Background: Neurokinin-1 receptor (NK1R) activation down-regulates norepinephrine transporter (NET).
Results: NET, NK1R, and protein kinase Cα exist in a physical complex and membrane microdomain-specific NET/NK1R/PKCα interaction is coupled to NK1R activation.
Conclusion: NET subcellular localization and regulation requires assembly of a much larger macromolecular complex.
Significance: How receptor/transporter interaction determines NET subcellular localization is crucial for transport regulation by neurokinin-1.
Keywords: Catecholamines, G Protein-coupled Receptors (GPCR), Monoamine Transporters, Plasma Membrane, Protein-protein Interactions, Internalization, Lipid Rafts, Regulation
Abstract
Neurokinin-1 receptor (NK1R) mediates down-regulation of human norepinephrine (NE) transporter (hNET) via protein kinase C (PKC). However, native NET regulation by NK1R and the mechanism by which NK1R targets NET among other potential effectors are unknown. Effect of NK1R activation on native NET regulation and NET/NK1R interaction were studied using rat brain synaptosomes expressing native NET and NK1R as well as human placental trophoblast (HTR) cells coexpressing WT-hNET or NK1R/PKC-resistant hNET-T258A,S259A double mutant (NET-DM) and hNK1R. The selective NK1R agonist, GR73632, and Substance-P (SP) inhibited NE transport and reduced plasma membrane expression of NET and NK1R. Pretreatment with the NK1R antagonist, EMEND (aprepitant) prevented these NK1R-mediated effects. Immunoprecipitation experiments showed that NET forms stable complexes with NK1R. In HTR cells, combined biotinylation and immunoprecipitation studies revealed plasma membrane localization of NET·NK1R complexes. Receptor activation resulted in the internalization of NET·NK1R complexes. Lipid raft and immunoprecipitation analyses revealed the presence of NET·NK1R complexes exclusively in non-raft membrane fractions under basal/unstimulated conditions. However, NK1R activation led to translocation of NET·NK1R complexes to raft-rich membrane fractions. Importantly, PKCα was found in association with raft-localized NET following SP treatment. Similar to WT-NET, PKC-resistant NET-DM was found in association with NK1R exclusively in non-raft fractions. However, SP treatment failed to translocate NET-DM·NK1R complexes from non-raft fractions to raft fractions. Collectively, these results suggest that NK1R forms physical complexes with NET and that the receptor-mediated Thr258 + Ser259 motif-dependent translocation of NET·NK1R complexes into raft-rich microdomains facilitates NET/NK1R interaction with PKCα to coordinate spatially restricted NET regulation.
Introduction
The norepinephrine (NE)3 transporter (NET) expressed on noradrenergic nerve terminals controls NE signaling by rapid clearance of the catecholamine (1–3). It is known that NET interacts with presynaptic signaling molecules such as syntaxin 1A (SYN1A), protein phosphatase 2A catalytic subunit (PP2Ac), PDZ domain-containing protein interacting with protein kinase C1 (PICK1), α-synculein, and focal adhesion protein Hic5 (reviewed recently in Ref. 4). Some of these NET interacting proteins modulate PKC-mediated NET regulation (5–8). Signals linked to G protein-coupled receptor (GPCR) activation are also known to regulate NET (9, 10). Studies have shown evidence for receptor activation regulating transporter and transporter modulation regulating receptor function (4, 11–13). These studies indicate a close interaction between GPCR and transporter proteins.
Our previous studies have demonstrated that NET in localization in lipid rafts and PKC activation either directly by phorbol ester or via SP activation of NK1R stimulates NET internalization (10, 14). We have identified a trafficking motif involved in NK1R/PKC-mediated NET down-regulation and established a close relationship between transporter phosphorylation and internalization (10, 14). Many GPCRs including NK1R mediate PKC signaling, and are known to redistribute signaling partner proteins when activated. NK1R is localized in lipid rafts (15, 16) and activation of NK1R stimulates translocation of PKC into lipid rafts, where it interacts with the receptor and causes its desensitization (15). This raises the interesting possibility that NET localized in the rafts may be found in association with NK1R. In addition, our studies also point to the importance of the Thr258/Ser259 motif in NK1R/PKC-mediated NET down-regulation (10). Here we addressed four important questions concerning NET regulation by NK1R in native and heterologous systems. (i) Does NK1R mediate NET regulation in native tissues? (ii) Does NET exist in association with NK1R and other signaling partners? (iii) How does NK1R receptor activation affect or modulate NET/NK1R association and NET function? and (iv) does the NET trafficking motif Thr258/Ser259 play a role in the regulation of NET/NK1R association and NET subcellular localization?
We report that NET exists in association with NK1R in rat brain synaptosomes and in HTR cells co-expressing hNET and hNK1R. We show that NET/NK1R association occurs on the plasma membrane and the NET·NK1R complex internalizes following receptor activation. Upon NK1R activation, NET·NK1R complexes translocate to raft-rich membrane subdomains and are found in association with plasma membrane recruited PKCα. We also show that the previously identified PKC-resistant Thr258/Ser259 motif in NET mediates NET/NK1R translocation to the rafts. These results suggest that subcellular localization of NET is established via regulated NET/NK1R interaction and raft translocation. The raft-specific regulation of receptor-modulated NET/NK1R association and translocation is of physiological significance. NK1R/PKC and NE signaling participates in a variety of cellular processes mediating stress response and pain modulation (17, 18). Moreover, both NE and NK1 signals are implicated in mental disorders such as depression and attention-deficit hyperactivity disorder (19–23).
EXPERIMENTAL PROCEDURES
Materials
Monoclonal antibodies to human NET and rat NET were from mAb Technologies Inc. (Stone Mountain, Atlanta, GA). His6 monoclonal antibody and antibodies to PKCα and flotillin-1 were from BD Biosciences (San Diego, CA). Polyclonal rat-NK1R antibody ADI-905-801 was from Enzo Life Sciences (Farmingdale, NY) and monoclonal human-NK1R was from R&D Systems Inc. (Minneapolis, MN). HA antibody was from Cell Signaling Technologies, Inc. (Danvers, MA) Calnexin antibody was from Millipore Biosciences (Temecula, CA). Ava[l-Pro9,N-MeLeu10]Substance P(7–11) also known as GR73632 was from R&D Systems Inc. 5-([(2R,3S)-2-((R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethoxy)-3-(4-fluorophenyl)morpholino]methyl)-1H-1,2,4-triazol 3(2H)-one) also known as EMEND (aprepitant) was from Merck (Whitehouse Station, NJ). All other chemicals were from Sigma or Fisher Scientific (Pittsburgh, PA), unless otherwise indicated.
Rat Brain Synaptosome Preparations
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Male Sprague-Dawley rats (150–200 g) were decapitated, and the brains were collected in ice-cooled dishes. Brain tissues from ventral striatum (VST) and prefrontal cortex (PFC) were dissected and collected in 10 volumes (w/v) of cold 0.32 m sucrose. The tissue was immediately homogenized using a Teflon-glass homogenizer and centrifuged at 1,000 × g for 10 min at 4 °C. The resulting supernatant was centrifuged at 12,000 × g for 20 min and the pellet was washed by resuspending in 0.32 m sucrose (24). The protein concentration was determined by DC protein assay (Bio-Rad) using bovine serum albumin as standard. Tissue was pooled from 2 to 4 rats based on the experiment conducted and all experiments were replicated at least three times.
NE Uptake Measurements in Synaptosomes
Rat VST synaptosomes suspended in KRH buffer, pH 7.4 (120 mm NaCl, 4.7 mm KCl, 2.2 mm CaCl2, 10 mm HEPES, 1.2 mm MgSO4, 1.2 mm KH2PO4, 5 mm Tris, and 10 mm d-glucose), were used for drug treatments followed by NE uptake. Rat VST synaptosomes (30 μg) were preincubated with vehicle or 10 μm EMEND at 37 °C for 10 min and then incubations were continued in the presence or absence of 10 μm GR73632 for 10 min in a total volume of 1 ml. The concentration of GR73632 was chosen based on previous reports (25, 26) and our initial concentration studies. Following drug treatments, NE uptake assay was performed as described previously (24) using 40 nm [3H]NE (35.0 Ci/mmol of l-[7,8-3H] norepinephrine (PerkinElmer Life Sciences) for 5 min. Synaptosomes were preincubated with the NET inhibitor desipramine (DMI) (100 μm) at 37 °C for 5 min followed by the addition of [3H]NE to determine the nonspecific NE uptake. Uptake was terminated by addition of 1 ml of ice-cold KRH buffer containing 100 μm DMI followed by rapid filtration over 0.3% polyethylenimine-coated GF-B filters on a Brandel Cell Harvester (Gaithersburg, MD). Filters were washed rapidly with 15 ml of cold PBS and radioactivity bound to filters was quantified by liquid scintillation counting using MicroBeta2 LumiJet (PerkinElmer Life Sciences Inc.). Nonspecific uptake was defined as uptake in the presence of 100 μm DMI and subtracted from total accumulation to yield specific NET-mediated NE uptake. Mean values of specific uptake ± S.E. of at least three separate experiments were determined.
Surface Biotinylation of Synaptosomes
Rat VST synaptosomes (300 μg) treated with drugs as described above (in the uptake assay) were subjected to surface biotinylation and isolation of avidin-bound and unbound fractions as described previously (24). Aliquots from total extracts (50 μl) and the entire eluted fractions were separated by SDS-PAGE (10%), transferred to membrane, and probed with mouse-NET antibody. This rodent NET-specific monoclonal antibody has been characterized for its suitability to identify rat and mouse NET protein by Western blotting, immunoprecipitations, and immunocytochemistry (27). Blots were stripped and reprobed with rat NK1R antibody and anti-calnexin antibody. Rat-NET proteins were visualized using ECL Plus reagent followed by exposure to Hyperfilm-ECL. Multiple exposures of immunoblots were taken to ensure that the band development on the film was within the linear range. Band densities were quantified by scanning, and analyzed using NIH ImageJ (version 1.62) software. Anti-calnexin antibody was used to validate the surface biotinylation of plasma membrane proteins. NET band densities from total and biotinylated (representing the surface pool) fractions were normalized using levels of calnexin in the total extract (24).
Cell Cultures and Transfections
HTR cells were cultured in a mixture of RPMI 1640 (Mediatech-Cellgro) supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). Cells seeded in 24-well cell culture plates (100,000 cells/well used for NE uptake), or 12- (200,000 cells/well for cell surface biotinylations) or 6-well plates (500,000 cells/well used for lipid raft isolations) were allowed to grow in an atmosphere of 95% air, 5% CO2. HTR cells were transfected with His-tagged WT-hNET cDNA or hNET-T258A/S259A (hNET-DM) cDNA in PCDNA3 plus HA-tagged hNK1R cDNA in pCIN4 vector (28) (1:2 ratio) using FuGENE 6 Transfection Reagent (Roche Diagnostics). The following DNA concentrations were used: 0.25 μg of hNET plus 0.5 μg of NK1R/well in 12-well plates or 0.5 μg of hNET plus 1.0 μg of NK1R/well in 6-well plates or 1.0 μg of hNET plus 2.0 μg of NK1R/well in 6-well plates. To normalize NET protein expression levels between WT and DM, the following DNA concentrations were used: 0.375 μg of hNET-DM plus 0.75 μg of NK1R/well in 12-well plates or 0.75 μg of hNET-DM plus 1.5 μg of NK1R/well in 6-well plates or 1.5 μg of hNET-DM plus 3.0 μg of NK1R/well in 6-well plates. In some immunoprecipitation experiments where cell extracts were mixed prior to NET immunoprecipitations, HTR cells were transfected with 0.5 μg of WT-hNET or 1.0 μg of NK1R or 0.75 μg of hNET-DM or 1.5 μg of NK1R. Cell cultures were maintained for 24 h prior to transfections and grown for 48 h prior to experiments.
NE Uptake Measurements in Transfected HTR Cells
Transfected HTR cells were treated with vehicle or 0.25 μm SP or 10 μm EMEND or EMEND plus SP for the indicated times as given elsewhere. Uptake measurements were performed by incubating the cells for 10 min at 37 °C with [3H]NE in 0.5 ml of Krebs-Ringer-HEPES (KRH) buffer, pH 7.4 (120 mm NaCl, 4.7 mm KCl, 2.2 mm CaCl2, 10 mm HEPES, 1.2 mm MgSO4, 1.2 mm KH2PO4, 5 mm Tris, and 10 mm d-glucose), containing 100 μm ascorbic acid and 100 μm pargyline. Assays were terminated by removing the radiolabel and rapid washing of the cells three times with 1 ml of ice-cold KRH buffer. Cells were solubilized in 0.5 ml of 1% SDS and the accumulated [3H]NE was quantified by liquid scintillation counting using MicroBeta2 LumiJet. Specific NE uptake was measured by subtracting the NE uptake measured in the presence of 1 μm DMI from the total NE uptake measured in the absence of DMI. Data are represented as the mean ± S.E. from three experiments performed in triplicates on different batches of trophoblast cultures.
Cell Surface Biotinylation of HTR Cells
To quantify the amount of plasma membrane NET and NK1R, transfected cells following drug treatments as indicated above were subjected to surface biotinylation followed by isolation of avidin-bound and unbound fractions as described previously (10, 14, 29–31). Aliquots from total cell lysates (40 μl) and unbound fractions (40 μl each), and all (50 μl) avidin-bound samples were analyzed by immunoblotting with hNET monoclonal antibody followed by reprobing with antibodies to NK1R. To validate the surface localization of biotinylated proteins, blots were striped and reprobed with anti-calnexin antibody (1:20,000 dilution). Band intensities were quantified using NIH ImageJ (version 1.62). Exposures were precalibrated to ensure quantitation within the linear range of the film and multiple exposures were taken to validate linearity of quantitation. Values of total, non-biotinylated and biotinylated NET or NK1R proteins were normalized using levels of calnexin immunoreactivity in total cell extract and values were averaged across three experiments.
Immunoprecipitations Using Synaptosomes
Rat PFC and VST synaptosomes were suspended in RIPA buffer containing protease inhibitors (1 μm pepstatin A, 250 μm PMSF, 1 mg/ml of leupeptin, 1 μg/ml of aprotinin) (32) by passing 10 times through a 25-gauge needle, and solubilized by gentle shaking on a nutator for 1 h at 4 °C. The clear supernatant obtained after centrifuging the solubilized synaptosomes at 25,000 × g for 30 min at 4 °C was subjected to immunoprecipitation with NET-specific antibody as given below. The supernatants were first precleared using Protein G-Sepharose (50 μl). NET protein was immunoprecipitated overnight at 4 °C by the addition of NET-specific antibody (NET05-2) with end-over-end continuous mixing, followed by a 2-h incubation with Protein G-Sepharose at 22 °C (room temperature). The immunoadsorbents captured by Protein G-Sepharose beads were washed with RIPA buffer and eluted by adding 50 μl of urea based sample buffer. The eluates were subjected to SDS-PAGE (10%), and the proteins were detected by immunoblotting with antibodies specific to rat/mouse NET and rat NK1R. Band densities were measured on multiple exposures to ensure quantitation within the linear range of the film using NIH Image software.
Immunoprecipitations Using HTR Cells
Transfected cells were treated as indicated above, and washed with cold PBS and lysed in 600 μl of RIPA buffer containing protease inhibitors. Extracts were centrifuged at 20,000 × g for 30 min at 4 °C and the supernatants containing 40–50 μg in 500 μl were precleared using 50 μl of Protein G- or A-Sepharose beads (32). Immunoprecipitations were carried out overnight at 4 °C by the addition of anti-His antibody (5 μl) to isolate NET or anti-NK1R antibody (5 μl) to isolate NK1R with end-over-end continuous mixing, followed by a 1.5-h incubation with Protein G- or A-Sepharose at 22 °C (room temperature). The immunoisolates were subjected to urea-based SDS-PAGE and transferred onto PVDF membranes. NET, NK1R, and other proteins were quantified by immunoblotting with specific antibodies.
Cell Surface Protein Biotinylation followed by Immunoprecipitation
To identify the cellular compartment where NET/NK1R association occurs, cell surface biotinylation was performed on NET + NK1R-transfected cells following treatments as indicated elsewhere. Biotinylated proteins were isolated by binding to monomeric avidin beads followed by elution using 500 μl of 2 mm d-biotin. Biotinylated and non-biotinylated proteins were used for immunoprecipitation of NET, as described above. NET immunoprecipitates from the cell surface and intracellular pools were subjected to SDS-PAGE followed by sequential immunoblotting with hNET and NK1R antibodies to analyze NET/NK1R associations as described above.
Lipid Raft Isolation
Transfected HTR cells treated as indicated elsewhere were used to isolate lipid rafts, as described earlier, with slight modifications (14, 33, 34). Cells were lysed in 1.5 ml of MBS (25 mm MES and 150 mm NaCl, pH 6.5) containing 0.1% Triton X-100 and a mixture of protease inhibitors (1 μm pepstatin A, 250 μm PMSF, 1 mg/ml of leupeptin, 1 μg/ml of aprotinin) using a Dounce homogenizer with 10 up and down strokes at 4 °C. Equal volumes of 80% (w/v) sucrose in MBS were added to the homogenates. Equal volumes of cell lysates (1 ml) in 40% sucrose were placed at the bottom of SW41 centrifuge tubes and overlaid successively with 4 ml of 30% sucrose and 3 ml of 5% sucrose. The tubes were centrifuged at 35,000 rpm for 18 h at 4 °C, and 1-ml fractions were collected from the top. Aliquots (50 μl) from each fraction were subjected to SDS-PAGE and Western blotting. In some experiments, equal volumes (500 μl) of collected fractions were used for NET immunoprecipitations followed by immunoblotting, as described above.
RESULTS
Down-regulation of NET Function and Surface Expression following NK1R Activation in Native and Heterologous Systems
Abundant expression of NK1R is shown in both human and rodent striatum (22, 35). Our recent study showed the expression of NET in rat PFC and VST (29). In addition, several studies have successfully used GR73632, a synthetic NK1R selective agonist to activate NK1R in vivo (36–38). Our previous study demonstrated that NK1R activation down-regulates NET via PKC in heterologous cells co-transfected/co-expressing with NK1R and NET (10). To examine whether such regulation is evident in the native NET expressing system, we explored the effect of NK1R activation on NET function and surface expression using rat VST synaptosomes. Treatment of rat VST synaptosomes with 10 μm GR73632 for 10 min resulted in significant reductions in NE transport and surface expression of rNET and rNK1R (Fig. 1). Specific NE uptake by rat VST synaptosomes was reduced by 20–25% following GR73632 treatment compared with vehicle treatment (Fig. 1A). Surface expression of rNET or rNK1R was also reduced by 45–50% following treatment with GR73632 compared with vehicle treatment (Fig. 1, B and C). In addition, the reductions in surface expression of rNET or rNK1R are reflected in parallel increases in the intracellular pools (Fig. 1, B and C). Furthermore, pretreatment with 10 μm EMEND (aprepitant), a NK1R antagonist, for 10 min completely blocked GR73632-induced down-regulation of NE transport and surface expression of rNET and rNK1R (Fig. 1, A–C). Importantly, EMEND alone did not have any significant effect on NE transport, rNET surface expression, or rNK1R surface expression (Fig. 1, A–C).
FIGURE 1.
NK1R activation by GR73632 leads to reduced NE transport and reduced surface expression of NET along with reduced NK1R surface expression in rat VST synaptosomes. Rat VST synaptosomes were preincubated with vehicle or 10 μm EMEND (Em) at 37 °C for 10 min. Incubations were continued in the presence or absence of 10 μm GR73632 for 10 min. A, NE uptake: drug-treated synaptosomes were used for NE uptake assays as described under “Experimental Procedures.” Data were derived from three separate experiments, each in triplicate are given as mean ± S.E. * indicates significant change (p < 0.01) in NE transport (one-way analysis of variance; Dunnett's test: F(4,8) = 10.99; p < 0.01). B and C, surface biotinylation: synaptosomes treated with drugs as above were biotinylated and biotinylated NET or NK1R were isolated and analyzed as described under “Experimental Procedures.” Total (T), non-biotinylated (UB), and biotinylated (B) rNET or rNK1R were analyzed using mNET monoclonal antibody or rNK1R polyclonal antibody. Representative blots show a rNET-specific band at ∼70 kDa (upper panel) and rNK1R at ∼50 kDa. Densities of biotinylated rNET (∼70 kDa) or rNK1R (∼50 kDa) from three separate experiments are given as mean ± S.E. (bar graphs). * indicates significant change (p < 0.05) in surface rNET or rNK1R immunoreactivity following SP treatment compared with vehicle-control (one-way analysis of variance; Dunnett's test: F(4,8) = 11.90, p = 0.01 for NET and F(4,8) = 17.71, p = 0.01 for NK1R). Calnexin immunoblots corresponding to the total are shown for equal protein loading. Densities of avidin unbound (intracellular) fractions of rNET (∼70 kDa) or rNK1R (∼50 kDa) from three separate experiments are given as mean ± S.E. (bar graphs). * indicates significant change (p < 0.05) in intracellular rNET or rNK1R immunoreactivity following GR73632 treatment compared with vehicle-control (one-way analysis of variance; Dunnett's test: F(4,8) = 7.121, p = 0.01 for NET and F(4,8) = 9.308, p = 0.01 for NK1R).
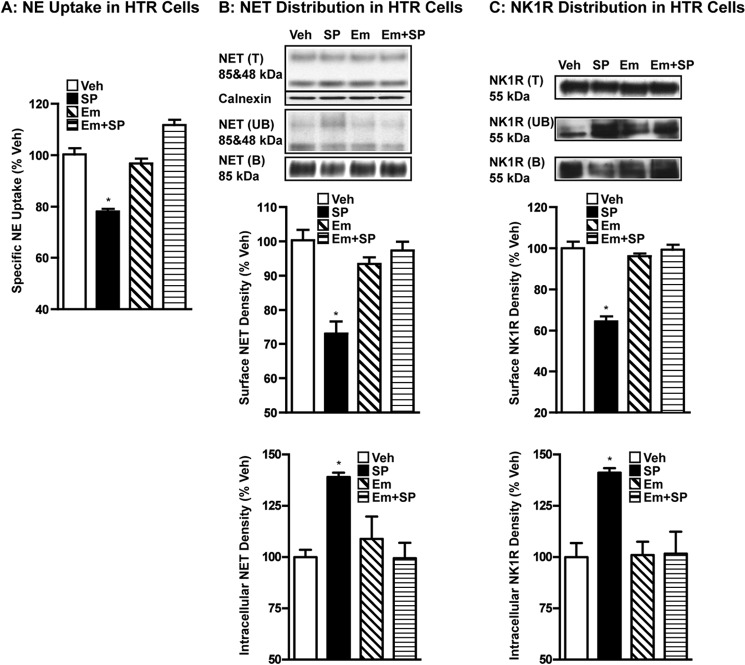
Similar to our previous heterologous study (10), 0.25 μm SP treatment of HTR cells expressing hNET and hNK1R resulted in significant reductions in NE transport and surface expression of hNET and hNK1R (Fig. 2). Specific NE uptake by transfected HTR cells was reduced by 20–25% following SP treatment compared with vehicle treatment (Fig. 2A). Cell surface expression of hNET or hNK1R was also reduced by 30–35% following treatment with SP compared with vehicle treatment (Fig. 1, B and C). These reductions in the surface expression of hNET or hNK1R are reflected in parallel increases in the intracellular pools (Fig. 2, B and C). Similar to results seen with VST synaptosomes, pretreatment with 10 μm EMEND (aprepitant), an NK1R antagonist, completely blocked SP-induced down-regulation of NE transport and cell surface expression of hNET and hNK1R (Fig. 2, A–C). EMEND alone did not have any significant effect on NE transport, hNET surface expression, or hNK1R surface expression (Fig. 2, A–C). Similar to SP, GR73632, a synthetic NK1R agonist, also inhibited NE uptake by HTR cells expressing hNET and NK1R, in a manner sensitive to EMEND pretreatment (data not shown).
FIGURE 2.
NK1R activation by SP leads to reduced NE transport and reduced cell surface expression of NET in parallel with reduced surface NK1R in transfected HTR cells. HTR cells coexpressing hNET and hNK1R were preincubated with vehicle or 10 μm EMEND (Em) at 37 °C for 10 min. Incubations were continued in the presence or absence of 0.25 μm SP for 15 min. A, NE uptake: drug-treated cells were used for NE uptake assays as described under “Experimental Procedures.” Data were derived from three separate experiments, each in triplicate are given as mean ± S.E. * indicates significant change (p < 0.01) in NE transport (one-way analysis of variance; Dunnett's test: F(4,8) = 53.12; p < 0.01). B and C, surface biotinylation: cells treated with drugs as above were biotinylated and biotinylated NET or NK1R were isolated and analyzed as described under “Experimental Procedures.” Total (T), non-biotinylated (UB), and biotinylated (B) hNET or hNK1R were analyzed using hNET monoclonal antibody or hNK1R antibody. Representative blots show hNET-specific bands at ∼85 and ∼48 kDa (upper panel) and the hNK1R band at ∼55 kDa. Densities of biotinylated NET (∼85 kDa) or NK1R (∼55 kDa) from three separate experiments are given as mean ± S.E. (bar graphs). * indicates significant change (p < 0.05) in surface hNET or hNK1R immunoreactivity following SP treatment compared with vehicle-control (one-way analysis of variance; Dunnett's test: F(4,8) = 19.28, p = 0.01 for hNET and F(4,8) = 50.95, p < 0.01 for hNK1R). Calnexin immunoblots corresponding to the total are shown for equal protein loading. Densities of avidin unbound fractions of NET (∼85 kDa) or NK1R (∼55 kDa) from three separate experiments are given as mean ± S.E. (bar graphs). * indicates a significant change (p < 0.05) in intracellular hNET or hNK1R immunoreactivity following SP treatment compared with vehicle-control (one-way analysis of variance; Dunnett's test: F(4,8) = 7.37, p = 0.01 for hNET and F(4,8) = 7.86, p < 0.01 for hNK1R).
Stable Association of NET with NK1R in Native and Heterologous Systems
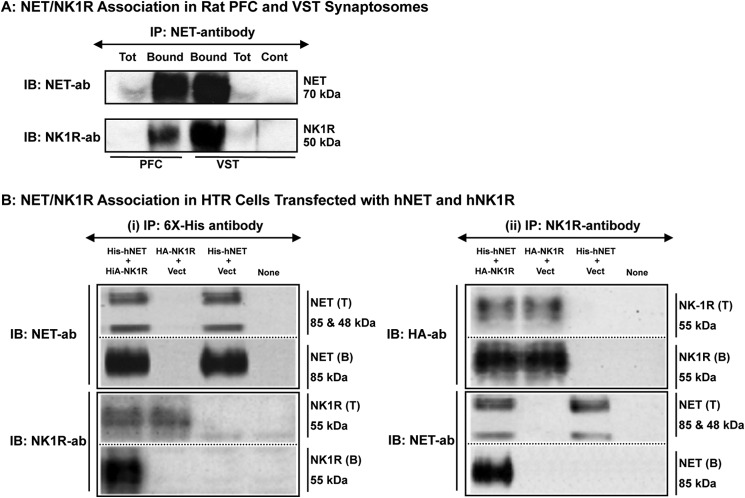
The results presented above (Figs. 1 and 2) along with our previous findings (10) suggested a possible association between NK1R activation and NET down-regulation. However, it is not known whether or not these two proteins physically interact to establish this regulation. Fig. 3A shows results from immunoprecipitation experiments using rat VST and PFC synaptosomes. Immunoblotting with mNET monoclonal antibody (mNET05-2) showed the presence of rNET in the immunoprecipitates isolated using the same NET antibody in both PFC and VST synaptosomal extracts. Reprobing of the same blots with monoclonal NK1R antibody revealed the presence of rNK1R in the immunoisolates, suggesting the presence of NET·NK1R physical complexes (Fig. 3A). No specific protein band corresponding to 70 or 50 kDa was found when Protein G-Sepharose beads were incubated with NET-antibody (NET05-2) alone, suggesting that no nonspecific IgG bands at these molecular weights corresponded to NET or NK1R (Fig. 3A). Similar results were obtained using our own NET-specific polyclonal antibody, which effectively pulled down NK1R when NET was immunoprecipitated from rat brain VST and PFC synaptosomes (data not shown).
FIGURE 3.
NET forms a stable association with NK1R. A, rat NET was immunoprecipitated from rat PFC or VST synaptosomes using mNET-monoclonal antibody. Equal protein extracts (300 μg in 500 μl) from synaptosomes were immunoprecipitated with mNET antibody (5 μl) to isolate rNET. Total extracts (T) as well as Protein G-Sepharose-bound immunoprecipitates (B) were subjected to SDS-PAGE followed by sequential immunoblotting (IB) with mNET-monoclonal antibody and rNK1R polyclonal antibody. The experiment shown was replicated in three independent experiments with similar results and representative rNET and rNK1R immunoblots (IB) are shown. Protein G-Sepharose beads were incubated with NET antibody (NET05-2) alone as a control to rule out nonspecific IgG bands. B, His-hNET and HA-hNK1R were coexpressed in HTR cells and either hNET or hNK1R was immunoprecipitated using (i) His6 antibody or (ii) NK1R antibody. (i) using His6 antibody: equal protein extracts (40–50 μg in 400 μl) were immunoprecipitated with His6 monoclonal antibody (5 μl) to isolate hNET. Total extracts (T) as well as Protein G-Sepharose-bound immunoprecipitates (B) were subjected to SDS-PAGE followed by sequential immunoblotting with hNET or and NK1R antibody. (ii) Using NK1R antibody: equal protein extracts (40–50 μg in 400 μl) were immunoprecipitated with anti-NK1R polyclonal antibody (5 μl) to isolate NK1R. Total extracts (T) and Protein A-Sepharose bound immunoprecipitates (B) were subjected to SDS-PAGE followed by immunoblotting with HA or hNET antibody. The experiment shown was replicated in three independent experiments with similar results and representative NET and NK1R immunoblots are shown.
Fig. 3B shows our results from immunoprecipitation experiments using HTR cells expressing His-hNET and HA-NK1R. Protein complexes of NET·NK1R were isolated by immunoprecipitation with His6 antibody (NET IP) (i) or NK1R-antibody (NK1R-IP) (ii).
NET-IP
Immunoblotting with hNET antibody showed the presence of hNET in total extracts from cells transfected with His-hNET + HA-NK1R or His-hNET + vector (Fig. 3B(i)). Immunoblotting with the hNET antibody showed the presence of hNET in NET immunoprecipitates (His immunoisolates) isolated from cells coexpressing His-hNET and HA-NK1R and from cells expressing only His-hNET, but not from cells expressing HA-NK1R (Fig. 3B(i)), suggesting specific NET isolation by the His6 antibody. Immunoblotting with NK1R antibody showed the presence of NK1R immunoreactivity in total extracts from cells transfected with His-hNET + HA-NK1R or HA-NK1R + vector (Fig. 3B(i)). Interestingly, immunoblotting with the NK1R antibody showed the presence of NK1R in NET immunoprecipitates isolated from cells coexpressing His-hNET and HA-NK1R but not in cells expressing only His-hNET or HA-NK1R (Fig. 3B(i)). Mock-transfected control cells did not show the presence of hNET or NK1R immunoreactivity. Thus, only cells coexpressing hNET and NK1R revealed the presence of NK1R in NET immunocomplexes isolated using His antibody (Fig. 3B(i)).
NK1R-IP
Immunoblotting with HA antibody showed the presence of NK1R (HA immunoreactivity) in total extracts from cells transfected with His-hNET + HA-NK1R or HA-NK1R + vector (Fig. 3B(ii)). Immunoblotting with HA antibody showed the presence of NK1R (HA immunoreactivity) in NK1R immunoprecipitates isolated from cells expressing His-hNET + HA-NK1R or HA-NK1R (Fig. 3B(ii)), suggesting specific NK1R isolation by NK1R antibody. Immunoblotting with hNET antibody showed the presence of hNET in the total extracts from cells transfected with His-hNET + HA-NK1R or His-hNET + vector (Fig. 3B). Importantly, immunoblotting with hNET antibody showed the presence of hNET in NK1R immunoprecipitates isolated from cells coexpressing His-hNET and HA-NK1R but not in cells expressing only His-hNET or HA-NK1R (Fig. 3B(ii)). Mock-transfected control cells showed neither hNET nor NK1R. Thus, only cells coexpressing hNET and NK1R revealed the presence of hNET in immunocomplexes isolated using NK1R antibody (Fig. 3B(ii)). These studies provided evidence that stable complexes of hNET with NK1R are formed in transporter/receptor-transfected cells and establish the presence of a specific association in this model.
Association of hNET with NK1R on the Plasma Membrane and Internalization of NET·NK1R Complexes following Receptor Activation by SP
It is known that SP-mediated activation induces NK1R internalization (15, 39). The data that NK1R activation reduces surface expression of NET as well as NK1R (Figs. 1 and 2) and that NET exists in association with NK1R (Fig. 3) prompted us to examine whether NET·NK1R complexes exist on the plasma membrane and internalize following NK1R activation. To identify subcellular localization of NET/NK1R interaction and internalization, we employed a combined biotinylation and co-immunoprecipitation strategy. NET immunoprecipitates isolated from the plasma membrane (biotinylated) and intracellular (non-biotinylated) proteins were subjected to Western blotting using hNET antibody and NK1R antibody. As shown in Fig. 2, following SP treatment, the surface expression of hNET as well as NK1R was decreased significantly by 5 min and beyond (Fig. 4A). Parallel time-dependent increases in intracellular hNET as well as intracellular NK1R suggested internalization of these proteins (Fig. 4A). In vehicle-treated cells, most of the NK1R immunoreactivity was found in NET immunoprecipitates isolated from the avidin-bound (biotinylated) fractions, whereas very little NK1R immunoreactivity was found in NET immunoprecipitates from the avidin unbound (non-biotinylated) fraction (Fig. 4B). However, SP treatment for 5, 15, and 30 min resulted in the reduction of NK1R immunoreactivity associated with the plasma membrane (biotinylated) hNET (Fig. 4B). In parallel, we observed a gradual time-dependent increase in NK1R immunoreactivity associated with intracellular (non-biotinylated) hNET (Fig. 4B). These results suggested that hNET exists in association with cell surface NK1R under basal or unstimulated conditions and internalizes together with the associated NK1R following receptor activation by SP.
FIGURE 4.

NK1R activation regulates NET/NK1R association and subcellular compartmentalization. HTR cells transfected with His-hNET and HA-hNK1R were treated with 0.25 μm SP for the indicated times and subjected to (A) cell surface biotinylation and isolation of avidin-bound (surface) and unbound (intracellular) fractions followed by (B) hNET immunoprecipitations (IP) as described under “Experimental Procedures.” A, biotinylation: representative blots from three independent biotinylation experiments show total (T), surface (avidin B), and intracellular (avidin UB) levels of hNET and NK1R. Note the time-dependent decreases in the surface levels of hNET and NK1R proteins with concomitant increases in the intracellular pools. B, immunoprecipitation: representative immunoblots from three independent experiments show co-immunoprecipitation of NK1R from surface and intracellular hNET. Note the robust NK1R association with surface hNET at 0 time/vehicle, which disappeared following SP treatment. In contrast, note the very little NK1R association with intracellular hNET at 0 time/vehicle that increased time dependently following SP treatment.
SP-induced Translocation of NET·NK1R Complexes from Non-raft Membrane Fractions to Raft Membrane Fractions
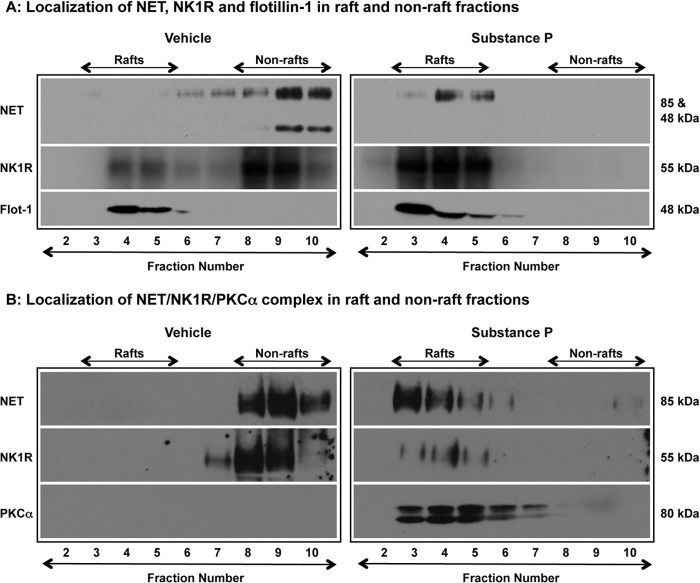
It is known that NK1R is localized in raft-rich membrane subdomains and NK1R activation results in plasma membrane mobilization of the receptor as well as PKCα (15). We have previously shown that NET is also localized in raft-rich membrane domains, and this localization is dynamically regulated by kinases (10, 14). Therefore, we next examined the localization of NK1R and PKCα in raft and non-raft membrane fractions and their interaction with hNET. Fig. 5A shows the distribution of hNET, NK1R, and flotillin-1 in raft and non-raft fractions. Following vehicle treatment, most of the hNET was found in non-raft fractions with little or none in the raft fractions (Fig. 5A). However, following SP treatment, hNET immunoreactivity was detected only in raft fractions (Fig. 5A). Fig. 5A also shows that in vehicle-treated cells, the majority of NK1R is localized in the non-raft fractions and little in the raft fractions. Following SP treatment, NK1R immunoreactivity was detected only in raft fractions (Fig. 5A). The immunoreactivity of flotillin-1, a raft-specific protein, was detected only in the raft fractions isolated from either vehicle-treated or SP-treated cells (Fig. 5A). Next, we examined whether NET/NK1R interactions occur in the raft or non-raft fractions and whether these interactions are altered by NK1R activation using the co-immunoprecipitation strategy using NET-specific antibody. Fig. 5B shows hNET immunoreactivity in the NET immunoprecipitates isolated from non-raft fractions following vehicle treatment. However, following SP treatment, hNET immunoreactivity was found in the NET immunoprecipitates isolated from raft fractions (Fig. 5B). Similar to hNET, NK1R immunoreactivity was found in the NET immunoprecipitates isolated from non-raft fractions following vehicle treatment and from raft-rich fractions following SP treatment (Fig. 5B). Thus, there was complete disappearance of NET·NK1R immune complexes from non-raft fractions with concomitant appearance in the raft-rich fractions following SP treatment (Fig. 5B). Following vehicle treatment, PKCα immunoreactivity was not detectable in NET immunoprecipitates suggesting the absence of PKCα association with hNET under basal conditions. However, following NK1R activation, PKCα immunoreactivity was found in the NET immunoprecipitates isolated from raft-rich fractions (Fig. 5B). These results indicated that NET localized in non-raft fractions forms stable complexes with NK1R, and receptor activation induces translocation of NET·NK1R complexes from non-raft fractions to raft-rich fractions, allowing the formation of NET·NK1R·PKCα ternary complexes.
FIGURE 5.
NK1R activation translocates NET·NK1R complexes from non-raft fractions to raft fractions. HTR cells transfected with His-hNET and HA-hNK1R were treated with vehicle or SP (0. 25 μm) for 15 min. Following treatments, the cells were solubilized in MBS containing 0.1% Triton X-100 and subjected to sucrose gradient centrifugation as described under “Experimental Procedures.” A, lipid raft analysis of NET, NK1R, and flotillin-1: equal aliquots (40 μl) of sucrose density gradient fractions (2–10) were subjected to SDS-PAGE followed by immunoblotting with antibodies to hNET, NK1R, and flotillin-1. Representative immunoblots from three independent experiments are shown. B, analysis of hNET/NK1R/PKCα associations in rafts and non-rafts: equal volumes (500 μl) of raft and non-raft fractions were used to isolate NET immunoprecipitates as described above and the immunoisolates were subjected to SDS-PAGE followed by sequential immunoblotting with antibodies to hNET, NK1R, and PKCα. Representative immunoblots from three separate experiments are shown.
Blunted Translocation of NET-DM·NK1R Complexes from Non-raft Fractions to Raft Fractions in Response to NK1R Activation
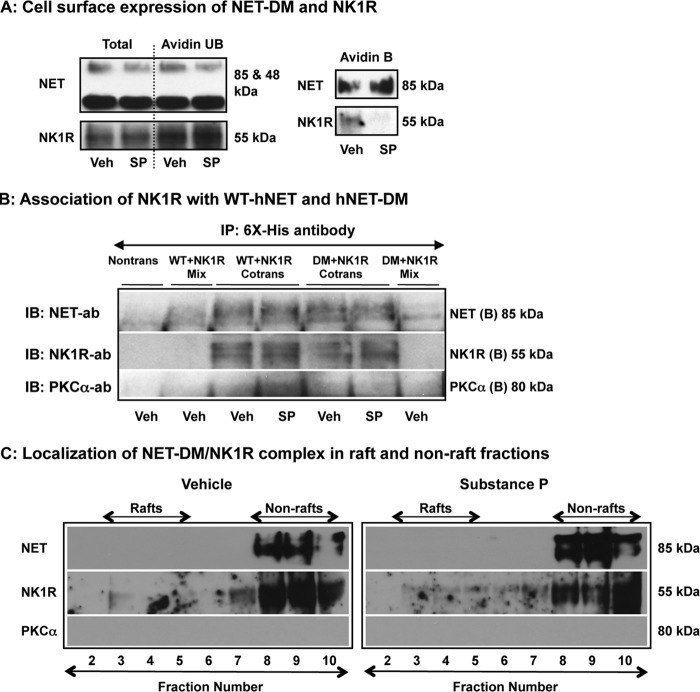
Our previous study demonstrated that the T258A,S259A double mutant is resistant to NK1R/PKC-mediated down-regulation and suggested a role for the Thr258/Ser259 motif in NET trafficking (10). In agreement with our previous observations (10), there was no reduction in the surface expression of hNET-DM following SP treatment (Fig. 6A). However, the surface expression of NK1R was reduced following SP treatment (Fig. 6A) suggesting intact NK1R activation and subsequent receptor internalization. Therefore, we conducted parallel immunoprecipitation experiments to examine whether hNET-DM interacts with NK1R similar to WT-hNET and whether there are any changes in the association of NK1R with WT-hNET or hNET-DM following SP treatment. NK1R was found in the NET immunoprecipitates isolated from WT-hNET + NK1R expressing cells as well as from hNET-DM + NK1R expressing cells. There were no significant changes in the interaction of NK1R with WT-hNET or hNET-DM (Fig. 6B). In addition, PKCα was found in the NET immunoprecipitates isolated from WT-hNET + NK1R expressing cells following SP treatment, but not following vehicle treatment. Interestingly, little or no PKCα was found in NET immunoprecipitates isolated from hNET-DM + NK1R expressing cells treated with vehicle or SP (Fig. 6B). We next examined whether NET-DM/NK1R interactions occur in raft or non-raft fractions and whether SP treatment alters NET-DM/NK1R distribution in these membrane compartments using NET-specific antibody. Similar to observations made in cells expressing hNET and NK1R (Fig. 5B), both hNET-DM and NK1R proteins were found in the immunoprecipitates isolated from non-raft fractions following vehicle treatment (Fig. 6C). Interestingly, the immunoreactivity of hNET-DM or NK1R in the NET immunoprecipitates isolated from non-raft fractions remained intact following SP treatment, and there was no hNET-DM or NK1R immunoreactivity in NET immunoprecipitates isolated from raft fractions (Fig. 6C). These data show no evidence of translocation of NET-DM·NK1R immunocomplexes from non-raft fractions into raft fractions following SP treatment (Fig. 6C). In addition, there was no detectable PKCα immunoreactivity in the NET immunoprecipitates isolated from either raft or non-raft fractions (Fig. 6C). These results indicate the existence of NET-DM·NK1R complexes in the non-raft fractions and impaired translocation of NET-DM/NK1R from non-raft compartments to raft-rich compartments following NK1R activation.
FIGURE 6.
NET-DM associates with NK1R, but fails to translocate to raft microdomains. HTR cells transfected with His-hNET-DM and HA-hNK1R were treated with vehicle or SP (0. 25 μm) for 15 min and used for surface biotinylation and lipid raft isolations. A, surface biotinylation: cell surface biotinylation was performed as described under “Experimental Procedures.” A representative blot from three experiments shows total (T), surface (avidin B), and intracellular (avidin UB) levels of hNET-DM and NK1R. B, immunoprecipitation: His-tagged WT-hNET or hNET-DM and HA-hNK1R were coexpressed in HTR cells and equal protein extracts (40–50 μg in 400 μl) were immunoprecipitated with His6 monoclonal antibody (5 μl) to isolate NET. The immunoprecipitates were subjected to SDS-PAGE followed by sequential immunoblotting (IB) with antibodies to hNET, NK1R, and PKCα. Representative immunoblots from three independent experiments are shown. C, analysis of hNET-DM/NK1R/PKCα associations in rafts and non-rafts: raft and non-raft fractions were isolated as described above and under “Experimental Procedures.” Equal volumes (500 μl) of raft and non-raft fractions were used to isolate NET immunoprecipitates as described above and the immunoisolates were subjected to SDS-PAGE followed by sequential immunoblotting with antibodies to hNET, NK1R, and PKCα. Representative immunoblots from three independent experiments are shown.
DISCUSSION
Monoamine transporters exist in association with several signaling molecules including presynaptic receptors, kinases, phosphatases, and SNARE proteins. Amine transport function is modulated by regulated interactions with these transporter-associated proteins (4). NET has been shown to physically associate with PP2Ac, syntaxin 1A, Hic-5, PICK-1, 14-3-3 proteins, and α-synuclein (5–8, 40, 41). However, the mechanism by which these interactions are coordinated to receptor-linked signaling is unclear. It is possible that membrane subdomains such as rafts may serve as a site at which distinct NET associations are acquired or stabilized. Previously we have demonstrated the expression of native NETs in rat placental trophoblasts (42), and that in placental trophoblasts, NET localizes in part to lipid raft-containing membrane subdomains (10, 14). Other recent studies demonstrated raft localization of NET in the brain and superior cervical ganglion neurons (27). Considerable evidence indicates that caveolae and/or lipid rafts serve as attachment platforms for various receptors including GPCRs and for a wide array of signaling and scaffolding proteins (39, 43). Our previous studies have demonstrated a role for lipid rafts in NK1R/PKC-mediated NET down-regulation (10, 14). Lipid rafts are hubs of signaling activity, and constitute several receptors, kinases, and other signaling proteins (44). The current report provides the first evidence that NET can form regulated detergent-resistant complexes with NK1R and PKCα in a manner that influences transporter membrane redistribution and subcellular localization.
We have previously demonstrated that NK1R activation leads to NET down-regulation in hNET/NK1R-cotransfected HTR cells (10). The present study extended this observation by demonstrating NK1R-mediated NET down-regulation in native NET-expressing brain synaptosomes as well as in heterologously expressing HTR cells. In addition, the current study demonstrates that reductions in NE transport and NET surface expression occur in parallel to reduced plasma membrane NK1R. Aprepitant, an NK1R-specific antagonist, blocks these NK1R-mediated effects, further substantiating the involvement of NK1R in NET down-regulation. However, in VST synaptosomes, the decrease in surface NET was larger compared with the decrease in NE uptake following NK1R activation. The presence of transporter substrate (NE) during uptake assays may have effects contributing to this discrepancy. A similar discrepancy but to a lesser extent was also observed in HTR cells. This may be due to differences in NET expression levels and/or lipid environments between native tissues and heterologous expression systems. Indeed, NE at a high concentration (20 μm) was found to block SP-induced inhibition of NE uptake in HTR cells.4 Amine transporter substrates are known to have effects of their own and also modulate transporter regulation by kinases and/or other signals (45, 46). Using pharmacological and genetic manipulations, several studies suggest a strong interaction between the SP/NK1 system and NE signaling (19–23). Factors such as protein/protein interactions and localization of signaling machinery could contribute to how NE signaling is regulated by neurokinins. To our knowledge, ours are the first studies to identify the interaction between NK1R and NET as an important determinant of NE signaling. More importantly, our results suggest that raft localization of NET·NK1R complexes, and subsequent interaction with membrane-recruited PKCα form the molecular basis for transporter internalization, presumably triggered by the phosphorylation step.
A full appreciation of the mechanisms by which activation of NK1R controls NET function and trafficking requires an understanding of whether regulation is indirect or is mediated by more confined, physical interactions that are regulated following an incoming signal. To date, compartmentalization mechanisms by which GPCRs can target one or more of these modulators to regulate NET without influencing other cytosolic and membrane effectors are unknown. Here, we provide evidence that NET forms stable complexes with NK1R in native as well as heterologous systems. Moreover, we find that the NK1R agonist, SP, can regulate the abundance of NET·NK1R complexes in a raft-dependent manner. Upon receptor activation, NET·NK1R complexes translocate to raft-rich membrane domains where NET and NK1R assemble with membrane recruited PKCα and form a ternary (NET·NK1R·PKCα) complex. It is possible that such highly regulated compartmentalized interactions allow the transporter to be placed in the right environment and at the right time for incoming signals to act upon. For example, the assembly of NET, NK1R, and PKCα in raft membrane domains may allow NET phosphorylation by PKCα, a key molecular determinant of transporter internalization. Protein kinases PKC and PKG are known to interact with the amine transporters DAT and serotonin transporter, and regulate their function (47, 48). Activation of PKC and NK1R results in reduced NET activity and surface expression (10, 14). Thus, the formation of NET·NK1R complexes as well as the regulation of membrane localization of NET·NK1R complexes may be an important aspect of NE transport regulation (current study).
Similar to hNET, PKC-resistant hNET-DM forms stable complexes with NK1R as evidenced by immunoisolation of hNET-DM·NK1R complexes. In addition, NET/NK1R association is not altered following NK1R activation. Neither WT-hNET nor hNET-DM interacts with PKCα under basal conditions. However, following NK1R activation, WT-hNET, but not the double mutant is found in association with PKCα. Translocation of the hNET-DM·NK1R complex from the non-raft to raft-rich fractions is not observed following NK1R activation. This suggests that the PKC motif, Thr258/Ser259 determines NK1R-mediated NET membrane redistribution and assembly with PKCα. Although hNET-DM failed to internalize following receptor activation, the internalization of NK1R was intact in cells expressing hNET-DM and NK1R. Both raft- as well as dynamin/clathrin-dependent pathways are implicated in NK1R internalization (15, 39). Therefore, the dynamin-dependent pathway might account for internalization of the NK1R pool that is not transporter or hNET-DM associated. The present study using the PKC-resistant NET mutant shows that although the NET/NK1R interaction is not disrupted by the T258A,S259A mutation, regulation of the raft-specific membrane localization of the NET·NK1R complex is determined by the trafficking motif Thr258/Ser259. In addition, the results indicate that the Thr258/Ser259 motif mediates SP-induced modulation of NET/NK1R interactions by placing the transporter in close proximity to incoming signals, such as PKCα, to introduce a molecular link between NET phosphorylation, internalization, and down-regulation. Thus, the Thr258/Ser259-linked spatiotemporal control of the NET/NK1R/PKCα interaction mediates NE transport regulation. The physical assembly of NET/NK1R/PKCα in rafts may serve as an important molecular event necessary for the transporter to be positioned in the “signalosome” for post-translational modification and eventual internalization. In this regard, raft-rich membrane domains serve as transit cargos for transporter internalization.
We have reported previously that native NETs expressed in rat placental trophoblasts are phosphorylated and down-regulated by PKC activation via the raft-mediated pathway (14). This effect requires the calcium-independent novel PKC isoform PKCϵ (14). Interestingly, although PKCϵ was found in association with hNET, the hNET/PKCα interaction was not altered following NK1R activation (data not shown). Our subsequent studies using heterologous expression systems identified that PKC is involved in NK1R-mediated down-regulation of hNET (10). However, the specific PKC isoform involved is not clear. By identifying the components of NK1R signaling where NET·NK1R complexes assemble with PKCα in raft-rich membrane domains, we believe that our data demonstrate a role for PKCα in NK1R-mediated NET regulation. Studies have shown that NK1R activation leads to plasma membrane recruitment of PKCα and concurrent translocation to raft-rich membrane compartments (15). Other studies have indicated PKCδ activation mediated by SP (49). Additional studies are needed to assess whether NET is regulated independently by a specific PKC isoform depending upon the incoming signal, and whether there is an overlap between PKC isoforms downstream of NK1R activation.
Several proteins including presynaptic receptors are known to interact with monoamine transporters (4, 50). Recently, DAT has been shown to interact with G-protein βγ subunits (51). Few studies have successfully identified the motifs in the transporter proteins that directly interact with transporter-associated proteins. The amino-terminal region of NET is known to directly interact with syntaxin 1A and PP2Ac (7, 41). The N-terminal regions of DAT and DAT intracellular loop 4 are known to interact with D2DRs (52). The C-terminal region of DAT is known to interact with PICK1 (6). Interestingly, PP2A has been shown to interact with NK1R (53). It is possible that in a signaling hub, NET may be transiently in association with NK1R via another anchoring protein such as β-arrestin or other signaling proteins such as PP2A and G-protein βγ subunits. Protein-protein interactions affect monoamine transporter trafficking, and it is known that amine transporters interact with several signaling proteins (4, 54). In this regard, the assembly of NET with NK1R/PKC-linked signals constitutes an important molecular mechanism among many facets of NET regulation. In the context of evidence presented here that NK1R and PKCα interact with NET, we propose that NET trafficking, localization, and phosphorylation require assembly of a much larger and regulated macromolecular complex in which compromised interactions could affect risk for disorders associated with altered NE signaling.
In summary, evidence is provided for the NET/NK1R interaction and the regulation of subcellular localization of NET·NK1R complexes in efficiently coupling the endogenous signals such as receptors and kinases. In addition, our findings provide a new framework for studying the functional importance of lipid rafts in the context of amine transporters and their interacting protein partners. Membrane-specific molecular interactions of NET/NK1R signify raft-specific signaling mechanisms underlying NET regulation by neurokinins and provide opportunities for future studies aimed at understanding the influence of neurokinins controlling NE signaling.
Acknowledgment
We gratefully acknowledge Dr. Sammanda Ramamoorthy for critical reading of the manuscript and valuable suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-GM081054 from the NIGMS and the start up fund from Virginia Commonwealth University (to L. D. J.).
L. D. Jayanthi, unpublished observations.
- NE
- norepinephrine
- NET
- norepinephrine transporter
- NK1R
- neurokinin-1 receptor
- HTR
- human placental trophoblast
- DAT
- dopamine transporter
- DMI
- desipramine
- VST
- ventral striatum
- PFC
- prefrontal cortex
- SP
- Substance-P
- GPCR
- G protein-coupled receptor.
REFERENCES
- 1. Iversen L. L. (1971) Role of transmitter uptake mechanisms in synaptic neurotransmission. Br. J. Pharmacol. 41, 571–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schömig E., Fischer P., Schönfeld C. L., Trendelenburg U. (1989) The extent of neuronal re-uptake of 3H-noradrenaline in isolated vasa deferentia and atria of the rat. Naunyn-Schmiedebergs Arch. Pharmacol. 340, 502–508 [DOI] [PubMed] [Google Scholar]
- 3. Trendelenburg U. (1991) The TiPs lecture. Functional aspects of the neuronal uptake of noradrenaline. Trends Pharmacol. Sci. 12, 334–337 [DOI] [PubMed] [Google Scholar]
- 4. Sager J. J., Torres G. E. (2011) Proteins interacting with monoamine transporters. Current state and future challenges. Biochemistry 50, 7295–7310 [DOI] [PubMed] [Google Scholar]
- 5. Bauman A. L., Apparsundaram S., Ramamoorthy S., Wadzinski B. E., Vaughan R. A., Blakely R. D. (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 20, 7571–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres G. E., Yao W. D., Mohn A. R., Quan H., Kim K. M., Levey A. I., Staudinger J., Caron M. G. (2001) Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30, 121–134 [DOI] [PubMed] [Google Scholar]
- 7. Sung U., Apparsundaram S., Galli A., Kahlig K. M., Savchenko V., Schroeter S., Quick M. W., Blakely R. D. (2003) A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J. Neurosci. 23, 1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sung U., Blakely R. D. (2007) Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol. Cell. Neurosci. 34, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apparsundaram S., Galli A., DeFelice L. J., Hartzell H. C., Blakely R. D. (1998) Acute regulation of norepinephrine transport. I. Protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J. Pharmacol. Exp. Ther. 287, 733–743 [PubMed] [Google Scholar]
- 10. Jayanthi L. D., Annamalai B., Samuvel D. J., Gether U., Ramamoorthy S. (2006) Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J. Biol. Chem. 281, 23326–23340 [DOI] [PubMed] [Google Scholar]
- 11. Kreibich A., Reyes B. A., Curtis A. L., Ecke L., Chavkin C., Van Bockstaele E. J., Valentino R. J. (2008) Presynaptic inhibition of diverse afferents to the locus ceruleus by κ-opiate receptors. A novel mechanism for regulating the central norepinephrine system. J. Neurosci. 28, 6516–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghisi V., Ramsey A. J., Masri B., Gainetdinov R. R., Caron M. G., Salahpour A. (2009) Reduced D2-mediated signaling activity and trans-synaptic up-regulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal 21, 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolan E. A., Kivell B., Jaligam V., Oz M., Jayanthi L. D., Han Y., Sen N., Urizar E., Gomes I., Devi L. A., Ramamoorthy S., Javitch J. A., Zapata A., Shippenberg T. S. (2007) D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 71, 1222–1232 [DOI] [PubMed] [Google Scholar]
- 14. Jayanthi L. D., Samuvel D. J., Ramamoorthy S. (2004) Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J. Biol. Chem. 279, 19315–19326 [DOI] [PubMed] [Google Scholar]
- 15. Monastyrskaya K., Hostettler A., Buergi S., Draeger A. (2005) The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J. Biol. Chem. 280, 7135–7146 [DOI] [PubMed] [Google Scholar]
- 16. Meyer B. H., Segura J. M., Martinez K. L., Hovius R., George N., Johnsson K., Vogel H. (2006) FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc. Natl. Acad. Sci. U.S.A. 103, 2138–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jasmin L., Tien D., Weinshenker D., Palmiter R. D., Green P. G., Janni G., Ohara P. T. (2002) The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc. Natl. Acad. Sci. U.S.A. 99, 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Min M. Y., Shih P. Y., Wu Y. W., Lu H. W., Lee M. L., Yang H. W. (2009) Neurokinin 1 receptor activates transient receptor potential-like currents in noradrenergic A7 neurons in rats. Mol. Cell Neurosci. 42, 56–65 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y. M., Xu F., Gainetdinov R. R., Caron M. G. (1999) Genetic approaches to studying norepinephrine function. Knockout of the mouse norepinephrine transporter gene. Biol. Psychiatry 46, 1124–1130 [DOI] [PubMed] [Google Scholar]
- 20. Lin Z., Madras B. K. (2006) Human genetics and pharmacology of neurotransmitter transporters. Handb. Exp. Pharmacol. 175, 327–371 [DOI] [PubMed] [Google Scholar]
- 21. Fisher A. S., Stewart R. J., Yan T., Hunt S. P., Stanford S. C. (2007) Disruption of noradrenergic transmission and the behavioural response to a novel environment in NK1R−/− mice. Eur. J. Neurosci. 25, 1195–1204 [DOI] [PubMed] [Google Scholar]
- 22. Yan T. C., Hunt S. P., Stanford S. C. (2009) Behavioural and neurochemical abnormalities in mice lacking functional tachykinin-1 (NK1) receptors. A model of attention deficit hyperactivity disorder. Neuropharmacology 57, 627–635 [DOI] [PubMed] [Google Scholar]
- 23. Yan T. C., McQuillin A., Thapar A., Asherson P., Hunt S. P., Stanford S. C., Gurling H. (2010) NK1 (TACR1) receptor gene “knockout” mouse phenotype predicts genetic association with ADHD. J. Psychopharmacol. 24, 27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuvel D. J., Jayanthi L. D., Manohar S., Kaliyaperumal K., See R. E., Ramamoorthy S. (2008) Dysregulation of dopamine transporter trafficking and function following abstinence from cocaine self-administration in rats. Evidence for differential regulation in Caudate-Putamen and nucleus accumbens. J. Pharmacol. Exp. Ther. 325, 293–301 [DOI] [PubMed] [Google Scholar]
- 25. Miyano K., Morioka N., Sugimoto T., Shiraishi S., Uezono Y., Nakata Y. (2010) Activation of the neurokinin-1 receptor in rat spinal astrocytes induces Ca2+ release from IP3-sensitive Ca2+ stores and extracellular Ca2+ influx through TRPC3. Neurochem. Int. 57, 923–934 [DOI] [PubMed] [Google Scholar]
- 26. Phenna S., Carpenter E., Peers C., Maudsley S., Gent J. P. (1996) Inhibition of Ca2+-sensitive K+ currents in NG 108–15 cells by substance P and related tachykinins. Br. J. Pharmacol. 119, 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthies H. J., Han Q., Shields A., Wright J., Moore J. L., Winder D. G., Galli A., Blakely R. D. (2009) Subcellular localization of the antidepressant-sensitive norepinephrine transporter. BMC Neurosci. 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Granas C., Ferrer J., Loland C. J., Javitch J. A., Gether U. (2003) N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J. Biol. Chem. 278, 4990–5000 [DOI] [PubMed] [Google Scholar]
- 29. Mannangatti P., Arapulisamy O., Shippenberg T. S., Ramamoorthy S., Jayanthi L. D. (2011) Cocaine up-regulation of the norepinephrine transporter requires threonine 30 phosphorylation by p38 mitogen-activated protein kinase. J. Biol. Chem. 286, 20239–20250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Annamalai B., Mannangatti P., Arapulisamy O., Ramamoorthy S., Jayanthi L. D. (2010) Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J. Neurochem. 115, 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Annamalai B., Mannangatti P., Arapulisamy O., Shippenberg T. S., Jayanthi L. D., Ramamoorthy S. (2012) Tyrosine phosphorylation of the human serotonin transporter. A role in the transporter stability and function. Mol. Pharmacol. 81, 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramamoorthy S., Giovanetti E., Qian Y., Blakely R. D. (1998) Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 273, 2458–2466 [DOI] [PubMed] [Google Scholar]
- 33. Becher A., White J. H., McIlhinney R. A. (2001) The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J. Neurochem. 79, 787–795 [DOI] [PubMed] [Google Scholar]
- 34. Foster J. D., Adkins S. D., Lever J. R., Vaughan R. A. (2008) Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 105, 1683–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mounir S., Parent A. (2002) The expression of neurokinin-1 receptor at striatal and pallidal levels in normal human brain. Neurosci. Res. 44, 71–81 [DOI] [PubMed] [Google Scholar]
- 36. Rupniak N. M., Carlson E. C., Harrison T., Oates B., Seward E., Owen S., de Felipe C., Hunt S., Wheeldon A. (2000) Pharmacological blockade or genetic deletion of substance P (NK(1)) receptors attenuates neonatal vocalisation in guinea pigs and mice. Neuropharmacology 39, 1413–1421 [DOI] [PubMed] [Google Scholar]
- 37. Rupniak N. M., Carlson E. J., Webb J. K., Harrison T., Porsolt R. D., Roux S., de Felipe C., Hunt S. P., Oates B., Wheeldon A. (2001) Comparison of the phenotype of NK1R−/− mice with pharmacological blockade of the substance P (NK1) receptor in assays for antidepressant and anxiolytic drugs. Behav. Pharmacol. 12, 497–508 [DOI] [PubMed] [Google Scholar]
- 38. Brocco M., Dekeyne A., Mannoury la Cour C., Touzard M., Girardon S., Veiga S., de Nanteuil G., deJong T. R., Olivier B., Millan M. J. (2008) Cellular and behavioural profile of the novel, selective neurokinin1 receptor antagonist, vestipitant. A comparison to other agents. Eur. Neuropsychopharmacol. 18, 729–750 [DOI] [PubMed] [Google Scholar]
- 39. Schmidlin F., Dery O., DeFea K. O., Slice L., Patierno S., Sternini C., Grady E. F., Bunnett N. W. (2001) Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J. Biol. Chem. 276, 25427–25437 [DOI] [PubMed] [Google Scholar]
- 40. Jeannotte A. M., Sidhu A. (2007) Regulation of the norepinephrine transporter by α-synuclein-mediated interactions with microtubules. Eur. J. Neurosci. 26, 1509–1520 [DOI] [PubMed] [Google Scholar]
- 41. Sung U., Jennings J. L., Link A. J., Blakely R. D. (2005) Proteomic analysis of human norepinephrine transporter complexes reveals associations with protein phosphatase 2A anchoring subunit and 14-3-3 proteins. Biochem. Biophys. Res. Commun. 333, 671–678 [DOI] [PubMed] [Google Scholar]
- 42. Jayanthi L. D., Vargas G., DeFelice L. J. (2002) Characterization of cocaine and antidepressant-sensitive norepinephrine transporters in rat placental trophoblasts. Br. J. Pharmacol. 135, 1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conner S. D., Schmid S. L. (2003) Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 162, 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simons K., Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 45. Zahniser N. R., Doolen S. (2001) Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters. Drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 92, 21–55 [DOI] [PubMed] [Google Scholar]
- 46. Chen R., Furman C. A., Gnegy M. E. (2010) Dopamine transporter trafficking. Rapid response on demand. Future Neurol. 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson L. A., Guptaroy B., Lund D., Shamban S., Gnegy M. E. (2005) Regulation of amphetamine-stimulated dopamine efflux by protein kinase Cβ. J. Biol. Chem. 280, 10914–10919 [DOI] [PubMed] [Google Scholar]
- 48. Steiner J. A., Carneiro A. M., Wright J., Matthies H. J., Prasad H. C., Nicki C. K., Dostmann W. R., Buchanan C. C., Corbin J. D., Francis S. H., Blakely R. D. (2009) cGMP-dependent protein kinase Iα associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol. Brain 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramnath R. D., Sun J., Adhikari S., Zhi L., Bhatia M. (2008) Role of PKC-δ on substance P-induced chemokine synthesis in pancreatic acinar cells. Am. J. Physiol. Cell Physiol. 294, C683–692 [DOI] [PubMed] [Google Scholar]
- 50. Bröer S., Gether U. (2012) The solute carrier 6 family of transporters. Br. J. Pharmacol. 167, 256–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garcia-Olivares J., Torres-Salazar D., Owens W. A., Baust T., Siderovski D. P., Amara S. G., Zhu J., Daws L. C., Torres G. E. (2013) Inhibition of dopamine transporter activity by G protein βγ subunits. PLoS One 8, e59788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee F. J., Pei L., Moszczynska A., Vukusic B., Fletcher P. J., Liu F. (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy J. E., Roosterman D., Cottrell G. S., Padilla B. E., Feld M., Brand E., Cedron W. J., Bunnett N. W., Steinhoff M. (2011) Protein phosphatase 2A mediates resensitization of the neurokinin 1 receptor. Am. J. Physiol. Cell Physiol. 301, C780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu C. B., Lindler K. M., Campbell N. G., Sutcliffe J. S., Hewlett W. A., Blakely R. D. (2011) Colocalization and regulated physical association of presynaptic serotonin transporters with A adenosine receptors. Mol. Pharmacol. 80, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]