Abstract
This minireview focuses on recent studies implicating class V myosins in organelle and macromolecule transport within neurons. These studies reveal that class V myosins play important roles in a wide range of fundamental processes occurring within neurons, including the transport into dendritic spines of organelles that support synaptic plasticity, the establishment of neuronal shape, the specification of polarized cargo transport, and the subcellular localization of mRNA.
Keywords: Cell Polarity; Endoplasmic Reticulum (ER); Ionotropic Glutamate Receptors (AMPA, NMDA); mRNA; Myosin; Neurons; PTEN; Rab Proteins; Receptor Recycling; Synaptic Plasticity
Class V Myosins Possess Features That Should Make Them Efficient Organelle Motors
Eukaryotic cells, including neurons, use molecular motors to transport and properly distribute their organelles. Indeed, the demands placed on motor-dependent organelle transport are greatly amplified in neurons relative to other cell types because of the enormous lengths of their axon and dendrites (1). Neurons use microtubule-dependent motors (i.e. kinesins and dynein) to drive long-range bidirectional transport of organelles within these processes. In contrast, short-range organelle movements in the periphery, such as within dendritic spines, appear to be driven by actin-based motors, i.e. myosins.
Among the ∼14 myosin classes in vertebrates, the class V myosins have so far received the most attention with regard to short-range organelle and macromolecule transport within neurons, as well as within other cell types (reviewed in Ref. 2). Mice and humans possess three class V myosin genes (MYO5A, MYO5B, and MYO5C), which encode the heavy chains of myosins Va, Vb, and Vc, respectively. Of these three, myosin Va is the most widely expressed isoform in the CNS, being present throughout virtually the entire CNS, including the cerebrum, hippocampus, and cerebellum (Allen Brain Atlas) (3, 4). Myosin Vb is present at significant levels in the hippocampus, as well as in other brain regions (5, 6). In contrast, myosin Vc is essentially undetectable in the CNS, consistent with the conclusion that it is epithelial cell-specific (7).
Importantly, myosins Va and Vb possess three features that should allow them to serve as effective organelle motors within neurons (Fig. 1A). First, both proteins are very processive (8), which should allow them to move organelles efficiently even when present at very low density on the organelle surface, the likely situation in vivo. Second, like other class V myosins, myosins Va and Vb possess very long lever arms, corresponding to their calmodulin-binding neck domains (Fig. 1A). This feature allows them to take an exceptionally large step (Fig. 1, A and C) (8). Moreover, because the size of their step (36 nm) matches the half-helical repeat of the actin filament, they can walk across the “top” of the actin filament rather than having to spiral around it. This feature may facilitate the transport of bulky cargos within crowded cellular environments. Third, both myosins Va and Vb exist in an equilibrium between two conformations (reviewed in Refs. 9 and 10). One is a folded conformation, in which the myosin cargo-binding globular tail domains are bound to their motor domains (Fig. 1, B and D). In this compact conformation, which is favored in the absence of cargo, the ATPase activity and actin affinity of their motor domains are strongly suppressed. Importantly, cargo addition drives both myosins into an extended, mechanochemically active conformation, either by trapping the myosin in the extended state and/or by inducing this state (Fig. 1A). This stunning feature, which is shared by processive organelle motors of the kinesin family (11), allows cargo availability to control the mechanochemical properties of myosins Va and Vb. In this way, cargo-free versions of these two myosins are prevented from walking for no purpose, and their recycling via diffusion upon completion of the transport step is promoted. Here, we review the current state of knowledge regarding the functions of myosins Va and Vb within neurons (for other pertinent recent reviews, see Refs. 2, 10, and 12–14).
FIGURE 1.
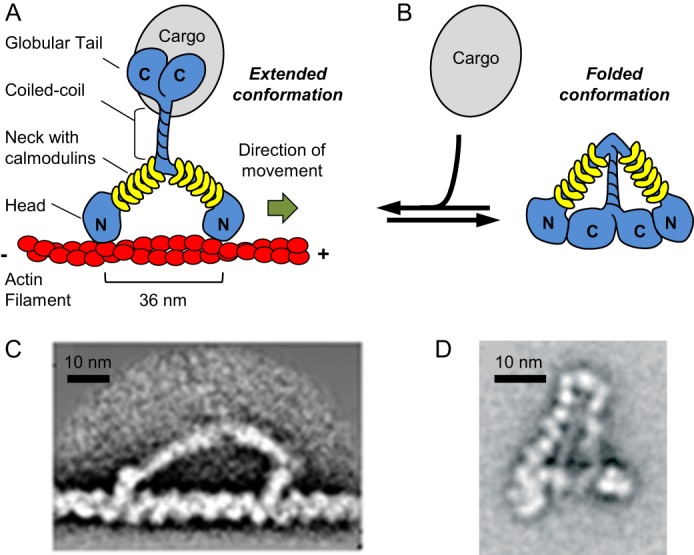
Structure of myosin Va and its regulation by cargo binding. A, schematic depicting myosin Va, which consists of a pair of heavy chains (blue; N and C termini are indicated) and six calmodulin light chains (yellow) per heavy chain. Each heavy chain harbors a head/motor domain for ATP hydrolysis and actin binding, as well as a neck domain/lever arm with associated calmodulins. The C-terminal tail domain comprises a coiled-coil region for heavy chain dimerization (alternatively spliced exons B, D, and F lead to insertions within this region) and a globular tail domain. Both the globular tail domain and exons D and F have been implicated in cargo binding. The extended, mechanochemically active conformation of cargo-bound myosin V is shown (cargo is indicated in gray). B, schematic of cargo-free myosin Va in its folded conformation. Black arrows indicate that myosin Va is in equilibrium between the folded and extended conformations and that cargo binding leads to myosin Va unfolding and activation. C, myosin Va attached to an actin filament as visualized via electron microscopy followed by image averaging. The image was adapted from Ref. 68 with permission. The head and neck regions are visible. D, myosin Va in its folded conformation as visualized via electron microscopy followed by image averaging. The myosin is shown in the same orientation as in B. The image was adapted from Ref. 69 with permission.
Myosin Vb Transports Recycling Endosomes into Dendritic Spines to Support Synaptic Plasticity
Studies in generic cells have established a close physical and functional connection between myosin Vb and recycling endosomes (REs),2 which function to return endocytosed plasma membrane proteins, such as receptors and channels, to the plasma membrane (15–17). Consistently, myosin Vb binds to Rab11, a RE-specific Rab GTPase that plays a critical role in regulating RE morphology and dynamics, and to FIP2, a Rab11 effector. Furthermore, the expression of a dominant-negative version of myosin Vb dramatically impairs RE organization and function.
Importantly, REs also exist at the base of and within dendritic spines, where they function to return endocytosed AMPA receptors (AMPARs) to the plasma membrane (18, 19). Indeed, AMPARs continuously undergo endocytosis from lateral regions of the spine head, transit through the RE compartment, and return to the spine plasma membrane via exocytosis. This is especially interesting because the control of the number of AMPARs at the spine postsynaptic density (PSD) is at the heart of synaptic plasticity and memory formation. Specifically, the stable insertion of additional AMPARs at the PSD drives an increase in synaptic strength (long-term potentiation (LTP)), whereas the stable reduction in AMPAR number at the PSD via receptor endocytosis drives a decrease in synapse strength (long-term depression (LTD)).
Several studies have provided strong support for the idea that exocytosis from a large pool of AMPARs present within intraspinal REs serves as a major source of AMPARs for LTP (18, 19). Moreover, the enlistment of these REs as an AMPAR source involves their translocation from the base of the spine into the spine head immediately following strong, LTP-inducing spine stimulation. Most importantly, a series of experiments demonstrated unequivocally that myosin Vb powers this translocation step (20). These included a chemical genetic approach using a transgenic mouse expressing myosin Vb that can be switched conditionally to rigor binding, which showed that the acute inhibition of myosin Vb motility and/or tightly linking the myosin to actin abolishes LTP (20). Finally, other experiments have shown that the myosin Vb-dependent translocation of REs into spines and the subsequent exocytosis from this compartment drive not only synaptic plasticity but also activity-dependent structural remodeling of the spine by providing lipid membrane and membrane proteins required for spine growth (20). In this way, REs serve to couple the structural and functional plasticity of spines.
Interestingly, the spike in intraspinal calcium concentration that occurs upon strong spine stimulation appears to unfold myosin Vb, thereby enhancing its interaction with Rab11 and FIP2 on the RE surface (20). This in turn enhances the frequency and robustness with which the myosin transports REs into the spine. Thus, myosin Vb appears to act as a calcium sensor to convert increases in spine calcium concentration produced by LTP-inducing stimuli into postsynaptic membrane transport required to generate LTP. It remains unclear, however, how the calcium-dependent activation of myosin Vb can avoid the dissociation of neck calmodulins and the concomitant loss of mechanochemical integrity that is seen in vitro when myosin V is exposed to micromolar calcium (9).
In another study, which used RNAi and the expression of dominant-negative constructs to abrogate myosin V function, the authors argued that it is in fact myosin Va and not myosin Vb that translocates REs into the spines of hippocampal neurons to support LTP (21). These findings are somewhat surprising, as previous studies in generic cells have identified a role only for myosin Vb in RE function. Moreover, extensive characterization of the electrophysiological properties of hippocampal neurons from dilute-lethal (dl; myosin Va-null) mice identified no defects (22). Perhaps myosin Va plays a more important role in driving LTP within cortical neurons, where it might be the predominant isoform. Efforts to define the relative contributions of myosins Va and Vb to RE dynamics in cortical neurons would be greatly facilitated by the development of a mouse model for microvillus inclusion disease, a rare human disorder caused by loss-of-function mutations in myosin Vb (23).
Myosin Va Transports the Endoplasmic Reticulum into the Dendritic Spines of Cerebellar Purkinje Neurons to Support Synaptic Plasticity
As the master neuron in the cerebellum, the Purkinje neuron is crucial for the control of both voluntary and involuntary motor coordination (24). An extensively studied form of synaptic plasticity that might contribute to cerebellar motor learning is LTD at parallel fiber-Purkinje neuron synapses. The reduction in synaptic strength that underlies LTD is created by the endocytosis of AMPARs following strong synaptic stimulation (25). The signaling pathway leading to AMPAR endocytosis requires the production of inositol trisphosphate (IP3), which occurs downstream of activation of G-protein-coupled metabotropic glutamate receptors (mGluRs) located at the edges of the PSD (26). IP3 is critical for LTD generation because it triggers, via the IP3 receptor, the release of calcium from tubules of the smooth endoplasmic reticulum (ER) that are present in all of the spines of the Purkinje neuron. The resulting rise in spine calcium concentration activates PKC, which phosphorylates AMPARs, thereby promoting their endocytosis. Importantly, the extension of the ER into the spines of this neuron does not occur in dl Purkinje neurons (27). Without this intraspinal calcium store, the local signaling pathway that drives LTD within the spine and LTD itself are abolished (28).
The absence of the ER in the spines of dl Purkinje neurons indicates a requirement for myosin Va in ER translocation into the spine. Indeed, a recent study provided clear evidence that myosin Va acts as a point-to-point organelle transporter to pull ER tubules into Purkinje neuron spines at a maximum velocity of 0.45 μm/s (29). Myosin Va was shown to concentrate at the tip of the ER tubule as it moves into the spine, and the rescue of dl Purkinje neurons with slow walking versions of myosin Va results in corresponding decreases in the velocity of ER movement into spines. This latter experiment has been argued to represent the acid test for proving that a particular motor moves a particular membrane organelle in vivo (2). In keeping with the paradigm mentioned above, the short-range, myosin Va-dependent transport of the ER into spines occurs downstream of long-range, microtubule-dependent transport of the ER within neuronal dendrites. Finally, this study showed that the myosin Va-mediated transport of the ER into spines is indeed required for the local rise in intraspinal calcium levels that is downstream of mGluR activation and required for LTD (29).
Interestingly, the neurological phenotype of the dl mouse (ataxia, opisthotonus, seizures, and juvenile lethality) is essentially identical to that of mice lacking the IP3 receptor (30). In contrast, mutant mice that only lack LTD at parallel fiber-Purkinje neuron synapses do not display such severe symptoms, and deficits in cerebellum-dependent motor learning could not be detected (31). These observations, together with the widespread expression of myosin Va in the brain, suggest that the myosin Va-dependent translocation and positioning of the IP3 receptor-laden ER might be of general importance for IP3 receptor-mediated calcium signaling throughout the CNS. Obtaining anatomical evidence for this may be difficult given that CNS neurons other than Purkinje neurons employ the spine apparatus to anchor the ER within spines (32). However, very short-range, myosin Va-dependent movements of the ER from the spine apparatus to the spine plasma membrane may occur. Such transport events could be important for gating TRPC (transient receptor potential cation) and/or Orai channels in the spine plasma membrane by STIM (stromal interaction module) in the ER membrane, analogous to the situation in non-neuronal cells (33, 34). The gating of these ion channels by the spine ER would likely impact synaptic plasticity throughout much of the CNS. Future studies should address these exciting possibilities and could be facilitated by further analyses of dilute-neurological (dn) mice, which harbor a missense mutation in myosin Va that greatly reduces the amount of the protein in tissues (35). These mice initially exhibit the same phenotype as dl mice but survive weaning to become slightly uncoordinated adults. Careful analyses of juvenile and adult dn mice have further underscored the direct correlation between myosin Va protein levels, ER localization to Purkinje neuron spines, LTD, and cerebellum-dependent motor coordination (36). Moreover, the dl and dn mice have shed further light on the pathophysiology of Griscelli syndrome type 1, a rare autosomal recessive disease in humans caused by MYO5A mutations (37).
Myosin Va and Other Forms of Synaptic Plasticity
Recent work has implicated myosin Va in the homeostatic scaling of synaptic strength by linking the myosin to the transport into the spines of guanylate kinase-associated protein (GKAP), a scaffold protein that links NMDA receptor-PSD-95 to Shank-Homer complexes and that controls homeostatic scaling (38). Interestingly, the interaction between myosin Va and GKAP is proposed to be bridged by DLC2 (DYNLL2), a small light chain that associates with the brain spliced isoform of myosin Va, with GKAP, and with several other proteins, including dynein (38–41). We note, however, that other studies are not consistent with this dimeric light chain serving as a cargo adaptor, instead indicating that it controls the local secondary structure of intrinsically dimeric proteins, such as myosin Va and the dynein intermediate chain (39–41).
Interestingly, myosin Va is also important at the postsynaptic side of neuromuscular junctions, where it functions in the recycling of nicotinic acetylcholine receptors required for plasticity (42) and in the targeting of PKA to the postsynaptic microdomain of these junctions (43). Finally, recent results obtained using the natural dominant-negative myosin Va mouse flailer reveal roles for myosin Va in the delivery of PSD components to the synapse and the regulation of LTD in cortical neurons (44).
Myosin Va Facilitates the Targeting of Transmembrane Proteins to Neuronal Dendrites
The establishment of the distinct structural and functional properties of axons and dendrites is driven by the specific transport of vesicles into these two compartments (1). With regard to how such specific transport is established, Arnold and co-workers (45) have shown that interfering with the function of myosin Va in cultured cortical neurons blocks dendritic targeting of several resident dendritic transmembrane proteins, such as the AMPAR subunit GluR1. This effect is specific to myosin Va, as interfering with the function of myosin Vb did not disrupt the dendritic targeting of GluR1.
A working model for how myosin Va steers transport vesicles into the dendrite was proposed based on two assumptions (45): (i) vesicles destined for the axon possess only kinesin, whereas those destined for the dendrite possess both kinesin and myosin Va; and (ii) the organization of actin filaments in the axon initial segment is unusual in that most barbed ends (the direction in which myosin Va moves) are pointing toward the cell body/soma. Given these assumptions, vesicles harboring axonal cargo that venture into the axon along microtubule tracks would be expected to proceed without interruption to the distal axon, whereas those harboring dendritic cargo would become disengaged from the microtubule track within the axon initial segment by the action of vesicle-bound myosin Va and would then transported back to the soma along the uniquely oriented actin filaments for another try at the dendrite. The net effect of this mechanism would be to prevent vesicles destined for the dendrite from proceeding beyond the axon initial segment, thereby biasing their movement toward the dendrite. Moreover, the absence within the dendrite of an actin organization-dependent “vesicle filter,” such as the one hypothesized to exist in the axon initial segment, would allow vesicles carrying dendritic cargo to proceed into the dendrite. Consistent with this model, dendritic cargo is present in the axon initial segment but not in the distal axon. Nevertheless, the strength of this model clearly rests on future efforts directed at testing its two main assumptions (46).
Arnold and co-workers (45) also showed that fusing channelrhodopsin-2 to the myosin Va-binding domain of melanophilin, a Rab27a effector that links myosin Va to Rab27a on the surface of melanosomes, causes this hybrid protein to target to the dendrites of pyramidal neurons in living mice. This interesting result is somewhat surprising, however, given that the tight interaction of melanophilin with myosin Va requires alternatively spliced exon F, a 27-residue exon that is absent in the spliced myosin Va isoform expressed in brain (47). Finally, it should be noted that the gross organization of the brain is normal in myosin Va-null mice. This observation argues either that another dendritic targeting mechanism compensates for loss of myosin Va or that defects in dendritic targeting caused by the loss of myosin Va are somehow not reflected in gross brain organization. Future studies should address these and other aspects of this model.
Interestingly, a recent study has revealed a role for myosin Vb in axon development (48). Knockdown/replacement experiments showed that the myosin Vb isoform containing alternatively spliced exon D is important for axon development in cultured hippocampal neurons and in neocortical neurons in vivo. Exon D promotes the binding of myosin Vb to Rab10, a small GTPase present on post-Golgi vesicles destined for the plasma membrane. Importantly, evidence suggests that the myosin Vb isoform containing exon D supports polarized axon development by promoting the fission of Rab10-positive carriers from the trans-Golgi network (48).
Myosin V Controls Neuronal Cell Size by Translocating PTEN to the Plasma Membrane
PTEN is a lipid phosphatase that antagonizes the myriad growth-promoting activities of PI3K by catalyzing the conversion of phosphatidylinositol 3,4,5-triphosphate in the plasma membrane, which is generated from phosphatidylinositol 4,5-bisphosphate by PI3K back to phosphatidylinositol 4,5-bisphosphate (49). Previous studies have established that the loss of PTEN function in many cell types, including neurons, causes an increase in cell size. Using immunoprecipitations and FRET, Eickholt and co-workers (50) showed that PTEN interacts directly with the myosin Va C-terminal cargo-binding domain. Dominant-negative inhibition of myosin Va function in wild-type hippocampal neurons by overexpression of the myosin cargo-binding domain resulted in an increase in soma size, i.e. it phenocopies the loss of PTEN function. Importantly, control experiments demonstrated that this effect was mediated by increased PI3K signaling. An increase in soma size was also observed in hippocampal neurons from dl mice but only after myosin Vb was knocked down, arguing that these two myosin V isoforms act redundantly to support PTEN function (note that the myosin Va tail domain would be expected to inhibit the function of both myosins Va and Vb in wild-type neurons in a dominant-negative fashion). Finally, introduction of the myosin Va tail domain into the brains of living mice by in utero electroporation resulted in neurons with larger somas. These core results suggest that class V myosins play a major role in supporting PTEN function in neurons, and Eickholt and co-workers (50) suggested that this role is the transport of PTEN by myosin V to its site of action at the plasma membrane. In the absence of myosin V, PTEN localization at the plasma membrane would be reduced, leading to unchecked PI3K signaling and cell hypertrophy.
Another interesting aspect of this study involves the specifics of myosin V interaction with PTEN (50). PTEN undergoes regulatory intramolecular folding, with the folded conformation exhibiting decreased membrane association, lower activity, and higher protein stability compared with the extended conformation. Phosphorylation of several residues within the PTEN C-terminal tail enhances folding. Intriguingly, myosin V interacts preferentially with the phosphorylated form of PTEN and so can compete effectively with the intramolecular folding reaction. One potential consequence of this is that myosin V can simultaneously translocate and unfold/activate PTEN, although evidence that the myosin Va-PTEN complex has increased lipid phosphatase activity was not presented. Interestingly, two kinases that phosphorylate PTEN (CK2 and GSK3β) were shown to act upstream of myosin V-mediated PTEN function and cell size regulation (50). Finally, residues in the myosin Va globular tail domain that are required for its interaction with the phosphorylated tail of PTEN were identified. Importantly, mutagenesis of these residues blocks the dominant-negative effect on soma size of the myosin cargo-binding domain (50).
Several aspects of this story merit further investigation. One question is why a myosin V-dependent mechanism for getting PTEN to the plasma membrane would be necessary because simple diffusion, coupled with the known PTEN membrane interaction domains, should be sufficient to achieve this. Considering that myosin Va-dependent movements in typical cortical regions of cells exhibit little directional persistence owing to the isotropic organization of the cortical actin cytoskeleton (51), one interesting possibility is that myosin V may serve primarily to tether PTEN at the membrane and possibly to regulate its conformation and hence activity there. This idea is consistent with the FRET results presented by Eickholt and co-workers (50), who notably did not provide direct evidence of myosin Va-driven PTEN transport. Alternately (or in addition), myosin V-dependent PTEN transport might take place in dendritic spines, where actin appears to be organized for efficient transport by this myosin. Because PTEN loss has also been shown to alter the migration of neurons and to increase dendritic arborization and spine density (49), it would be interesting to determine if the loss of myosin Va alters these neuronal properties as well. Relevant to this, however, the dendritic arborization of cultured Purkinje neurons (29) and the density of spine-like protrusions of cultured hippocampal neurons (52) are decreased rather than increased upon interfering with myosin Va function. The latter study further showed that myosin Va binds the Rac1 activator RILPL2, which exhibits, in a myosin Va-dependent manner, a positive effect on the formation of spine-like protrusions in hippocampal neurons (52).
Myosin Va and Neuronal mRNA Transport
Studies have shown unequivocally that the yeast class V myosin Myo4p transports mRNAs (in a complex with mRNA-binding proteins) from the mother cell to the bud tip (reviewed in Refs. 2 and 53). Evidence is growing that myosin Va may serve a similar function in vertebrate cells (54), including neurons, where mRNA transport and localization could be especially important for establishing polarity and creating functionally distinct internal compartments. The best indication of this to date is the work of Yoshimura et al. (55), who presented evidence that myosin Va facilitates, in a calcium-regulated manner, the transport of the mRNA-binding protein TLS (translocated in liposarcoma) and its associated mRNA Ndl-1 (which encodes an actin-stabilizing protein required for dendritic spine development) into the spines of cultured hippocampal neurons. Tagged versions of myosin Va and TLS co-localized significantly within spines, and TLS accumulation in spines was suppressed by RNAi-mediated knockdown of endogenous myosin Va, as well as by overexpression of a dominant-negative myosin Va tail construct. Moreover, TLS translocation into spines was impeded in dl neurons, albeit to a lesser extent than in knockdown cells. Interestingly, TLS and its bound mRNA are translocated into spines in response to mGluR activation, and this response is impaired in neurons with compromised myosin Va function (55). These observations suggests that the myosin Va-dependent transport of TLS and its bound mRNA may play a significant role upstream of localized protein synthesis in regulating synaptic function, including plasticity. Finally, as with several other myosin V cargos (reviewed in Ref. 2), the long-range transport of mRNA-protein complexes within dendrites that precedes the local, myosin Va-dependent transport within actin-rich spines is driven by microtubule-dependent motors.
Interestingly, myosin Va may also regulate, in a negative fashion, the microtubule-dependent transport of the mRNA-binding protein ZBP1 (zipcode binding protein 1) within axons (56). Specifically, myosin Va was shown to interact and co-localize with ZBP1 in hippocampal neurons, and the abrogation of myosin Va function by RNAi-mediated knockdown or the overexpression of a dominant-negative myosin Va tail construct resulted in enhancement of the anterograde transport and accumulation of ZBP1 within axons. One interpretation of this result is that the myosin controls a pool of ZBP1 within the actin-rich cortex that supplies the protein for its kinesin-dependent axonal transport (56).
Other Functions Attributed to Myosin V in the Nervous System
Myosin V has also been implicated in a range of other cellular functions in neurons. For example, myosin Va modulates the axonal transport of neurofilaments (57), tetanus-containing endosomes (58), large dense-core vesicles (LDCVs) (59), and synaptic vesicles (60). In Drosophila axons, myosin V acts to decrease microtubule-based transport of mitochondria (61). Moreover, myosin Va is involved in oligodendrocyte morphogenesis and myelination (62). Finally, myosin Va has been linked to the maturation and exocytosis of neuropeptide-containing LDCVs (63, 64). Importantly, Váradi and co-workers (65) recently presented evidence that the brain spliced isoform of myosin Va is recruited to LDCVs via receptor complexes containing Rab27a and either granuphilin-a/b or rabphillin-3A. This further underscores the widespread role played by Rab GTPases in the recruitment of class V myosins to organelles (2).
Taken together, a picture emerges in which myosins Va and Vb each support multiple distinct functions within neurons that together encompass a diverse array of fundamental processes, including neuronal development, axonal transport, dendritic spine structure, and synaptic plasticity (Fig. 2). Notably, these two class V myosins are capable of multitasking because they are able to interact with multiple cargos. Importantly, patients with Griscelli syndrome type 1 suffer from severe neurological impairment due to MYO5A mutation (37), and the MYO5B gene might be associated with an increased susceptibility to bipolar disorder (66) and schizophrenia (67). Given this, it is very important to continue efforts to dissect the neuronal functions of these two myosins, as such efforts should help explain how these motors contribute to brain function in both health and neurological/psychiatric disease.
FIGURE 2.
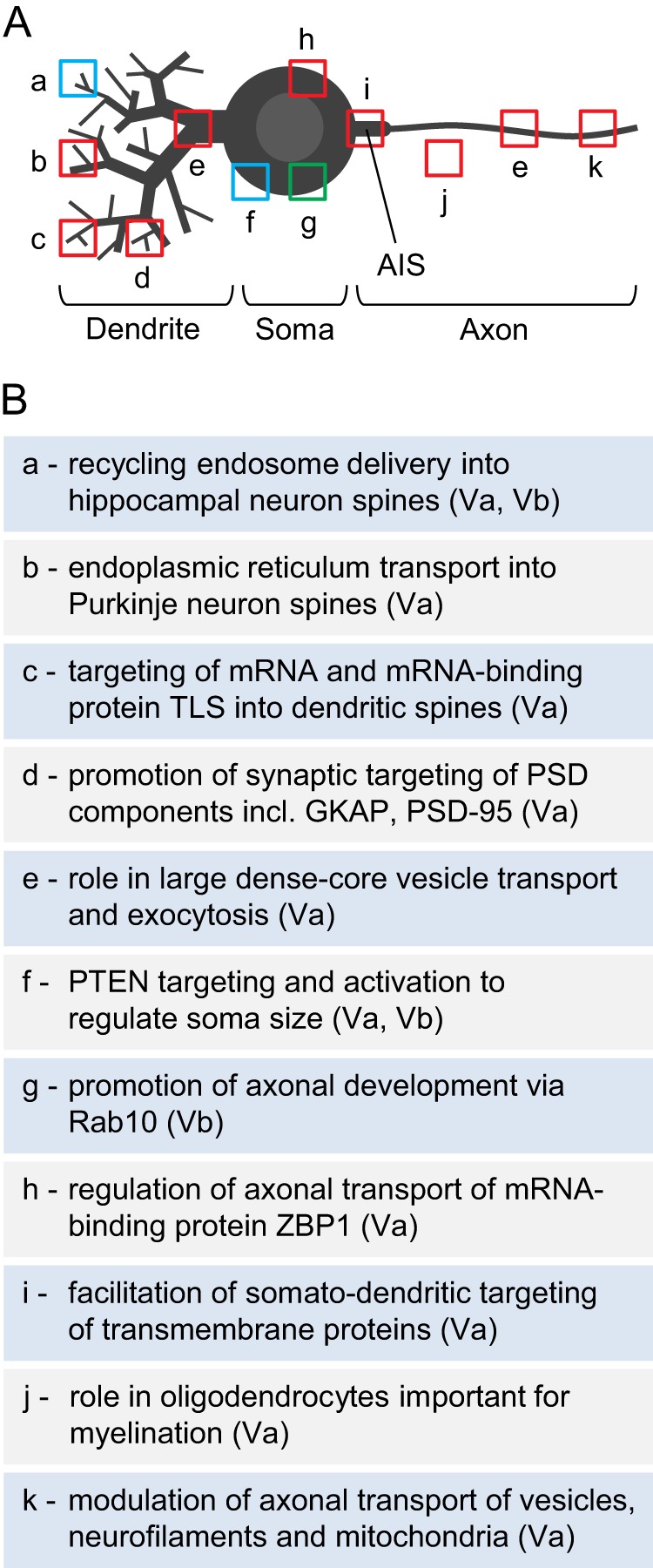
Myosin V functions in neurons. In A, sites of myosin V function are indicated by boxes overlaid on the schematic view of a neuron: red, myosin Va; green, myosin Vb; blue, both isoforms. The letters refer to the physiologic roles attributed to myosin V, which are listed in B. The specific isoform involved is indicated in parentheses in B. AIS, axon initial segment. See text for references.
Acknowledgment
We thank John Trinick for providing the image shown in Fig. 1C.
This work was supported by Marie Curie FP7 Integration Grant PCIG11-GA-2012-321905 within the Seventh European Union Framework Programme (to W. W.).
- RE
- recycling endosome
- AMPAR
- AMPA receptor
- PSD
- postsynaptic density
- LTP
- long-term potentiation
- LTD
- long-term depression
- IP3
- inositol trisphosphate
- mGluR
- metabotropic glutamate receptor
- ER
- endoplasmic reticulum
- GKAP
- guanylate kinase-associated protein
- LDCV
- large dense-core vesicle.
REFERENCES
- 1. Kapitein L. C., Hoogenraad C. C. (2011) Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol. Cell. Neurosci. 46, 9–20 [DOI] [PubMed] [Google Scholar]
- 2. Hammer J. A., 3rd, Sellers J. R. (2012) Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 13, 13–26 [DOI] [PubMed] [Google Scholar]
- 3. Tilelli C. Q., Martins A. R., Larson R. E., Garcia-Cairasco N. (2003) Immunohistochemical localization of myosin Va in the adult rat brain. Neuroscience 121, 573–586 [DOI] [PubMed] [Google Scholar]
- 4. Mercer J. A., Seperack P. K., Strobel M. C., Copeland N. G., Jenkins N. A. (1991) Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349, 709–713 [DOI] [PubMed] [Google Scholar]
- 5. Zhao L. P., Koslovsky J. S., Reinhard J., Bähler M., Witt A. E., Provance D. W., Jr., Mercer J. A. (1996) Cloning and characterization of myr 6, an unconventional myosin of the dilute/myosin-V family. Proc. Natl. Acad. Sci. U.S.A. 93, 10826–10831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lisé M. F., Wong T. P., Trinh A., Hines R. M., Liu L., Kang R., Hines D. J., Lu J., Goldenring J. R., Wang Y. T., El-Husseini A. (2006) Involvement of myosin Vb in glutamate receptor trafficking. J. Biol. Chem. 281, 3669–3678 [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez O. C., Cheney R. E. (2002) Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991–1004 [DOI] [PubMed] [Google Scholar]
- 8. Sellers J. R., Veigel C. (2006) Walking with myosin V. Curr. Opin. Cell Biol. 18, 68–73 [DOI] [PubMed] [Google Scholar]
- 9. Sellers J. R., Thirumurugan K., Sakamoto T., Hammer J. A., 3rd, Knight P. J. (2008) Calcium and cargoes as regulators of myosin 5a activity. Biochem. Biophys. Res. Commun. 369, 176–181 [DOI] [PubMed] [Google Scholar]
- 10. Trybus K. M. (2008) Myosin V from head to tail. Cell. Mol. Life Sci. 65, 1378–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verhey K. J., Hammond J. W. (2009) Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10, 765–777 [DOI] [PubMed] [Google Scholar]
- 12. Bridgman P. C. (2009) Myosin motor proteins in the cell biology of axons and other neuronal compartments. Results Probl. Cell Differ. 48, 91–105 [DOI] [PubMed] [Google Scholar]
- 13. Rudolf R., Bittins C. M., Gerdes H. H. (2011) The role of myosin V in exocytosis and synaptic plasticity. J. Neurochem. 116, 177–191 [DOI] [PubMed] [Google Scholar]
- 14. Kneussel M., Wagner W. (2013) Myosin motors at neuronal synapses: drivers of membrane transport and actin dynamics. Nat. Rev. Neurosci. 14, 233–247 [DOI] [PubMed] [Google Scholar]
- 15. Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W., Jr., Mercer J. A., Bähler M., Goldenring J. R. (2001) Myosin Vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provance D. W., Jr., Gourley C. R., Silan C. M., Cameron L. C., Shokat K. M., Goldenring J. R., Shah K., Gillespie P. G., Mercer J. A. (2004) Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic. Proc. Natl. Acad. Sci. U.S.A. 101, 1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swiatecka-Urban A., Talebian L., Kanno E., Moreau-Marquis S., Coutermarsh B., Hansen K., Karlson K. H., Barnaby R., Cheney R. E., Langford G. M., Fukuda M., Stanton B. A. (2007) Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J. Biol. Chem. 282, 23725–23736 [DOI] [PubMed] [Google Scholar]
- 18. Park M., Penick E. C., Edwards J. G., Kauer J. A., Ehlers M. D. (2004) Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 [DOI] [PubMed] [Google Scholar]
- 19. Park M., Salgado J. M., Ostroff L., Helton T. D., Robinson C. G., Harris K. M., Ehlers M. D. (2006) Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z., Edwards J. G., Riley N., Provance D. W., Jr., Karcher R., Li X. D., Davison I. G., Ikebe M., Mercer J. A., Kauer J. A., Ehlers M. D. (2008) Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Correia S. S., Bassani S., Brown T. C., Lisé M. F., Backos D. S., El-Husseini A., Passafaro M., Esteban J. A. (2008) Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat. Neurosci. 11, 457–466 [DOI] [PubMed] [Google Scholar]
- 22. Schnell E., Nicoll R. A. (2001) Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J. Neurophysiol. 85, 1498–1501 [DOI] [PubMed] [Google Scholar]
- 23. Müller T., Hess M. W., Schiefermeier N., Pfaller K., Ebner H. L., Heinz-Erian P., Ponstingl H., Partsch J., Röllinghoff B., Köhler H., Berger T., Lenhartz H., Schlenck B., Houwen R. J., Taylor C. J., Zoller H., Lechner S., Goulet O., Utermann G., Ruemmele F. M., Huber L. A., Janecke A. R. (2008) MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163–1165 [DOI] [PubMed] [Google Scholar]
- 24. Hansel C., Linden D. J., D'Angelo E. (2001) Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4, 467–475 [DOI] [PubMed] [Google Scholar]
- 25. Steinberg J. P., Takamiya K., Shen Y., Xia J., Rubio M. E., Yu S., Jin W., Thomas G. M., Linden D. J., Huganir R. L. (2006) Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49, 845–860 [DOI] [PubMed] [Google Scholar]
- 26. Kano M., Hashimoto K., Tabata T. (2008) Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2173–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takagishi Y., Oda S., Hayasaka S., Dekker-Ohno K., Shikata T., Inouye M., Yamamura H. (1996) The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci. Lett. 215, 169–172 [DOI] [PubMed] [Google Scholar]
- 28. Miyata M., Finch E. A., Khiroug L., Hashimoto K., Hayasaka S., Oda S. I., Inouye M., Takagishi Y., Augustine G. J., Kano M. (2000) Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 29. Wagner W., Brenowitz S. D., Hammer J. A., 3rd (2011) Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat. Cell Biol. 13, 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsumoto M., Nakagawa T., Inoue T., Nagata E., Tanaka K., Takano H., Minowa O., Kuno J., Sakakibara S., Yamada M., Yoneshima H., Miyawaki A., Fukuuchi Y., Furuichi T., Okano H., Mikoshiba K., Noda T. (1996) Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature 379, 168–171 [DOI] [PubMed] [Google Scholar]
- 31. Schonewille M., Gao Z., Boele H. J., Veloz M. F., Amerika W. E., Simek A. A., De Jeu M. T., Steinberg J. P., Takamiya K., Hoebeek F. E., Linden D. J., Huganir R. L., De Zeeuw C. I. (2011) Reevaluating the role of LTD in cerebellar motor learning. Neuron 70, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jedlicka P., Vlachos A., Schwarzacher S. W., Deller T. (2008) A role for the spine apparatus in LTP and spatial learning. Behav. Brain Res. 192, 12–19 [DOI] [PubMed] [Google Scholar]
- 33. Lee K. P., Yuan J. P., Hong J. H., So I., Worley P. F., Muallem S. (2010) An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 584, 2022–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartmann J., Henning H. A., Konnerth A. (2011) mGluR1/TRPC3-mediated synaptic transmission and calcium signaling in mammalian central neurons. Cold Spring Harb. Perspect. Biol. 3, a006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J. D., Mermall V., Strobel M. C., Russell L. B., Mooseker M. S., Copeland N. G., Jenkins N. A. (1998) Molecular genetic dissection of mouse unconventional myosin-VA: tail region mutations. Genetics 148, 1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyata M., Kishimoto Y., Tanaka M., Hashimoto K., Hirashima N., Murata Y., Kano M., Takagishi Y. (2011) A role for myosin Va in cerebellar plasticity and motor learning: a possible mechanism underlying neurological disorder in myosin Va disease. J. Neurosci. 31, 6067–6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ménasché G., Fischer A., de Saint Basile G. (2002) Griscelli syndrome types 1 and 2. Am. J. Hum. Genet. 71, 1237–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin S. M., Zhang N., Hansen J., Gerges N. Z., Pak D. T., Sheng M., Lee S. H. (2012) GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat. Neurosci. 15, 1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner W., Fodor E., Ginsburg A., Hammer J. A., 3rd (2006) The binding of DYNLL2 to myosin Va requires alternatively spliced exon B and stabilizes a portion of the myosin's coiled-coil domain. Biochemistry 45, 11564–11577 [DOI] [PubMed] [Google Scholar]
- 40. Barbar E. (2008) Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry 47, 503–508 [DOI] [PubMed] [Google Scholar]
- 41. Rapali P., Szenes Á., Radnai L., Bakos A., Pál G., Nyitray L. (2011) DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS J. 278, 2980–2996 [DOI] [PubMed] [Google Scholar]
- 42. Röder I. V., Petersen Y., Choi K. R., Witzemann V., Hammer J. A., 3rd, Rudolf R. (2008) Role of myosin Va in the plasticity of the vertebrate neuromuscular junction in vivo. PLoS ONE 3, e3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Röder I. V., Choi K. R., Reischl M., Petersen Y., Diefenbacher M. E., Zaccolo M., Pozzan T., Rudolf R. (2010) Myosin Va cooperates with PKA RIα to mediate maintenance of the endplate in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 2031–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshii A., Zhao J. P., Pandian S., van Zundert B., Constantine-Paton M. (2013) A myosin Va mutant mouse with disruptions in glutamate synaptic development and mature plasticity in visual cortex. J. Neurosci. 33, 8472–8482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis T. L., Jr., Mao T., Svoboda K., Arnold D. B. (2009) Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat. Neurosci. 12, 568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu K., Zhong G., Zhuang X. (2013) Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu X. S., Rao K., Zhang H., Wang F., Sellers J. R., Matesic L. E., Copeland N. G., Jenkins N. A., Hammer J. A., 3rd (2002) Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 4, 271–278 [DOI] [PubMed] [Google Scholar]
- 48. Liu Y., Xu X. H., Chen Q., Wang T., Deng C. Y., Song B. L., Du J. L., Luo Z. G. (2013) Myosin Vb controls biogenesis of post-Golgi Rab10 carriers during axon development. Nat. Commun. 4, 2005. [DOI] [PubMed] [Google Scholar]
- 49. van Diepen M. T., Eickholt B. J. (2008) Function of PTEN during the formation and maintenance of neuronal circuits in the brain. Dev. Neurosci. 30, 59–64 [DOI] [PubMed] [Google Scholar]
- 50. van Diepen M. T., Parsons M., Downes C. P., Leslie N. R., Hindges R., Eickholt B. J. (2009) Myosin V controls PTEN function and neuronal cell size. Nat. Cell Biol. 11, 1191–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson S. R., Ali M. Y., Trybus K. M., Warshaw D. M. (2009) Random walk of processive, quantum dot-labeled myosin Va molecules within the actin cortex of COS-7 cells. Biophys. J. 97, 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lisé M. F., Srivastava D. P., Arstikaitis P., Lett R. L., Sheta R., Viswanathan V., Penzes P., O'Connor T. P., El-Husseini A. (2009) Myosin-Va-interacting protein, RILPL2, controls cell shape and neuronal morphogenesis via Rac signaling. J. Cell Sci. 122, 3810–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heym R. G., Niessing D. (2012) Principles of mRNA transport in yeast. Cell. Mol. Life Sci. 69, 1843–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCaffrey M. W., Lindsay A. J. (2012) Roles for myosin Va in RNA transport and turnover. Biochem. Soc. Trans. 40, 1416–1420 [DOI] [PubMed] [Google Scholar]
- 55. Yoshimura A., Fujii R., Watanabe Y., Okabe S., Fukui K., Takumi T. (2006) Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr. Biol. 16, 2345–2351 [DOI] [PubMed] [Google Scholar]
- 56. Nalavadi V. C., Griffin L. E., Picard-Fraser P., Swanson A. M., Takumi T., Bassell G. J. (2012) Regulation of zipcode binding protein 1 transport dynamics in axons by myosin Va. J. Neurosci. 32, 15133–15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alami N. H., Jung P., Brown A. (2009) Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J. Neurosci. 29, 6625–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lalli G., Gschmeissner S., Schiavo G. (2003) Myosin Va and microtubule-based motors are required for fast axonal retrograde transport of tetanus toxin in motor neurons. J. Cell Sci. 116, 4639–4650 [DOI] [PubMed] [Google Scholar]
- 59. Bittins C. M., Eichler T. W., Hammer J. A., 3rd, Gerdes H. H. (2010) Dominant-negative myosin Va impairs retrograde but not anterograde axonal transport of large dense core vesicles. Cell. Mol. Neurobiol. 30, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bridgman P. C. (1999) Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J. Cell Biol. 146, 1045–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pathak D., Sepp K. J., Hollenbeck P. J. (2010) Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 30, 8984–8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sloane J. A., Vartanian T. K. (2007) Myosin Va controls oligodendrocyte morphogenesis and myelination. J. Neurosci. 27, 11366–11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bittins C. M., Eichler T. W., Gerdes H. H. (2009) Expression of the dominant-negative tail of myosin Va enhances exocytosis of large dense core vesicles in neurons. Cell. Mol. Neurobiol. 29, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kögel T., Bittins C. M., Rudolf R., Gerdes H. H. (2010) Versatile roles for myosin Va in dense core vesicle biogenesis and function. Biochem. Soc. Trans. 38, 199–204 [DOI] [PubMed] [Google Scholar]
- 65. Brozzi F., Diraison F., Lajus S., Rajatileka S., Philips T., Regazzi R., Fukuda M., Verkade P., Molnár E., Váradi A. (2012) Molecular mechanism of myosin Va recruitment to dense core secretory granules. Traffic 13, 54–69 [DOI] [PubMed] [Google Scholar]
- 66. Sklar P., Smoller J. W., Fan J., Ferreira M. A., Perlis R. H., Chambert K., Nimgaonkar V. L., McQueen M. B., Faraone S. V., Kirby A., de Bakker P. I., Ogdie M. N., Thase M. E., Sachs G. S., Todd-Brown K., Gabriel S. B., Sougnez C., Gates C., Blumenstiel B., Defelice M., Ardlie K. G., Franklin J., Muir W. J., McGhee K. A., MacIntyre D. J., McLean A., VanBeck M., McQuillin A., Bass N. J., Robinson M., Lawrence J., Anjorin A., Curtis D., Scolnick E. M., Daly M. J., Blackwood D. H., Gurling H. M., Purcell S. M. (2008) Whole-genome association study of bipolar disorder. Mol. Psychiatry 13, 558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen Y., Tian L., Zhang F., Liu C., Lu T., Ruan Y., Wang L., Yan H., Yan J., Liu Q., Zhang H., Ma W., Yang J., Li K., Lv L., Zhang D., Yue W. (2013) Myosin Vb gene is associated with schizophrenia in Chinese Han population. Psychiatry Res. 207, 13–18 [DOI] [PubMed] [Google Scholar]
- 68. Oke O. A., Burgess S. A., Forgacs E., Knight P. J., Sakamoto T., Sellers J. R., White H., Trinick J. (2010) Influence of lever structure on myosin 5a walking. Proc. Natl. Acad. Sci. U.S.A. 107, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thirumurugan K., Sakamoto T., Hammer J. A., 3rd, Sellers J. R., Knight P. J. (2006) The cargo-binding domain regulates structure and activity of myosin 5. Nature 442, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]


