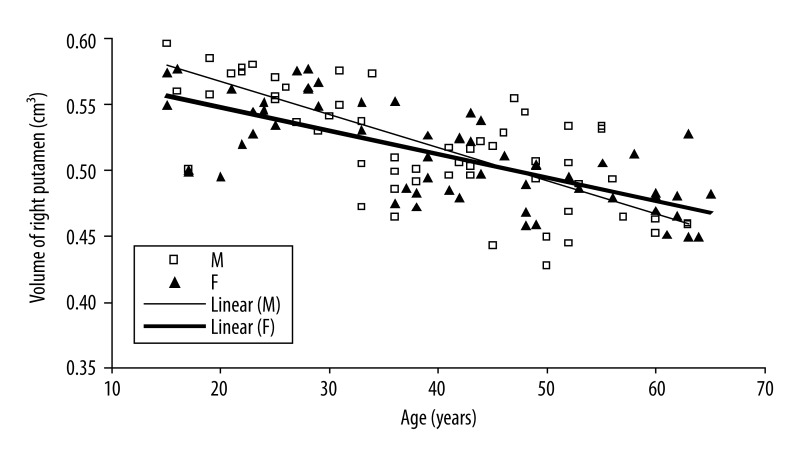
There exist significant negative correlations between age and caudate volume in male group (right side: r=−0.003, P-value <0.001; left side: r=−0.002, P-value <0.001) and putamen volume (right side: r=−0.002, P-value <0.001; left side: r=−0.003, P-value <0.001) (Figures 2 and 3).
Figure 2.
Scatter plot and linear regression showing a negative correlation between age and right caudate nucleus in men (open squares, solid regression line) and women (filled triangles, solid regression line).
Figure 3.
Scatter plot and linear regression showing a negative correlation between age and left caudate nucleus in men (open squares, solid regression line) and women (filled triangles, solid regression line).
In female group, there exist significant negative correlations between age and caudate volume or putamen volume (right side and left side: r=−0.002, P-value <0.001) (Figures 2 and 3).
In both groups, the correlation between total putamen volume and age was the same as the correlation between caudate volume and age (r=−0.004).
In male group, the negative correlation between age and volumes of caudate and putamen nucleus were stronger than in female group (P-value <0.001). However, there were significant differences between age groups in the volumes of caudate and putamen nucleus.
Older men and women revealed significantly lower total cerebral hemispheric brain volume or striatum volume than younger men and women (Table 1). These differences were observed after normalization by volume of the intracranial volume (ICV). Men in the young group had significantly higher total normalized volumes for the caudate nucleus (0.86 vs. 0.78 cm3, respectively, P-value <0.001) and the putamen (1.07 vs. 0.96 cm3, respectively, P-value <0.001) than the old group. In comparison to the old group, men in the young group had higher measured normalized volumes of the right (0.44 vs. 0.40 cm3, respectively, P-value <0.001) and left (0.42 vs. 0.38 cm3, respectively, P-value <0.001) caudate nucleus.
A similar pattern of volumetric differences was found for the putamen nucleus. Compared to the group of old men, men in the young group had higher measured normalized volumes of the right (0.55 vs. 0.50 cm3, respectively, P-value <0.001) and left (0.53 vs. 0.47 cm3, respectively, P-value <0.001) putamen nucleus (Figures 4 and 5). In women, the young group had significantly higher total measured normalized volumes for the caudate (0.84 vs. 0.75 cm3, respectively, P-value <0.001) and putamen (1.05 vs. 0.96 cm3, respectively, P-value <0.001) nucleus (Figures 4 and 5).
Figure 4.
Scatter plot and linear regression showing a negative correlation between age and right putamen nucleus in men (open squares, solid regression line) and women (filled triangles, solid regression line).
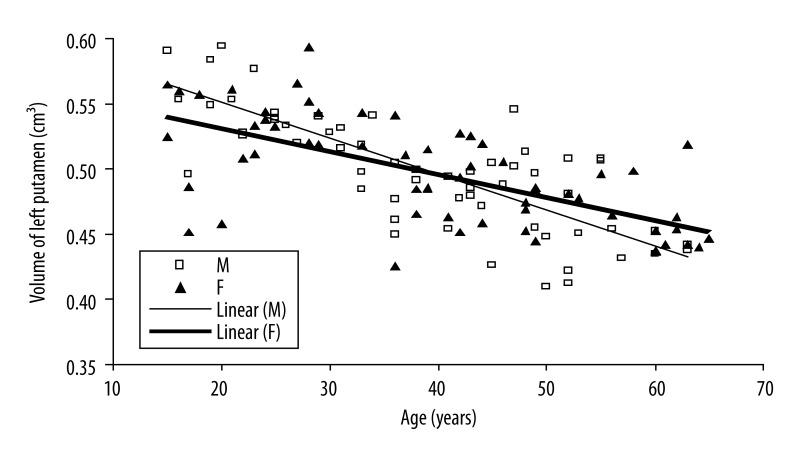
Figure 5.
Scatter plot and linear regression showing a negative correlation between age and left putamen nucleus in men (open squares, solid regression line) and women (filled triangles, solid regression line).
Besides, measured normalized volumes of the right caudate (0.43 vs. 0.38 cm3, respectively, P-value <0.001), left caudate (0.41 vs. 0.36 cm3, respectively, P-value <0.001), right putamen (0.53 vs. 0.49 cm3, respectively, P-value <0.001) and left putamen (0.52 vs. 0.47 cm3, respectively, P-value <0.001) were higher in the young group. The age-related reduction of the total normalized volume of the putamen was the same as for the caudate (4%).
Effect of gender on striatum volume
The descriptive statistics for the volume of the right or left caudate and putamen nucleus by age×sex×group are presented in Table 1. Men had higher right (4.09 vs. 3.90 cm3, respectively, P-value =0.63) and left (3.9 vs. 3.7 cm3, respectively, P-value =0.55) caudate volumes than women. Also, men revealed higher right putamen volumes (5.08 vs. 4.90 cm3, respectively, P-value =0.97) and left putamen volumes (4.87 vs. 4.75 cm3, respectively, P-value =1.00) than women. In two age groups, there were no statistically significant volume differences between two genders for the right and left caudate and putamen nuclei (P-value >0.05). These results were also true when volumes of the caudate and putamen nuclei were expressed as a percent of cerebral hemispheric volume or normalized by the ICV. Furthermore, the rate of reduction in the striatum volume (age×sex) was used to assess the degree of volume shrinkage in the range of 15–65 years. A significant age-related decrease in the volumes of striatum for men and women was observed.
The observed volume reduction was 4.9% and 4.5% for men, and 3.3% and 2.9% for women in the right and left caudate by one step increase in age (Figures 2 and 3). The age-related volume shrinkage in men was 5.3% and 5.5%, and 3.4% and 3.3% in women for the right and left putamen, respectively (Figures 4 and 5). The overall reduction rate was lower for women than for men and the correlation of gender and age was significant (for caudate P-value =0.036 and for putamen P-value =0.008) suggesting that the rates of caudate and putamen volume decrease with age were different for two genders.
Asymmetry of striate nucleus
Gender- and age-related asymmetry differences were determined using 120 right-handed subjects in the age range of 15–65 years. The index of hemispheric asymmetry was computed as the absolute percentage difference:
The results of the age×sex×hemispheric asymmetry analysis showed a trend toward rightward asymmetry in the caudate and putamen nucleus in both genders (Table 1). That trend was significant only for the putamen between young and old men (P-value =0.015). For both genders, the volumes of caudate and putamen nucleus were higher in the right nucleus as compared to the left one. In men, the asymmetry in young and old groups was 3.93% and 3.85% for the caudate, and 3.01% and 5.40%, for the putamen, respectively (Table 1). The rightward asymmetry in the volume of the caudate nucleus was stronger in the younger group than in the older one but that asymmetry was not significant (P-value >0.05). The rightward asymmetry in the volume of the putamen nucleus was stronger in the older men than in the younger group (P-value =0.014). The asymmetry in young and old women was 4.58% and 4.45% for the caudate and 3.04% and 3.60% for the putamen nucleus, respectively (Table 1). There were no correlations between age×sex×hemispheric asymmetry in female group, which suggests that the hemispheric differences in caudate and putamen nuclei in women were not affected by age. The age-related asymmetry was higher in males than females, but gender-related asymmetric differences were not significant (P-value =0.102).
Discussion
In this study, striatum (caudate and putamen nucleus) volume was measured and evaluated by age, gender and hemispheric differences in healthy subjects (15–65 yrs) which was discussed below. Quantitative analysis indicated that the volume of striate nucleus decreased with aging of both genders. Moreover, it was observed that the age-related decrease in the volume of striate nucleus was steeper in men; on the other hand, the loss of striatum tissue due to aging in men was greater than in women. A trend for rightward asymmetry in the volume of the putamen and caudate nucleus was observed in both genders.
Effect of age
Age was negatively correlated with striatum volume and bilateral atrophies of the putamen and caudate nucleus in both genders.
This age-related atrophy of the striatum volume not only confirmed previous reports [14,21,22] but also demonstrated that the striatum was more vulnerable to shrinkage with age in men than in women. The results of this study are consistent with the results of a study by Kokkalainen and indicated an age-related decrease in the striatum volume. Moreover, shape variability was used and showed that the volume decrease was not uniform [14]. In this research, we used volume analysis that obtained reliable results.
Older men and women had significantly smaller striate nuclei than younger subjects. This difference was observed after normalization of nucleus volume to ICV, suggesting that the striatum volume is more affected by age-related changes than brain hemispheres. The precise etiology of age-related decline in the striatum volume is still unknown. The reduction or atrophy in striatum structure could be mediated by several processes, including neuronal and glial cell death (especially dopaminergic neurons), restricted blood flow and progressive iron deposition [9,23–26]. Some researchers suggested that neuronal structures of striatum are sensitive to free radical reactions and oxidative phosphorylation that lead to a variety of neurodegenerative diseases and decrease in the volume of striatum during life-span [9]. Also Matochik et al. suggested that the striate nucleus atrophy could result from age-related changes in the integrity of dopaminergic system. Accordingly, they demonstrated that the globus pallidus, which receives less dopaminergic input than the striatum, should show less volume reduction [25]. In another study, Yu ZQ et al. showed a reduction in dopamine content in the striatum of elderly rats and induction of degeneration and death of neurons, which might offset the motor coordination ability observed in the elderly subjects [26].
Unfortunately, MRI studies are unable to identify the mechanisms that are responsible for volume reduction, but in combination with advanced software techniques they can provide complementary information to potential mechanisms of age-related decline in the volume of the striatum. Regardless of the underlying mechanisms, our results have several implications for age-related atrophy of the striatum. First, we believe that this volumetric analysis of striate nucleus will help to assess neurodegenerative diseases. Second, the mechanisms might favor the disruption of motor function and cognition in elderly that are symptoms of age-related shrinkage of striate nucleus. Third, age–related decline was more rapid in men; such accelerated age-related decline in men might be the reason why men are more probable candidates for movement disorders such as Parkinson’s disease.
Effect of gender
There are few published reports on the effects of gender differences on age-related changes in striatum. In this study, the effect of age on striatum atrophy was similar for men and women; there exist significant gender-related changes in the striatum volume and the total volume of striate nucleus in men is greater than in women.
Some authors indicated that the age-related changes in the putamen were found only in men whereas others stated that they were found both in men and women but women had a significantly larger putamen nucleus than men [27]. The discrepancy between our data and the data reported by Nunnemann and Xu might be ascribed to the sub-regions of striate nucleus. Nunnemann and et al. analyzed only the posterior region of the putamen and Xu did not divide the basal ganglia into the caudate and the putamen nuclei [11,27].
The cause of gender differences in striatum atrophy with aging is still unclear and might be attributed to external and internal factors [11,17]. Sex hormones may substantially affect brain structures. For example, intranasal administration of progesterone and testosterone increases dopamine level and enhances the dopaminergic activity in the neostriatum of male rats [28,29]. Such gender-related differences in hormone status may be responsible for age-related changes of the striatum in men and women.
Asymmetry of striate nucleus
Our study indicates a consistent asymmetry of the human striatum. The hemispheric asymmetry of striate nuclei (right greater than left) appeared in all age groups. Previous studies reported different results on hemispheric asymmetries of the striatum [22,30,31]. For example, Gunning-Dixon demonstrated a leftward asymmetry in the caudate (2.7%) and rightward asymmetry in the putamen nucleus (8.2%) in both sexes [22]. Besides, Yamashita K et al. showed that the trend for rightward asymmetry in caudate volume and the asymmetry index increased with age [31]. Moreover, Xu et al. showed a trend for rightward asymmetry in the volume of caudate and putamen nucleus in both sexes [30] while Ahn et al. showed no significant asymmetry in the striate nucleus [32]. Besides, this study showed that elder men exhibited significant age-related asymmetry in the putamen volume which was not observed in women. According to us, if more subjects had been included in the above mentioned studies and normalized nucleus volumes to ICV had been used, that trend would have become more reliable and disappeared.
In this study, gender-related asymmetry in the putamen nucleus was confined to older subjects and hemispheric asymmetry was not observed in younger subjects. Based on this finding, there was no age- or gender-related asymmetry in the caudate nucleus. It is also clear that hemispheric asymmetries are affected by several factors that may give rise to anatomical and functional lateralization of the brain such as motor activity, brain damage, genetic factors and aging [11,15,18,19]. For example, the results of animal studies have indicated a higher number of dopamine receptors in the right striatum, a rightward asymmetry in the striatum volume and reduced asymmetry in dopamine receptors with normal aging [33,34].
Conclusions
Our results confirmed age-related decline and rightward volume asymmetry of the striate nucleus. These data from normal subjects provide useful information for interpretation of changes in the striatum structure in neurode-generative conditions and for understanding behavioral changes with aging. One of our limitations was no access to 3D imaging facilities in our study which would surely allow for a more precise determination of absolute volume; However, it is worth noticing that our goal was not exact determination of the volume of the nucleus in both genders and that we tried to present the trend in volume change of the nuclei with normal aging in order to suggest a mechanism for the above mentioned neurodegenerative diseases. Our data might be used as ethnic data on age-related shrinkage of those nuclei. Besides, we did not include teenagers (below 15 yrs) in our study and it is necessary to conduct such a study to find out age and gender effects on the volume of the nuclei.
References:
- 1.Ahn S, Lee SK. Diffusion tensor imaging: exploring the motor networks and clinical applications. Korean J Radiol. 2011;12(6):651–61. doi: 10.3348/kjr.2011.12.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alheid G, Switzer R, Heimer L. Basal ganglia. In: Paxinos G, editor. The human nervous system. San Diego: Academic Press; 1990. pp. 438–532. [Google Scholar]
- 3.Pfefferbaum A, Mathalon DH, Sullivan EV, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 4.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130(4):203–15. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ge Y, Grossman RI, Babb JS, et al. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. Am J Neuroradiol. 2002;23(8):1327–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Coffey CE, Wilkinson WE, Parashos IA, et al. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42(3 Pt 1):527–36. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y, Grossman RI, Babb JS, et al. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. Am J Neuroradiol. 2002;23(8):1334–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CD, Chebrolu H, Wekstein DR, et al. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28(7):1075–87. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Cherubini A, Peran P, Caltagirone C, et al. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48(1):29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 75–78. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Kobayashi S, Yamaguchi S, et al. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. 2000;21(1):112–18. [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JS, Bruss J, Brown CK, et al. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26(9):1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 79–82. [DOI] [PubMed] [Google Scholar]
- 13.Gur RC, Gunning-Dixon FM, Turetsky BI, et al. Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry. 2002;10(1):72–80. [PubMed] [Google Scholar]
- 14.Koikkalainen J, Hirvonen J, Nyman M, et al. Shape variability of the human striatum – effects of age and gender. Neuroimage. 2007;34(1):85–93. doi: 10.1016/j.neuroimage.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Raz N, Rodrigue KM, Kennedy KM, et al. Differential aging of the human striatum: longitudinal evidence. Am J Neuroradiol. 2003;24(9):1849–56. [PMC free article] [PubMed] [Google Scholar]
- 16.Riello R, Sabattoli F, Beltramello A, et al. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin Exp Res. 2005;17(4):329–36. doi: 10.1007/BF03324618. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Sachdev PS, Wen W, et al. Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. Neuroimage. 2007;36(3):691–99. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 18.Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26(7):819–25. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 19.Szabo CA, Lancaster JL, Xiong J, et al. MR imaging volumetry of subcortical structures and cerebellar hemispheres in normal persons. Am J Neuroradiol. 2003;24(4):644–47. [PMC free article] [PubMed] [Google Scholar]
- 20.Miyahira Y, Yu J, Hiramatsu K, et al. [Brain volumetric MRI study in healthy elderly persons using statistical parametric mapping] Seishin Shinkeigaku Zasshi. 2004;106(2):138–51. [PubMed] [Google Scholar]
- 21.Brabec J, Kraseny J, Petrovicky P. Volumetry of striatum and pallidum in man – anatomy, cytoarchitecture, connections, MRI and aging. Sb Lek. 2003;104(1):13–65. [PubMed] [Google Scholar]
- 22.Gunning-Dixon FM, Head D, McQuain J, et al. Differential aging of the human striatum: a prospective MR imaging study. Am J Neuroradiol. 1998;19(8):1501–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Pickrell AM, Fukui H, Wang X, et al. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J Neurosci. 2011;31(27):9895–904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder SL, Hopp KM, Ward H, et al. Mineralization of the deep gray matter with age: a retrospective review with susceptibility-weighted MR imaging. Am J Neuroradiol. 2008;29(1):176–83. doi: 10.3174/ajnr.A0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matochik JA, Chefer SI, Lane MA, et al. Age-related decline in striatal volume in monkeys as measured by magnetic resonance imaging. Neurobiol Aging. 2000;21(4):591–98. doi: 10.1016/s0197-4580(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 26.Yu ZQ, Liu MY, Ren QX, et al. Dopamine content in the striatum and expression changes of Bad and Bcl-2 in elderly rats with abnormal behavior. Neurochem Res. 2011;36(12):2333–38. doi: 10.1007/s11064-011-0558-3. [DOI] [PubMed] [Google Scholar]
- 27.Nunnemann S, Wohlschlager AM, Ilg R, et al. Accelerated aging of the putamen in men but not in women. Neurobiol Aging. 2009;30(1):147–51. doi: 10.1016/j.neurobiolaging.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 28.de Souza Silva MA, Mattern C, Topic B, et al. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur Neuropsychopharmacol. 2009;19(1):53–63. doi: 10.1016/j.euroneuro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 29.de Souza Silva MA, Topic B, Huston JP, et al. Intranasal administration of progesterone increases dopaminergic activity in amygdala and neostriatum of male rats. Neuroscience. 2008;157(1):196–203. doi: 10.1016/j.neuroscience.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Xu JF, Xu XJ, Wang QD, et al. [MRI scan of striatum volume in healthy adults] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010;39(2):130–35. doi: 10.3785/j.issn.1008-9292.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita K, Yoshiura T, Hiwatashi A, et al. Volumetric asymmetry and differential aging effect of the human caudate nucleus in normal individuals: a prospective MR imaging study. J Neuroimaging. 2011;21(1):34–37. doi: 10.1111/j.1552-6569.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahn MS, Breeze JL, Makris N, et al. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. J Affect Disord. 2007;104(1–3):147–54. doi: 10.1016/j.jad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Giardino L. Right-left asymmetry of D1- and D2-receptor density is lost in the basal ganglia of old rats. Brain Res. 1996;720(1–2):235–38. doi: 10.1016/0006-8993(96)00144-8. [DOI] [PubMed] [Google Scholar]
- 34.Larisch R, Meyer W, Klimke A, et al. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998;19(8):781–87. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]