Background: Dopamine D2R-mediated ERK activation regulates dopaminergic neuronal development.
Results: D2R activation induces shedding of heparin-binding EGF by activating a disintegrin and metalloproteinase (ADAM) 10 or 17, causing EGFR transactivation in mesencephalic neurons.
Conclusion: D2R-mediated ERK activation regulates mesencephalic dopaminergic neuron development via EGFR transactivation through ADAM10/17.
Significance: Dopaminergic system alteration through D2R-ADAM-EGFR signaling maybe associated with dopamine-related neurological/psychiatric disorders.
Keywords: ADAM, ADAMTS, Dopamine Receptors, Epidermal Growth Factor Receptor (EGFR), ERK, Signal Transduction, Dopaminergic Neuron Development
Abstract
Dopamine D2 receptor (D2R)-mediated extracellular signal-regulated kinase (ERK) activation plays an important role in the development of dopaminergic mesencephalic neurons. Here, we demonstrate that D2R induces the shedding of heparin-binding epidermal growth factor (EGF) through the activation of a disintegrin and metalloprotease (ADAM) 10 or 17, leading to EGF receptor transactivation, downstream ERK activation, and ultimately an increase in the number of dopaminergic neurons and their neurite length in primary mesencephalic cultures from wild-type mice. These outcomes, however, were not observed in cultures from D2R knock-out mice. Our findings show that D2R-mediated ERK activation regulates mesencephalic dopaminergic neuron development via EGF receptor transactivation through ADAM10/17.
Introduction
Abnormal dopaminergic activity in the midbrain is implicated in several neuropsychiatric diseases, such as Parkinson disease, drug addiction, and schizophrenia. Novel treatments for these disorders, therefore, hinge upon a deeper understanding of the mechanisms underlying the development of dopaminergic neurons. Several previous studies demonstrate that the development of dopaminergic neurons requires a complex network of transcription factors and signaling pathways (1–6). The dopamine D2 receptor (D2R)3 plays a crucial role in the development of dopaminergic neurons by serving as an autoreceptor on midbrain neurons (7–9). We previously found that in the absence of D2R, later stages of dopaminergic neuronal development, including terminal differentiation, were blunted, and the expression of proteins required for dopaminergic neuron development, Nurr1 and Ptx3, was altered (7).
The development of dopaminergic neurons also involves D2R-mediated ERK activation (7–9). Recently, we reported that Wnt5a interacts with D2R to promote the differentiation of dopaminergic neurons and that this regulation is dependent on ERK signaling (9). Wnt5a-mediated ERK activation is regulated not only by D2R but also by EGF receptor (EGFR) signaling, suggesting that stimulation of D2Rs by Wnt5a activates ERK phosphorylation via EGFR, thereby promoting dopaminergic neuron development (9). Therefore, EGFR signaling appears to serve as a final transducer for the D2R-mediated ERK signaling that controls dopaminergic neuron development.
We investigated the cross-talk between D2R and EGFR to clarify the role of EGFR signaling in the regulation of ERK activation during the development of dopaminergic neurons. We found that D2R-mediated transactivation of EGFR is critical not only for D2R-mediated ERK activation but also for D2R-mediated control of dopaminergic neuron development.
EXPERIMENTAL PROCEDURES
Animal Preparation and Mesencephalic Neuronal Cell Culture
All experiments were performed with wild-type and dopamine D2 receptor knock-out (D2R−/−) mice. D2R−/− mice (B6;129S2-Drd2tmllow) were purchased from the Induced Mutant Resource at the Jackson Laboratory and produced from heterozygous D2R+/− mice. Primary mesencephalic neuronal cultures were prepared as described previously (7–9). To visualize morphological features immunocytochemically, dopamine (DA) neurons were treated from days 2 to 4 with 1 μm quinpirole (Tocris) or 1 μm pramipexole (Sigma) every 12 h and with EGF (20 ng/ml; PeproTech) every 24 h, in the presence or absence of pretreatment with 10 μm AG1478 (Calbiochem) for 1 h and with 10 μm GM6001 for 1 h during the experiment. For Western blot analysis, on the 4th day in vitro, DA neurons were incubated with Neurobasal medium without B27 supplement (Invitrogen) and treated with various experimental reagents for the time periods indicated. All of the experiments were approved by the Institutional Animal Care and Use Committee of Korea University.
Western Blot Analysis of p-ERK
For Western blot analysis, on the 4th day in vitro, DA neurons were incubated with Neurobasal medium without B27 supplement for 2 h. After treatment with 10 μm quinpirole or 10 μm pramipexole or 20 ng/ml EGF for 10 min in the presence or absence of pretreatment with haloperidol (1 μm for 10 min), AG1478 (10 μm for 1 h), or GM6001 (10 μm for 1 h), DA neurons were washed with ice-cold PBS and treated with lysis buffer on ice. Cells were then centrifuged at 13,000 × g for 10 min at 4 °C. Protein (∼30 μg) was separated on 10% SDS-PAGE. Protein was blotted onto pre-wetted polyvinylidene difluoride nitrocellulose membranes. Mouse monoclonal anti-p-ERK (1:3000; Cell Signaling), rabbit monoclonal anti-ERK (1:5000; Santa Cruz Biotechnology), rabbit polyclonal antibody against a disintegrin and metalloprotease 10 (anti-ADAM10, 1:1000; Millipore), rabbit polyclonal anti-ADAM17 (anti-TACE, 1:1000; Calbiochem), rabbit polyclonal anti-pEGFR (1:1000; Cell Signaling), and rabbit polyclonal anti-EGFR (1:2000; Santa Cruz Biotechnology) were used as primary antibodies. Specific bands were detected by enhanced chemiluminescence (ECL; Amersham Biosciences) and analyzed using the LAS3000 image analysis system (Fuji, Tokyo, Japan).
Immunocytochemistry
DA neurons were fixed and incubated with rabbit polyclonal anti-tyrosine hydroxylase (TH) (1:7500; Pel-Freez). Neurons were then stained according to avidin-biotin immunocytochemical procedures (Vector Laboratories). The number of cells, number of neurites, and neurite length were analyzed as described previously (7–9).
Immunfluorescence Histochemistry
For immunohistochemical studies, the heads of wild-type mouse embryos (E15.5) were fixed in 4% paraformaldehyde for 4 h at 4 °C and then soaked in 20% sucrose for 48 h. Whole heads were mounted in a cryostat maintained at −20 °C and serially sectioned into 10-μm slices. Sections were placed on glass slides, coated with 0.5% gelatin and 0.5% chromium potassium sulfate. Sections were washed in 0.1 m PBS, pH 7.4, 3 times for 10 min each, and then placed in 0.1 m PBS solution containing 0.3% H2O2 and 50% methanol for 20 min. Sections were incubated in a solution of 0.2% Triton X-100 in 0.1 m PBS for 30 min and then overnight in rabbit polyclonal anti-ADAM10 (1:200; Abcam) or rabbit polyclonal anti-ADAM17 (1:200; Abcam) with chicken polyclonal anti-TH (1:500; Abcam) at 4 °C. After washes with PBS containing 0.2% Triton X-100, the sections were incubated at room temperature for 1 h with Alexa Fluor 568 goat anti-rabbit IgG (1:200; Invitrogen) for ADAM10 or ADAM17 and Alexa Fluor 488 goat anti-chicken IgG (1:200; Invitrogen) in PBS containing 0.2% Triton X-100. After rinsing in PBS, the sections were mounted in Vectashield (Vector Laboratories) to prevent fading of the immunofluorescence stain. Sections were examined on a BX51 microscope (Olympus, Tokyo, Japan) with a DP72 camera (Olympus) for low magnification and on an LSM700 confocal laser scanning microscope (Carl Zeiss, Jena, Germany) for high magnification.
Oligomeric siRNA Transfection
A pair of oligonucleotides, 5′-UCUUCCAUCAAUGACAGACCCTT-3′ (antisense) and 5′-GGGUCUGUCAUUGAUGG AAGATT-3′ (sense) to silence ADAM10 and 5′-GGCAGACUUUAGAUGCUUCUUTT-3′ (antisense) and 5′-AAGAAGCAUCUAAAGUCUGCCTT-3′(sense) to silence ADAM17 (Invitrogen) was prepared. A pair of oligonucleotides was designed to silence green fluorescence protein (GFP): 5′-GUUCAUCGUGUCCGGCGAGTT-3′ (sense) and 5′-CUCGCCGGACACGCUGAACTT-3′ (antisense), and this pair of oligonucleotides was used as a negative control for siRNA transfection. Primary mesencephalic cells were plated at 3.75 × 105 cells/well on 6-well plates or at 2.5 × 105 cells/coverslip on 18 × 18-mm coverslips precoated with 50 μg/ml poly-d-lysine and 2 μg/ml laminin (Sigma). On the 3rd day in vitro, primary cultured cells were treated with 20 nm duplex oligonucleotides and 3.0 μl of Lipofectamine RNAiMax (Invitrogen), or a 15 nm concentration of each duplex oligonucleotide and 4.5 μl of Lipofectamine RNAiMax for double knockdown of ADAM10 and ADAM17. After 48 h, cells were treated with 10 μm quinpirole for 10 min for analysis of p-ERK activation by D2R. To determine the morphological features of DA neurons using immunocytochemistry, neurons were treated for 48 h with EGF (20 ng/ml) every 24 h or 1 μm quinpirole every 12 h for the duration of the experiment.
RNA Isolation and RT-PCR
Total RNA was isolated from mesencephalic neurons with TRIzol reagent (Invitrogen). RNA was purified with RQ1 RNase-free DNase treatment. First-strand cDNA was synthesized by reverse transcription using SuperScript II reverse transcriptase (Invitrogen). Primers for candidate genes were designed to amplify a PCR fragment from the following transcripts: mouse HB-EGF forward, 5′-GCTGAGATCATGGTGTCAGG-3′ and reverse, 5′-GCAGCTTCCACCAACG-3′; mouse EGF forward, 5′-ATGAAGCTGCTGCCGTCGGT-3′ and reverse, 5′-TCAGTGGGAGCTAGCCACGC-3′; mouse β-actin forward, 5′-ACTATTGGCAACGAGCGGTT-3′ and reverse, 5′-TGTCAGCAATGCCTGGGTACAT-3′. The PCR products were resolved on a 1.5% agarose gel and visualized by staining with ethidium bromide.
Enzyme Immunoassay
The growth medium of mesencephalic neurons was exchanged with phenol red-free DMEM (HyClone; Thermo Scientific) 2 h before drug treatment. After treatment with quinpirole (10 μm) or phorbol 12-myristate-13-acetate (PMA, 100 ng/ml), supernatants were collected. Cultured cells were washed with ice-cold PBS and treated with lysis buffer on ice. Cells were then centrifuged at 13,000 × g for 10 min at 4 °C for Western blot analysis. Supernatants were used in ELISA for HB-EGF quantification (R&D Systems) according to the manufacturer's instructions. The working concentrations of capture antibody and detection antibody were 400 ng/ml and 100 ng/ml, respectively. Total protein in the supernatant was quantified by the Bradford assay.
Preparation of Concentrated Conditioned Media
Primary mesencephalic cells were plated at 3.75 × 105 cells/well on 6-well plates, and on the 4th day in vitro, the DA neurons were incubated with 1 ml of Neurobasal medium without B27 supplement for 2 h after washing twice with 0.1 m PBS. After treatment with quinpirole for 2 h, conditioned media were harvested and clarified by centrifugation at 4000 × g for 5 min. Conditioned media were concentrated 10-fold using an Ultracel YM-3 membrane with a 3-kDa molecular mass cut-off. Concentrated conditioned media were stored at −80 °C.
Statistical Analysis
For statistical analysis, a two-sample comparison was performed using Student's t test, and multiple comparisons were made using two-way ANOVA or one-way ANOVA followed by a Bonferroni test.
RESULTS
D2R-mediated ERK Activation via EGFRs in Mesencephalic Neuronal Cells
We first examined the involvement of EGFR signaling in D2R-mediated activation of ERK in mesencephalic neuronal cells. ERK activation has been found to occur in response to D2R stimulation in mesencephalic dopaminergic neurons from wild-type (WT) mice, and ERK signaling is significantly decreased in TH-positive neurons in D2R−/− mice (7). Mesencephalic neurons from WT and D2R−/− mice were pretreated with the EGFR inhibitor AG1478, and D2R-mediated ERK activation was evaluated. In mesencephalic neurons from WT mice, treatment with the D2R agonist quinpirole resulted in ERK activation, which was suppressed by pretreatment with AG1478 (Fig. 1A). In mesencephalic neurons from D2R−/− mice, ERK activation was absent following quinpirole treatment and further suppressed by pretreatment with AG1478 (Fig. 1A).
FIGURE 1.

ERK activation induced by D2R or EGFR stimulation in cultured mesencephalic neurons from WT and D2R−/− mice. A, mesencephalic neuronal cells from WT (n = 6) and D2R−/− (n = 6) mice were treated with or without AG1478 (10 μm) and with or without haloperidol (1 μm). B, mesencephalic neuronal cells from WT or D2R−/− mice were treated with EGF (20 ng/ml) or quinpirole (10 μm) or pramipexole (10 μm) for 5 min to 2 h. C and D, mesencephalic neuronal cells were treated with quinpirole (WT, n = 6; D2R−/−, n = 5) and EGF (WT, n = 7; D2R−/−, n = 8) with or without GM6001 (10 μm). Representative Western blots and quantitative relative intensity analysis from Western blots of phospho-ERK and ERK levels from WT and D2R−/− mice are shown. Mean values ± S.E. (error bars) are shown for WT and D2R−/− mesencephalic neurons. *, p < 0.05; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells; Quin, quinpirole; Halo, haloperidol.
We also examined the effect of the D2R antagonist haloperidol on EGF-induced ERK activation. Previously, we demonstrated that haloperidol treatment completely blocks the effects of quinpirole in cultured mesencephalic neurons from WT mice, but does not change the number of TH-positive neurons and neurite extensions in neurons from D2R−/− mice (7). In the present study, we found that EGF-induced ERK activation was not affected by haloperidol treatment (Fig. 1A). These results suggest that D2R-mediated ERK activation depends on EGFR signaling and that dopamine, acting through D2R, transactivates EGFR to stimulate ERK signaling in mesencephalic neuronal cells.
As additional evidence of EGFR activation by D2R, we examined tyrosine phosphorylation on EGFR upon treatment with quinpirole. In mesencephalic neurons from WT mice, quinpirole treatment produced an immediate increase in the tyrosine phosphorylation of EGFR starting from 5 min, and this was strongly maintained up to 30 min (Fig. 1B). EGFR phosphorylation was also observed upon treatment with another D2R agonist, pramipexole (Fig. 1B), supporting the hypothesis that D2R can transactivate EGFR.
The phosphorylation profile of EGFR upon treatment with quinpirole or pramipexole was comparable with those obtained after stimulation with EGF, showing phosphorylation at up to 30 min of treatment in mesencephalic neurons (Fig. 1B). Interestingly, even though EGFR phosphorylation diminished after 1 h, ERK phosphorylation was maintained even after 2 h; this was also seen with quinpirole- or pramipexole-induced ERK activation (Fig. 1B). However, in mesencephalic neurons from D2R−/− mice, treatment with quinpirole or pramipexole did not induce EGFR phosphorylation, whereas EGF treatment induced receptor phosphorylation in these cells (Fig. 1B).
It has been suggested that the transactivation of receptor tyrosine kinase by G protein-coupled receptors, in particular D2R, is mediated by matrix metalloproteinases (MMPs) (10, 11). We therefore examined the involvement of MMPs in EGFR transactivation by D2R. Treatment with the broad spectrum MMP inhibitor GM6001 led to a complete inhibition of quinpirole-induced ERK activation in cultured neurons from WT mice, but no such changes were observed in cultured neurons from D2R−/− mice (Fig. 1C). Treatment with GM6001 did not alter EGF-induced ERK activation in primary mesencephalic cultures from WT or D2R−/− mice (Fig. 1D). These findings indicate that D2R stimulation transactivates EGFR through MMPs.
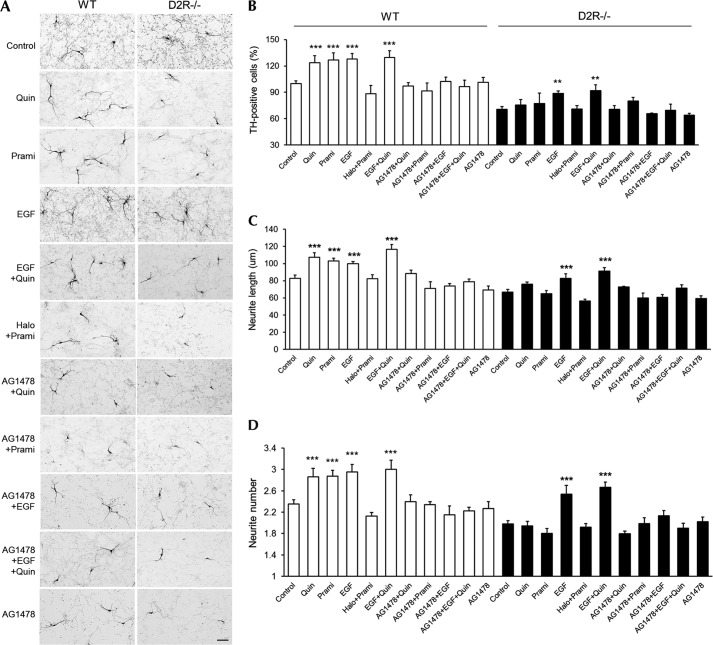
Next, we investigated whether D2R-induced EGFR activation regulates the development of dopaminergic mesencephalic neurons. Primary mesencephalic cultures from WT and D2R−/− mice were prepared and treated with quinpirole, EGF, or quinpirole + EGF. As demonstrated previously (7), the number of detected TH-immunoreactive neurons was substantially lower in D2R−/− mice than in WT mice. Furthermore, quinpirole increased the number of TH-positive neurons, the average length of neuritis, and the arborization of neuronal processes in cultures from WT mice, but these changes were not detected in cultures from D2R−/− mice (Fig. 2). EGF and EGF + quinpirole had effects similar to quinpirole alone on the number of TH-positive neurons and neurite morphology in mesencephalic neurons from WT mice. Treatment with the EGFR inhibitor AG1478, however, completely blocked the effects of quinpirole, EGF, and EGF + quinpirole on the number of TH-positive cells in mesencephalic neurons from WT mice (Fig. 2, A and B). In contrast, in cultures from D2R−/− mice, although EGF and EGF + quinpirole slightly enhanced the development of TH-positive neurons compared with the control condition, these effects were substantially lower than those observed in mesencephalic neurons from WT mice (Fig. 2). Treatment with pramipexole in mesencephalic neurons also induces an enhancement of dopaminergic neuronal development, and this effect was blocked by haloperidol or AG1478, thus showing a result similar to that in quinpirole treatment in these neuronal cells (Fig. 2). These results indicate that the developmental influence of dopamine via D2R is dependent on EGFR, but in the absence of D2R, the effect of EGF is attenuated.
FIGURE 2.
Inhibition of D2R agonist-induced dopaminergic neuronal development by EGFR inhibitor in cultured mesencephalic neurons. A, treatment with quinpirole (1 μm), pramipexole (1 μm), EGF (20 ng/ml), EGF plus quinpirole, haloperidol (1 μm) plus quinpirole, AG1478 (10 μm) plus quinpirole, AG1478 plus pramipexole, AG1478 plus EGF, AG1478 plus EGF plus quinpirole, and only AG1478 with a control group of mesencephalic neuronal cultures from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 50 μm. B–D, effects of the treatments on the numbers of TH neurons (B), neurite length (C), and neurite number (D) in mesencephalic neuronal cultures from WT and D2R−/− mice. Only visualized TH neurons were analyzed. Mean values ± S.E. (error bars) are shown for WT (n ≥ 5) and D2R−/− (n ≥ 4) mesencephalic neurons. **, p < 0.01; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells; Quin, quinpirole; Prami, pramipexole.
We also examined whether MMPs regulate the development of dopaminergic mesencephalic neurons. Pretreatment with the MMP inhibitor GM6001 led to a complete blockade of quinpirole-induced ERK activation but had no significant effect on EGF-induced ERK activation (Fig. 3). These results indicate that MMP is involved in the regulation of dopaminergic neuronal development by stimulating D2R-mediated activation of EGFR signaling.
FIGURE 3.
Inhibition of D2R agonist-induced dopaminergic neuronal development by metalloprotease inhibitor in cultured mesencephalic neurons. A, treatment with quinpirole (1 μm), EGF (20 ng/ml), GM6001 (10 μm) plus quinpirole, GM6001 plus EGF, GM6001 plus EGF plus quinpirole, and only GM6001 with a control group on mesencephalic neuronal cultures from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 50 μm. B–D, effects of treatments on the numbers of TH neurons (B), neurite length (C), and neurite number (D) in mesencephalic neuronal cultures from WT and D2R−/− mice. Only visualized TH neurons were analyzed. Mean values ± S.E. (error bars) are shown for mesencephalic neurons from WT (n = 7) and D2R−/− (n = 6) mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells. Quin, quinpirole.
Role of ADAMs in D2R-mediated Dopaminergic Neuron Development
To identify the MMPs participating in D2R-mediated EGFR transactivation, we assessed the expression of some ADAMs in the embryonic mouse midbrain. At E15.5, ADAM10 and ADAM17 were co-localized with TH-positive neurons in the substantia nigra and the ventral tegmental area of the midbrain as evidenced by double immunofluorohistochemical labeling (Fig. 4, B and C). Therefore, we set out to investigate the role of ADAM10 and ADAM17 in D2R-regulated dopaminergic neuron development.
FIGURE 4.

Double-immunohistochemical staining of TH with ADAM10 or ADAM17 in the mesencephalon of an E15. 5 embryo brain. A, immunohistochemical image shows TH staining in the mesencephalon of an E15.5 embryo brain. Scale bar, 100 μm. B and C, in the substantia nigra (SN) and ventral tegmental area (VTA), immunohistochemical images of TH staining (green) with ADAM10 (B) or ADAM17 (C) (red) are shown. Scale bar, 50 μm (upper panel) and 5 μm (lower panel).
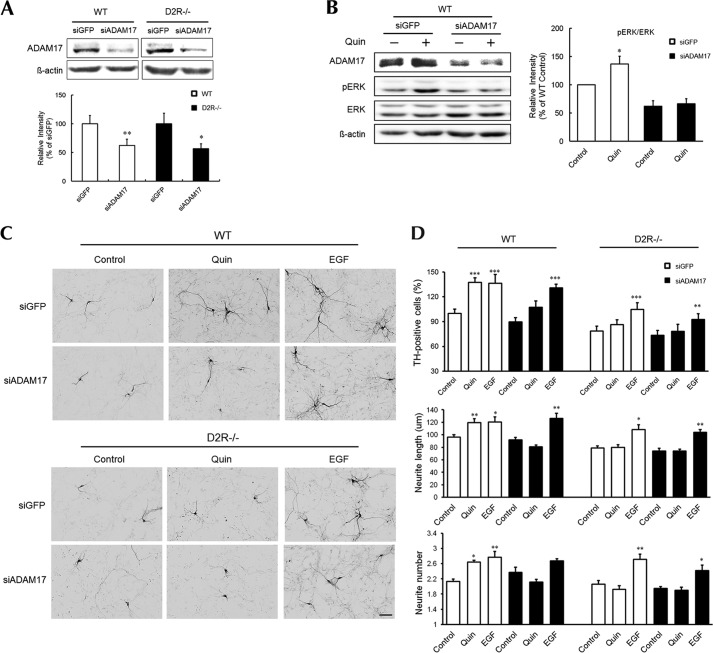
First, we evaluated whether ADAM10 regulates dopaminergic neuron development by modulating D2R-mediated ERK signaling. Using oligomeric siRNA against ADAM10 (siADAM10), we reduced the expression of ADAM10 by ∼50% in both WT and D2R−/− mice mesencephalic neuronal cultures (Fig. 5A). To determine whether ADAM10 knockdown affects D2R-mediated ERK activation, control siGFP- and siADAM10-transfected cells from WT mice were stimulated with quinpirole, and ERK phosphorylation was assessed by Western blotting (Fig. 5B). Quinpirole-induced ERK phosphorylation was completely attenuated in siADAM10-transfected cells. Transfection with siGFP did not alter quinpirole-induced ERK activation (Fig. 5B).
FIGURE 5.
D2R-mediated ERK activation and dopaminergic neuronal development after knockdown of ADAM10 in mesencephalic neurons. A, representative immunoblots of ADAM10 from cell lysates of mesencephalic neurons transfected with 20 nm oligomeric siRNA against GFP (siGFP) or ADAM10 (siADAM10). B, representative Western blot and quantitative relative intensity analysis from Western blots of phospho-ERK and ERK levels of siGFP- and siADAM10-transfected mesencephalic neuronal cells from WT mice. Cells were treated with 10 μm quinpirole. Mean values ± S.E. (error bars) are shown for siGFP- and siADAM10-treated cells (n = 6). *, p < 0.05; **, p < 0.01; results of unpaired Student's t test for control versus drug-treated cells. C, treatment with quinpirole (1 μm), EGF (20 ng/ml) with a control group of siADAM10-transfected mesencephalic neurons from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 50 μm. D, effects of treatments on the numbers of TH neurons, neurite length, and neurite number in mesencephalic neuronal cultures from WT and D2R−/− mice. Only visualized TH neurons were analyzed. Mean values ± S.E. are shown for mesencephalic neurons from WT (n = 7) and D2R−/− (n = 6) mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells. Quin, quinpirole.
Next, we tested whether ADAM10 knockdown also affects the D2R-mediated development of dopaminergic mesencephalic neurons. Treatment with siADAM10 significantly blunted the quinpirole-induced enhancement of neuronal development in primary mesencephalic cultures from WT mice (Fig. 5, C and D), with no quinpirole-induced increase in number of TH-positive neurons. Treatment with siADAM10 also blunted the quinpirole-induced enhancement in neurite number and extension in mesencephalic neurons from WT mice (Fig. 5, C and D). ADAM10 knockdown, however, had no effect on the EGF-induced increase in the number or morphological complexity of TH-positive neurons in mesencephalic neurons from WT or D2R−/− mice (Fig. 5, C and D). Reduction of ADAM10 did not affect the number of TH-positive neurons or neurite length in mesencephalic neurons from D2R−/− mice (Fig. 5, C and D), and treatment with siGFP did not alter the effect of quinpirole on the number of TH-positive neurons or neurite length in mesencephalic neurons from WT or D2R−/− mice (Fig. 5, C and D).
In parallel to ADAM10 knockdown, we also examined the effect of ADAM17 knockdown on D2R-regulated ERK activation and dopaminergic neuron development. Using oligomeric siRNA against ADAM17 (siADAM17), we reduced the expression of ADAM17 by ∼60% in WT and by approximately 50% in mesencephalic neuronal cultures from D2R−/− mice (Fig. 6A). Knockdown of ADAM17 completely attenuated quinpirole-induced ERK activation in mesencephalic neurons from WT mice (Fig. 6B). Treatment with siADAM17 also significantly suppressed the number of TH-positive neurons and the number and length of their neurites in response to quinpirole treatment in mesencephalic neurons from WT mice, whereas no such effect was observed in cultures from D2R−/− mice (Fig. 6, C and D). Similar to knockdown of ADAM10, knockdown of ADAM17 did not alter the effect of EGF on the number or morphological complexity of TH-positive neurons in mesencephalic neurons from WT or D2R−/− mice (Fig. 6, C and D).
FIGURE 6.
D2R-mediated ERK activation and dopaminergic neuronal development after knockdown of ADAM17 in mesencephalic neurons. A, representative immunoblots of ADAM17 from cell lysates of mesencephalic neurons transfected with 20 nm oligomeric siRNA against GFP (siGFP) or ADAM17 (siADAM17). B, representative Western blot and quantitative relative intensity analysis from Western blots of phospho-ERK and ERK levels of siGFP- and siADAM17-transfected mesencephalic neuronal cells from WT mice. Cells were treated with 10 μm quinpirole. Mean values ± S.E. (error bars) are shown for siGFP- and siADAM17-treated cells (n = 4). *, p < 0.05 and **, p < 0.01 represent the result of unpaired Student's t test for control versus drug-treated cells. C, treatment with quinpirole (1 μm) and EGF (20 ng/ml) in a control group of siADAM17-transfected mesencephalic neurons from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 50 μm. D, effects of treatments on the numbers of TH neurons, neurite length, and neurite number in mesencephalic neuronal cultures from WT and D2R−/− mice. Only visualized TH neurons were analyzed. Mean values ± S.E. are shown for mesencephalic neurons from WT (n = 5) and D2R−/− (n = 6) mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells. Quin, quinpirole.
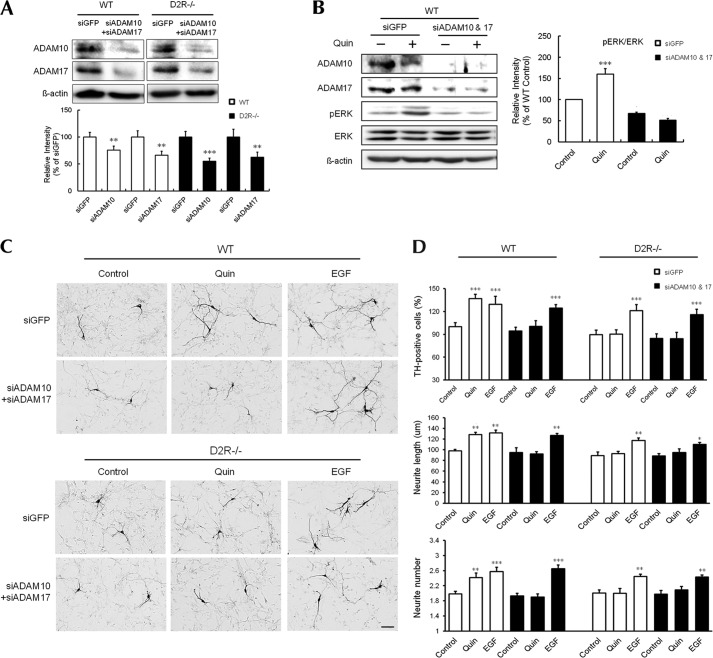
To determine whether the effects of ADAM10 and ADAM17 knockdown are additive, we examined D2R-regulated ERK activation and dopaminergic neuron development after treatment with both ADAM10 and ADAM17 siRNA. Double knockdown of ADAM10/17 significantly reduced the expression of both ADAM10 and ADAM17 by 55–65% in mesencephalic neurons from both mice (Fig. 7A). Double knockdown of ADAM10/17 significantly suppressed quinpirole-induced ERK activation and enhancements in the number, neurite number, and neurite extension of TH-positive neurons in mesencephalic primary cultures from WT mice, whereas no such enhancement by quinpirole was observed in D2R−/− mice (Fig. 7, C and D). This double knockdown of ADAM10/17 did not suppress the EGF-induced increase in the number or morphological complexity of TH-positive neurons in mesencephalic neurons from WT or D2R−/− mice (Fig. 7, C and D). These results suggest that ADAM10 and ADAM17 are activated by D2R through a common signaling pathway that stimulates EGFR and triggers ERK activation, thereby regulating dopaminergic neuron development.
FIGURE 7.
D2R-mediated ERK activation and TH neuronal development after double knockdown of ADAM10 and ADAM17 in mesencephalic neurons. A, representative immunoblots of ADAM10 and ADAM17 from cell lysates of mesencephalic neurons transfected with oligomeric siRNA against GFP (siGFP) or each ADAM10 (siADAM10) and ADAM17 (siADAM17). B, representative Western blot and quantitative relative intensity analysis from Western blot of phospho-ERK and ERK levels of siGFP- or siADAM10/17-transfected mesencephalic neuronal cells from WT mice. Cells were treated with quinpirole. Mean values ± S.E. (error bars) are shown for siGFP and siADAM10/17 (n = 4). **, p < 0.01 and ***, p < 0.001 represent the results of unpaired Student's t test for control versus drug-treated cells. C, treatment with quinpirole or EGF with a control group of siADAM10/17-transfected mesencephalic neuronal cells from WT and D2R−/− mice. TH neurons were visualized using immunocytochemistry. Scale bar, 50 μm. D, effects of treatments on the numbers of TH neurons, neurite length, and neurite number in mesencephalic neuronal cultures from WT and D2R−/− mice. Only visualized TH neurons were analyzed. Mean values ± S.E. are shown for mesencephalic neurons from WT (n = 6) and D2R−/− (n = 6) mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ANOVA followed by a Bonferroni test for control versus drug-treated cells. Quin, quinpirole.
Activation of D2R Induces the Release of HB-EGF in Mesencephalic Neuronal Cells
ADAM10 and ADAM17 are thought to be major sheddases, with ADAM10 involved in the shedding of EGF, HB-EGF, and betacellurin, and ADAM17 involved in the shedding of TGF-α, amphiregulin, and HB-EGF (12–14). As both ADAM10 and ADAM17 commonly mediate pro-HB-EGF shedding via different EGFR ligands (13–15), we presumed that the activation of D2R could induce HB-EGF shedding. Indeed, when we assessed the levels of EGF or HB-EGF expression in mesencephalic neurons from WT and D2R−/− mice by RT-PCR analysis, we observed that only HB-EGF mRNA was expressed in both groups of mice (Fig. 8A).
FIGURE 8.

Release of HB-EGF induced by D2R stimulation. A, HB-EGF and EGF mRNA expression was amplified by RT-PCR. B, mesencephalic neuronal cells from WT (n = 4) and D2R−/−(n = 4) mice were treated with PMA (100 ng/ml) or quinpirole (10 μm) for 10 min. C, siGFP- or siADAM10/17-transfected mesencephalic neuronal cells from WT (n = 6) and D2R−/− (n = 7) mice were treated with quinpirole. D, mesencephalic neuronal cells from WT mice (n = 6) were treated with quinpirole for 30 min to 12 h (left). Concentrations of HB-EGF in culture supernatants were measured by ELISA. Mesencephalic neuronal cells from D2R−/− mice were treated with or without control concentrated conditioned media and quinpirole-treated concentrated conditioned media (right). Mean values ± S.E. (error bars) are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001 for control versus drug-treated cells. &&, p < 0.01; ANOVA followed by a Bonferroni test for siGFP- versus siADAM10/17-transfected cells. Quin, quinpirole; CM, conditioned media. E, proposed mechanism for D2R-dependent dopaminergic neuronal development, involving ADAM10, ADAM17, HB-EGF, and EGFR signaling coupled with ERK activation. Activated D2R can phosphorylate ERK, resulting in EGFR transactivation through HB-EGF. This signaling can promote dopamine neuron development.
To examine HB-EGF release in response to D2R stimulation, we treated mesencephalic neuronal cultures from WT and D2R−/− mice with quinpirole and measured the release of HB-EGF into the cell medium by an ELISA. Quinpirole treatment induced an increase in HB-EGF release in cultures from WT mice but had no such effect on cultures from D2R−/− mice (Fig. 8B). Treatment with PMA, which stimulates the shedding of HB-EGF (13, 16), induced HB-EGF release in cultures from both WT and D2R−/− mice (Fig. 8B).
To investigate whether D2R-mediated release of HB-EGF involves ADAM10/17 in mesencephalic neurons, we performed double knockdown of ADAM10 and ADAM17 by transfecting cells with both siADAM10 and siADAM17. In mesencephalic neurons from WT mice, siADAM10/17 treatment completely attenuated quinpirole-induced HB-EGF release, compared with siGFP treatment (Fig. 8C). However, no such effect was observed in cultures from D2R−/− mice, showing that quinpirole-induced HB-EGF was absent and not affected by siADAMs in the absence of D2R (Fig. 8C). Next, we tested whether the HB-EGF released from quinpirole-treated mesencephalic cultures of WT mice could then recover the ability to induce ERK activation in mesencephalic cultures from D2R−/− mice. We harvested and measured the amount of HB-EGF in the media from cultures of mesencephalic neurons from WT mice after quinpirole treatment for different times and observed that the accumulation of HB-EGF was maximal after 2 h of treatment with quinpirole (Fig. 8D, left). We thus collected the media after 2 h of quinpirole treatment, and the media were concentrated 10-fold. When we treated mesencephalic neurons of D2R−/− mice with these conditioned media, we found that this treatment induces not only ERK activation but also the phosphorylation of EGFR, whereas treatment with control nontreated conditioned media had no such effect (Fig. 8D, right). Thus, the specific activation of EGFR and ERK with concentrated quinpirole-treated conditioned media collected from mesencephalic neurons of WT mice strongly suggests that activation of D2R is indeed able to induce the shedding of HB-EGF and subsequent EGFR activation for further ERK activation. Taken together, these findings suggest that D2R activation induces HB-EGF shedding via ADAM activation in mesencephalic neuronal cells, resulting in EGFR signaling and ERK activation and thereby regulating dopaminergic neuron development (Fig. 8E).
DISCUSSION
In the present study, we determined that dopamine D2R-mediated ERK activation via transactivation of EGFR through ADAMs plays a crucial role in the development of mesencephalic dopaminergic neurons. Dopamine-producing cells are generated within the embryonic ventral midbrain, and this developmental programming has been shown to require a complex network of transcription factors and signaling pathways, such as Nurr1, Ptx3, Lmx1a, and others (2–6). Recent reports show that by generating induced pluripotent stem cells overexpressing specific transcription factors, it is possible to cause them to differentiate into dopaminergic neuronal cells or even directly induced neuronal cells (1, 17). Indeed, the generation of induced dopamine neurons derived from somatic cells has also been reported (17), showing that a combination of 3 transcription factors, Mash1, Nurr1, and Lmx1a, can rapidly and efficiently induce the development of dopaminergic neuronal cells from mouse and human fibroblasts. These reprogrammed dopaminergic cells show dopamine release and pacemaker activity that can be modulated via D2R (17). This indicates that the presence of D2R is crucial for the proper function of dopaminergic neurons in vivo and that D2R-mediated signaling serves to maintain this homeostatic regulation of the final differentiation environment of dopaminergic neurons, thereby contributing to the sustainability of dopaminergic neurons. Although fine-tuning of this differentiation mechanism is not well understood, one can hypothesize that D2R, as a unique dopaminoceptive molecule in dopamine neurons during development, seems to be essential as a provider of a secure environment for dopaminergic neuron development.
Upon dopaminergic tone, D2R would employ some specific signaling pathway to control dopaminergic neuronal development; we have demonstrated that D2R-mediated ERK signaling is a critical signaling pathway for this regulation. We observed that in mesencephalic neurons, ERK phosphorylation at p42 is stronger than at p44; this has been also reported in our previous study (7, 8). However, in other types of cells, such as immortalized cell lines, Chinese hamster ovary cells, or human embryonic kidney 293 cells, in which D2R-mediated ERK activation is achieved by stably transfecting D2R, almost equal amounts of p42/p44 phosphorylated ERK are observed (11, 18), showing differences in ERK activation by D2R depending on different cell types.
As is known for other GPCRs, the activation of ERKs by D2R includes the transactivation of receptor tyrosine kinases such as the PDGF receptor or EGFR depending on cell type and possibly the activity of an MMP (9, 11, 18). Here, we have demonstrated that treatment with the D2R agonist quinpirole as well as pramipexole produced an immediate increase in the tyrosine phosphorylation of EGFR in mesencephalic neuronal cells (Fig. 1B), supporting the hypothesis that D2R can transactivate EGFR. Pramipexole is known to bind selectively and with high affinity to D2R and with highest affinity to D3R. However, because D3R is barely expressed in the rodent midbrain (19), our observed results can be attributed to the specific binding of pramipexole to D2R in mesencephalic neuronal cells.
Our present study is the first demonstration that stimulation of D2R can induce EGFR transactivation through ADAM10 and ADAM17 and HB-EGF shedding, thus implicating ADAMs in dopaminergic neuronal development. Their co-expression with TH-positive neurons in the embryonic mesencephalon indicates that these ADAMs can be involved in dopaminergic neuronal development. Using siADAM10 and siADAM17, we found that ADAM10 and ADAM17 can mediate D2R-induced ERK activation and D2R-mediated dopaminergic neuronal development, strongly supporting HB-EGF as a common substrate of ADAMs in this midbrain neuronal culture. Indeed, quinpirole, a D2R agonist, could induce HB-EGF release into the culture medium of mesencephalic neurons in WT mice within a time frame comparable with that for ERK signaling, whereas this release was not observed in D2R−/− mice.
HB-EGF promotes the survival of dopaminergic neurons (20), suggesting that the absence of HB-EGF may result in hypofunctioning of the dopaminergic system. In conditional knock-out mice in which HB-EGF is reduced in the ventral forebrain, prefrontal cortex dopamine levels are significantly lower than in control mice (21), and these knock-out mice display behavioral abnormalities associated with psychiatric disorders. These reports suggest that HB-EGF signaling plays an important role within the dopaminergic system. In this context, we imagine that the D2R-mediated ERK signaling that controls dopaminergic neuron development involves a complex cascade of signaling mechanisms. Specifically, the binding of dopamine to D2R could induce the activation of ADAMs and the shedding of HB-EGF, thereby triggering the activation of EGFR and leading to ERK signaling (Fig. 8E). Because EGF and EGFR signaling contribute to dopaminergic neuron survival and dopamine-mediated neurogenesis (22–26), our findings suggest that the enhancement of morphological and neurochemical development of cultured midbrain dopaminergic neurons by EGF and EGFR could be under the control of D2R signaling.
In this regard, we imagine that perturbations of the dopaminergic system leading to alterations in EGFR signaling could be associated with neurological and psychiatric disorders. In line with this hypothesis, an up-regulation of ErbB1 receptors has been found in the striatum of patients with schizophrenia, suggesting a potential link between impaired EGF signaling and the pathology of this disease (27, 28). Iwakura et al. reported that the ErbB1 protein is depleted in the striatum of patients with Parkinson disease (29). Therefore, dysfunction of the dopaminergic system, especially in D2R signaling, maybe associated with critical alterations in EGFR pathways, which may contribute to dopamine-related neurological or psychiatric disorders.
Because EGFR transactivation occurs not only in the same cell in which the EGFRs reside but also affects neighboring cells that detect released HB-EGF (12), we suspect that the signal from D2R to EGFR not only propagates laterally along the cell membrane but also coordinates the responses of surrounding cells via cleaved HB-EGF, thereby potentiating EGFR signaling. It will therefore be valuable to further explore how D2R-ADAM-EGFR signaling impacts D2R-dependent dopaminergic pathologies to develop novel therapeutic strategies for these diseases.
Acknowledgments
We thank members of our laboratories for technical assistance and discussion.
This work was supported by Research Grants 2011K000273 from the Brain Research Center of the 21st Century Frontier Research Program, 2011-0015678 from the National Research Foundation of Korea, funded by the Korea government (MEST), and ST100079 from the Seoul R&BD Program.
- D2R
- dopamine D2 receptor
- ADAM
- a disintegrase and metalloprotease
- DA
- dopamine
- EGFR
- epidermal growth factor receptor
- En
- embryonic day number
- HB-EGF
- heparin-binding epidermal growth factor
- MMP
- matrix metalloproteinase
- PMA
- phorbol 12-myristate-13-acetate
- TH
- tyrosine hydroxylase.
REFERENCES
- 1. Lindvall O., Kokaia Z. (2009) Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol. Sci. 30, 260–267 [DOI] [PubMed] [Google Scholar]
- 2. Smidt M. P., Burbach J. P. (2007) How to make a mesodiencephalic dopaminergic neuron. Nat. Rev. Neurosci. 8, 21–32 [DOI] [PubMed] [Google Scholar]
- 3. Perrone-Capano C., Da Pozzo P., di Porzio U. (2000) Epigenetic cues in midbrain dopaminergic neuron development. Neurosci. Biobehav. Rev. 24, 119–124 [DOI] [PubMed] [Google Scholar]
- 4. Simon H. H., Bhatt L., Gherbassi D., Sgadó P., Alberí L. (2003) Midbrain dopaminergic neurons: determination of their developmental fate by transcription factors. Ann. N.Y. Acad. Sci. 991, 36–47 [PubMed] [Google Scholar]
- 5. Riddle R., Pollock J. D. (2003) Making connections: the development of mesencephalic dopaminergic neurons. Brain Res. Dev. Brain Res. 147, 3–21 [DOI] [PubMed] [Google Scholar]
- 6. Perlmann T., Wallén-Mackenzie A. (2004) Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 318, 45–52 [DOI] [PubMed] [Google Scholar]
- 7. Kim S. Y., Choi K. C., Chang M. S., Kim M. H., Kim S. Y., Na Y. S., Lee J. E., Jin B. K., Lee B. H., Baik J. H. (2006) The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J. Neurosci. 26, 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S. Y., Lee H. J., Kim Y. N., Yoon S., Lee J. E., Sun W., Choi E. J., Baik J. H. (2008) Striatal-enriched protein tyrosine phosphatase regulates dopaminergic neuronal development via extracellular signal-regulated kinase signaling. Exp. Neurol. 214, 69–77 [DOI] [PubMed] [Google Scholar]
- 9. Yoon S., Choi M. H., Chang M. S., Baik J. H. (2011) Wnt5a-dopamine D2 receptor interactions regulate dopamine neuron development via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 286, 15641–15651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liebmann C. (2011) EGF receptor activation by GPCRs: a universal pathway reveals different versions. Mol. Cell. Endocrinol. 331, 222–231 [DOI] [PubMed] [Google Scholar]
- 11. Wang C., Buck D. C., Yang R., Macey T. A., Neve K. A. (2005) Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J. Neurochem. 93, 899–909 [DOI] [PubMed] [Google Scholar]
- 12. Yan Y., Shirakabe K., Werb Z. (2002) The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J. Cell Biol. 158, 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higashiyama S., Nanba D. (2005) ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim. Biophys. Acta 1751, 110–117 [DOI] [PubMed] [Google Scholar]
- 15. Ohtsu H., Dempsey P. J., Eguchi S. (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 291, C1–10 [DOI] [PubMed] [Google Scholar]
- 16. Huovila A. P., Turner A. J., Pelto-Huikko M., Kärkkäinen I., Ortiz R. M. (2005) Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 30, 413–422 [DOI] [PubMed] [Google Scholar]
- 17. Caiazzo M., Dell'Anno M. T., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T. D., Menegon A., Roncaglia P., Colciago G., Russo G., Carninci P., Pezzoli G., Gainetdinov R. R., Gustincich S., Dityatev A., Broccoli V. (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 [DOI] [PubMed] [Google Scholar]
- 18. Kim S. J., Kim M. Y., Lee E. J., Ahn Y. S., Baik J. H. (2004) Distinct regulation of internalization and mitogen-activated protein kinase activation by two isoforms of the dopamine D2 receptor. Mol. Endocrinol. 18, 640–652 [DOI] [PubMed] [Google Scholar]
- 19. Diaz J., Ridray S., Mignon V., Griffon N., Schwartz J. C., Sokoloff P. (1997) Selective expression of dopamine D2 receptor mRNA in proliferative zones during embryonic development of the rat brain. J. Neurosci. 17, 4282–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farkas L. M., Krieglstein K. (2002) Heparin-binding epidermal growth factor-like growth factor (HB-EGF) regulates survival of midbrain dopaminergic neurons. J. Neural Transm. 109, 267–277 [DOI] [PubMed] [Google Scholar]
- 21. Oyagi A., Oida Y., Kakefuda K., Shimazawa M., Shioda N., Moriguchi S., Kitaichi K., Nanba D., Yamaguchi K., Furuta Y., Fukunaga K., Higashiyama S., Hara H. (2009) Generation and characterization of conditional heparin-binding EGF-like growth factor knockout mice. PLoS One 4, e7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moses D., Teper Y., Gantois I., Finkelstein D. I., Horne M. K., Drago J. (2006) Murine embryonic EGF-responsive ventral mesencephalic neurospheres display distinct regional specification and promote survival of dopaminergic neurons. Exp. Neurol. 199, 209–221 [DOI] [PubMed] [Google Scholar]
- 23. Inoue H., Lin L., Lee X., Shao Z., Mendes S., Snodgrass-Belt P., Sweigard H., Engber T., Pepinsky B., Yang L., Beal M. F., Mi S., Isacson O. (2007) Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson's disease models. Proc. Natl. Acad. Sci. U.S.A. 104, 14430–14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Keeffe G. C., Tyers P., Aarsland D., Dalley J. W., Barker R. A., Caldwell M. A. (2009) Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc. Natl. Acad. Sci. U.S.A. 106, 8754–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwakura Y., Zheng Y., Sibilia M., Abe Y., Piao Y. S., Yokomaku D., Wang R., Ishizuka Y., Takei N., Nawa H. (2011) Qualitative and quantitative re-evaluation of epidermal growth factor-ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: target-derived neurotrophic signaling (part 1). J. Neurochem. 118, 45–56 [DOI] [PubMed] [Google Scholar]
- 26. Iwakura Y., Wang R., Abe Y., Piao Y. S., Shishido Y., Higashiyama S., Takei N., Nawa H. (2011) Dopamine-dependent ectodomain shedding and release of epidermal growth factor in developing striatum: target-derived neurotrophic signaling (part 2). J. Neurochem. 118, 57–68 [DOI] [PubMed] [Google Scholar]
- 27. Goldstein M., Deutch A. Y. (1992) Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 6, 2413–2421 [PubMed] [Google Scholar]
- 28. Futamura T., Toyooka K., Iritani S., Niizato K., Nakamura R., Tsuchiya K., Someya T., Kakita A., Takahashi H., Nawa H. (2002) Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol. Psychiatry 7, 673–682 [DOI] [PubMed] [Google Scholar]
- 29. Iwakura Y., Piao Y. S., Mizuno M., Takei N., Kakita A., Takahashi H., Nawa H. (2005) Influence of dopaminergic lesion on epidermal growth factor-ErbB1 signals in Parkinson's disease and its model: neurotrophic implication in nigrostriatal neurons. J. Neurochem. 93, 974–983 [DOI] [PubMed] [Google Scholar]