Background: TftG is a YCII superfamily dehydrochlorinase that catalyzes conversion of 5-chlorohydroxyhydroquinone to hydroxybenzoquinone.
Results: The TftG crystal structure in complex with product analog 2,5-dihydroxybenzoquinone illustrated the catalytic residues and mechanism.
Conclusion: A His-Asp dyad and other conserved signature residues are implicated for catalysis and substrate binding.
Significance: This is the first elucidation of a YCII superfamily protein mechanism, which helps explain their obscure nature.
Keywords: Biodegradation, Enzyme Catalysis, Enzyme Mechanisms, Isothermal Titration Calorimetry, X-ray Crystallography, YCII Superfamily, Chlorophenol, Dehydrochlorinase
Abstract
TftG, 5-chloro-2-hydroxyhydroquinone (5-CHQ) dehydrochlorinase, is involved in the biodegradation of 2,4,5-trichlorophenoxyacetate by Burkholderia phenoliruptrix AC1100. It belongs to the YCII superfamily, a group of proteins with largely unknown function. In this work, we utilized structural and functional studies, including the apo-form and 2,5-dihydroxybenzoquinone binary complex crystal structures, computational analysis, and site-directed mutagenesis, to determine the dehydrochlorination mechanism. The His-Asp dyad, which initiates catalysis, is strongly conserved in YCII-like proteins. In addition, other catalytically important residues such as Pro-76, which orients the His-Asp catalytic dyad; Arg-17 and Ser-56, which form an oxyanion hole; and Asp-9, which stabilizes the oxyanion hole, are among the most highly conserved residues across the YCII superfamily members. The comprehensive characterization of TftG helps not only for identifying effective mechanisms for chloroaromatic dechlorination but also for understanding the functions of YCII superfamily members, which we propose to be lyases.
Introduction
2,4,5-Trichlorophenol (2,4,5-TCP)3 is a toxic recalcitrant pollutant introduced into the environment through its extensive use as a herbicide, insecticide, and wood preservative (1, 2). Similar to other chlorophenols, the major toxicity of 2,4,5-TCP results from its ability to uncouple mitochondrial oxidative phosphorylation, leading to subsequent convulsions, hyperthermia, and possible death (3). 2,4,5-TCP is a product of the biodegradation of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) (4, 5).
Early investigation of Burkholderia phenoliruptrix illustrated that it could utilize 2,4,5-T as a sole carbon source. A 2,4,5-T-negative mutant, PT88, was also characterized and found to accumulate 5-chloro-2-hydroxyhydroquinone (5-CHQ) when grown in the presence of glucose and 2,4,5-T (4, 6). Complementation of PT88 for growth on 2,4,5-T as a sole source of carbon identified a cluster of genes (tftEFGH) essential for the metabolism of the 5-CHQ intermediate (4–6). The revealed biodegradation pathway of 2,4,5-T B. phenoliruptrix begins with the conversion of 2,4,5-T to 2,4,5-TCP by the oxygenase enzyme encoded by the tftA and tftB genes. 2,4,5-TCP is subsequently converted to 5-CHQ (4, 6, 7) by TftC and TftD. We previously reported the crystal structures and reaction mechanisms of TftC and TftD, which are reductase and monoxygenase enzymes, respectively (8). 5-CHQ is then dehydrochlorinated into hydroxybenzoquinone (HBQ) by the enzyme TftG. The resulting HBQ is enzymatically reduced to hydroxyhydroquinone by a quinone reductase and then to β-ketoadipate by TftH and TftE, respectively (4). β-Ketoadipate, a common metabolite in the biodegradation of aromatic compounds, is further channeled into the TCA cycle for complete mineralization (Fig. 1).
FIGURE 1.

Conversion of 2,4,5-trichlorophenoxy acetic acid to 2-hydroxybenzoquinone. TftAB converts 2,4,5-trichlorophenoxy acetic acid to 2,4,5-TCP. TftCD converts 2,4,5-TCP to 2,5-dichlorobenzoquinone (not shown), which is reduced to 2,5-DCHQ. TftD further dechlorinates 2,5-DCHQ to 5-chloro-2-hydroxybenzoquinone (not shown), which is reduced to 5-chloro-2-hydroxyhydroquinone, the substrate of TftG. TftG catalyzes the dehydrochlorination of 5-chloro-2-hydroxyhydroquinone to the product 2-hydroxybenzoquinone, which subsequently enters the TCA cycle after several previously established enzymatic steps. This figure was produced using ChemDraw (version 12.0.2).
TftG, a relatively small (11,166 Da) protein, catalyzes aromatic dehydrochlorination, but its mechanism has been unknown. Sequence analysis suggests that TftG belongs to the YCII superfamily. All members of YCII share a conserved His-Asp dyad, of which the putative role has been suggested. This rapidly expanding family also contains strongly conserved Arg and Ser residues. The structural genomics effort has determined several crystal structures from this family, which confirmed similar placement of these residues, but functions of those proteins still remain unclear. Thus, a structural and mechanistic understanding of TftG, a critical enzyme in the biodegradation of 2,4,5-T, is useful not only for future informed bioremediation strategies, but also in better understanding the largely uncharacterized YCII superfamily. Here, we report the crystal structures for both apo-form and the binary complex of TftG with the product analog 2,5-dihydroxybenzoquinone (2,5-DHBQ). This information, together with accompanying enzymatic assays for site-directed mutants, illustrates the biological function and potential reaction mechanism, which sheds light on the functions of YCII superfamily enzymes.
EXPERIMENTAL PROCEDURES
Chemicals
Chemicals were obtained from Sigma Aldrich or Fisher Scientific. Crystallization screens were obtained from Hampton Research.
Cloning and Enzyme Purification
The tftG gene was cloned into the pET30-LIC vector and expressed as a C-terminal His6 fusion protein. 100 ml of Luria broth supplemented with 30 μg/ml kanamycin was inoculated with a freezer stock of pET30A TftG in BL21(DE3) cells and incubated overnight at 37 °C with constant shaking at 250 rpm. The 100-ml culture was then used to inoculate 1.5 liter of Luria broth medium. TftG expression was induced by addition of isopropyl β-d-thiogalactopyranoside to 0.3 mm final concentration at mid-log phase (A600 ∼ 0.6). Following induction, the cells were further incubated for 12 h at 22 °C with constant shaking at 250 rpm. The cells were then harvested by centrifugation (3,000 × g), after which the pellet was frozen to promote cell lysis. The pellet was thawed at room temperature and suspended in a minimal volume of lysis buffer (50 mm Tris, 300 mm NaCl, 20 mm imidazole, and 1 mm dithiothreitol, pH 8.0). The cell suspension was sonicated five times for 10 s each using a model 450 Sonifier® (Branson Ultrasonics), and the resulting lysate cleared by centrifugation (20,000 × g for 30 min).
Lysate was applied to a nickel-nitrilotriacetate column and washed with several column volumes of lysis buffer. Elution buffer consisted of lysis buffer supplemented with 250 mm imidazole. Eluted fractions containing TftG were combined, concentrated, and buffer-exchanged into 20 mm Tris (pH 8.5) with 1 mm DTT by ultrafiltration in an Amicon 8050 cell with a 5-kDa cut-off polyethersulfone membrane (Millipore), loaded onto a Mono QTM GL10/100 anion-exchange column (GE Healthcare), and eluted at 200 mm NaCl with a linear NaCl gradient of 0 to 2 m NaCl using a preparative HPLC (Akta Explorer, GE Healthcare). Fractions containing TftG were pooled, concentrated, and exchanged into 20 mm Tris (pH 7.5) with 1 mm DTT. Final homogeneity of purified TftG was estimated at >99%.
Selenium auxotroph B834 cells were also transformed with the same tftG pET30-LIC plasmid. Expression and purification of selenium-substituted TftG was carried out by the same protocol as native TftG.
Data Collection and Structure Determination
TftG at 50 mg/ml in 20 mm Tris, 1 mm DTT, pH 7.5, was crystallized at 4 °C using the hanging drop vapor-diffusion method. 1.5 μl of TftG was mixed with 1.5 μl of a reservoir solution containing 30% (w/v) polyethylene glycol 1500 and equilibrated against the same reservoir solution. This condition was used for both native TftG and selenium-substituted TftG crystals. Diffraction-quality crystals appeared after 3 weeks. Selenomethionyl-TftG diffraction data were collected on a Rigaku FR-E+ Superbright rotating anode dual wavelength x-ray source. Cryoprotectant consisted of 20% (v/v) glycerol in reservoir buffer. Initial data collection for selenium-single wavelength anomalous dispersion phasing was conducted using chromium radiation (2.29 Å) at 100 K and processed using d*TREK (9). Experimental single wavelength anomalous dispersion phasing was conducted using PHENIX AutoSol followed by one cycle of model building in PHENIX Autobuild (10), resulting in an electron density map in which 94 of 100 residues were correctly modeled. Higher resolution (1.75 Å) apo-form data were collected at the Advanced Light Source beamline 8.2.1. A binary complex crystal of TftG with 2,5-dihydroxybenzoquinone was formed by soaking an apo-form TftG crystal with 2,5-dihydroxybenzoquinone and followed by cryoprotection in 30% (w/v) polyethylene glycol 1500 supplemented with 20% glycerol. Binary complex diffraction data were also collected at the Advanced Light Source (BL8.2.1) and processed using HKL2000 (11). The atomic model provided by the selenomethionyl-TftG chromium data set was used for refinement with the Advanced Light Source data described above. The TftG-Apo structure had 100% of residues in Ramachandran favored regions. Selenomethionyl-TftG had 99.19% of residues in preferred regions and 0.84% in allowed regions with no outliers. The TftG·2,5-DHBQ binary complex structure had 97.94% of residues in favored regions, 1.03% in allowed, and 1.03% outliers. The coordinates for TftG-apo, selenomethionyl-TftG, and TftG·2,5-DHBQ binary complex have been deposited in the Protein Data Bank (PDB) under codes 4LBH, 4LBI, and 4LBP, respectively.
Multi-angle Light Scattering and Isothermal Titration Calorimetry (ITC)
The weight average molecular mass of TftG was measured by combined size exclusion chromatography and multi-angle laser light scattering as described previously (12). Briefly, 200 μg of TftG was loaded onto a BioSep-SEC-S 2000 column (Phenomenex) and eluted isocratically with a flow rate of 0.5 ml min−1. The eluate was passed through a tandem UV detector (Gilson), Optilab digital signal processing interferometric refractometer (Wyatt Technology), and a Dawn EOS laser light scattering detector (Wyatt Technology). Scattering data were analyzed using the Zimm fitting method with software (ASTRA) provided by the instrument manufacturer.
Isothermal titration calorimetric reactions were carried out in a VP-ITC instrument (MicroCal). The protein was prepared for ITC by extensive buffer exchanging into titration buffer (20 mm Tris, pH 8.5). The concentration of protein in the calorimetric reaction cell was diluted to 100 μm. All titrations were performed at 25 °C with a stirring speed of 300 rpm and 29 injections (10-μl each). Ligands were diluted into the same titration buffer and injected into the cell containing TftG solution, and the heats of binding were recorded. Ligands were also titrated against buffer to account for heats of dilution. Ligand concentrations were adjusted to obtain significant heats of binding. All samples were degassed prior to titration.
Atomic Absorption Spectroscopy
A standard curve of ZnCl2 (0–100 μm, 20 mm Tris, pH 7.5) was generated using a Shimadzu AA-6200 atomic absorption spectrophotometer at the atomic absorption wavelength of zinc (213.9 nm). TftG samples were measured at 20, 40, 60, and 80 μm protein concentration to detect any bound zinc.
Enzyme Assays of Mutant TftG
TftG mutants (R17A, H24A, S56A, D75N, and H96A) were generated using a QuikChange Lightning site directed mutagenesis kit (Stratagene) and transformed into XL-10 Gold ultra-competent cells. DNA sequencing was used to confirm mutation, and the resulting plasmids were transformed into BL21(DE3) expression cells. Purification of mutant TftG was the same as that of wild type. 5-CHQ was enzymatically produced by TftD as described previously (13). Briefly, purified TftD (1 mg/ml) and Escherichia coli flavin reductase (5 μg/ml) was incubated with 100 μm 2,4,5-TCP in the presence of 10 μm FAD, 2 mm NADH, and 2 mm ascorbate in 100 μl of 40 mm potassium phosphate buffer at 25 °C for 10 min. Flavin reductase utilized NADH to reduce FAD to FADH2, and TftD oxidized 2,4,5-TCP to 5-CHQ with O2 and FADH2 as the co-substrates. The complete conversion of 2,4,5-TCP to 5-CHQ was confirmed by HPLC analysis. Then, the reaction mixture was aliquoted to 20 μl each, and 1 μl of TftG was added and mixed. The reaction was incubated at 25 °C for 1 min, and 20 μl of 10% acetic acid in acetonitrile was added to stop the reaction. After centrifugation to remove protein precipitates, the supernatant was analyzed by HPLC for 5-CHQ consumption. Assays were run in triplicate, and specific activities were calculated for TftG and its mutants.
Circular Dichroism Spectroscopy
CD spectroscopy was utilized to investigate the effect of mutations on secondary structure. CD spectra for each wild-type and mutant TftG protein was measured from 200 to 300 nm wavelength using an AVIV 202SF spectropolarimeter (AVIV Biomedical) at 25 °C. The TftG samples were prepared in 5 mm potassium phosphate (pH 7.0) at a protein concentration of 10 μm.
Quantum Mechanics/Molecular Mechanics Calculations
Unless otherwise stated, all calculations were performed in the gas phase using Gaussian 09 (G09) (14) and utilized double-ζ correlation consistent basis sets (15) with augmented functions on all atoms but hydrogen (hereafter referred to as aVDZ). ONIOM (16) calculations were assisted by the Toolkit to Assist ONIOM (17) The residues allowed to move during optimizations were Ile-5, Arg-7, Asp-9, Arg-17, Ile-18, Tyr-21–Leu-25, Lys-32, Ile-35, Gly-38–Pro-40, Met-53, Gly-55–Leu-58, Phe-71, Val-72, Asp-75–Phe-77, Leu-82, Phe-83, Gly-94–Asp-98; all but the carbon of an additional C-terminal N-methyl cap were allowed to move. 5-Chloro-2-hydroxyhydroquinone was generated in GaussView (version 3.09) (18) and optimized with tight convergence criteria, followed by a frequency calculation, at the B3LYP (19–21)/aVDZ level of theory. Ligand harmonic stretch and bend AMBER molecular mechanics (MM) force field (22) parameters were generated from these frequencies using the parafreq utility (23); chlorine non-bonded parameters were taken from the parmpol12 parameter set as found in AmberTools 13 (22), and dihedrals/improper torsions involving chlorine were assigned by analogy with the hydroxyl OH atom type. The geometry-optimized ligand was inserted into the N-methyl-capped TftG product·analog complex crystal structure with Lys-95 converted to Ala, and hydrogen atoms were added using the PHENIX ReadySet! program (10), with His-24 as Nδ-His and His-96 doubly protonated; all other histidines were kept singly protonated. This model was converted into a dimer via symmetry operations, followed by optimization of its key residues (listed above), the ligand, and all hydrogen atoms using PM7 semi-empirical theory (25) with the MOZYME linear-scaling method (26) as implemented in MOPAC2012 (27). Restrained electrostatic potential charges (28) were then fitted to the MOZYME-optimized Arg-7/His-24/Asp-75 and Asp-9/Arg-17/Ser-56 trios via Merz-Kollman-Singh (29) electrostatic potential fitting (overlay 6/33 = 2) at the CAM-B3LYP (30)/aVDZ level of theory, generating the ONIOM input structure. Hydrogen atom geometries of the input structure were first reoptimized using a two-layer ONIOM scheme with PM6 semi-empirical theory (31) for the model system (defined as the ligand and the side chains of Arg-7, Arg-17, Asp-9, Asp-75, Asp-96, His-24, His-96, Ser-56, and Tyr-21) and the AMBER MM force field for the real system. Following this, the ligand and key residues were optimized using a three-layer ONIOM scheme with CAM-B3LYP/aVDZ for the model system, PM6 for the intermediate system, and the AMBER force field for the real system. Residues included in the model and intermediate systems, with their approximations shown as (model/intermediate), were Arg-7 and Arg-17 (N-methylguanidinium/N-propylguanidinium); Asp-9 and Asp-75 (acetate/propanoate); Tyr-21 (phenol/4-ethylphenol); His-24 (1H-imidazole/4-propyl-1H-imidazole); Ser-56 (ethanol/ethanol); the Pro-76/Phe-77 peptide bond (N-methylacetamide, intermediate layer only); and the C-terminal tail (His-96 as imidazolium and Asp-98 as acetate/His-93 as Ala plus Gly-94 through the N-methyl cap in its entirety).
RESULTS
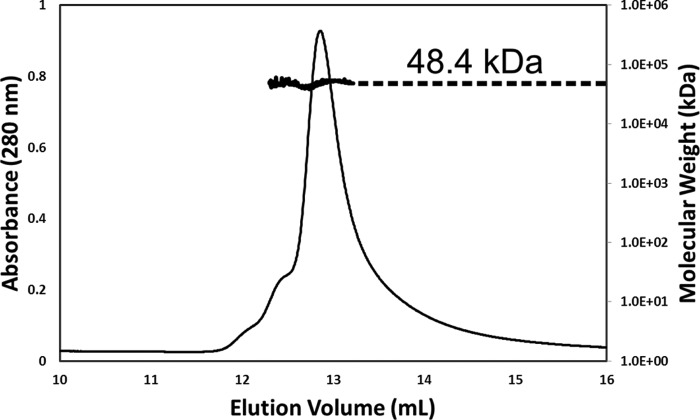
The apo-form TftG crystallized in a hexagonal space group with one molecule in the asymmetric unit (Table 1). A crystallographic symmetry operation of monomeric TftG generated a tetramer, which was consistent with the tetrameric nature of TftG in solution (Fig. 2). To obtain a binary structure of TftG, a product analog, 2,5-DHBQ, was diffused into an apo-form crystal by a soaking approach.
TABLE 1.
Data collection and refinement statistics
Values in parentheses are for the highest-resolution shell.
| TftG-SeMET | TftG-Apo | TftG binary complex | |
|---|---|---|---|
| Data collection | |||
| Space group | C2221 | P622 | P622 |
| Cell dimensions | |||
| a, b, c (Å) | 135.24, 158.01, 50.39 | 90.32, 90.32, 51.26 | 90.20, 90.20, 50.92 |
| α, β, γ | 90.00, 90.00, and 90.00° | 90.00, 90.00, and 120.00° | 90.00, 90.00, and 120.00° |
| Resolution (Å) | 23.61–2.21 | 50.00–1.75 | 50.00–1.87 |
| Rsym | 0.040 (0.193) | 0.077 (0.266) | 0.089 (0.226) |
| I/σI | 28.7 (6.3) | 53.96 (12.08) | 66.22 (20.65) |
| Completeness (%) | 77.3 (13.5) | 99.8 (96.1) | 99.8 (99.6) |
| Redundancy | 6.23 (3.30) | 20.6 (17.3) | 19.7 (15.9) |
| Refinement | |||
| Resolution (Å) | 23.61–2.21 | 45.17–1.75 | 45.09–1.87 |
| No. reflections | 21,232 | 12826 | 10,571 |
| Rwork/Rfree | 0.1704/0.2070 | 0.1859/0.2165 | 0.1887/0.2194 |
| No. of atoms | |||
| Protein | 2992 | 748 | 783 |
| Ligand/ion | 0 | 7 | 10 |
| Water | 272 | 79 | 78 |
| B-factors | |||
| Protein | 29.60 | 27.30 | 27.20 |
| Ligand/ion | N/A | 44.30 | 28.40 |
| Water | 32.90 | 35.50 | 33.30 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.007 | 0.008 | 0.016 |
| Bond angles | 1.05° | 1.08° | 1.20° |
FIGURE 2.
Oligomeric nature of TftG in solution. Elution profile for TftG protein (2 mg ml−1) was monitored with multi-angle laser light scattering and was shown as absorbance (left-Y axis) and molecular weight (right-Y axis) versus elution volume (ml). The solid line represents changes in absorption at 280 nm. The thick black cluster in the middle of the peaks indicated the calculated molecular mass, which was extended to the right y axis for ease of interpretation. The average molecular weight for the TftG peak was indicated.
Global Structure
Apo-form TftG resembled a ferredoxin-like core fold. Each subunit consisted of four β-strands (β1–β4) arranged in an antiparallel β-sheet that was flanked by three opposing α-helices (α1, α3, and α4) (Fig. 3a). The tetrameric assembly of TftG establishes through the interaction of two dimeric α-β-barrels (Fig. 3, b and c). Hydrogen bonding between the β4 strands (residues 84–88) from two different dimeric α-β-barrels forms a continuous eight-stranded anti-parallel β-sheet. There are also several electrostatic interactions such as Lys-8-Glu-89 and Arg-90 with the carbonyl of Gly-47, which stabilize the tetramer.
FIGURE 3.

Ribbon diagram representing the crystal structure of TftG. a, TftG monomer. Secondary structural elements have been numbered sequentially as α1–α4 and β1–β4. N and C refer to the N- and C-terminal regions, respectively. α-Helices were shown in red, and β-strands were shown in green. The product analog 2,5-dihydroxybenzoquinone in the active site is illustrated in orange. b and c, TftG tetramer. Two orientations illustrating arrangement of the TftG tetramer and binding sites with 2,5-dihydroxybenzoquinone represented as a molecular surface. This figure was generated by open source PyMOL (version 1.1r1).
A structural alignment between the apo-form and binary complex with 2,5-DHBQ illustrated no large structural deviations (root mean square deviation, 0.193 Å). One noticeable structural difference was the improved electron density corresponding to residues 95–98 in the binary complex structure due to its hydrogen bonding with the substrate.
ITC
ITC was employed to find any suitable substrate analog for obtaining binary complex crystals. Tested ligands included hydroquinone, 5-chlorohydroquinone, catechol, and 2,5-DHBQ. The only compound with significant affinity was 2,5-dihydroxybenzoquinone (Fig. 4) with Kd of 11.4 μm, which led us to pursue a binary complex. The association of 2,5-DHBQ with TftG had a considerable enthalpic contribution, ΔH = −6.5 kcal mol−1, likely due to the observed hydrogen bonds between ligand and residues of TftG, which was later confirmed in the binary crystal structure. In addition, the positive entropic contribution, ΔS = 0.79 cal mol−1 degree−1 was observed, suggesting the liberation of water molecules from the active site upon ligand association.
FIGURE 4.

Measurement of substrate/substrate analog binding through ITC experiments. The trend of heat released by serial injections of substrates into TftG was monitored. 2,5-Dihydroxybenzoquinone (diamond) showed a typical heat-releasing pattern. The solid line represents the least square fit of the data. Hydroquinone (square), 5-chlorohydroquinone (circle), and catechol (triangle) did not show any significant affinity to TftG.
Active Site
As indicated in Figs. 3 and Fig. 5a, a distinct solvent accessible pocket was noticed between β2-β3 and α1 in apo-form TftG. Coincidentally, the Fo − Fc maps calculated from the 2,5-DHBQ-soaked crystal diffraction data showed clear electron density for 2,5-DHBQ in this pocket. The polar residues comprising the pocket are Arg-17, His-24, Ser-56, Asp-75, His-96, and Asp-98. The C-terminal residues of the adjacent subunit, His-96 and Asp-98, close off the active site trapping the ligand inside (Fig. 5b).
FIGURE 5.

TftG active site and ligand binding. a, molecular surface of TftG-Apo tetramer. The apo-form structure illustrated a distinct solvent accessible pocket (shown in the black squares), which functions as the active site in TftG. This figure was generated by open source PyMOL (version 1.1r1). b, active site of TftG·2,5-dihydroxybenzoquinone binary complex. Shown is a 2Fo − Fc map covering substrate and hydrogen bonded residues at a contour level of 1.0σ. c, computational results for position of 5-CHQ. Shown is an overlay of the crystallographic and three-layer ONIOM-optimized active site catalytic residues and substrate. Carbons are shown in gray for the crystallographic structure with bound 2,5-DHBQ and light blue for the ONIOM-optimized protein and 5-CHQ sites. Catalytic residue-substrate hydrogen bond distances are shown.
His-24 and Asp-75 were proximate to each other, establishing a catalytic dyad, such that the Asp-75 Oδ1 was hydrogen bonded to the His-24 Nδ1. The His-24 Nϵ2 atom also established a hydrogen bond with the 2-hydroxyl group of 2,5-DHBQ. In addition, the side chains of Arg-17 and Ser-56 were both within hydrogen bonding distance to each other and to 2,5-DHBQ at its 1-carbonyl oxygen. The His-96 Nϵ2 atom was within hydrogen bonding distance of either the 4-carbonyl oxygen or the 5-hydroxyl group of 2,5-DHBQ. Asp-98 was within hydrogen bonding distance of the 4-carbonyl oxygen of 2,5-DHBQ (Fig. 5b). A hydrogen bond network continued away from the His-24-Asp-75 and Arg-17-Ser-56 dyads, including a salt bridge between the side chains of Arg-7 and Asp-75, and another between the side chains of Asp-9 and Arg-17.
Quantum Mechanics/Molecular Mechanics (QM:MM) Substrate Modeling
Quantum mechanics/molecular mechanics was employed to investigate placement of the native substrate 5-CHQ and surrounding catalytic residues. The r.m.s.d between the crystal structure and three-layer ONIOM-optimized structures was 0.3 Å. The orientations of the key catalytic residues and other residues forming the active site pocket showed little change, with the major changes being due to movement of the C-terminal tail. The position of 5-CHQ had similar orientation to the crystallographic position of the product analog 2,5-DHBQ with the chlorine atom facing out of the binding pocket toward the C-terminal tail (Fig. 5c).
Activity Assays and CD Spectra of TftG Mutants
Activity assays of R17A, H24A, S56A, and H96A site-directed mutants were performed to test their potential catalytic roles (Table 2). The mutant D75N could not be tested due to solubility issues. The H24A mutant exhibited the least amount of activity, which was <1% specific activity relative to wild type. R17A and H96A also displayed quite low activity (∼1%), and S56A retained 5% activity.
TABLE 2.
Specific activity of TftG active site mutations
| Enzyme | Specific activity | % Specific activity (relative to WT) |
|---|---|---|
| μmol min−1 mg−1 | ||
| WT | 66.877 ± 0.993 | 100 |
| R17A | 1.102 ± 0.057 | 1.6% |
| H24A | 0.420 ± 0.046 | <1.0% |
| S56A | 3.422 ± 0.330 | 5.1% |
| H96A | 0.938 ± 0.068 | 1.4% |
The overall secondary structure as evaluated by CD spectra appeared unperturbed relative to wild-type for all mutants except H24A mutant (Fig. 6). The crystal structure illustrated the residue His-24 was located in α1 and Asp-75 was located at the C-terminal end of α3. The side chains of those residues, contributed from two different helices, were within hydrogen bond distance and thus appeared to be important for structural integrity of the active site pocket. Considering the location of the functional imidazole ring of His-24 and its mutant CD data, it is likely that His-24 could be important for both structural and catalytic roles.
FIGURE 6.

CD spectra for TftG wild-type and mutants. The CD spectra were recorded from 200 to 300 nm for wild-type (diamonds), R17A (triangles), H24A (filled circles), S56A (empty circles) and H96A (squares) using an AVIV 202SF spectropolarimeter (AVIV Biomedical) at 25 °C at a concentration of 10 μm.
DISCUSSION
Structural Homologs of TftG
TftG is a recently discovered dehydrochlorinase enzyme that catalyzes a cofactor-independent dehydrochlorination of 5-CHQ to produce 2-hydroxy-1,4-benzoquinone. There has been a critical gap in structural and mechanistic information regarding the dechlorination of chlorocatechols such as 5-CHQ, hampering an overall understanding of their catabolic mechanism.
To firmly establish a classification of TftG and to find any structural homologs, a DALI (32) search was performed against deposited structures in the PDB. The search found several proteins with similar structures but relatively low sequence identity to TftG. All of the matches had promiscuous or unknown functional roles. The highest match was the HI0828 protein of unknown function from Haemophilus influenza (PDB code 1MWQ) (33), with a Z-score of 14.3 and 27% sequence identity. The second highest match was 5-chloromuconolactone dehalogenase of Rhodococcus opacus 1Cp (PDB code 3ZNU) (34), with a Z-score of 10 but displayed lower sequence identity (23%). Another protein of unknown function, TM1266 from Thermotoga maritima (PDB code 2NZC), shared reasonable structural similarity with a Z-score of 8.5 and a sequence identity of 11%. All of the following proteins in the DALI search list had substantially decreased Z-scores and sequence identity, which included a bacterial actinorhodin biosynthesis monooxygenase (ActVa-Orf6) (35), bacterial muconalactone isomerase (36) and the C-terminal domain of archaeal LprA (37).
Detailed structural investigation of TftG with a manual superposition of HI0828 protein (PDB code 1MWQ) illustrated a similar active site pocket as shown in TftG with the exception of Arg at position 21 instead of Tyr. However, HI0828 has a zinc ion at the active site, and a phosphohistidine has been proposed to be involved in activity. Atomic absorption spectroscopy was conducted to test for the presence of zinc ions in TftG; however, no zinc ions were indicated at all tested concentrations of TftG. The lack of any zinc ion in the crystal structure of TftG and supporting atomic absorption evidence shows that zinc is not involved in TftG catalysis. It is possible that the presence of zinc ion in the HI0828 active site is an artifact from the crystallization condition and HI0828 could be a dehalogenase. However, the HI0828 quaternary structure is a dimer, whereas the TftG quaternary structure is a tetramer (Fig. 7a). Thus, our speculation about HI0828 from Haemophilus influenza needs to be tested.
FIGURE 7.

Monomeric and oligomeric state of known dehydrochlorinases. a, TftG; b, 5-chloromuconolactone dehalogenase of R. opacus 1Cp (PDB code 3ZNU); and c, LinA from Sphingobium japonicum (PDB code 3A76). This figure was generated by open source PyMOL (version 1.1r1).
The next match in our DALI search, 5-chloromuconolactone dehalogenase (PDB code 3ZNU) belongs to the muconolactone δ-isomerase (pfam02426) superfamily. Members of this protein family (EC 5.3.3.4) are involved in the metabolism of catechols. 5-Chloromuconolactone dehalogenase has a ferredoxin-like fold similar to TftG, but it does not have a conserved His-Asp dyad distancing itself from TftG and YCII superfamily. Instead, it has a conserved Glu-27 acting as a catalytic base. The oligomeric structure of 5-chloromuconolactone isomerase was also quite different, existing as a decamer (Fig. 7b).
Although it was not listed in our DALI search due to low level of structural similarity with TftG, LinA from Sphingobium japonicum catalyzes a dechlorination reaction for γ-hexachlorocyclohexane (38, 39). LinA belongs to the SnoaL-4 superfamily (cl17707), which are all polyketide cyclases that share the SnoaL fold. LinA exists as a homotrimer, and each subunit forms a cone-shaped α+β-barrel fold. Although LinA and TftG share little similarity (18% sequence similarity and 4.6 Å root mean square deviation in their Cα positions), manual Cα chain alignment of LinA and TftG displayed an approximately similar location of the active site. In addition, the exceedingly hydrophobic substrate-binding pocket of LinA contains the same proposed catalytic dyad of His-73 and Asp-25. The carboxyl group of Asp-25 of LinA has been proposed to abstract a proton from imidazole side chain of His-73, thereby increasing the basicity of the imidazole nitrogen (38). The imidazole nitrogen then acts as a catalytic base, abstracting a proton from the carbon of γ-hexachlorocyclohexane ring, ultimately leading to a spontaneous loss of chlorine from the ring (38). The oligomeric structure of LinA was a trimer, further setting it apart from TftG and 5-chloromuconolactone isomerase (Fig. 7c).
LinA, TftG, and 5-chloromuconolactone dehalogenase belong to different enzyme superfamilies and use different substrates. TftG catalyzes proton abstraction from an oxygen atom of an aromatic compound, whereas 5-chloromuconolactone dehydrohalogenase and LinA catalyze proton abstraction from a carbon atom of non-aromatic substrates.
Sequence Alignment with Other YCII Family Members
To further understand the relation of TftG to other members of the YCII superfamily, we conducted a BLAST (24) search of all non-redundant protein sequences. The results revealed many YCII-like proteins, some with high identity (40–45%), but all were of unknown function. Inspection of a sequence alignment of the top 100 proteins illustrated a conserved signature sequence of residues that were both implicated in TftG catalysis and conserved throughout the YCII superfamily. An alignment of the top 20 highest sequence identity proteins illustrates this signature sequence (Fig. 8). As expected, the His-24-Asp-75 dyad was conserved throughout the family. In addition, Pro-76, which is responsible for stabilizing and orienting the catalytic dyad in TftG, was conserved. Another set of residues conserved across the YCII superfamily were Asp-9, Arg-17, and Ser-56. Both Arg-17 and Ser-56 were responsible for forming an oxyanion hole critical for catalysis in TftG. Asp-9 in TftG electrostatically interacts with Arg-17, ultimately keeping Arg-17 in the proper orientation for oxyanion charge stabilization on the substrate. In summary, Asp-9, Arg-17, His-24, Ser-56, Asp-75, and Pro-76 are conserved throughout the YCII superfamily. Expansion of the BLAST search/sequence alignment to include dozens of lower identity proteins from YCII superfamily still maintains those residues, and thus this conservation of Asp-9, Arg-17, His-24, Ser-56, Asp-75, and Pro-76 is likely quite important for functions throughout the YCII superfamily and could serve as its signature.
FIGURE 8.

Sequence alignment of TftG BLAST search results. The top 20 sequences were aligned with TftG, illustrating several conserved residues (shown in boldface type) throughout the YCII superfamily. Residues that were conserved and implicated in TftG catalysis were highlighted yellow. M. magneticum, Magnetospirillum magneticum; M. magnetotacticum, Magnetosprillum magnetotacticum; M. gryphiswaldense, Magnetospirillum gryphiswaldense; R. rubrum, Rhodospirillum rubrum; P. gallaeciensis, Phaeobacter gallaeciensis; R. bacterium, Rhodobacteraceae bacterium; R. sphaeroides, Rhodobacter sphaeroides; R. capsulatus, Rhodobacter capsulatus; S. meliloti, Sinorhizobium meliloti; S. fredii, Sinorhizobium fredii; L. aggregata, Labrenzia aggregata; A. brasilense, Azospirillum brasilense.
Catalytic Mechanism of TftG for 5-CHQ
The crystal structure of the 2,5-DHBQ binary complex of TftG in combination with the QM:MM results for 5-CHQ, and the site-directed mutagenesis illustrated a plausible catalytic mechanism for TftG. The resting state of TftG likely contains water in the active site as supported by our ITC results and the presence of PEG in its apo-form crystal structure. The QM:MM results confirmed that 5-CHQ would bind in a near identical angular orientation to 2,5-DHBQ as expected from the relatively tight fit of the TftG active site. The computational results also illustrated the 1- and 2-hydroxyl groups of 5-CHQ oriented toward His-24 and the Arg-17/Ser-56, respectively. Upon association of 5-CHQ, all water molecules are liberated from the active site, and there is a concomitant closure of the active site by the C-terminal residues of the neighboring subunit. The His-24-Asp-75 catalytic dyad is responsible for starting the reaction through general base catalysis. The imidazole nitrogen of His-24 abstracts a proton from the 2-hydroxyl group of 5-CHQ, leading to intramolecular hydrogen bonding between the 2-oxygen and 1-hydroxyl group, stabilizing the resulting oxyanion at the 1-position, which is further stabilized by an existing oxyanion hole formed by Arg-17 and Ser-56 (Fig. 9, I). The other two mutants, R17A and S56A, displayed 1.6 and 5.1% specific activity, respectively. The higher specific activity of S56A relative to R17A is probably due to the fact that Arg-17 plays a more dominant role in stabilizing the oxyanion at the 1-position of 5-CHQ.
FIGURE 9.
Proposed mechanism for dechlorination of 5-CHQ. The resting state of the enzyme contains water in the active site of TftG. I, upon 5-CHQ association, water is liberated from the active site, and the C-terminal arm closes. The catalytic dyad His-24-Asp-75 begins the reaction through general base catalysis as the imidazole nitrogen of His-24 abstracts a proton from the 2-hydroxyl group of 5-CHQ, leading to intramolecular hydrogen bonding between the 2-oxygen and 1-hydroxyl group and formation of an oxyanion at the 1-position, which is stabilized by an oxyanion hole formed by Ser-56 and Arg-17. II, ring protonation occurs at the 5-chloro position of 5-CHQ by the general acid His-96 followed by deprotonation of the 4-hydroxyl group of 5-CHQ. III, electrons from the 1-position oxyanion move causing the spontaneous loss of chloride. IV, the chloride ion leaves following a proton transfer from Asp-98, the C-terminal arm opens, and the product 2-hydroxy-1,4-benzoquinone is released with subsequent active site regeneration.
Ring protonation occurs at the 5-chloro position of 5-CHQ by the general acid role of His-96, being followed by deprotonation of the 4-hydroxyl group of 5-CHQ (Fig. 9, II). This was consistent with the specific activity of H96A (1.4%). The QM:MM result also illustrated the 5-chloro position of 5-CHQ oriented toward His-96. The resulting movement of electrons from the 1-oxyanion causes a spontaneous loss of chloride ion (Fig. 9, III). Opening of the C-terminal arm then allows for release of the product 2-hydroxy-1,4-benzoquinone. The chloride ion leaves as HCl following a proton transfer from Asp-98 and the active site of TftG is regenerated (Fig. 9, IV).
Summary
TftG is a novel dehydrochlorinase enzyme. The conserved His-Asp dyad proves to be catalytic performing a unique dehydrochlorination reaction, together with the neighboring polar residues. The active site residues implicated in TftG catalysis form a signature sequence conserved throughout the YCII superfamily. Considering the unique topology and conservation of active site residues in TftG, it is very likely that the proteins in YCII superfamily conduct lyase reactions (e.g. dehydrochlorination) for hydroquinones or related structural analogs.
Acknowledgments
We thank B. Webb and M. Nissen for help with initial crystallization trials and preparation of selenomethionyl-derivatized TftG.
This work was supported by the National Science Foundation (MCB 1021148 and DBI 0959778) and the M. J. Murdock Charitable Trust.
The atomic coordinates and structure factors (codes 4LBH, 4LBI, and 4LBP) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- 2,4,5-TCP
- 2,4,5-trichlorophenol
- 2,4,5-T
- 2,4,5-trichlorophenoxyacetic acid
- 5-CHQ
- 5-chloro-2-hydroxyhydroquinone
- HBQ
- hydroxybenzoquinone
- 2,5-DHBQ
- 2,5-dihydroxybenzoquinone
- ITC
- isothermal titration calorimetry
- MM
- molecular mechanics
- QM
- quantum mechanics
- PDB
- Protein Data Bank.
REFERENCES
- 1. U. S. Environmental Protection Agency (1999) Integrated risk information system (IRIS) on 2,4,6-trichlorophenol. National Center for Environmental Assessment, Office of Research and Development, U. S. Environmental Protection Agency, Washington, D. C [Google Scholar]
- 2. Czaplicka M. (2004) Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 322, 21–39 [DOI] [PubMed] [Google Scholar]
- 3. Kintz P., Tracqui A., Mangin P. (1992) Accidental death caused by the absorption of 2,4-dichlorophenol through the skin. Arch. Toxicol. 66, 298–299 [DOI] [PubMed] [Google Scholar]
- 4. Zaborina O., Daubaras D. L., Zago A., Xun L., Saido K., Klem T., Nikolic D., Chakrabarty A. M. (1998) Novel pathway for conversion of chlorohydroxyquinol to maleylacetate in Burkholderia cepacia AC1100. J. Bacteriol. 180, 4667–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daubaras D. L., Danganan C. E., Hubner A., Ye R. W., Hendrickson W., Chakrabarty A. M. (1996) Biodegradation of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia strain AC1100: evolutionary insight. Gene 179, 1–8 [DOI] [PubMed] [Google Scholar]
- 6. Sangodkar U. M., Chapman P. J., Chakrabarty A. M. (1988) Cloning, physical mapping and expression of chromosomal genes specifying degradation of the herbicide 2,4,5-T by Pseudomonas cepacia AC1100. Gene 71, 267–277 [DOI] [PubMed] [Google Scholar]
- 7. Xun L., Wagnon K. B. (1995) Purification and properties of component B of 2,4,5-trichlorophenoxyacetate oxygenase from Pseudomonas cepacia AC1100. Appl. Environ. Microbiol. 61, 3499–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Webb B. N., Ballinger J. W., Kim E., Belchik S. M., Lam K. S., Youn B., Nissen M. S., Xun L., Kang C. (2010) Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:FAD oxidoreductase (TftC) of Burkholderia cepacia AC1100. J. Biol. Chem. 285, 2014–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pflugrath J. (1999) The finer things in x-ray diffraction data collection. Acta Crystallogr. D Biol. Crystallogr. 55, 1718–1725 [DOI] [PubMed] [Google Scholar]
- 10. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Otwinowski Z., Minor W. (1997) Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 12. Youn B., Moinuddin S. G., Davin L. B., Lewis N. G., Kang C. (2005) Crystal structures of apo-form and binary/ternary complexes of Podophyllum secoisolariciresinol dehydrogenase, an enzyme involved in formation of health-protecting and plant defense lignans. J. Biol. Chem. 280, 12917–12926 [DOI] [PubMed] [Google Scholar]
- 13. Gisi M. R., Xun L. (2003) Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Bacteriol. 185, 2786–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J., J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J. (2009) Gaussian 09, Revision C. 01, Gaussian, Inc., Wallingford, CT [Google Scholar]
- 15. Peterson K. A., Dunning T. H. (2002) Accurate correlation consistent basis sets for molecular core-valence correlation effects: the second row atoms Al-Ar, and the first row atoms B-Ne Revisited. J. Chem. Phys. 117, 10548–10560 [Google Scholar]
- 16. Dapprich S., Komáromi I., Byun K. S., Morokuma K., Frisch M. J. (1999) A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. 461–462, 1–21 [Google Scholar]
- 17. Tao P., Schlegel H. B. (2010) A toolkit to assist ONIOM calculations. J. Comput. Chem. 31, 2363–2369 [DOI] [PubMed] [Google Scholar]
- 18. Frisch M. (2004) GaussView, Version 3, Guassian, Inc.,Wallingford, CT [Google Scholar]
- 19. Becke A. D. (1993) Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648 [Google Scholar]
- 20. Lee C., Yang W., Parr R. G. (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 37, 785. [DOI] [PubMed] [Google Scholar]
- 21. Miehlich B., Savin A., Stoll H., Preuss H. (1989) Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chemical Physics Letters 157, 200–206 [Google Scholar]
- 22. Case D. A., Darden T. A., Cheatham I., T.E., Simmerling C. L., Wang J., Duke R. E., Luo R., Walker R. C., Zhang W., Merz K. M., Roberts B., Hayk S., Roitberg A., Seabra G., Swails J., Goetz A. W., Kolossvai I., Wong K. F., Paesani F., Vanicek J., Wolf R. M., Liu J., X., W., Brozell S. R., Steinbrecher T., Gohlke H., Cai Q., Ye X., Wang J., Hsieh M.-J., Cui G., Roe D. R., Mathews D. H., Seetin M. G., Salomon-Ferrer R., Sagui C., Babin V., Luchko T., Gusarov S., Kovalenko A., Kollman P. A. (2012) AMBER 12, University of California, San Francisco, CA [Google Scholar]
- 23. Burger S. K., Lacasse M., Verstraelen T., Drewry J., Gunning P., Ayers P. W. (2012) Automated Parametrization of AMBER Force Field Terms from Vibrational Analysis with a Focus on Functionalizing Dinuclear Zinc(II) Scaffolds. J. Chem. Theor. Comput. 8, 554–562 [DOI] [PubMed] [Google Scholar]
- 24. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stewart J. J. (2013) Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 19, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart J. J. (2009) Application of the PM6 method to modeling proteins. J. Mol. Model. 15, 765–805 [DOI] [PubMed] [Google Scholar]
- 27. Stewart J. J. (2012) MOPAC2012, Stewart Computational Chemistry, Colorado Springs, CO [Google Scholar]
- 28. Bayly C. I., Cieplak P., Cornell W., Kollman P. A. (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 [Google Scholar]
- 29. Besler B. H., Merz K. M., Kollman P. A. (1990) Atomic charges derived from semiempirical methods. J. Comput. Chem. 11, 431–439 [Google Scholar]
- 30. Yanai T., Tew D. P., Handy N. C. (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chemical Physics Letters 393, 51–57 [Google Scholar]
- 31. Stewart J. J. (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. Journal of Molecular modeling 13, 1173–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holm L., Sander C. (1993) Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 [DOI] [PubMed] [Google Scholar]
- 33. Willis M. A., Song F., Zhuang Z., Krajewski W., Chalamasetty V. R., Reddy P., Howard A., Dunaway-Mariano D., Herzberg O. (2005) Structure of YciI from Haemophilus influenzae (HI0828) reveals a ferredoxin-like α/β-fold with a histidine/aspartate centered catalytic site. Proteins 59, 648–652 [DOI] [PubMed] [Google Scholar]
- 34. Roth C., Gröning J. A., Kaschabek S. R., Schlömann M., Sträter N. (2013) Crystal structure and catalytic mechanism of chloromuconolactone dehalogenase ClcF from Rhodococcus opacus 1CP. Mol. Microbiol. 88, 254–267 [DOI] [PubMed] [Google Scholar]
- 35. Sciara G., Kendrew S. G., Miele A. E., Marsh N. G., Federici L., Malatesta F., Schimperna G., Savino C., Vallone B. (2003) The structure of ActVA-Orf6, a novel type of monooxygenase involved in actinorhodin biosynthesis. EMBO J. 22, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katti S. K., Katz B. A., Wyckoff H. W. (1989) Crystal structure of muconolactone isomerase at 3.3 A resolution. J. Mol. Biol. 205, 557–571 [DOI] [PubMed] [Google Scholar]
- 37. Leonard P. M., Smits S. H., Sedelnikova S. E., Brinkman A. B., de Vos W. M., van der Oost J., Rice D. W., Rafferty J. B. (2001) Crystal structure of the Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus. EMBO J. 20, 990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okai M., Kubota K., Fukuda M., Nagata Y., Nagata K., Tanokura M. (2010) Crystal structure of γ-hexachlorocyclohexane dehydrochlorinase LinA from Sphingobium japonicum UT26. J. Mol. Biol. 403, 260–269 [DOI] [PubMed] [Google Scholar]
- 39. Nagata Y., Mori K., Takagi M., Murzin A. G., Damborský J. (2001) Identification of protein fold and catalytic residues of γ-hexachlorocyclohexane dehydrochlorinase LinA. Proteins 45, 471–477 [DOI] [PubMed] [Google Scholar]