Background: How to differentiate embryonic stem cells into specific neuronal types is a key question.
Results: Lack of Cyp26a1 results in increased ALDH1a2 and Hoxc6, markers of lateral motor column identity.
Conclusion: The cytochrome P450 enzyme Cyp26a1 plays a critical role in specifying motor neuron columnar subtypes.
Significance: Blocking Cyp26a1 has therapeutic potential in regenerative medicine, neurodegenerative diseases, and cancer therapies.
Keywords: Cytochrome P450, Differentiation, Neurological Diseases, Retinoid, Stem Cells
Abstract
The ability to differentiate embryonic stem cells (ESCs) into specific cell types is critical for improved regenerative medicine strategies, cancer chemotherapeutic approaches, and regimens to combat chronic diseases associated with aging. Subclasses of motor neurons (MNs) are generated at different positions along the rostrocaudal axis of the spinal cord, and the signals that specify MN subtype fates remain poorly defined. We show here that the cytochrome P450 enzyme Cyp26a1, which metabolizes all-trans-retinoic acid (RA) and thereby reduces RA levels, plays a crucial role in specifying MN columnar subtypes. Lack of Cyp26a1 in ESCs during differentiation to spinal MNs increases Aldh1a2 (RALDH2) and Hoxc6, markers of the Hox-dependent, lateral motor column (LMC) subtype identity. In contrast, Lhx3, a marker for median motor column identity, showed lower expression in Cyp26a1−/−-derived MNs compared with WT. Without Cyp26a1, an increase in intracellular RA concentration plus sonic hedgehog agonist treatment confer an LMC fate on differentiating MNs. Our data suggest a strategy for increasing LMC-type MNs from ESCs by blocking Cyp26a1 in cell replacement/ESC differentiation therapy to treat neurodegenerative diseases, such as amyotrophic lateral sclerosis.
Introduction
The degeneration of specific motor neuron (MN)3 subtypes is the underlying factor for a large number of neurological disorders, including amyotrophic lateral sclerosis (ALS) (1). Neurodegenerative disorders are suited for embryonic stem cell and stem cell replacement therapy because they often target individual classes of neurons that are restricted to defined regions of the central nervous system (CNS). For instance, the neurodegenerative disorder ALS results in the loss of upper and lower motor neurons and leads to progressive paralysis and death (2, 3). One possible therapy for ALS is the differentiation of embryonic stem cells (ESCs) or induced pluripotent stem cells into MNs (4–6).
It is generally accepted that ESCs must be directed to differentiate into discrete, neuronal phenotypes that specifically match the properties of the degenerated neurons (7–9). However, many ESC studies have simply classified neurons by their neurotransmitter phenotype (10). This classification is often insufficient because subpopulations of neurons that express a given transmitter can vary remarkably with respect to size, ion channels, receptors, projection patterns, and function (9). Therefore, for transplantation studies, it is necessary to direct ESCs to differentiate into distinct, neuronal subtypes. Cultured wild type (WT) ESCs generally differentiate into MNs that express high Lhx3, a LIM homeodomain (HD) protein characteristic of the median motor column (MMC) (9).
Evidence in the literature suggests that limb innervations would be greater if ESCs are differentiated to lateral motor column (LMC) MNs. For example, WT ESC-derived MNs, treated with all-trans-retinoic acid (RA) and sonic hedgehog (Shh) and transplanted into the developing chick neural tube, express Lhx3, migrate to the MMC, and exhibit functional characteristics of endogenous MMC MNs (9, 11). However, little is known about how to differentiate ESCs into LMC neurons, the question we address here.
RA is a key signaling molecule that can induce the differentiation of ESCs (12) and promote the differentiation of various types of cancer cells (13). To test the function of the cytochrome p450 enzyme Cyp26a1 (Gene ID: 13082) in specifying MNs, we subjected Cyp26a1−/− ESCs (14) to an MN differentiation protocol (15) and assessed the molecular phenotype of the resulting MNs. Our results show a significant role for Cyp26a1 and RA in the specification of MN subtype identities and also provide insight into how Cyp26a1 inhibition could be used for cell differentiation therapies to generate a reliable source of specified LMC MNs. We show that treatment of ESCs that lack Cyp26a1 with an Shh agonist and RA leads to the differentiation of MNs specific to the LMC.
EXPERIMENTAL PROCEDURES
Cell Culture and Chemicals
AB1 WT and Cyp26a1−/− murine ESCs, cultured in monolayer on gelatin-coated culture dishes, were maintained as described previously (16). 1 × 103 units/ml leukemia inhibitory factor (LIF2010, Millipore-Chemicon (Temecula, CA)) was added prior to use. ESCs were cultured in ADFNK medium to form embryoid bodies (EBs) and subsequently HB9+ motor neurons as described (15). ADFNK medium is made as follows: 250 ml of Advanced DMEM/F-12 (catalog no. 12634-028, Invitrogen), 250 ml of Neurobasal medium (catalog no. 21103-049, Invitrogen), 5.6 ml of penicillin/streptomycin (catalog no. 15070-063, Invitrogen), 5.6 ml of 200 mm l-glutamine (catalog no. 25030-081, Invitrogen); 400 μl of 1:100 diluted 2-mercaptoethanol in PBS (catalog no. M6250, Sigma-Aldrich); 56 ml of knock-out replacement serum (catalog no. 10828-028, Invitrogen). All-trans-retinoic acid (Sigma) was dissolved in 100% ethanol and diluted in the appropriate cell medium to obtain final concentrations. The sonic hedgehog agonist (Hh-Ag1.3 (SHH), Curis, Cambridge, MA) was dissolved in DMSO and diluted in ADFNK medium (15) to obtain the final concentration. Doxycycline was from Sigma-Aldrich. All experiments involving retinoids were performed in dim light.
The “rescue” ESC lines were generated as follows. The plasmid pTAm-N, derived from the pTA-N plasmid, encodes the mutant tetracycline transactivator (rtTA). This transactivator is under the control of the tetracycline operator (tet-OP), which is linked to a TATA box-containing minimum promoter sequence. Thus, in the presence of tetracycline (or doxycycline) the transcription of the transactivator gene is turned on. A second plasmid, pUHD-10-3, was used to clone the full-length mCyp26a1 cDNA (1,735 bp) into the multiple cloning site using the EcoRI and BamHI restriction sites. The pUHD-10-3 plasmid contains heptamerized tet-operators upstream in two different orientations and a CMV minimal promoter, including a TATA box. Once the mCyp26a1 full-length cDNA was cloned into pUHD-10-3, a diagnostic restriction digest and subsequent DNA sequencing were used to determine that the mCyp26a1 cDNA was inserted in the correct orientation into the plasmid. The Cyp26a1−/− ESCs were transfected with the pTAm-N and pUHD-10-3/mCyp26a1 plasmids using Lipofectamine 2000 (catalog no. 11668-019, Invitrogen). Individual cell colonies, after selection, were screened for both rtTA and mCyp26a1 mRNA levels with or without 1 μg/ml doxycycline and/or with or without 1 μm RA for 24 h via semiquantitative PCR. The cell colonies with the greatest doxycycline inducibility were characterized further (see “Results”).
RNA Isolation and Reverse Transcription and Real-time PCR (qPCR) Analysis
EBs were harvested by collecting each sample in a 15-ml conical tube and allowing EBs to settle by gravity. The supernatant was removed, the EBs were resuspended in 1 ml of TRIzol (Invitrogen), and total cellular RNA was extracted according to the manufacturer's protocol. RNA was quantified by measuring the optical density at 260 nm. RNA (1 μg) was reverse-transcribed using qScript cDNA SuperMix in accordance with the manufacturer's guidelines (Quanta Biosciences) and then diluted 1:10 with H2O. A reverse-transcription reaction without the reverse transcriptase (RT) enzyme was used as a negative control for genomic DNA template. Real-time PCR was performed as described (17) with specific primer pairs (see Table 1). All qPCR analyses were performed in triplicate and were from three or more independent, biological experimental repeats starting with new cell cultures.
TABLE 1.
Primers
All primers were designed around introns.
| Primer | Forward sequence (5′ → 3′) | Reverse sequence (5′ → 3′) | Product size |
|---|---|---|---|
| bp | |||
| cyp26a1 | AAACATTGCAGATGGTGCTTCAG | CGGCTGAAGGCCTGCATAATCAC | 272 |
| cyp26b1 | GTCGTGAGTGTCTCGGATGCTA | TGGACTGTGTCATCAAGGAGGT | 143 |
| hoxc6 | CCAGGACCAGAAAGCCAGTA | CCGAGTTAGGTAGCGGTTGA | 166 |
| hoxc8 | CCTCCGCCAACACTAACAGT | CAAGGTCTGATACCGGCTGT | 140 |
| nkx6.1 | AAACACACCAGACCCACGTT | GGAACCAGACCTTGACCTGA | 145 |
| isl1 | TCATCCGAGTGTGGTTTCAA | CCATCATGTCTCTCCGGACT | 154 |
| shh | TCCAAAGCTCACATCCACTG | GGGACGTAAGTCCTTCACCA | 132 |
| pax6 | AACAACCTGCCTATGCAACC | ACTTGGACGGGAACTGACAC | 206 |
| hoxc9 | CGCAGCTACCCGGACTACAT | AGCGCTTCTTCCTTGTGGA | 184 |
| bmp5 | ATCACTGTGACCAGCAACCA | AAGTACCTCGCTTGCCTTGA | 180 |
| isl2 | CTCTGCCGAGCTGACCAC | CTCGTTGAGCACAGTCCGTA | 189 |
| lhx3 | CAGACCCAGGGGAAGTTCAG | GAAACGGTCCAAGATGTGCT | 195 |
| foxp1 | GCAGCTCCTCCTTCAGCA | ATAGCCACTGACACGGGAAC | 195 |
| olig2 | CCGAAAGGTGTGGATGCTTA | GATGGGCGACTAGACACCAG | 186 |
| hb9 | CTCATGCTCACCGAGACTCA | TCAGCTCCTCTTCCGTCTTC | 169 |
| oct4 | GAGGAGTCCCAGGACATGAA | AGATGGTGGTCTGGCTGAAC | 154 |
| aldh1a2 | GACTTGTAGCAGCTGTCTTCACT | TCACCCATTTCTCTCCCATTTCC | 160 |
| lim1 | ATCCTGGACCGTTTCCTCTT | AACCAGATCGCTTGGAGAGA | 198 |
Fixation, Cryosectioning, and Immunostaining
EBs were fixed, sectioned, and processed for immunocytochemistry as described (15). For immunostaining, slides were thawed at room temperature for ∼10 min. Ice-cold 1× PBS (1 ml) was pipetted on each slide, and slides were allowed to stand for 5 min. PBS was aspirated off of each slide, with care taken not to disturb the EBs. Sections were circled on each slide using an ImmunoPen. Bovine serum albumin (BSA) (2%) was pipetted on each circled area, as a blocking step, for 30 min at room temperature. The BSA was removed from each section by aspiration. The primary antibody was diluted in 2% BSA to the appropriate concentration, and ∼100 μl was pipetted on each section. Antibodies were as follows: HB9, 1:500 (Developmental Studies Hybridoma Bank, Iowa City, IA), 81.5c10; Aldh1a2, 1:100 (18); Hoxc6, 1:100 (Abcam, Cambridge, MA), ab41587; vesicular acetylcholine transporter (VAChT), 1:100 (Abcam), ab68984. Sections were incubated at room temperature for 1 h. Slides were then moved to 4 °C overnight and kept in a humid environment. The antibody was aspirated off of slides, and each slide was washed twice with 1× PBS for 5 min for each wash. Slides were incubated with fluorophore-conjugated secondary antibody (goat anti-mouse, AlexaFluor594; goat anti-rabbit 488, 1:300 (Invitrogen)) for 1 h at room temperature. Slides were then washed twice with 1× PBS for 5 min each time while being protected from light. One drop of mounting medium with DAPI (Vectashield H-1500, Vector Laboratories) was placed on each slide, and coverslips were placed over each section. Fluorescence images were obtained using a TE2000 inverted microscope (Nikon, Melville, NY). For cell counts, at least three sections from each block were examined from each of three independent, biological repeats.
Retinoid Extraction and High Performance Liquid Chromatography (HPLC) Analysis
Motor neurons generated from WT or Cyp26a1−/− ESCs treated with 1 μm RA and 1 μm Hh-Ag1.3 were harvested on day 7 for HPLC analysis. The plates were scraped, and all medium from each plate was transferred to a 15-ml conical tube. Cells were allowed to settle by gravity, and an aliquot of 0.5 ml (from the 10 ml) of ADFNK medium was saved from each sample for extraction of retinoids. The remaining medium was removed, and cells (EBs) were washed twice with 4 ml of PBS (allowing EBs to settle by gravity between each wash). After the final wash, PBS was removed, and 0.5 ml of fresh PBS was added to each sample for harvesting. The retinoids were extracted from 350 μl of organic phase from both the cell and medium samples of each sample in a dark environment as described (19, 20). The HPLC was performed using a Waters Millennium system (Waters Corp., Milford, MA). Each sample (100 μl) was loaded on an analytical 5-μm reverse-phase C18 column (Vydac, Hesperia, CA) and eluted at a flow rate of 1.5 ml/min. Two mobile phase gradient systems were used as described (19, 20). Retinoids were detected at a wavelength of 340 nm. Retinoids were identified by an exact match of the retention time of an unknown peak with the retinoid standard. The concentrations of retinoids were calculated on the basis of their peak areas, volume of the sample, concentration of known standards in each sample, and cell number. A separate plate for each cell line was counted on day 7 using an electron particle counter (Coulter Z, Beckman Coulter, Inc. (Fullerton, CA)) to determine cell number.
Statistical Analysis
The mean values ± S.E. were determined for each of the data sets and plotted as error bars in the graphs using GraphPad Prism version 4.0 software (San Diego, CA). Student's t test was used to determine differences between two groups. Differences with a p value of <0.05 were considered to be statistically significant.
RESULTS
Cyp26a1−/− Embryonic Stem Cells (ESCs) Can Differentiate to MN Progenitors Despite an Altered Class I HD Transcription Factor Profile
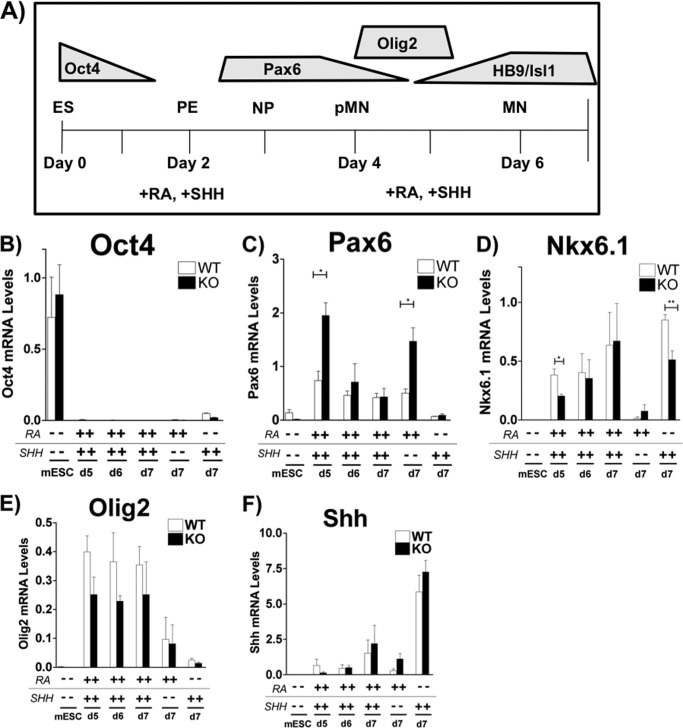
To examine the role of RA metabolism in spinal motor neuron differentiation and subsequent motor column specification, we utilized murine wild type (WT) and Cyp26a1−/− ESCs (14) in a previously established differentiation protocol (15) (Fig. 1A). Briefly, ESCs were cultured as EBs, and on day 2 of the differentiation protocol, the EBs were treated with 1 μm RA and 1 μm sonic hedgehog agonist (Hh Ag-1.3, indicated as SHH throughout) (7, 21). RA was previously shown to promote neural and repress mesodermal gene expression in cultured ESCs (22). Purmorphamine (23) could substitute for Hh Ag-1.3 (data not shown). On day 5, either the EBs were collected for analysis or the medium was changed and the EBs were allowed to grow for 1–2 more days, with the last harvest on day 7. We determined that the WT and Cyp26a1−/− ESCs were undergoing differentiation to spinal motor neurons by collecting the cells at different time points and assaying for the appropriate developmental markers. We also determined that the cell numbers of WT and Cyp26a1−/− ESCs treated with this protocol for 7 days with RA and SHH were very similar (not shown). We examined the mRNA levels of Oct4, an ESC marker (24), at the onset of differentiation and confirmed that both cell lines had comparable mRNA levels of this transcription factor (Fig. 1B) and were thus starting from a pluripotent state.
FIGURE 1.
Cyp26a1−/− murine ESCs differentiate to pMN despite an altered class I HD profile. A, time line of ESC differentiation, showing expression of key developmental markers. ES, embryonic stem cells; PE, primitive ectoderm; NP, neural plate; pMN, progenitor motor neuron. B, qPCR analysis of oct4 mRNA to confirm ESC status. C, qPCR analysis of Pax6, a class I HD transcription factor, transcripts. Cyp26a1−/− knockout (KO) cells at day 5 exhibit a 2.6 ± 0.3-fold (p = 0.0146) higher pax6 mRNA level as compared with WT. D, qPCR analysis of nkx6.1, a class II HD transcription factor, transcripts. Day 5 WT neural progenitors express 1.9 ± 0.2-fold (p = 0.026) higher nkx6.1 mRNA levels than Cyp26a1−/− neural progenitors. E, qPCR analysis of olig2, a pMN marker. No statistically significant differences in olig2 expression were detected at any time point in the WT or Cyp26a1−/− cells. F, qPCR analysis of shh transcript levels. These qPCR data are from at least three independent biological repeats (n ≥ 3), run in triplicate in each experiment, and all qPCR data are normalized to 36b4 mRNA as a control. KO, mCyp26a1−/− ESCs; SHH, treatment with sonic hedgehog agonist, Hh Ag-1.3). y axes, arbitrary units; note differences in y axes in different panels. Error bars, S.E.
Because RA plays a role in establishing the MN progenitor domain by regulating the class I HD transcription factors and the Cyp26a1−/− murine ESCs lack the ability to eliminate RA through further metabolism, we assayed for the transcripts of HD and basic helix-loop-helix transcription factors that delineate sets of neural progenitor cells positioned along the dorsoventral axis of the developing neural tube (25–28). Prospective MN progenitors (pMNs) are distinguished by their class I (e.g. Pax6) (Fig. 1A) and class II (e.g. Nkx6.1) HD protein profiles (25). The primary function of Pax6 and Nkx6.1 within pMNs is to prevent the expression of transcription factors capable of repressing Olig2 expression (27). Olig2, a basic helix-loop-helix protein, acts as a transcriptional repressor to direct the expression of downstream HD regulators of MN identity via HB9 and LIM proteins (27–31). Day 5 WT pMNs expressed 1.9 ± 0.2-fold (p = 0.026) higher levels of nkx6.1 transcript than day 5 Cyp26a1−/− pMNs (Fig. 1D), whereas the day 5 Cyp26a1−/− pMNs displayed a 2.6 ± 0.3-fold (p = 0.015) higher level of pax6 mRNA compared with WT pMNs (Fig. 1C). The increase in pax6 transcript levels was also seen in the RA-only control samples (Fig. 1C), suggesting that the Cyp26a1−/− cells have higher pax6 mRNA levels as a result of higher intracellular levels of RA and that this effect is independent of the actions of SHH. The differences in pax6 and nkx6.1 mRNA levels were not associated with altered Olig2 expression (Fig. 1E), most likely because these HD proteins are not required for the transcriptional activation of olig2 (26, 32, 33).
We examined the sonic hedgehog transcript level to determine if the lack of Cyp26a1 function altered the levels of this key signaling molecule during MN progenitor formation. We show that the shh transcript level is not changed by the Cyp26a1−/− mutation (Fig. 1F). Thus, the lack of Cyp26a1 does not alter the ability of the Cyp26a1−/− ESCs to differentiate to MN progenitors.
Cyp26a1−/− ESCs Can Differentiate into HB9+ Spinal MNs
We analyzed the expression of HB9, an HD transcription factor selectively and persistently expressed by somatic MNs that acts to consolidate the identity of postmitotic MNs (34–36). By day 7, the peak of MN differentiation in this protocol, both the WT and Cyp26a1−/− RA plus SHH-treated cells expressed HB9 transcripts (Fig. 2A). We confirmed these findings at the HB9 protein level via immunocytochemistry (Fig. 3A, HB9, red images in all panels). We determined that >40% of WT and >40% of Cyp26a1−/− cells at day 7 were HB9-positive (Fig. 3A). By comparison, the 7-day RA-only (negative control)-treated WT and Cyp26a1−/− cells did not express HB9 mRNA (Fig. 2A) or HB9 protein (Fig. 3B, HB9, red images in all panels). These data indicate that the combination of RA plus SHH is required for HB9 expression.
FIGURE 2.
Cyp26a1−/−-derived MNs have an altered retinoid profile compared with WT derived MNs. A and B, qPCR analysis of Hb9, a motor neuron marker, and VAChT, vesicular acetylcholine transporter, indicative of their cholinergic transmitter status. No significant differences in Hb9 or VAChT mRNA levels were detected between the RA plus SHH-treated WT and Cyp26a1−/− cells at day 7, the peak of MN differentiation. C, aldh1a2 mRNA levels in WT versus KO MNs. There was an 8.6 ± 2.5-fold (p = 0.0123) higher aldh1a2 mRNA level in the Cyp26a1−/− MNs as compared with WT MNs at day 7. D, cyp26a1 mRNA levels at various time points during MN differentiation. E, HPLC analysis of retinoids from both cells and media of WT and Cyp26a1−/− MNs (day 7). A 92.4 ± 25.1-fold (p = 0.0219) higher amount of total RA was detected in the Cyp26a1−/− MNs as compared with WT MNs at day 7. R. palmitate, retinyl palmitate. F, cyp26b1 mRNA levels at day 7 during MN differentiation. No differences were seen between WT and Cyp26a1−/− MNs. These data are from at least three biological repeats (n > 3), and all qPCR data, in triplicate for each experiment, are normalized to 36b4 mRNA. y axes, arbitrary units; note differences in y axes in different panels.
FIGURE 3.
Immunocytochemistry of key protein markers identifies Cyp26a1−/− MNs as LMC MNs. A, HB9 protein (all panels, red fluorescence), ALDH1a2 (top panels, green fluorescence), Hoxc6 (middle panels, green fluorescence), VAChT (bottom panels, green fluorescence), and DAPI (all panels, blue fluorescence). The “merge” in each set of panels shows overlap of HB9, DAPI, and ALDH1a2 (top panels), Hoxc6 (middle panels), and VAChT (bottom panels). WT (left) and cytochrome P450 Cyp26a1−/− ESCs were treated with RA and SHH, and representative pictures were taken at day 7. B, panels are as described in A except that WT (left) and Cyp26a1−/− ESCs (right) were treated only with RA (negative control), and representative pictures were taken at day 7. These data are representative of data from three independent, biological repeats. Magnification is ×200 in all pictures.
These MNs also expressed VAChT, a cholinergic marker, indicating that they had activated the enzymatic pathways for producing acetylcholine, the neurotransmitter released by MNs during formation of functional synapses (37) (Figs. 2B and 3A, bottom panels, green images). Thus, Cyp26a1−/− ESCs are capable of differentiating into HB9+ spinal motor neurons that express VAChT, a marker for functional spinal motor neurons.
RA and SHH (Hh Ag-1.3) Cooperate to Induce Aldh1a2 in Cyp26a1−/− ESCs
To define the contribution of retinoid signaling to the motor neuron differentiation process, we examined the expression of aldehyde dehydrogenase 1a2 (ALDH1a2, RALDH2), an important marker for LMC neurons (38) and a key enzyme in the synthesis of active retinoids (39–41). Aldh1a2 expression is initiated during the early phase of MN generation at brachial levels of the spinal cord and distinguishes developing LMC neurons from other somatic or visceral MNs (38). The aldh1a2 mRNA level was 2.4-fold higher at day 6 and 8.6 ± 2.5-fold (p = 0.01) higher in the Cyp26a1−/−-derived day 7 MNs as compared with the WT (Fig. 2C). Thus, the maximal difference occurs at day 7 compared with earlier time points. We confirmed the Aldh1a2 expression in the Cyp26a1−/− MNs at the protein level via immunocytochemistry (Fig. 3A, top panels, green images). Aldh1a2 expression correlates with the highest HB9 mRNA and protein levels at day 7 (Figs. 2 (A and C) and 3A). aldh1a2 induction in the Cyp26a1−/− MNs depends on the combined actions of RA and SHH, because neither treatment alone induced aldh1a2 to comparable levels (Fig. 2C).
To test further the role of Cyp26a1, we generated “rescue” lines in which the full-length murine cyp26a1 cDNA driven by a doxycycline-inducible promoter was reintroduced into the Cyp26a1−/− ESCs. In the “rescue” line, Cyp26a1−/−tet:cyp26a1-21, the cyp26a1 mRNA was increased by 2.2-fold in day 6 RA plus SHH plus doxycycline (1 μg/ml)-treated versus RA plus SHH-treated cells. Similarly, the ALDH1a2 mRNA level was decreased in the Cyp26a1−/−tet:cyp26a1-21 line by 1.8-fold when doxycycline was included along with RA plus SHH. These data indicate that the lack of Cyp26a1 results in much higher ALDH1a2 mRNA levels (Fig. 2C). Moreover, they show that the phenotype can be partially reversed when Cyp26a1 activity is partially restored relative to the Cyp26a1 level in RA-treated WT ESCs. These data suggest that although both WT and Cyp26a1−/− ESCs are capable of differentiating to MNs, only the Cyp26a1−/−-derived MNs differentiate to MNs of LMC identity, as measured by their high Aldh1a2 levels.
Cyp26a1−/− ESCs Generate Spinal Motor Neurons with an Altered Retinoid Profile
We next measured both the intracellular and medium retinoid contents of day 7 MNs via HPLC analyses. The Cyp26a1−/− MNs had a significantly higher amount of total moles (intracellular plus medium) of RA (92.4 ± 25.1-fold, p = 0.022) as compared with WT (Fig. 2E). The WT MNs displayed increased cyp26a1 mRNA levels and protein (data not shown) in response to RA and peak cyp26a1 mRNA levels at days 6 and 7 (Fig. 2D). Thus, cyp26a1 mRNA is expressed during the differentiation of WT ESCs to MNs, demonstrating that Cyp26a1 is a major RA-metabolizing enzyme in these MNs. Furthermore, the lack of Cyp26a1 leads to an increased intracellular RA concentration (Fig. 2E).
Cyp26b1 displays substrate specificity similar to that of Cyp26a1 (42); Cyp26b1 is expressed at thoracic but not brachial levels of the spinal cord (43). cyp26b1 transcript levels were similar in WT and Cyp26a1−/− cells on day 7 of the differentiation protocol (Fig. 2F).
Cyp26a1 Is Necessary for the Establishment of the Proper Hoxc Expression Profile
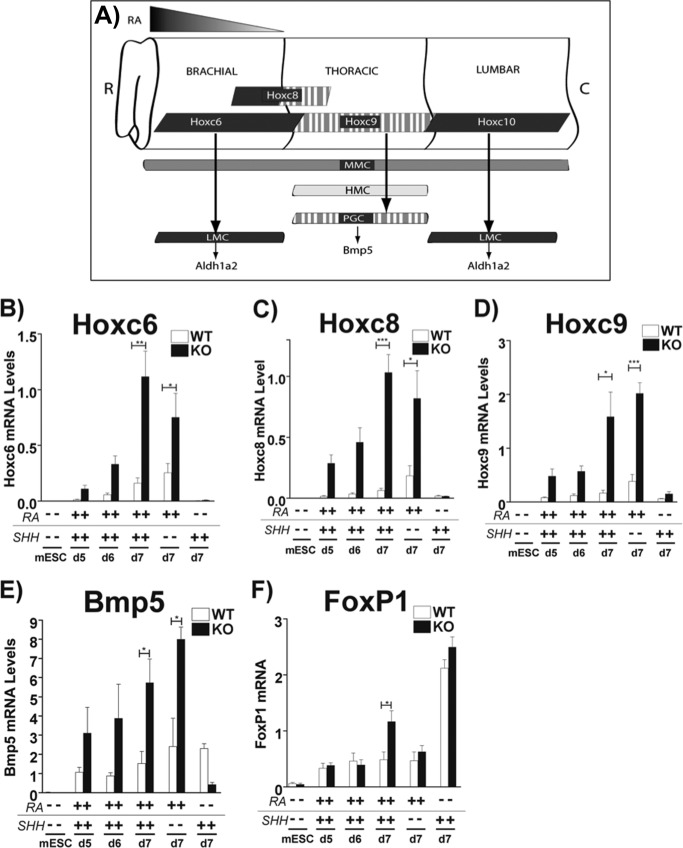
Because Aldh1a2 expression and RA concentration were significantly higher in the day 7 spinal MNs derived from Cyp26a1−/− ESCs, we next determined if there were changes in the expression of Hoxc proteins, critical determinants of spinal motor neuron columnar identity (44). hoxc6 and hoxc8 transcripts are highly co-expressed by brachial level LMC MNs, and this expression coincides with aldh1a2 expression (45) (Fig. 4A). Cyp26a1−/−-derived day 7 MNs expressed 7 ± 1.4-fold higher (p = 0.0016) levels of hoxc6 and 16.4 ± 2.4-fold higher (p < 0.0001) hoxc8 transcripts than WT (Fig. 4, B and C), suggesting that Cyp26a1−/− MNs have a greater propensity to differentiate to a brachial LMC identity. We also confirmed the increased expression of HoxC6 at the protein level in Cyp26a1−/− MNs by immunocytochemistry (Fig. 3A, middle panels, green images). We determined that ∼5% of WT and ∼80% of Cyp26a1−/− MNs were HoxC6-positive at day 7 (Fig. 3A). No hoxc10 transcripts were detected, indicating that differentiation to a lumbar LMC identity (Fig. 4A) does not take place (data not shown).
FIGURE 4.
Cyp26a1−/− MNs have an altered Hoxc expression profile. A, rostrocaudal domains of Hoxc expression in MNs, with respect to molecularly defined MN columns (modified from Ref. 45). HMC, hypaxial motor column; R, rostral; C, caudal. B, Cyp26a1−/− MNs exhibit 7 ± 1.4-fold (p = 0.0016) higher levels of hoxc6 mRNA as compared with WT MNs upon RA plus SHH treatment. The Cyp26a1−/− MNs also exhibited 3 ± 0.8-fold (p = 0.0301) higher hoxc6 mRNA levels compared with WT MNs at day 7 with RA treatment alone. C, hoxc8 mRNA expression was 16.4 ± 2.4-fold (p < 0.0001) higher in the Cyp26a1−/− MNs as compared with WT MNs at day 7. The Cyp26a1−/− MNs also exhibited 4.5 ± 1.2-fold (p = 0.0242) higher hoxc8 mRNA levels compared with WT MNs at day 7 with RA treatment alone. D, Cyp26a1−/− MNs exhibit 9.5 ± 2.7-fold (p = 0.0154) higher levels of hoxc9 mRNA compared with WT MNs at day 7. The Cyp26a1−/− MNs also exhibited 5.3 ± 0.5-fold (p = 0.0005) higher hoxc9 mRNA levels compared with WT MNs at day 7 with RA treatment alone. E, bmp5 mRNA expression levels were 3.7 ± 0.8-fold (p = 0.0390) higher in Cyp26a1−/− MNs at day 7 compared with WT MNs. The Cyp26a1−/− MNs also exhibited 3.3 ± 0.2-fold (p = 0.0257) higher bmp5 mRNA levels compared with WT MNs at day 7 with RA treatment alone. F, foxP1 mRNA was 2.4 ± 0.4-fold (p = 0.0211) higher in the Cyp26a1−/− MNs compared with WT MNs at day 7. These data are from at least three biological repeats, and all qPCR data, from triplicate samples, are normalized to 36b4 mRNA. y axes, arbitrary units; note differences in y axes in different panels. Error bars, S.E.
Because RA is a known caudalizing factor during embryogenesis, we wanted to determine if other, more caudally expressed hoxc genes, such as hoxc9, exhibited an altered mRNA expression profile in the Cyp26a1−/− as compared with the WT MNs. hoxc9 expression by MNs is restricted to thoracic levels and coincides with the expression of Bmp5, a transforming growth factor-β family member (45, 46). Together, Hoxc9 and Bmp5 distinguish preganglionic column (PGC) MNs from other thoracic level MNs. The Cyp26a1−/−-derived day 5, 6, and 7 MNs expressed higher hoxc9 (9.5 ± 2.7-fold, p = 0.0154) and bmp5 (3.7 ± 0.8-fold, p = 0.039) mRNA levels as compared with WT (Fig. 4, D and E), indicative of a PGC subtype identity. The differences in hoxc9 mRNA levels between the Cyp26a1−/− and WT were maximal at day 7 (Fig. 4, D and E).
We next determined whether expression of FoxP1, a putative cofactor required for deployment of Hox programs in LMC and PGC MNs, was altered (47, 48). foxP1 mRNA level was 2.4 ± 0.4-fold higher (p = 0.02) in day 7 RA plus SHH-treated Cyp26a1−/− as compared with WT MNs (Fig. 4F). We suggest that the lack of Cyp26a1 function alters the Hox-controlled molecular programs that specify motor column subtypes via FoxP1, which acts downstream of the cross-repressive Hox network and engages this network to activate columnar-specific programs (47). This increased FoxP1 expression, as well as the increased intracellular RA level in the Cyp26a1−/− MNs, suggest that FoxP1 acts in an RA-dependent manner.
Our data show that the lack of Cyp26a1 function results in increased expression of Hoxc mRNA and proteins in day 7 MNs. However, hoxc6, hoxc8, and hoxc9 mRNA levels were also 3 ± 0.8-fold (p = 0.03), 4.5 ± 1.2-fold (p = 0.02), and 5.3 ± 0.5-fold (p = 0.0005) higher, respectively, at day 7 in the Cyp26a1−/− versus WT cells treated with RA alone (Fig. 4, B–D). This hoxc expression was independent of HB9 because HB9 mRNA and protein levels were low to absent in the Cyp26a1−/− and WT cells treated with RA alone (Figs. 2A and 3B).
The Lack of Cyp26a1 Function Results in an LMC Subtype Identity
We next assessed whether the mRNA levels of the LIM homeodomain transcription factors that delineate motor neuron columnar subtypes at different rostrocaudal positions were changed because of the altered Aldh1a2 and Hoxc expression profiles (Fig. 5A). The expression of Aldh1a2 by LMC neurons directs lateral LMC (LMC(l)) divisional identity, as defined by co-expression of Isl2 and Lim1 (49). LMC MNs are located only at the limb levels of the spinal cord. The Cyp26a1−/− MNs expressed 2.4 ± 0.4-fold higher (p = 0.01) lim1 mRNA levels at day 7 as compared with WT, suggesting that the Cyp26a1−/− cells were differentiating to MNs specific for the lateral subtype of the LMC (LMC(l)) (Fig. 5E). However, there was no significant difference in the isl2 transcript levels in Cyp26a1−/− versus WT cells (Fig. 5C). The characterization of the Cyp26a1−/− MNs as an LMC MN subtype is also supported by the high transcript levels of foxP1 (Fig. 4F), a marker selectively expressed in MNs of the Hox-sensitive LMC and PGC (47, 50, 51). Additionally, the Cyp26a1−/− MNs showed a 2 ± 0.4-fold higher (p = 0.02) isl1 mRNA level as compared with the WT MNs at day 7 (Fig. 5B). The LIM homeodomain transcription factor, Isl1, is required for the survival and specification of spinal motor neurons; all LMC neurons initially express Isl1, and its expression is maintained in the medial division of the LMC (LMC(m)) (34, 49, 52). Taken together, these molecular data indicate that Cyp26a1−/−-derived MNs differentiate to an LMC subtype identity as compared with MNs derived from WT ESCs, which differentiate to an MMC identity. Treatment of WT ESCs with higher exogenous RA concentrations (e.g. 3 μm RA) did not result in a LMC identity (data not shown), indicating that inhibition of Cyp26a1 is necessary for LMC subtype differentiation.
FIGURE 5.
The LIM HD code is altered in the Cyp26a1−/− MNs. A, LIM HD code of motor columns. LMC(m), medial subtype of lateral motor column; LMC(l), lateral subtype of lateral motor column; MMC(m), medial subtype of median motor column. B, isl1 mRNA expression was 2 ± 0.4-fold (p = 0.021) higher in the Cyp26a1−/− MNs compared with WT MNs at day 7. In contrast, WT MNs exhibited 2.93 ± 0.2-fold (p = 0.0001) higher isl1 mRNA levels compared with Cyp26a1−/− MNs at day 7 with SHH treatment alone. C, isl2 mRNA is expressed in both WT and Cyp26a1−/− MNs at day 7. D, lhx3 mRNA, a marker for the MMC, is 2.4 ± 0.4-fold (p = 0.0298) higher in the WT MNs compared with Cyp26a1−/− MNs at day 7. E, lim1 mRNA is 2.4 ± 0.4-fold (p = 0.012) higher in the Cyp26a1−/− MNs compared with the WT MNs at day 7. The Cyp26a1−/− MNs also exhibited 2.45 ± 0.5-fold (p = 0.0396) higher Lim1 mRNA levels compared with WT MNs at day 7 with RA treatment alone. These data are from at least three biological repeats, and all qPCR data are normalized to 36b4 mRNA. y axes, arbitrary units; note differences in y axes in different panels. Error bars, S.E. F, summary diagram of WT and Cyp26a1−/− ESC differentiation into various columns of motor neurons (modified from Jessell et al. (56)). The LMC MNs are generated only at forelimb and hindlimb levels and send axons into limb mesenchyme. The MMCs innervate the dorsal axial musculature. WT ESCs express Cyp26a1 so that they have lower RA levels (Fig. 2E) and higher RA polar metabolite levels. The Cyp26a1−/− ESCs have sustained, higher intracellular RA levels (Fig. 2E) and very low levels of polar metabolites of RA. These differences lead to differences in the types of MNs. The thicker arrow indicates sustained, higher [RA].
We then addressed the question of whether the Cyp26a1−/− MNs also expressed lhx3, a LIM homeobox gene specific for the MMC (Fig. 5A). The motor neurons that innervate axial muscles are located in the MMC and are present at all segmental levels of the spinal cord (53). The majority of WT ESC-derived MNs have been reported to express high levels of Lhx3, indicating that WT ESC-derived MNs are of the MMC subtype identity (9, 54, 55). Cyp26a1−/− MNs expressed 2.4 ± 0.46-fold lower (p = 0.03) levels of lhx3 mRNA than WT MNs on days 6 and 7 (Fig. 5D). These data suggest that the Cyp26a1−/− MNs differentiate to an LMC subtype rather than an MMC subtype.
DISCUSSION
Roles of RA in Spinal MN Subtype Diversification
Inductive signals, such as RA, from the axial and paraxial mesoderm operate along the dorsoventral and rostrocaudal axes of the neural tube (46, 56–58). Although all MNs arise from the same progenitor domain in the ventral spinal cord, one major feature of MN diversification at different rostrocaudal levels of the spinal cord is the formation of distinct columnar classes that can be delineated by specific Hoxc and LIM HD protein expression profiles (44, 49, 59–62) (Figs. 4A and 5A). Expression of Hoxc6 segregates with the brachial (forelimb) LMC MNs, and expression of Hoxc9 segregates with thoracic level PGC MNs, and thus, the LMC and PGC are considered Hox-dependent motor columns (45, 50).
RA is required for normal patterning of Hox gene expression along the rostrocaudal body axis (44), but the precise mechanism by which Hox-dependent subtypes are generated within discrete areas of the spinal cord is not fully understood. RA is also required to specify the brachial LMC, composed of MNs that extend axons into the limb, because Aldh1a2 is expressed at strikingly higher levels adjacent to the brachial level spinal cord (63). Aldh1a2 is later expressed by LMC neurons themselves (38, 39), and this motor neuronal source of retinoids directs the diversification of LMC neurons into medial and lateral subtypes (38). In contrast, MNs that innervate axial muscles are located in the Hox-independent MMC and are present at all segmental levels of the spinal cord, including the limb levels (Fig. 4A) (62). How the distinction between LMC, PGC, and MMC MNs is achieved remains an important question, which we have addressed here.
Cyp26a1 Function Is Not Necessary for the Formation of MN Progenitors or MNs
Our data demonstrate that a lack of Cyp26a1 function leads to increased levels of the class I HD transcription factor, Pax6. Despite this increase in pax6 mRNA (Fig. 1C), neither Nkx6.1, a class II HD transcription factor, nor Olig2, an MN progenitor marker, was expressed at a different level in Cyp26a1−/− MNs compared with WT MNs (Fig. 1, D and E). Within the MN lineage, the transition from a class I and II HD defined progenitor domain to MN progenitor status is marked by Olig2 expression (27, 28). Retinoid signaling is necessary for promoting the transition of Pax6+, Nkx6.1+ ventral progenitors to Olig2+ MN progenitors (64). Our data show that although the lack of Cyp26a1 function led to 2.6 ± 0.3-fold (p = 0.015) higher Pax6 transcript levels and resulted in an altered ventral progenitor domain profile compared with WT, the ability of Cyp26a1−/− ESCs to become MN progenitors and subsequently HB9+ MNs was not altered as compared with WT ESCs (Fig. 2A).
RA and Shh have crucial and complementary roles in directing the transcriptional cascade that specifies MN progenitor identity. Cyp26a1−/− MNs do not have alterations in shh mRNA as compared with WT (Fig. 1F), suggesting that RA does not act directly on shh during MN progenitor boundary formation. The lack of Cyp26a1 function and resulting increase in intracellular RA levels (Fig. 2E) do not prevent Cyp26a1−/− ESCs from developing into MN progenitors, suggesting that increased levels of RA in the Cyp26a1−/− pMNs at this stage do not inhibit MN differentiation.
Cyp26a1 Function Is Necessary for the Differentiation of murine ESCs to MMC MNs
We show that the Cyp26a1−/− MNs have a greater ability to differentiate to an LMC(l) subtype identity, as assessed by the 8.6 ± 2.5-fold (p = 0.012) higher aldh1a2 mRNA levels, the 7 ± 1.4-fold (p = 0.0016) higher hoxc6 mRNA levels, and the 2.4 ± 0.4-fold (p = 0.01) higher lim1 transcript levels in these cells compared with WT MNs (Figs. 2C, 4B, and 5E). The Cyp26a1−/− MNs also exhibit increased expression of markers characteristic of a PGC subtype identity, namely 9.5 ± 2.7-fold (p = 0.015) higher hoxc9 transcripts and 3.7 ± 0.8-fold (p = 0.039) higher bmp5 transcripts (Fig. 4, D and E). WT ESCs differentiate to MNs that express high Lhx3, a LIM HD protein characteristic of the MMC (9). Our data confirm these findings and show that the WT MNs express 2.4 ± 0.4-fold (p = 0.0298) higher lhx3 transcripts compared with the Cyp26a1−/− MNs at day 7 (Fig. 5D). Thus, our data suggest that Cyp26a1 is necessary for the development of the Hox-independent MMC MN subtype.
There were no significant differences in the isl2 mRNA levels between the two cell lines at day 7 (Fig. 5C). However, isl2 is expressed in both the MMC and LMC (Fig. 5A). We observed higher isl1 mRNA levels in the Cyp26a1−/− MNs, characteristic of an LMC identity, as well (Fig. 5B). Our data demonstrate that lack of Cyp26a1 function in ESCs drives these cells to differentiate to MNs of an LMC and PGC subtype identity and that this effect is dependent on the cooperative actions of RA plus SHH, because neither RA alone- nor SHH alone-treated MNs induced HB9 or subsequent markers for specific motor columns (Figs. 2A and 3B).
The Lack of Cyp26a1 Function Increases Hoxc Expression at All Rostrocaudal Levels
The profile of Hoxc protein expression by postmitotic MNs is a major determinant of their columnar fate (Fig. 4A). Hoxc6 is able to function as a postmitotic determinant of brachial LMC identity (45), whereas Hoxc9 is required for the generation of PGC thoracic motor column neurons (65). Additionally, the cross-repressive interactions between Hoxc6 and Hoxc9 in postmitotic Hb9+ and Isl1+ MNs consolidate the distinct Hox profiles of LMC and PGC MNs (45).
Our data demonstrate that a lack of Cyp26a1 function results in increases in hoxc6, hoxc8, and hoxc9 mRNA levels (Fig. 4, B–D) and Hoxc6 protein (Fig. 3A). Although RA from the paraxial mesoderm has been shown to regulate Hoxc expression at brachial levels (44), and retinoids in part antagonize the FGF gradient (66, 67), the mechanisms underlying this process remain unclear. Here we show that the lack of Cyp26a1 function alters this mechanism and results in a pan-up-regulation of Hoxc expression, including Hoxc9, a global repressor of other Hox genes (65) (Fig. 4D). Despite the increase in hoxc9 mRNA levels in the Cyp26a1−/− MNs, hoxc6 did not undergo the repression reported previously (45, 65) (Fig. 4B). Our data suggest that lack of Cyp26a1 and the resultant sustained increase in intracellular RA concentration can override the cross-repressive interaction between Hoxc6 and Hoxc9. Additionally, the increases in hoxc9 and bmp5 mRNAs in the Cyp26a1−/− MNs indicate that there are also MNs of a PGC subtype identity in the day 7 Cyp26a1−/− MN population (Fig. 4, D and E). Thus, a lack of Cyp26a1 function drives ESCs to differentiate to MNs characteristic of the Hox-dependent motor columns, LMC and PGC, and not MNs of the Hox-independent MMC. Further, these data suggest that the sustained, increased intracellular RA concentration, as seen in the Cyp26a1−/− MNs, is needed to achieve the generation of these Hox-dependent columns, whereas the metabolizing ability of Cyp26a1 is necessary for the development of the Hox-independent MMC, as seen in the WT MNs (Fig. 4).
Recently, a reduction in the polycomb group protein Bmi1, via in ovo electroporation of shRNA into the developing chick spinal cord, was shown to switch LMC MNs to PGC MNs via derepression of Hoxc9; this points to the critical role of Hoxc9 in cell fate specification (68). This increase in Hoxc9 expression, via depletion of Bmi1, not only resulted in changes in molecular identity but also changes in peripheral connectivity (i.e. instead of bypassing the sympathetic ganglia and projecting to the limb, the axons in Bmi1-depleted LMC MNs innervated sympathetic ganglia).
Use of ESCs in Neurodegenerative Diseases
The use of ESCs in patients will depend to a great extent on the ability of the ESCs to differentiate into disease-relevant cell types. Induced pluripotent stem cells were shown to differentiate into HB9+ MNs when treated as EBs in cell culture with RA and a sonic hedgehog agonist (5). WT ESCs, treated with RA and SHH to differentiate them into MNs, migrated to the MMC and functioned like endogenous MMC MNs (9, 11). Furthermore, ESC-derived MNs sent axons that reached target muscles and partially reversed paralysis in a rat model of virus-induced MN death (69). Although we used a genetic approach in this study to block Cyp26a1 function, specific inhibitors of Cyp26a1 could potentially be used to enhance differentiation of ESCs to brachial LMC MNs that extend axons into the limbs. Thus, our findings could ultimately provide ALS patients and others with neurodegenerative diseases with improved use of their limbs.
Acknowledgments
We thank Dr. John Wagner for comments on the manuscript. We thank Curis, Inc., for generously providing the Hh agonist Hh-Ag1.3. We thank Carlos Rodriguez for assistance with the RA metabolism assays and Tamara Weissman for editorial assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA43796 (to L. J. G.). This work was also supported by Weill Cornell funds.
- MN
- motor neuron
- ALS
- amyotrophic lateral sclerosis
- EB
- embryoid body
- ESC
- embryonic stem cell
- HD
- homeodomain
- LMC
- lateral motor column
- MMC
- median motor column
- PGC
- preganglionic column
- qPCR
- quantitative real-time PCR
- RA
- all-trans-retinoic acid
- pMN
- MN progenitor
- VAChT
- vesicular acetylcholine transporter
- SHH
- sonic hedgehog agonist
- Shh and shh
- sonic hedgehog protein and transcript, respectively.
REFERENCES
- 1. Ludolph A. C., Brettschneider J., Weishaupt J. H. (2012) Amyotrophic lateral sclerosis. Curr. Opin. Neurol. 25, 530–535 [DOI] [PubMed] [Google Scholar]
- 2. Pasinelli P., Brown R. H. (2006) Molecular biology of amyotrophic lateral sclerosis. Insights from genetics. Nat. Rev. Neurosci. 7, 710–723 [DOI] [PubMed] [Google Scholar]
- 3. Bruijn L. I., Miller T. M., Cleveland D. W. (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 [DOI] [PubMed] [Google Scholar]
- 4. López-González R., Kunckles P., Velasco I. (2009) Transient recovery in a rat model of familial amyotrophic lateral sclerosis after transplantation of motor neurons derived from mouse embryonic stem cells. Cell Transplant. 18, 1171–1181 [DOI] [PubMed] [Google Scholar]
- 5. Dimos J. T., Rodolfa K. T., Niakan K. K., Weisenthal L. M., Mitsumoto H., Chung W., Croft G. F., Saphier G., Leibel R., Goland R., Wichterle H., Henderson C. E., Eggan K. (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 [DOI] [PubMed] [Google Scholar]
- 6. Egawa N., Kitaoka S., Tsukita K., Naitoh M., Takahashi K., Yamamoto T., Adachi F., Kondo T., Okita K., Asaka I., Aoi T., Watanabe A., Yamada Y., Morizane A., Takahashi J., Ayaki T., Ito H., Yoshikawa K., Yamawaki S., Suzuki S., Watanabe D., Hioki H., Kaneko T., Makioka K., Okamoto K., Takuma H., Tamaoka A., Hasegawa K., Nonaka T., Hasegawa M., Kawata A., Yoshida M., Nakahata T., Takahashi R., Marchetto M. C., Gage F. H., Yamanaka S., Inoue H. (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 4, 145ra104. [DOI] [PubMed] [Google Scholar]
- 7. Miles G. B., Yohn D. C., Wichterle H., Jessell T. M., Rafuse V. F., Brownstone R. M. (2004) Functional properties of motoneurons derived from mouse embryonic stem cells. J. Neurosci. 24, 7848–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harper J. M., Krishnan C., Darman J. S., Deshpande D. M., Peck S., Shats I., Backovic S., Rothstein J. D., Kerr D. A. (2004) Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc. Natl. Acad. Sci. U.S.A. 101, 7123–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soundararajan P., Miles G. B., Rubin L. L., Brownstone R. M., Rafuse V. F. (2006) Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J. Neurosci. 26, 3256–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Björklund L. M., Isacson O. (2002) Regulation of dopamine cell type and transmitter function in fetal and stem cell transplantation for Parkinson's disease. Prog. Brain Res. 138, 411–420 [DOI] [PubMed] [Google Scholar]
- 11. Soundararajan P., Fawcett J. P., Rafuse V. F. (2010) Guidance of postural motoneurons requires MAPK/ERK signaling downstream of fibroblast growth factor receptor 1. J. Neurosci. 30, 6595–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gudas L. J., Wagner J. A. (2011) Retinoids regulate stem cell differentiation. J. Cell Physiol. 226, 322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang X. H., Gudas L. J. (2011) Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 6, 345–364 [DOI] [PubMed] [Google Scholar]
- 14. Langton S., Gudas L. J. (2008) CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Dev. Biol. 315, 331–354 [DOI] [PubMed] [Google Scholar]
- 15. Wichterle H., Peljto M. (2008) Differentiation of mouse embryonic stem cells to spinal motor neurons. Curr. Protoc. Stem Cell Biol. Chapter 1, Unit 1H, 1.1–1H.1.9 [DOI] [PubMed] [Google Scholar]
- 16. Chen A. C., Gudas L. J. (1996) An analysis of retinoic acid-induced gene expression and metabolism in AB1 embryonic stem cells. J. Biol. Chem. 271, 14971–14980 [DOI] [PubMed] [Google Scholar]
- 17. Laursen K. B., Wong P. M., Gudas L. J. (2012) Epigenetic regulation by RARα maintains ligand-independent transcriptional activity. Nucleic Acids Res. 40, 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Touma S. E., Perner S., Rubin M. A., Nanus D. M., Gudas L. J. (2009) Retinoid metabolism and ALDH1A2 (RALDH2) expression are altered in the transgenic adenocarcinoma mouse prostate model. Biochem. Pharmacol. 78, 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen A. C., Guo X., Derguini F., Gudas L. J. (1997) Human breast cancer cells and normal mammary epithelial cells. Retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res. 57, 4642–4651 [PubMed] [Google Scholar]
- 20. Guo X., Gudas L. J. (1998) Metabolism of all-trans-retinol in normal human cell strains and squamous cell carcinoma (SCC) lines from the oral cavity and skin. Reduced esterification of retinol in SCC lines. Cancer Res. 58, 166–176 [PubMed] [Google Scholar]
- 21. Frank-Kamenetsky M., Zhang X. M., Bottega S., Guicherit O., Wichterle H., Dudek H., Bumcrot D., Wang F. Y., Jones S., Shulok J., Rubin L. L., Porter J. A. (2002) Small-molecule modulators of Hedgehog signaling. Identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bain G., Ray W. J., Yao M., Gottlieb D. I. (1996) Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem. Biophys. Res. Commun. 223, 691–694 [DOI] [PubMed] [Google Scholar]
- 23. Hu B. Y., Zhang S. C. (2009) Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 4, 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niwa H., Miyazaki J., Smith A. G. (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 24, 372–376 [DOI] [PubMed] [Google Scholar]
- 25. Briscoe J., Ericson J. (2001) Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 11, 43–49 [DOI] [PubMed] [Google Scholar]
- 26. Ericson J., Rashbass P., Schedl A., Brenner-Morton S., Kawakami A., van Heyningen V., Jessell T. M., Briscoe J. (1997) Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169–180 [DOI] [PubMed] [Google Scholar]
- 27. Novitch B. G., Chen A. I., Jessell T. M. (2001) Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31, 773–789 [DOI] [PubMed] [Google Scholar]
- 28. Mizuguchi R., Sugimori M., Takebayashi H., Kosako H., Nagao M., Yoshida S., Nabeshima Y., Shimamura K., Nakafuku M. (2001) Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron 31, 757–771 [DOI] [PubMed] [Google Scholar]
- 29. Lu Q. R., Sun T., Zhu Z., Ma N., Garcia M., Stiles C. D., Rowitch D. H. (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 [DOI] [PubMed] [Google Scholar]
- 30. Takebayashi H., Nabeshima Y., Yoshida S., Chisaka O., Ikenaka K. (2002) The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 12, 1157–1163 [DOI] [PubMed] [Google Scholar]
- 31. Zhou Q., Anderson D. J. (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73 [DOI] [PubMed] [Google Scholar]
- 32. Sander M., Paydar S., Ericson J., Briscoe J., Berber E., German M., Jessell T. M., Rubenstein J. L. (2000) Ventral neural patterning by Nkx homeobox genes. Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 14, 2134–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vallstedt A., Muhr J., Pattyn A., Pierani A., Mendelsohn M., Sander M., Jessell T. M., Ericson J. (2001) Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron 31, 743–755 [DOI] [PubMed] [Google Scholar]
- 34. Pfaff S. L., Mendelsohn M., Stewart C. L., Edlund T., Jessell T. M. (1996) Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84, 309–320 [DOI] [PubMed] [Google Scholar]
- 35. Arber S., Han B., Mendelsohn M., Smith M., Jessell T. M., Sockanathan S. (1999) Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674 [DOI] [PubMed] [Google Scholar]
- 36. Thaler J., Harrison K., Sharma K., Lettieri K., Kehrl J., Pfaff S. L. (1999) Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron 23, 675–687 [DOI] [PubMed] [Google Scholar]
- 37. de Castro B. M., De Jaeger X., Martins-Silva C., Lima R. D., Amaral E., Menezes C., Lima P., Neves C. M., Pires R. G., Gould T. W., Welch I., Kushmerick C., Guatimosim C., Izquierdo I., Cammarota M., Rylett R. J., Gomez M. V., Caron M. G., Oppenheim R. W., Prado M. A., Prado V. F. (2009) The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell Biol. 29, 5238–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sockanathan S., Jessell T. M. (1998) Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 94, 503–514 [DOI] [PubMed] [Google Scholar]
- 39. Zhao D., McCaffery P., Ivins K. J., Neve R. L., Hogan P., Chin W. W., Dräger U. C. (1996) Molecular identification of a major retinoic-acid-synthesizing enzyme, a retinaldehyde-specific dehydrogenase. Eur. J. Biochem. 240, 15–22 [DOI] [PubMed] [Google Scholar]
- 40. Niederreither K., Subbarayan V., Dollé P., Chambon P. (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448 [DOI] [PubMed] [Google Scholar]
- 41. Mic F. A., Haselbeck R. J., Cuenca A. E., Duester G. (2002) Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129, 2271–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White J. A., Ramshaw H., Taimi M., Stangle W., Zhang A., Everingham S., Creighton S., Tam S. P., Jones G., Petkovich M. (2000) Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc. Natl. Acad. Sci. U.S.A. 97, 6403–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abu-Abed S., MacLean G., Fraulob V., Chambon P., Petkovich M., Dollé P. (2002) Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech. Dev. 110, 173–177 [DOI] [PubMed] [Google Scholar]
- 44. Liu J. P., Laufer E., Jessell T. M. (2001) Assigning the positional identity of spinal motor neurons. Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron 32, 997–1012 [DOI] [PubMed] [Google Scholar]
- 45. Dasen J. S., Liu J. P., Jessell T. M. (2003) Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature 425, 926–933 [DOI] [PubMed] [Google Scholar]
- 46. William C. M., Tanabe Y., Jessell T. M. (2003) Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development 130, 1523–1536 [DOI] [PubMed] [Google Scholar]
- 47. Dasen J. S., De Camilli A., Wang B., Tucker P. W., Jessell T. M. (2008) Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell 134, 304–316 [DOI] [PubMed] [Google Scholar]
- 48. Rousso D. L., Gaber Z. B., Wellik D., Morrisey E. E., Novitch B. G. (2008) Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron 59, 226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsuchida T., Ensini M., Morton S. B., Baldassare M., Edlund T., Jessell T. M., Pfaff S. L. (1994) Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79, 957–970 [DOI] [PubMed] [Google Scholar]
- 50. Dasen J. S., Jessell T. M. (2009) Hox networks and the origins of motor neuron diversity. Curr. Top. Dev. Biol. 88, 169–200 [DOI] [PubMed] [Google Scholar]
- 51. Peljto M., Dasen J. S., Mazzoni E. O., Jessell T. M., Wichterle H. (2010) Functional diversity of ESC-derived motor neuron subtypes revealed through intraspinal transplantation. Cell Stem Cell 7, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liang X., Song M. R., Xu Z., Lanuza G. M., Liu Y., Zhuang T., Chen Y., Pfaff S. L., Evans S. M., Sun Y. (2011) Isl1 is required for multiple aspects of motor neuron development. Mol. Cell Neurosci. 47, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kania A., Johnson R. L., Jessell T. M. (2000) Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell 102, 161–173 [DOI] [PubMed] [Google Scholar]
- 54. Wichterle H., Lieberam I., Porter J. A., Jessell T. M. (2002) Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397 [DOI] [PubMed] [Google Scholar]
- 55. Peljto M., Wichterle H. (2011) Programming embryonic stem cells to neuronal subtypes. Curr. Opin. Neurobiol. 21, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jessell T. M. (2000) Neuronal specification in the spinal cord. Inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20–29 [DOI] [PubMed] [Google Scholar]
- 57. Livet J., Sigrist M., Stroebel S., De Paola V., Price S. R., Henderson C. E., Jessell T. M., Arber S. (2002) ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35, 877–892 [DOI] [PubMed] [Google Scholar]
- 58. Tanabe Y., Jessell T. M. (1996) Diversity and pattern in the developing spinal cord. Science 274, 1115–1123 [DOI] [PubMed] [Google Scholar]
- 59. Landmesser L. (1978) The development of motor projection patterns in the chick hind limb. J. Physiol. 284, 391–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hollyday M. (1980) Organization of motor pools in the chick lumbar lateral motor column. J. Comp. Neurol. 194, 143–170 [DOI] [PubMed] [Google Scholar]
- 61. Hollyday M. (1980) Motoneuron histogenesis and the development of limb innervation. Curr. Top. Dev. Biol. 15, 181–215 [DOI] [PubMed] [Google Scholar]
- 62. Gutman C. R., Ajmera M. K., Hollyday M. (1993) Organization of motor pools supplying axial muscles in the chicken. Brain Res. 609, 129–136 [DOI] [PubMed] [Google Scholar]
- 63. Sockanathan S., Perlmann T., Jessell T. M. (2003) Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron 40, 97–111 [DOI] [PubMed] [Google Scholar]
- 64. Novitch B. G., Wichterle H., Jessell T. M., Sockanathan S. (2003) A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40, 81–95 [DOI] [PubMed] [Google Scholar]
- 65. Jung H., Lacombe J., Mazzoni E. O., Liem K. F., Jr., Grinstein J., Mahony S., Mukhopadhyay D., Gifford D. K., Young R. A., Anderson K. V., Wichterle H., Dasen J. S. (2010) Global control of motor neuron topography mediated by the repressive actions of a single hox gene. Neuron 67, 781–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Diez del Corral R., Storey K. G. (2004) Opposing FGF and retinoid pathways. A signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays 26, 857–869 [DOI] [PubMed] [Google Scholar]
- 67. Duester G. (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Golden M. G., Dasen J. S. (2012) Polycomb repressive complex 1 activities determine the columnar organization of motor neurons. Genes Dev. 26, 2236–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deshpande D. M., Kim Y. S., Martinez T., Carmen J., Dike S., Shats I., Rubin L. L., Drummond J., Krishnan C., Hoke A., Maragakis N., Shefner J., Rothstein J. D., Kerr D. A. (2006) Recovery from paralysis in adult rats using embryonic stem cells. Ann. Neurol. 60, 32–44 [DOI] [PubMed] [Google Scholar]