Background: Human milk contains hyaluronan (HA).
Results: Milk HA concentration is highest immediately after delivery. Treatment of epithelium with physiologic levels of milk-derived HA increases intracellular expression of β-defensin (in vitro and in vivo) and resistance to Salmonella.
Conclusion: Milk HA enhances functional antimicrobial defense mechanisms of the intestinal epithelium.
Significance: Milk HA may be a mediator of maternal protection of newborns.
Keywords: Cd44, Defensins, Epithelium, Host Defense, Hyaluronate, Toll-like Receptors (TLR), Salmonella Infection, Human Milk
Abstract
Breast-feeding is associated with enhanced protection from gastrointestinal disease in infants, mediated in part by an array of bioactive glycan components in milk that act through molecular mechanisms to inhibit enteric pathogen infection. Human milk contains hyaluronan (HA), a glycosaminoglycan polymer found in virtually all mammalian tissues. We have shown that synthetic HA of a specific size range promotes expression of antimicrobial peptides in intestinal epithelium. We hypothesize that hyaluronan from human milk also enhances innate antimicrobial defense. Here we define the concentration of HA in human milk during the first 6 months postpartum. Importantly, HA isolated from milk has a biological function. Treatment of HT-29 colonic epithelial cells with human milk HA at physiologic concentrations results in time- and dose-dependent induction of the antimicrobial peptide human β-defensin 2 and is abrogated by digestion of milk HA with a specific hyaluronidase. Milk HA induction of human β-defensin 2 expression is also reduced in the presence of a CD44-blocking antibody and is associated with a specific increase in ERK1/2 phosphorylation, suggesting a role for the HA receptor CD44. Furthermore, oral administration of human milk-derived HA to adult, wild-type mice results in induction of the murine Hβ D2 ortholog in intestinal mucosa and is dependent upon both TLR4 and CD44 in vivo. Finally, treatment of cultured colonic epithelial cells with human milk HA enhances resistance to infection by the enteric pathogen Salmonella typhimurium. Together, our observations suggest that maternally provided HA stimulates protective antimicrobial defense in the newborn.
Introduction
Numerous positive health outcomes are associated with breast-feeding in infants (1–4), particularly regarding gastrointestinal infection. Grulee et al. (5) conducted the first major evaluation of morbidity and mortality among 20,061 breast-fed and artificially fed infants in 1934, reporting as much as 50% reduction in gastrointestinal infection incidence among breast-fed infants. Modern epidemiologic studies reinforced and expanded upon these findings (1, 6), indicating that breast-feeding confers remarkably enhanced protection from both gastrointestinal and respiratory infections, including Salmonella infection (7).
In addition to nutrients, breast-feeding supplies a wide array of bioactive components that enhance both innate and adaptive immunity in the neonatal gastrointestinal tract. Milk components act as critical stimuli in the ontology of intestinal immune education and microflora development (4), supplying passive defense mediators (8, 9), growth hormones (10), prebiotics (11–14), and immunomodulators (15).
The best characterized protective milk component is soluble IgA (15). However, milk also contains an abundant and extraordinarily diverse array of glycans, including oligosaccharides, glycolipids, glycoproteins, mucins, glycosaminoglycans, and other complex carbohydrates, which provide infant protection (4, 16, 17). The ways in which human milk glycans shape innate gastrointestinal defense are diverse (16, 17), and include prebiotic function (11–14), antiadhesive antimicrobial activity (18–20), and intestinal epithelial cell modulation (21–24). Induction of altered gene expression in intestinal epithelium by human milk oligosaccharides results in enhanced protection from pathogenic Escherichia coli infection through modulation of epithelial cell surface glycans (21), and milk lactose induces the expression of antimicrobial peptide LL-37 in cultured epithelium (24), suggesting that direct effects of human milk glycans on intestinal epithelial cells may contribute significantly to the protection from gastrointestinal infection associated with breast-feeding.
Among the known glycan components of both human and bovine milk are abundant glycosaminoglycans (GAGs),2 large linear polysaccharide polymers containing amino sugars. Hyaluronan (HA) is a GAG usually found as a high molecular weight polymer and consists of repeating disaccharides of N-acetyl-β-d-glucosamine and β-d-glucuronic acid. Unlike other GAGs, HA is synthesized without a protein core and is not sulfated, nitrosylated, or phosphorylated in vivo (25). A recent study determined that HA is one of the GAGs contained in milk (26). Milk GAGs may play a significant role in enhancing intestinal defense against pathogens, as suggested by inhibition of HIV engagement with host receptor CD4 by chondroitin sulfate derived from human milk (27). However, the specific function of milk HA has not been reported previously.
HA is found in every tissue of the body, primarily in the form of high molecular weight polymers (∼107 Da), and plays a fundamental role in tissue homeostasis (28). Current evidence demonstrates that fragmented HA polymers generated in damaged or inflamed tissue act as endogenous “danger signals,” or “damage-associated molecular patterns” (29–31), triggering localized innate defense responses. Endogenous fragmented HA is thought to be recognized in much the same way as the conserved pathogen-associated molecular patterns, such as LPS and peptidoglycan, via Toll-like receptors (TLRs) (31, 32).
HA fragments play a role in enhancing innate epithelial defense independent of the proinflammatory immunomodulation characteristic of macrophage (33), chondrocyte (34), or endothelial cell activation (35) by low molecular weight HA or the stimulation of TLR4 by bacterial pathogen-associated molecular patterns (36). A polydispersed HA fragment preparation of polymers of less than 750 kDa injected intraperitoneally protects wild-type mice in a TLR4-dependent manner from a microflora-mediated epithelial damage model of colitis (37) or from the epithelium-depleting effects of radiation (38). Low molecular weight HA has been also been shown to induce elevated expression of antimicrobial defensin proteins that may contribute to enhanced epithelial defense in the intestine (39), skin (40), and vagina (41).
Defensins are small cationic peptides that play a critical role in the preservation of epithelial barrier integrity in the presence of continuous microbial challenges. These antimicrobial peptides are expressed by gastrointestinal, urogenital, and pulmonary epithelium, skin, and the ocular surface (42, 43). Defensins have direct antimicrobial activity against a wide range of human pathogens and commensals, including both Gram-positive and Gram-negative bacteria, virus, fungi, and protozoa (44). Interestingly, microbes stimulate the expression of inducible β-defensins 2, 3, and 4 in epithelium through the interaction of a variety of pathogen-associated molecular patterns with TLRs (45–49). TLR4 regulates the expression of human β-defensin 2 (HβD2) in epithelium following stimulation with LPS (48). The same cell surface receptor, TLR4, mediates the induction of HβD2, without an accompanying increase in inflammatory cytokine production, in human keratinocytes exposed to low molecular weight HA (40) and vaginal epithelium (41). Our group has recently demonstrated the TLR4-dependent induction of murine HβD2 ortholog in colonic epithelium in vivo following the administration of synthetic, specific sized HA (39). Therefore, it is becoming increasingly clear that low to intermediate molecular weight HA is an endogenous ligand capable of promoting enhanced antimicrobial defense of epithelial barriers through TLR4-dependent pathways.
Despite the growing evidence of the significant role of HA in bolstering innate defense of the intestine (37–39), an endogenous source of HA responsible for mediating innate defense remains unknown. In light of the recent report that human milk contains HA (26) and our finding that that HA promotes expression of the antimicrobial peptide HβD2 in intestinal mucosa (39), we hypothesized that innate epithelial antimicrobial defense is enhanced in the intestinal epithelium by HA supplied in breast milk. We have determined the concentration range of HA in human breast milk collected from a cohort of 44 mothers who provided multiple samples during the first 6 months after delivery. Our data confirm the previous finding that human milk contains HA (26, 50) and demonstrate that milk HA concentration is highest in the critical first weeks after birth and decreases in the population to a steady-state level over the next 2 months. Accordingly, using physiologic levels of HA, we demonstrate two independent parameters of enhanced epithelial antimicrobial defense that are specifically enhanced by HA purified from human milk: 1) HA-dependent induction of the antimicrobial peptide HβD2 in cultured human colonic epithelial cells and in murine colonic mucosa following oral administration of a milk HA preparation and 2) HA-dependent protection from intracellular infection by Salmonella typhimurium in cultured intestinal epithelial cells pretreated with milk-derived HA. Furthermore, the in vivo induction of the murine HβD2 ortholog is dependent upon expression of the cell surface receptors TLR4 and CD44.
EXPERIMENTAL PROCEDURES
Human Milk Sample Collection
Multiple, dated human breast milk samples were provided by 44 unique donors between January 2011 and December 2012. All donors provided informed consent in accordance with a protocol approved by the Cleveland Clinic Institutional Review Board, and provided de-identified samples that were assigned a code number corresponding to postpartum day of milk collection. All samples were stored at −20 °C.
Isolation of HA from Milk
Donated human milk samples with a minimum volume of 50 ml were boiled for 10 min to heat-inactivate endogenous milk enzymes. Digestion of milk protein content was completed using Proteinase K (Roche Applied Science) added to the sample at a concentration of 0.6 mg/ml and incubated at 60 °C for a minimum of 18 h. Following proteolysis, milk samples were cooled at 4 °C for 24 h to enhance physical separation of milk lipid content. The bulk of milk fats were removed from the sample with a sterile spatula before centrifugation at 35,000 × g for 10 min at 4 °C to further separate the remaining lipid content, which was removed from the surface of the sample by suction. Milk salts and small molecules, including the peptide products of proteolysis, were removed by overnight dialysis against ultrapure sterile H2O using dialysis membrane cassettes with a maximum pore size of 2 kDa (Thermo Scientific, Rockford, IL). Following additional boiling to ensure sterility, NaCl was added to the dialyzed aqueous milk fraction to a final concentration of 0.05 m NaCl. Ammonium cation-based anion exchange maxicolumns (Thermo Scientific) were equilibrated to 0.05 m NaCl before sample loading by centrifugation in a swinging bucket rotor at 500 × g for 5 m. Sample-loaded anion exchange columns were washed with 5 volumes of 0.1 m NaCl to remove weak cations or positively charged or nonspecifically bound content. Sample elution was completed by loading the columns with 0.7 m NaCl elution buffer with centrifugation at 500 × g for 5 min. Eluted content was again dialyzed for the removal of NaCl for downstream cell culture compatibility before slowly precipitating HA content in 95% isopropyl alcohol overnight at 4 °C. Following final HA precipitation by centrifugation at 30,000 × g for 30 min at 4 °C, the supernatant was discarded, and the sample was dehydrated by centrifugation under vacuum. Precipitated HA isolates were rehydrated in sterile H2O and adjusted to 10 μg/ml HA concentrated stock solutions following determination of HA yield by an enzyme-linked sorbent assay (ELSA). Wash and elution buffer salt concentrations used in the HA isolation scheme were determined empirically for optimal elution of purified, lyophilized HA with an average molecular mass of 4.7, 35, or 2000 kDa (Lifecore Biomedical, LLC, Chaska, MN) as verified by HA ELSA assay.
Quantification of HA by Enzyme-linked Sorbent Assay (ELSA)
A 1-ml portion of each donated milk sample was stored separately from the time of donation at −20 °C and labeled to indicate the donor and sample number, indexed to the postpartum day in a database. A total of 1710 unique milk samples were assayed undiluted in triplicate and quantified by an ELSA kit according to the manufacturer's protocol (Echelon Biosciences Inc., Salt Lake City, UT). HA was similarly quantified in human milk HA preparations prepared as described above, with appropriate dilutions applied to account for the increased concentration of HA in the isolate. Mass-independent detection of HA was verified in the ELSA, using known quantities of purified, lyophilized HA with average molecular mass of 4.7, 35, 74, or 2000 kDa (Lifecore Biomedical, LLC, Chaska, MN). Specificity of the HA ELSA was verified by demonstrating that nonsulfated chondroitin was not detected. (Amsbio, LLC).
Fluorophore-assisted Carbohydrate Electrophoresis
Fluorophore-assisted carbohydrate electrophoresis was used to assess the purity of milk HA preparations and to quantify carbohydrate constituents. A complete description of this method has been published previously (51). A complete protocol is available online (see the Cleveland Clinic Programs of Excellence in Glycosciences Web site).
Cell Culture
HT-29 epithelial cells, a line initially derived from a human intestinal tumor, were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS) and incubated at 37 °C in a humidified environment containing 5% CO2. Stock cultures were split at a ratio of 1:20 once per week.
Experimental Cultures
HT29 cells were released from stock cultures by dissociation with solution containing 0.05% trypsin and 0.53 mm EDTA in phosphate-buffered saline for 1.5 min. Collected cells were washed and plated at a 1:15 area/area ratio in 12-well plates (BD Biosciences) and grown until 70–80% confluent (3 days). On the day of the experiment, growth medium was removed, and the HT-29 cells were treated with fresh RPMI containing 10% FBS without or with human milk HA preparations at the concentrations (0.001–5 μg/ml HA) and times (0–48h) specified in the figure legends. In specific experiments, mouse monoclonal IgG antibody against human CD44 (clone A3D8, Sigma-Aldrich) or nonspecific mouse IgG (Sigma) was supplied at 2 μg/ml in RPMI medium containing 10% FBS for 1 h prior to the addition of cell culture treatments. In experiments that included commercial, purified HA, 35-kDa HA (HA-35, Lifecore Biomedical, LLC, Chaska, MN) was supplied at 350 μg/ml in RPMI medium containing 10% FBS as reported previously (39).
Hylauronidase Digestion of Human Milk HA Preparations
HA content of human milk preparations was specifically degraded to tetra- and hexasaccharides (52, 53) by incubating 10 μg/ml milk HA preparation with 0.25 unit/ml Streptomyces hyalurolyticus hyaluronidase (EC 4.2.2.1; Seikagaku Corp.) for 16 h at 60 °C and subsequently heat-inactivated by boiling for 10 min prior to use as a cell treatment. Complete enzymatic digestion of hyaluronan in human milk HA isolates using the above procedure was verified by ELSA.
Detection of HβD2 by Immunoblot (Western) Analysis
Whole cell lysates from HT-29 cells were isolated for Western blotting in the following lysis buffer: 300 mm NaCl, 50 mm Tris, pH 8.0, 0.5% Nonidet P-40, 1 mm EDTA, 10% glycerol, protease inhibitor for mammalian tissue P8340 (Sigma-Aldrich). Cell lysate proteins were separated by SDS-PAGE using precast Tricine-based 10–20% gradient gels (Invitrogen). Separated proteins were transferred at 4 °C to PVDF membrane (Millipore Corp., Billerica, MA) by an electroblotting apparatus (Bio-Rad) at 110 V for 60 min. In cases where analysis of numerous replicate samples or multiple protein targets of differing molecular weight was required, a multistrip Western blotting protocol was used to increase quantitative output and improve signal consistency (54). PVDF membranes were blocked in Odyssey Blocking Buffer (LI-COR) diluted to 50% concentration in Tris-buffered saline (TBS) for 60 m. The membrane was incubated with rabbit polyclonal antibody against HβD2 (Abcam, Cambridge, MA) at 1:750 in the blocking buffer, and the primary antibody was followed by biotin-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) at 1:30,000 in blocking buffer and finally horseradish peroxidase (HRP)-conjugated streptavidin (GE Healthcare) at 1:100,000 in TBS alone. Membrane-bound GAPDH protein expression was detected by incubation with rabbit polyclonal antibody against GAPDH at 1:5000 (Abcam, Cambridge, MA) followed by HRP-conjugated donkey polyclonal anti-rabbit IgG (1:20,000; GE Healthcare). All washing steps were conducted in Tris-buffered saline with 0.1% Tween 20. Protein bands were visualized using ECL prime chemiluminescent development (GE Healthcare) and detection by BioMax XAR scientific imaging film (Carestream Health Inc., Rochester, NY). Differences in chemiluminescent signal intensity were quantified using the ImageScanner III with the ImageQuantTL version 7.0 software package (GE Healthcare).
Real-time Quantitative PCR
HT-29 cells were cultured and treated with human milk HA preparations as indicated above. Cell lysates were collected for real-time quantitative PCR analysis using the Cells-to-cDNA kit (Invitrogen) according to the manufacturer's instructions. Briefly, cultured epithelium were released by dissociation with solution containing 0.05% trypsin and 0.53 mm EDTA in phosphate-buffered saline for 1.5 min before being resuspended in 1 ml of sterile PBS at 4 °C. Trypsinized cells were centrifuged at 1200 × g for 5 min at 4 °C before removal of the supernatant and the addition of lysis buffer. Following digestion of genomic DNA by DNase, reverse transcription using oligo(dT) primers and M-MLV RTase was completed in accordance with the manufacturer's instructions. The cDNA product was stored at −20 °C prior to quantitative PCR analysis. Validated primers with associated 6-carboxyfluorescein fluorogenic probes for DEFB4 (defensin β-4; Assay ID Hs00175474_m1) and 18 S rRNA (Assay ID Hs99999901_s1) were purchased from Applied Biosystems (Invitrogen). The real-time PCR amplifications were performed in 25-μl reaction volumes containing TaqMan gene expression Master Mix, primers, and fluorogenic probes (Invitrogen) and cDNA generated by the Cells-to-cDNA kit. All reactions were performed with four replicate reactions using a Bio-Rad C1000 Touch Thermal Cycler with attached CFX96 real-time system. The real-time PCR conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 60 s. Real-time detection data were analyzed using Bio-Rad CFX Manager 2.1 software.
Detection of Murine HβD2 Ortholog by Fluorescence Histochemistry
Descriptions of fluorescence histochemistry and confocal microscopy were provided previously (55). For tissue section staining, fixed, paraffin-embedded mouse colon sections were deparaffinized and incubated in a blocking solution of Hanks' balanced salt solution (HBSS) containing 2% FBS for >30 min followed by overnight incubation at 4 °C in a solution of rabbit polyclonal antibody against MuβD3 (Santa Cruz Biotechnology, Inc.) at 1:100 dilution in HBSS with 2% FBS. Coverslips were washed three times with HBSS before incubation in a solution containing Alexa-568-tagged goat anti-rabbit IgG (1:1000) (Invitrogen) in HBSS with 2% FBS for 1 h at 25 °C. The coverslips were washed an additional three times in HBSS. After washing, Vectashield mounting medium (Vector Labs) containing 4′,6-diamidino-2-phenylindole (DAPI), which fluorescently labels DNA, was used to adhere coverslips to antibody-labeled colon sections. Slides were stored at −20 °C until imaged. Confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope (Leica, Heidelberg, Germany).
Evaluation of Murine HβD2 Ortholog Expression in Nursing Animals
Six pups of conventionally housed wild-type adult C57BL/6 mice were euthanized on the day of birth, 10 days postpartum (during nursing), or after weaning (>4 weeks). 0.5-cm cross-sections were collected at regular intervals along the entire length of the bowel, fixed in Histochoice (AMRESCO, Inc., Solon, OH), and paraffin-embedded. Representative cross-sections of the mouse intestine were deparaffinized and stained for MuβD3, the murine ortholog of HβD2, as described above.
In Vivo Induction of Murine HβD2 Ortholog by Human Milk HA
Age and sex-matched adult C57BL/6 were purchased from Jackson Laboratory (Bar Harbor, ME) and housed by conventional methods. All treatments were conducted according to Institutional Animal Care and Use Committee-approved protocols. Nine mice were gavage-fed once per day 0.25 ml of water alone (control) or 1 μg of milk HA or an equivalent, donor-matched quantity of hyaluronidase-treated milk HA preparation suspended in 0.25 ml of water once daily for three consecutive days. Mice were sacrificed 16–18 h after the final gavage treatment, and 0.5-cm cross-sections of the proximal colon descending from the ileocecal junction, the transverse colon equidistant to the ileocecal junction and rectum, and the last 0.5 cm of the distal colon were excised from each mouse, fixed in Histochoice (AMRESCO, Inc., Solon, OH), and paraffin-embedded. Representative cross-sections of the mouse distal colon were deparaffinized and stained for MuβD3, the murine ortholog of HβD2, as described above.
Evaluating the Role of TLR4 and CD44 in HA-35-induced HβD2 Expression in Vivo
Age-matched male C57/Bl6 (“wild-type”), B6.129(Cg)-CD44tm1Hbg/J (CD44−/−), and B6.B10ScN-Tlr4lps-del/JthJ (TLR4−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed by conventional methods. All treatments were conducted according to Institutional Animal Care and Use Committee-approved protocols. Five mice of each of the three genotypes were gavage-fed tap water alone (250 ml) or a solution containing milk HA preparation in tap water (1 μg of HA in 250 ml) once daily for three consecutive days. Mice were sacrificed 16–18 h after the final gavage treatment, and a 2-cm section of the proximal colon descending from the ileocecal junction was excised from each mouse, opened, pressed flat between two layers of paper towel, fixed overnight in Histochoice (AMRESCO, Inc., Solon, OH), and paraffin-embedded. Longitudinal sections of the mouse proximal colon were deparaffinized and stained for MuβD3 as described above.
Quantization of Histological Observations
Individual sections were labeled in a random fashion so as to shield the microscopist from knowledge of mouse genotype or treatment status. Ideal fluorescent signal exposure times for capturing the complete range of MuβD3 staining intensity were determined at the outset of the experiment by a survey of 10 random sections and were held constant for subsequent image capture. Four MuβD3-stained fields of colonic epithelial structures as well as one unstained control from each animal were digitally captured from longitudinal sections of each of the 30 mouse proximal colon samples. Captured fields were selected on the basis of epithelial tissue morphology as determined by DAPI staining. Each of the 150 images (four stained fields and one unstained field from each of the 30 mice) were graded on a 0–4 scale by a panel of five blinded researchers, with a grade of 0 indicating no MuβD3 staining and a grade of 4 corresponding to peak MuβD3 staining intensity (39). The mean scores given by the evaluating panel for each of the 150 images were calculated for each mouse before treatment and genotype status were revealed according to a key.
Fluorescent Immunohistological Detection of TLR4 and CD44
HT-29 cultures grown on glass coverslips were subjected to various cell culture treatments as indicated above and fixed in ice-cold methanol for 8 min and air-dried. Coverslips were then incubated in a blocking solution of HBSS containing 2% FBS for >30 min, followed by overnight incubation at 4 °C in a solution of rabbit polyclonal antibody against TLR4 (Abcam, Cambridge, MA) at 1:100 dilution and mouse monoclonal antibody against CD44 at 1:100 dilution (Sigma-Aldrich) in HBSS with 2% FBS. Coverslips were washed three times with HBSS before incubation in a solution containing biotin-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) at 1:1000 in blocking buffer for 1 h. Following another set of washes, coverslips were incubated in Alexa-568-tagged donkey anti-mouse IgG (1:1000) and Alexa-488 streptavidin (1:500; Invitrogen) in HBSS with 2% FBS for 1 h at 25 °C. The coverslips were washed an additional three times in HBSS and mounted to slides in Vectashield containing DAPI (Vector Labs). Slides were stored at −20 °C until imaged. Confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope.
Quantification of Total Phosphorylated JNK1/2/3, p38 MAPK, and ERK1/2 by ELISA
HT-29 cells cultured and experimentally treated according to the methods described above were harvested 30 min after experimental treatment according to the manufacturer's protocol (Phosphotracer, Abcam, Cambridge, MA) in cell lysis buffer containing phosphatase inhibitors. The volume of assayed cell lysate was normalized according to protein content as measured by a Bio-Rad protein assay. Two replicate experiments, containing three biological replicates per treatment group, with duplicate measurements of each sample, were assayed according to the manufacturer's protocol (Phosphotracer, Abcam, Cambridge, MA).
Salmonella Infection of Cultured Epithelium
HT-29 colonic epithelial cells were cultured in 48-well tissue culture plates (Corning Inc.) according to the protocol described above. 24 h prior to infection, HT-29 cells were treated with new medium containing 0.5 μg/ml milk HA or an equivalent quantity of donor-matched hyaluronidase-treated milk HA preparation in RPMI with 10% FBS or medium alone. Salmonella enterica serovar Typhimurium SL1344 (gift of Dr. Mary O'Riordian, University of Michigan, Ann Arbor, MI) was cultured overnight in LB broth at 30 °C with shaking at 200 rpm before dilution at 1:7 in new LB broth the next day and further cultured until A600 = 0.5 or ∼3.5 × 108 cfu/ml, with multiplicity of infection (MOI) = 10. 24 h after the addition of milk HA or control treatments, HT-29 epithelial cells were washed twice with fresh RPMI medium, followed by infection with new medium containing ∼4.7 × 107 cfu/ml Salmonella taken from the 1:7 LB broth culture. Following a 30-min infection period, the cell culture medium containing Salmonella was removed, and HT-29 epithelium was washed twice with sterile PBS before the addition of medium containing gentamicin at 50 ng/ml (Sigma-Aldrich) for an additional 1 h. HT-29 cells were then washed twice and lysed in ice-cold PBS containing 1% Triton X-100. Dilutions of cell lysates were spread on sterile LB agar plates and cultured overnight at 30 °C. Colony-forming units were counted on the following day. The experimental design included no fewer than 6 replicate culture wells per treatment, and 3 technical replicates per culture well and was separately replicated with three separate milk HA isolates derived from unique donors.
Statistical Analysis
Statistical difference between treatment groups was evaluated where appropriate by unpaired two-tailed Student's t test, and all error bars were drawn to indicate the S.E. A paired two-tailed Student's t test was used to evaluate the difference between sigmoidal dose-response relationships. Differences were considered significant when p < 0.05. Statistical analysis, including linear regression analysis of milk HA concentration over days postpartum generated from the analysis of donated milk samples by ELSA, was performed using R version 2.12.1 for Mac OS X (R Foundation for Statistical Computing; available on the World Wide Web). Graphing was completed using R version 2.12.1 for Mac OS X or GraphPad Prism version 4.0c.
RESULTS
Human Milk HA Concentrations Are Greatest in the First 60 Days Postpartum
Although human milk is known to contain HA (26), variation in concentration between individuals and over time after delivery is not known. We examined milk HA concentrations during the first 6 months postpartum among a group of 44 (41 Caucasian, 2 Asian, 1 African-American) mothers from the Cleveland metropolitan area who donated milk samples at daily intervals starting in the first week after giving birth. The HA concentration was determined in unprocessed milk samples using a competitive ELSA. As a whole, HA concentrations were greatest in the immediate postpartum period, with a mean concentration of 755.01 ± 94.15 ng/ml within the first week after birth, and decreased at a linear rate over the first 60 days postpartum (m = −10.9 ± 1.2 ng/ml/day, R2 = 0.11, p < 1.0 × 10−16) (Fig. 1). The mean HA concentration in samples collected during the first 60 days postpartum was 452.34 ± 19.58 ng/ml. Average HA concentrations were essentially static at 215.74 ± 5.77 ng/ml at time points greater than 60 days after birth (m = −0.4 ± 0.1 ng/ml/day, R2 = 0.04, p = 1.4 × 10−9) and persisted up to 1 year.
FIGURE 1.
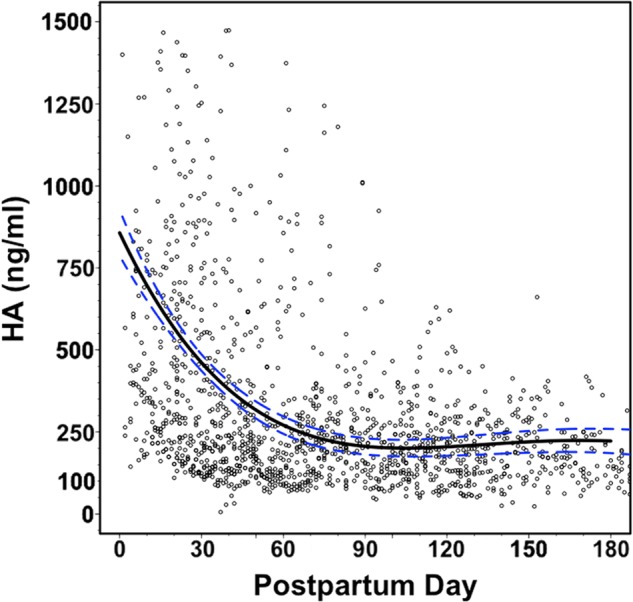
Distribution of milk HA concentration by postpartum day in 1710 human milk samples collected from 44 unique donors during the first 6 months after delivery. A fourth degree polynomial regression curve was fitted to the entire data set and is indicated by the solid black line, with the 95% confidence interval of the curve represented by the dashed blue lines (R2 = 0.17, p = 6.1 × 10−67).
Milk HA Specifically Induces HβD2 Expression
Our group previously demonstrated that synthetically produced HA promotes enhanced expression of HβD2 in intestinal mucosa (39). However, the function of hyaluronan contained in milk relevant to epithelial antimicrobial function was unknown. We hypothesized that hyaluronan from human breast milk would induce HβD2 expression in intestinal epithelium. To this end, we developed a method to purify HA from the proteins, lipids, and sugars and charged carbohydrate polymers (described under “Experimental Procedures”) in the chemically complex milk. The concentration of HA in the milk HA isolates was determined by ELSA, with an average HA yield of 55% relative to unprocessed whole milk samples. Analysis of HA polymer size in milk preparations by agarose gel electrophoresis (56) revealed broad polydispersity, between 1 × 105 and 2 × 106-Da polymers.3 Our milk-HA preparations also contained chondroitin as a contaminant (Table 1) that we were unable to specifically remove from our batch preparations. This is consistent with the human milk analysis of Coppa et al., who reported an excess of undersulfated chondroitin in human milk (26).
TABLE 1.
Carbohydrate constituents of milk HA preparations
Shown are concentrations of milk carbohydrates present in milk HA isolates used for the presented biological assays as measured by fluorophore-assisted carbohydrate electrophoresis. Results represent the mean concentration ± S.E. of each saccharide species relative to 1 μg/ml HA preparation (e.g. 0.5 μg/ml HA contains 3.27 μg/ml chondroitin and 0.08 μg/ml maltose, etc.) from five donor-unique milk HA preparations used in the biological assays presented in Figs. 2–7. Note that three species of di- and trisulfated chondroitin could not be individually resolved and are quantified under the term “other chondroitins.” NA, not applicable.
| Saccharide species | Relative concentration | S.E. |
|---|---|---|
| μg/μg HA | ||
| Chondroitin | 6.53 | 0.98 |
| ΔDiOS | 5.49 | 0.75 |
| ΔDi4S | 0.28 | 0.03 |
| ΔDi6S | 0.71 | 0.09 |
| ΔDi2S | <0.01 | |
| Other chondroitin | 0.04 | 0.01 |
| ΔDi2,6S | NA | NA |
| ΔDi2,4,6S | NA | NA |
| Δ6S-Di2,4S | NA | NA |
| Heparan sulfate | 0.94 | 0.14 |
| ΔDi-2S-U-6S-G-NS | 0.33 | 0.11 |
| ΔDi-U-G-NS | 0.26 | 0.06 |
| ΔDi-U-G-NAc | 0.22 | 0.05 |
| ΔDi-2S-U-G-NS | 0.08 | 0.02 |
| ΔDi-6S-G-NS | 0.06 | 0.03 |
| ΔDi-U-6S-G-NS | <0.01 | |
| Other sugars | 0.29 | 0.18 |
| Maltose | 0.16 | 0.10 |
| Maltotriose | 0.13 | 0.10 |
| Maltotetrose | <0.01 | |
| GalNAc | <0.01 | |
| 6S-GalNAc | <0.01 | |
| 4S-GalNAc | <0.01 | |
| Glucose | <0.01 | |
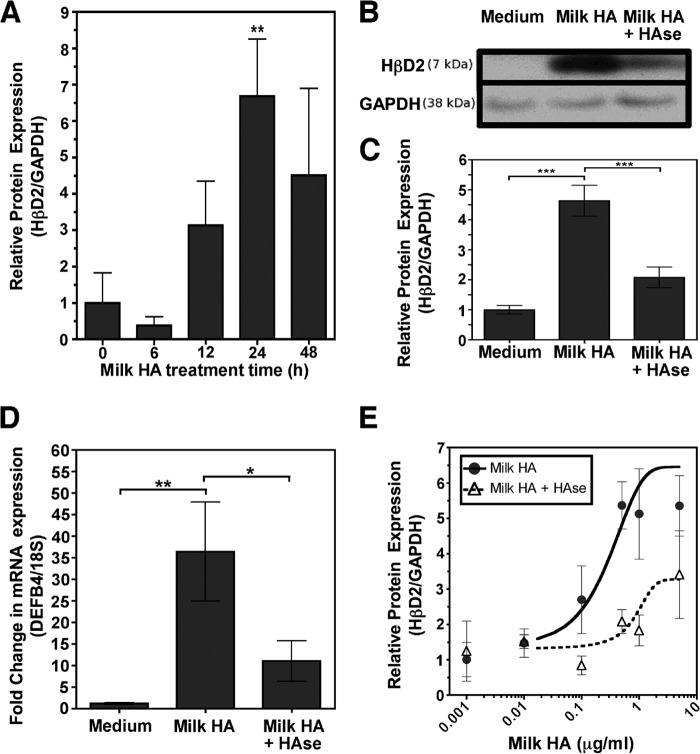
We tested the ability of our milk HA preparations to promote expression of HβD2 protein in the human intestinal epithelial cells. Cultured HT-29 cells were treated with 500 ng/ml HA derived from pooled milk HA preparations obtained from three individual donors, and HβD2 protein was measured in cell lysates by an immunoblot assay (Fig. 2A). Induction of HβD2 protein expression relative to GAPDH occurs within 12 h following treatment with milk HA isolates. Maximal HβD2 protein expression in milk HA-treated HT-29 cells was observed at 24 h after initial treatment (p = 0.006 versus 0 h), with elevated HβD2 protein levels still present at 48 h post-treatment. To confirm that increased HβD2 protein expression were specifically induced by HA in the milk preparations, HT-29 cells were treated for 24 h with medium alone, milk HA isolates (0.5 μg/ml HA), or donor-matched milk HA isolates that were predigested with a substrate-specific hyaluronidase derived from S. hyalurolyticus. Fig. 2, B and C, demonstrates a highly significant 4.5-fold increase in normalized HβD2 protein expression relative to both treatment with medium alone and treatment with hyaluronidase-digested milk HA isolates (p < 0.0001 and p = 0.0008, respectively). However, hyaluronidase-digested milk HA preparations still retained the ability to induce a 2-fold increase in HβD2 protein expression relative to treatment with medium alone (p = 0.0101). Furthermore, we evaluated the effects of milk HA on expression of the gene encoding the HβD2 peptide, DEFB4 (Fig. 2D). Quantitative real-time PCR analysis revealed notable induction of DEFB4 mRNA expression in HT-29 cells treated for 24 h with milk HA (500 ng/ml HA) relative to treatment with medium alone (p = 0.0073). The effect of milk HA on DEFB4 expression was mostly dependent upon the presence of HA, as indicated by the significant reduction in induced DEFB4 in HT-29 cells treated with hyaluronidase-digested milk HA (p = 0.04 versus milk HA + HAse; Fig. 2D). The data presented in Fig. 2, C and D, represent the collective results of identical experiments conducted with three separate milk HA isolates derived from unique donors. Given that milk HA concentration varies over time and between individuals (Fig. 1), we evaluated the induction of HβD2 protein expression in vitro following milk HA isolate treatment over a dose range of 0.001–5 μg/ml HA for 24 h (Fig. 2E). Matched aliquots of milk HA preparations were predigested with S. hyalurolyticus hyaluronidase and tested across the same dilution range to specifically determine the contribution of HA to the dose-response relationship of milk HA preparations to HβD2 protein induction in HT-29 intestinal epithelium. Milk HA treatment resulted in significantly increased HβD2 protein expression at a concentration range of 0.5–5.0 μg/ml HA. Importantly, HβD2 induction was significantly reduced in cells treated with hyaluronidase-digested milk isolates at doses greater than 0.1 μg/ml HA (EC50 = 5.94 μg/ml versus 0.03 μg/ml, respectively, with p = 0.04), although HβD2 protein expression was increased above background levels at doses greater than 0.5 μg/ml HA (p = 0.01 versus control).
FIGURE 2.
A, average densitometric quantification of immunoblots from four individual experiments in which the abundance of HβD2 protein relative to GAPDH protein was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated with 0.5 μg/ml milk HA for the time intervals indicated (0–48 h). B, representative Western blot demonstrating HβD2 protein expression relative to GAPDH in the whole cell lysates of HT-29 cells treated for 24 h with medium alone, medium containing milk HA isolate containing 0.5 μg/ml HA, or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase at 0.25 unit/ml for 16 h at 60 °C. C, average densitometric quantification of immunoblots from four individual experiments, each separately using three donor-unique milk HA isolates in which the abundance of HβD2 protein relative to GAPDH protein was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated for 24 h with medium alone, 0.5 μg/ml milk HA, or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. D, combined real-time quantitative PCR results from four individual experiments, each separately using three donor-unique milk HA isolates in which the abundance of DEFB4 mRNA relative to 18 S rRNA was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated for 24 h with medium alone, 0.5 μg/ml milk HA, or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. Significance of differences in normalized HβD2 or DEFB4 expression was evaluated by comparison of each time point with control treatment, unless otherwise indicated by brackets, using Student's t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. E, average densitometric quantification of immunoblots from six individual experiments in which the abundance of HβD2 protein relative to GAPDH protein was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated for 24 h with 0.001–5 μg/ml milk HA or medium containing 0.001–5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. Sigmoidal dose-response relationships are indicated, with the solid black curve corresponding to milk HA isolate treatment (EC50 = 0.03 μg/ml) and the dashed black line corresponding to hyaluronidase-digested milk HA treatment (EC50 = 5.94 μg/ml), with the observed difference between treatment responses being statistically significant by two-sided Student's t test analysis (p = 0.04). Error bars, S.E.
Cumulatively, these findings (Fig. 2) indicate that HA contained in human milk specifically enhances induction of DEFB4 transcription and HβD2 protein expression in HT-29 colonic epithelium. These effects of milk HA are both time- and concentration-dependent.
Oral Administration of Human Milk HA Promotes in Vivo Expression of the Murine HβD2 Ortholog in Intestinal Epithelium
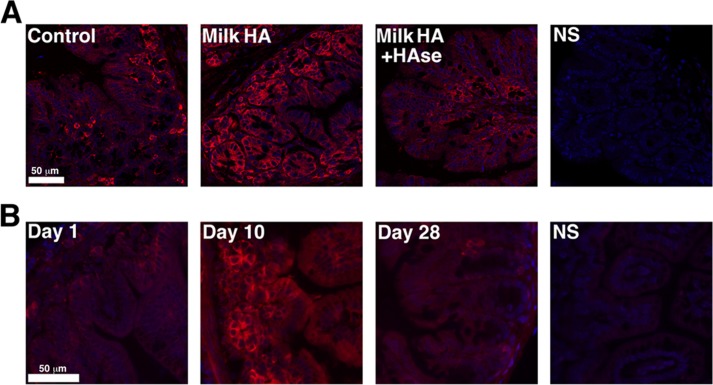
After observing that milk HA greatly enhances the induction of HβD2 protein expression in vitro, we next tested whether isolated human milk HA could promote expression of the murine HβD2 ortholog in colonic mucosa in adult mice. Nine age- and sex-matched adult wild-type C57BL/6 mice were segregated equally into the following treatment groups: control, milk HA, or donor-matched milk HA that was predigested with S. hyalurolyticus hyaluronidase. All animals were gavage-fed 0.25 ml of water without (control) or containing isolated milk HA (1 μg of HA) or an equivalent mass of hyaluronidase-digested milk HA once daily for 3 consecutive days. Fixed proximal or transverse colon tissue sections were analyzed for MuβD3, the murine ortholog of HβD2 (57), using immunostaining and fluorescence microscopy. Blinded analysis of intestinal tissue staining by microscopy revealed enhanced epithelial MuβD3 protein expression in colonic mucosa of mice fed milk HA compared with control-treated animals or those receiving hyaluronidase-degraded milk HA, with the greatest MuβD3 induction observed in the mucosa of the proximal colon (Fig. 3A). Representative images shown in Fig. 3A are of the median staining intensity of each of the respective treatment groups.
FIGURE 3.
A, representative fluorescent micrographs of epithelium in proximal colonic cross-sections from adult C57BL/6 wild-type mice. The mice were given single daily doses of 1 μg of milk HA in 0.25 ml of water (Milk HA) or 1 μg of milk HA that had been predigested with S. hyalurolyticus hyaluronidase in 0.25 ml of water (Milk HA + HAse) or water alone (Control) for 3 consecutive days. B, representative fluorescent micrographs of epithelium in intestinal cross-sections from newborn (day 1), nursing (day 10), or weaned (28 days) mice. The mouse ortholog of HβD2, MuβD3, is fluorescently immunolabeled (red), and nuclei are stained with DAPI (blue). NS, an immunostaining control in which no MuβD3 antibody was utilized.
The Intestinal Epithelium of Nursing Mice Expresses the HβD2 Ortholog
Because milk HA isolates specifically promote the expression of MuβD3 in adult mice, we hypothesized that this observation may reflect a physiologic process that occurs in nursing young. To test this in the mouse model, we examined expression of MuβD3 in the intestinal mucosa of healthy, wild-type C57BL/6 mice immediately after birth, 10 days after birth, and 5 days after weaning (Fig. 3C). Analysis of immunofluorescently stained MuβD3 protein in cross-sectioned intestinal mucosa revealed that expression was greatly increased in the intestinal mucosa in the period between the first and tenth day after birth, during which time the animals were deriving complete nutrition from the mother. After weaning, base-line MuβD3 protein expression was relatively reduced in intestinal mucosal tissue, suggesting a correlation between milk consumption and intestinal mucosal MuβD3 expression.
In Vivo Induction of Murine HβD2 by Milk HA Is both CD44- and TLR4-dependent
Among the known HA cell surface receptors, TLR4 has been implicated repeatedly in the induction of augmented innate epithelial defense by HA in both in vitro and in vivo experimental models (37–40). In addition, CD44 is reported to mediate a wide variety of HA-dependent signaling responses (29, 33, 34, 58, 59). Therefore, CD44 and TLR4 were evaluated as candidate receptors mediating the HA-enhanced induction of murine HβD2 ortholog following oral administration of human milk HA in vivo. Adult wild-type, CD44−/−, and TLR4−/− C57BL/6 mice were gavage-fed 0.25 ml of water alone (control) or containing milk HA (1 μg of HA) once daily for three consecutive days, with 10 mice per genotype divided equally between the two treatment groups. Mice were sacrificed 16–18 h after the final gavage treatment. Proximal colon tissue was fixed and cut in longitudinal sections for MuβD3 immunofluorescent staining. Fig. 4A presents representative images of MuβD3 protein expression in immunostained murine proximal colonic epithelium of both control and milk HA-treated animals from each of the three genotypes. Blinded analysis of fluorescent microscopy images utilizing an immunostain scoring system (see “Experimental Procedures”) revealed that significant induction of MuβD3 protein expression occurred in the colonic epithelium of wild-type animals following oral administration of the milk HA preparation relative to control-treated wild-type mice (p = 0.009; Fig. 4B). However, expression of MuβD3 in the colonic mucosa of TLR4−/− or CD44−/− animals was unchanged after oral administration of milk HA relative to control-treated animals of each genotype (p = 0.387 and 0.311, respectively). Significantly lower MuβD3 staining intensity was observed in TLR4−/− and CD44−/− mice following oral administration of milk HA relative to milk HA-treated wild-type mice (p = 0.007 and 0.0002, respectively; Fig. 4B). Thus, genetic deletion of either TLR4 or CD44 is sufficient to abrogate the induction of MuβD3 expression in the colonic epithelium following oral administration of milk HA.
FIGURE 4.
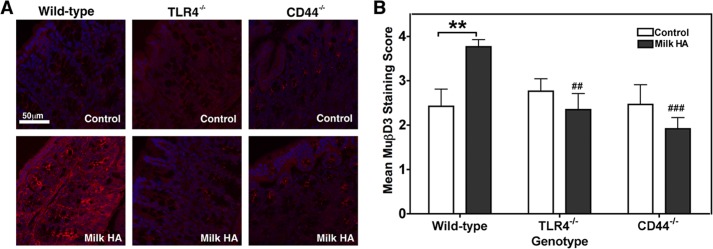
A, representative fluorescent micrographs of epithelium in proximal colon cross-sections from adult C57BL/6 wild-type, TLR4−/−, or CD44−/− mice. The mice were given single daily doses of milk HA containing 1 μg of HA in 0.25 ml of water or water alone (Control) by oral administration for 3 consecutive days. MuβD3 is fluorescently immunolabeled (red), and nuclei are stained with DAPI (blue). B, average scored MuβD3 staining intensity of proximal colon tissue sections from wild-type, TLR4−/−, or CD44−/− mice given single daily doses of 1 μg of milk HA in 0.25 ml of water or water alone (Control) by oral administration for 3 consecutive days. Average MuβD3 staining intensity score represents 5 mice/group, with 4 stained sections/mouse, as judged by a blinded panel of five researchers on a scale of 0–4 with a score of 4 corresponding to peak MuβD3 staining. Mean staining intensity score ± S.E. (error bars) of nonspecifically stained sections (1 section/mouse) was 0.26 ± 0.06. Significance of differences in mean MuβD3 staining intensity was evaluated using a two-tailed Student's t test. **, p < 0.01 for the comparison indicated by the bracket; ## and ###, p < 0.01 or p < 0.001, respectively, for the comparison with wild-type mice receiving milk HA.
Anti-CD44 Antibody Abrogates the Induction of HβD2 by Milk HA, but Not HA-35, in Cultured Epithelium
Recent work published by our group (39) has demonstrated that oral administration of a biosynthetic HA preparation with an average molecular mass of 35 kDa (HA-35) promotes the induction of murine HβD2 in vivo in a manner that is dependent upon expression of TLR4 and independent of CD44. However, the induction of murine HβD2 ortholog in the intestinal epithelium by HA isolated from human milk requires both TLR4 and CD44 (Fig. 4). Given the apparent difference between the receptor requirements of HA-35 and human milk HA for the induction of murine defensin in vivo, we set out to evaluate the role of CD44 in HβD2 induction by HA-35 and milk HA using a more defined, in vitro system. Cultured HT-29 intestinal epithelium was treated with medium alone; medium containing 350 μg/ml HA-35, the active concentration determined in our previous publication (39); or medium containing 0.5 μg/ml milk HA for 24 h. Additional HT-29 cultures were pretreated for 1 h with 2 μg/ml nonspecific mouse immunoglobulin or 2 μg/ml mouse anti-human CD44 IgG prior to the addition of medium, HA-35, or milk HA (Fig. 6A). Whereas milk HA and HA-35 resulted in significant induction of HβD2 protein expression relative to treatment with medium alone, pretreatment with anti-CD44 antibody abrogated the induction of HβD2 by milk HA. Significantly, induction of HβD2 by HA-35 was robust even in the presence of anti-CD44. Nonspecific IgG had no independent effect on HβD2 protein expression and did not inhibit the induction of HβD2 by either milk HA or HA-35.
FIGURE 6.
A, average densitometric quantification of immunoblots from four individual experiments in which the abundance of HβD2 protein relative to GAPDH protein was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated with medium alone, medium containing 350 μg/ml of a synthetic HA preparation with an average molecular mass of 35 kDa (HA-35), or medium containing 0.5 μg/ml milk HA for 24 h. Where indicated, HT-29 cultures were pretreated for 1 h with 2 μg/ml nonspecific mouse immunoglobulin (IgG) or 2 μg/ml mouse anti-human CD44 IgG (CD44) prior to the addition of medium, HA-35, or milk HA. B, relative quantification of total phosphorylated ERK1 and ERK2 by ELISA in cell lysates of HT-29 cells treated for 30 min with medium alone, medium containing 350 μg/ml HA-35, 0.5 μg/ml milk HA, or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. C, relative quantification of total phosphorylated JNK1, JNK2, and JNK3 by ELISA in cell lysates of HT-29 cells treated for 30 min with medium alone, medium containing 350 μg/ml HA-35, 0.5 μg/ml milk HA, or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. D, relative quantification of total phosphorylated p38 MAPK by ELISA in cell lysates of HT-29 cells treated for 30 min with medium alone, medium containing 350 μg/ml HA-35, 0.5 μg/ml milk HA or medium containing 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase. Significance of differences in protein expression was evaluated by comparison of each time point with control treatment (medium alone), unless otherwise indicated by brackets, using Student's t test. *, p < 0.05; **, p < 0.01. Error bars, S.E.
Milk HA Does Not Promote Significant Membrane Colocalization of CD44 and TLR4
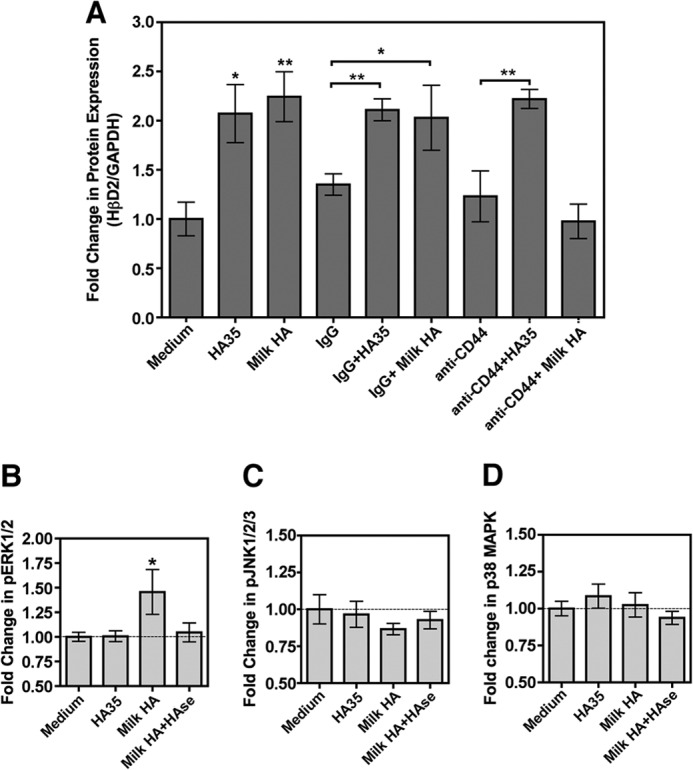
Proposed mechanistic schemes for the integration of CD44- and TLR4-dependent effects of HA include the colocalization of TLR4 and CD44 in the cell membrane subsequent to exposure to fragmented HA, evidence of which has been provided in a macrophage model (31). To evaluate this in our system, cultured HT-29 epithelium was treated with medium alone, medium containing 0.5 μg/ml milk HA or milk HA that had been predigested with S. hyalurolyticus hyaluronidase, or medium containing 350 μg/ml HA-35 for 30 min (Fig. 5). Fluorescent immunolabeling of CD44 (red) and TLR4 (green) and microscopy revealed no apparent difference between treatment groups in the relative arrangement of CD44 and TLR4 in the epithelial cell membrane. Whereas CD44 expression was predominantly associated with the cell membrane, TLR4 staining was diffusely cytoplasmic. Image analysis revealed that the arrangement of CD44 relative to TLR4 did not differ significantly from that predicted by random chance alone (data not shown). However, we observed an increased presence of aggregated CD44 immunolabeling in the cell membrane of HT-29 cells treated with intact milk HA relative to treatment with medium alone, consistent with the induction of CD44 clustering (indicated by white arrows). This pattern of CD44 arrangement in the cell membrane was not observed in HA-35-treated epithelium.
FIGURE 5.
Cellular localization of CD44 and TLR4 in cultured HT-29 cells following 30-min HA treatment. Representative fluorescent micrographs of cultured HT-29 epithelium after 30-min treatment with medium alone, medium containing 0.5 μg/ml milk HA or 0.5 μg/ml milk HA that had been predigested with S. hyalurolyticus hyaluronidase, or medium containing 350 μg/ml hyaluronan with an average molecular mass of 35 kDa (HA-35). CD44 is fluorescently immunolabeled in red, and TLR4 is indicated by the green staining, whereas the nuclei are stained with DAPI (blue).
Milk HA Specifically Enhances Phosphorylation of ERK1/2 in Vitro
Regulation of HβD2 expression at the level of signal transduction occurs predominantly through ERK, JNK, and MAPK (49), and these pathways have also been associated with HA signaling (TLR4 through JNK and MAPK; CD44 through ERK) (40, 60–62).We examined whether milk HA and HA35 signal HBD2 expression via similar pathways. Cultured HT-29 epithelium was treated with medium alone, medium containing 350 μg/ml HA-35, or medium containing 0.5 μg/ml milk HA or milk HA that had been predigested with S. hyalurolyticus hyaluronidase for 30 min, and cell lysates were assayed for total phosphorylated ERK1/2, total phosphorylated JNK1/2/3, and phosphorylated p38 MAPK by ELISA (Figs. 5, B–D, respectively). The relative abundance of total phosphorylated ERK1 and ERK2 was significantly increased by treatment with milk HA relative to treatment with medium alone, but no increase in phosphorylated ERK1/2 was observed in HT-29 cells treated with hyaluronidase-digested milk HA or high concentrations of HA-35 (Fig. 6B). Although no statistically significant difference in phospho-JNK1/2/3 was detected, treatment with milk HA was associated with relative inhibition of JNK1/2/3 phosphorylation in HT-29 epithelium (Fig. 6C). Total phosphorylated p38 MAPK was unchanged in HT-29 cells, regardless of treatment (Fig. 6D). Thus, milk HA specifically enhances ERK1/2 phosphorylation, consistent with HA-CD44 signaling (61).
Milk HA Enhances Resistance to Intracellular Salmonella Infection in Intestinal Epithelium
Inhibition of growth and infection by pathogenic organisms by direct interaction with human milk glycans with varying structures and chemical properties, including GAGs, has been previously reported (16–20, 27). One previous report also suggests that specific human milk oligosaccharides may directly protect intestinal epithelial cells from gastrointestinal infection (21). Based on our data indicating that human milk HA promotes induction of antimicrobial defense in intestinal mucosa (Figs. 2–5), we hypothesized that human milk HA also enhances functional resistance of human colonic epithelium to the intracellular enteric pathogens. To test this hypothesis, we utilized an in vitro infection assay (63), which employs Salmonella, a significant source of morbidity in infants (7), as the model organism. Confluent HT-29 colonic epithelial monolayers were treated for 24 h with medium alone, medium containing 500 ng/ml milk HA preparation, or donor-matched milk HA that was predigested with S. hyalurolyticus hyaluronidase. After recovery of intracellular Salmonella, pretreatment of HT-29 cells with milk HA resulted in a highly significant decrease (−24.6 ± 7.5%) in intracellular Salmonella recovered relative to epithelial cells pretreated with medium alone (p < 0.0001) or hyaluronidase-digested milk HA (p < 0.0001; Fig. 7). Importantly, the data presented in Fig. 7 represent the combined results of identical experiments conducted with three distinct isolates of milk HA derived from unique donors. These data indicate that milk HA enhances functional resistance to intracellular Salmonella infection.
FIGURE 7.
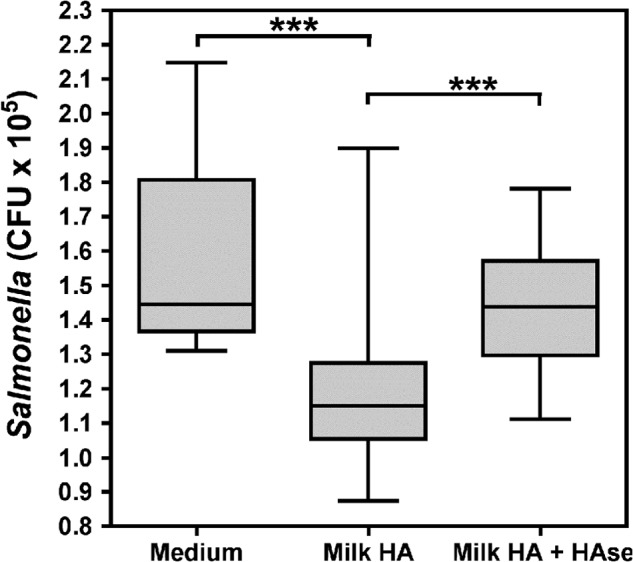
A box plot representing the number of colony-forming units (CFU) of S. enterica serovar Typhimurium SL1344 collected from host HT-29 epithelium subject to pretreatment for 24 h with 0.5 μg/ml milk HA or 0.5 μg/ml milk HA predigested with S. hyalurolyticus hyaluronidase or medium alone. Horizontal lines indicate median colony-forming units with interquartile range denoted by the shaded box and the full range of observations represented by the error bars. Significance of differences in colony-forming units was evaluated as indicated by the brackets using Student's t test. ***, p < 0.001.
DISCUSSION
Our recent report indicating that HA promotes expression of the antimicrobial peptide HβD2 in intestinal epithelium (39) and the observation that human milk contains HA (26) resulted in our hypothesis that HA supplied in human breast milk enhances innate intestinal epithelial antimicrobial defense. Our data support the finding of Coppa et al. (26) that human milk contains HA while further defining the time-dependent change in milk HA concentration from the start of lactation through the first 6 months postpartum. In addressing our hypothesis, we have demonstrated two parameters of epithelial antimicrobial defense that are greatly augmented by HA supplied in breast milk. First, cultured human colonic epithelial cells or the intestinal mucosa of wild-type mice treated with human milk HA at physiologically relevant concentrations exhibit dramatic induction of HβD2 expression that is significantly reduced by predigestion with a substrate-specific hyaluronidase and is specifically associated with increased ERK1/2 phosphorylation. Similar induction of murine HβD2 ortholog is observed in the intestinal mucosa of nursing mice relative to newborn or weaned animals. The induction of murine HβD2 ortholog by milk HA is dependent upon both HA cell surface receptors TLR4 and CD44 in vivo. Second, cultured colonic epithelial cells exhibit enhanced resistance to infection with the enteric pathogen Salmonella following pretreatment with milk HA isolates, an effect that is also abolished by hyaluronidase digestion. Taken together, our data indicate that HA naturally supplied in human breast milk contributes significantly to the induction of antimicrobial defense in intestinal epithelium.
Analysis of 1710 unique milk samples collected from a cohort of 44 mothers revealed high concentrations of HA in the initial weeks of lactation (Fig. 1). Despite wide variation between individual donors, HA concentrations in milk consistently decreased over the first 8 weeks postpartum, reaching a steady-state concentration by the third month after birth. Importantly, all samples evaluated contained detectable levels of HA, adding to the recent findings of Coppa et al. (26, 50), who utilized pooled milk samples collected from a limited cohort to suggest that HA is a universally expressed component of human milk. The use of a competitive ELSA that was highly specific to HA but independent of HA polymer size3 allowed for reproducible quantification of HA in whole milk samples without biochemical manipulation of the sample. Numerous bioactive components of human milk are present in high concentrations in the early stages of lactation, including other GAGs (50), sIgA (9), and fatty acids (64). Given the critical nature of the immediate postnatal period and the high susceptibility of newborns to infection (1, 7), it is perhaps unsurprising to find that HA in milk is also present at higher concentrations in the first weeks after birth. Maternal genetics and nutrition probably contribute to the intradonor variation in HA content we have observed.
Milk HA treatment exhibited both time-and dose-dependent induction of HβD2 protein in cultured HT-29 epithelium (Figs. 2, A and E), with maximal activity at 500 ng/ml, the approximate mean concentration of HA present in milk within the first 30 days postpartum (Fig. 1). In addition, transcription of the gene encoding the HβD2 peptide, DEFB4, was specifically and significantly up-regulated by milk HA at physiologic concentration (Fig. 2), indicating that the increased expression of HβD2 peptide is mediated by transcriptional activation. Numerous reports have indicated that the signaling properties of HA, including the induction of HβD2 (39), are highly size-specific (25). Size analysis of the HA we isolated from milk indicates broad polydispersity, containing 104- to 106-Da polymers and with polymer size distribution varying widely between individuals.3 Future studies may be required to determine the size-specific bioactivity of naturally occurring milk HA. Importantly, the milk HA preparations isolated from unique donors independently exhibited similar HβD2- and DEFB4-inducing activity at similar concentrations (Fig. 2), indicating that the expression of bioactive HA in milk is likely to be widespread among the general population. In addition, although previous work suggests that HA acts as a specific inducer of HβD2 in intestinal epithelium independent of other ligands (39), we cannot exclude the possibility that additional contaminant milk glycans contribute to or potentiate induction of HβD2 by milk HA. Analysis of the milk HA preparations indicates that undersulfated chondroitin is the predominant non-HA component in the isolates (Table 1). Indeed, hyaluronidase-treated milk preparations promoted some, albeit greatly reduced, induction of HβD2 protein and DEFB4 transcription at physiologically relevant concentrations (Fig. 2). However, substantially increased doses of hyaluronidase-treated milk HA isolates were required to achieve effects comparable with treatment with milk preparations containing intact HA. The observed EC50 of hyaluronidase-treated milk isolates is 200-fold higher than the HA-containing milk preparation (Fig. 2E). Clearly, the presence of HA in milk isolates contributes significantly to HβD2 induction. In addressing our hypothesis that HA in milk enhances innate intestinal epithelial antimicrobial defense, evaluating induction of antimicrobial peptide HβD2 by milk HA presented in context with other milk carbohydrates has merit in that it perhaps more nearly replicates the physiologic setting of milk HA presentation to the neonatal intestinal mucosa.
The intestinal mucosa of nursing mice expresses enhanced MuβD3 peptide relative to newborn or weaned animals (Fig. 3C), suggesting that milk components contribute to induction of MuβD3. We have previously detected HA in mouse milk,3 and together these observations, this indicates that mice are a relevant animal model for the evaluation of the hypothesis that milk HA specifically enhances epithelial antimicrobial MuβD3 expression in vivo. Oral administration of human milk HA to adult C57BL/6 mice resulted in substantial induction of the murine HβD2 ortholog in the mucosa of the proximal colon relative to administration of control or hyaluronidase degraded human milk preparations (Fig. 3, A and B), recapitulating the induction of MuβD3 observed in nursing mice (Fig. 3C). Consistent with our in vitro observations, induction of murine HβD2 ortholog in vivo by the human milk HA preparation is substantially reduced in the absence of intact hyaluronan, suggesting that the presence of HA in the milk contributes significantly to defensin induction. In addition, up-regulated expression of MuβD3 in the murine intestinal epithelium is seen distally in the transverse colonic mucosa (data not shown), suggesting that human milk HA retains bioactivity within the digestive tract in the context of both murine and microflora-driven catabolic activity. Previous work by Balogh et al. (65) has demonstrated that 87–96% of radiolabeled HA is recovered in the feces of rats following oral administration of a high molecular weight HA preparation, and the diffusion of HA through the paracellular junctions of cultured intestinal epithelium decreases rapidly with increasing molecular weight (66). In light of these reports and our own observation that milk HA promotes HβD2 expression in isolated cultures of human colonocytes (Figs. 2 and 5), it appears highly unlikely that the effect of oral administration of milk HA is mediated through the circulation and instead involves a direct interaction at the luminal surface of the intestinal epithelium.
Induction of the murine HβD2 ortholog following oral administration of milk HA is dependent upon expression of cell surface receptors TLR4 and CD44. Expression of MuβD3 was significantly enhanced in the intestinal mucosa of wild-type animals following oral administration of human milk HA. In contrast, MuβD3 expression was similar to control treatment in both TLR4- and CD44-deficient animals following administration of the milk HA preparation for 3 days (Fig. 4). Prior work in animal models has principally implicated TLR4 in HA-dependent modulation of intestinal defense (37, 38), particularly following oral administration of HA (39, 67), and the induction of HβD2 in cultured keratinocytes is dependent upon TLR4 (40). Our results suggest that TLR4 is an essential cell surface receptor for the induction of murine HβD2 ortholog by milk HA (Fig. 4), a finding that is consistent with our previous report that induction of MuβD3 by synthetic HA is TLR4-dependent (39). However, CD44-dependent induction of defense effectors has been reported in monocytes and macrophages (31, 58, 59) and renal epithelium (29); CD44 is required for the induction of murine HβD2 ortholog following oral administration of milk HA (Fig. 4); and anti-CD44 antibodies specifically abrogate the induction of HβD2 in cultured human epithelial cells (Fig. 6A). To our knowledge, this is the first report to suggest a role for CD44 in the regulation of defensin expression. Thus, both TLR4 and CD44 are required in the response to milk HA in vivo, and genetic deletion of either putative HA receptor is sufficient to abrogate the induction of MuβD3.
These findings indicate differing regulation of the induction of HβD2 by milk HA or synthetic HA-35, because genetic deletion of CD44 (39) or application of anti-CD44 antibody (Fig. 6A) did not significantly inhibit the induction of HβD2 by HA-35. Differences in receptor membrane dynamics or the differing activation of signal transduction mediators by the two HA preparations may account for the apparent differences in CD44 dependence. Previous work by Taylor et al. (31) suggests a mechanism by which TLR4 and CD44 complexes, colocalized in the cell membrane, cooperatively regulate the macrophage response to HA fragments generated in sterile injury through a shared signal transduction pathway. Recognition of milk HA and subsequent signal transduction resulting in MuβD3 expression may utilize similar TLR4-CD44 receptor complexes expressed on the intestinal epithelial surface. However, analysis of CD44 and TLR4 localization in the cell membrane of HT-29 cells treated with milk HA or HA-35 resulted in no observable difference in the relative distribution of CD44 and TLR4. Interestingly, aggregation of CD44 epitopes within the epithelial cell membrane appeared to be increased in the presence of milk HA but not HA-35 (Fig. 5). An alternate explanation of the observed phenomena may include separate signal transduction pathways independently activated by TLR4 and CD44 capable of acting in a complementary manner to enhance defensin expression in the presence of broadly polydispersed milk HA. Although TLR4-dependent induction of HβD2 following stimulation with bacterial pathogen-associated molecular patterns acts through the canonical TLR signal transduction mediators IRAK, TRAF6, and JNK to promote translocation of the transcription factor AP-1 and engagement of the DEFB4 promoter (36, 49), HβD2 expression is also regulated by other ligands via the MAPK/ERK signal transduction pathway (49, 68). Engagement of HA with CD44 has repeatedly been demonstrated to result in specific MAPK/ERK activation (60, 61) in a mechanism that is potentially dependent on HA size (62), and milk HA may induce HβD2 expression in a CD44-dependent manner through this pathway. Analysis of HT-29 cell lysates for the relative abundance of phosphorylated isoforms of ERK1/2, JNK1/2/3, and p38 MAPK using an ELISA panel (Fig. 6, B–D) revealed an increase in total phosphorylated ERK1/2 following treatment with milk HA, but not HA-35 or hyaluronidase-digested milk HA (Fig. 6D). Future studies will be required to distinguish between cooperative and complementary signal transduction mechanisms regulating the TLR4- and CD44- dependent response to milk HA; however, our results indicate that ERK1/2 activation is a specific consequence of the application of broadly polydispersed milk HA.
Treatment of cultured colonic epithelium with human milk HA enhances resistance to infection by the enteric pathogen Salmonella (Fig. 7), providing functional evidence in support of the hypothesis that endogenous human milk HA enhances antimicrobial defense of intestinal epithelium. Although numerous examples of the direct inhibition of pathogen-host binding by interaction with human milk glycans have been reported (16, 18–20, 27), experimental conditions included the removal of medium containing human milk HA prior to the introduction of Salmonella, suggesting that the observed reduction in Salmonella infection occurs due to modification of host epithelial cells. Infection resistance resulting from modulation of epithelium by human milk glycans has not been extensively studied; however, previous studies suggest at least two potential mechanisms for milk HA-induced antimicrobial protection. First, treatment of cultured Caco-2 epithelium with sialyllactose, a human milk oligosaccharide, results in the down-regulated expression of specific glycan epitopes on the surface of the host epithelium correlating with a reduction in the binding of pathogenic E. coli (21). Milk HA may act similarly to enhance resistance to Salmonella in HT-29 epithelium through the modulation of surface receptor expression.
Second, peak milk HA-dependent expression of antimicrobial HβD2 peptide occurred at the same time point as peak milk HA-dependent protection against Salmonella. This implies a potential role for HβD2 in the observed resistance to intracellular infection. Accumulation of intracellular defensins has been demonstrated to inhibit replication of the obligate intracellular pathogen Listeria monocytogenes in macrophages (69), and a similar mechanistic link between HβD2 and resistance to intracellular Salmonella may exist in milk HA-treated intestinal epithelium. Although questions regarding the mechanism of milk HA-mediated Salmonella infection resistance remain, our results suggest that milk HA may contribute to the reduced incidence of enteric Salmonella infection associated with breast-feeding in human infants (7). Numerous prior reports have implicated HA in modulation of the immune response (25, 31, 59); however, this is the first report to our knowledge to suggest a role for endogenous HA in resistance to enteric infection through direct effects on epithelium.
Our observations are consistent with prior publications indicating that human milk contains HA (26, 50) while providing the first evidence that this HA has biological functions at physiologic concentrations. At least two previous reports have indicated that oral administration of HA results in altered function of innate (39) or adaptive (62) components of intestinal defense; however, it was previously unclear if these observations reflected an endogenous physiologic process. The role of human milk glycans, including HA, in generating intestinal homeostasis in breast-fed infants in the presence of diverse and persistent microbial challenges is incompletely understood. Milk contains a diverse bacterial community (70), seeding the developing gastrointestinal microbiome along with maternally and environmentally acquired species and interacting with the antigen-naive adaptive mucosal immune system (71). The combined effect of milk probiotics, prebiotics, antibiotics, immunomodulators, and microbial transfer is evident in the observation that breast-fed infants exhibit dramatically different gastrointestinal microbial communities in comparison with artificially fed infants (72–74). Given the robust correlation between breast-feeding and gastrointestinal health (1, 4, 5, 16), the role of breast milk in establishing the microbial-epithelial interface may be of critical importance to lifelong gastrointestinal health (75), potentially accounting in part for the reduced lifetime risk of inflammatory bowel disease (2), obesity (76, 77), and allergic disease (78, 79) associated with breast-feeding. The bioactivity of milk components relative to the function of intestinal epithelium, such as the induction of HβD2 in intestinal epithelium by milk HA, could have a profound effect in shaping intestinal microbial ecology through the modulation of epithelial substrates and the generation of selective niches (80). Altered expression of α-defensins in the intestine has been shown to result in significant shifts in microbial species distribution (81), and our data demonstrate that the effect of milk HA on intestinal epithelium alters the interaction of host epithelium with at least one relevant component of the human microbiome, S. enterica. Future studies will probably enhance understanding of the complex dynamic regulating host epithelium and microbial interactions and the role of dietary components in shaping the relationship between enterocyte and bacterium. Of more immediate practical relevance, the supplementation of artificial formulas with HA among other glycan components of human milk may reduce the incidence of enteric infection and diarrhea among susceptible infants (16).
Acknowledgments
We thank the study participants and the staff of the Cleveland Clinic Children's Hospital, particularly Shirley Leaser, RN, IBCLC, for generous and enthusiastic assistance in the recruitment of new mothers for our study cohort. Dr. John Peterson is greatly appreciated for microscopy assistance, and we thank Dr. Mark Lauer for assisting with carbohydrate analysis. Finally, we thank Artin Soroosh, Melissa Michaud, and Ryan Verbic for diligently documenting and processing numerous donated human milk samples.
This work was supported, in whole or in part, by National Institutes of Health Grants HD061918 and HL17147 (to C. A. de la M.) and R01DK82437 (to C. M.).
D. R. Hill, H. K. Rho, S. P. Kessler, R. Amin, C. R. Homer, C. McDonald, M. K. Cowman, and C. A. de la Motte, unpublished data.
- GAG
- glycosaminoglycan
- HA
- hyaluronan
- HβD2
- human β-defensin 2
- TLR
- Toll-like receptor
- ELSA
- enzyme-linked sorbent assay
- MuβD3
- murine β-defensin 3
- HBSS
- Hanks' balanced salt solution.
REFERENCES
- 1. Howie P. W., Forsyth J. S., Ogston S. A., Clark A., Florey C. D. (1990) Protective effect of breast feeding against infection. BMJ 300, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klement E., Cohen R. V., Boxman J., Joseph A., Reif S. (2004) Breastfeeding and risk of inflammatory bowel disease. A systematic review with meta-analysis. Am. J. Clin. Nutr. 80, 1342–1352 [DOI] [PubMed] [Google Scholar]
- 3. Anderson J. W., Johnstone B. M., Remley D. T. (1999) Breast-feeding and cognitive development. A meta-analysis. Am. J. Clin. Nutr. 70, 525–535 [DOI] [PubMed] [Google Scholar]
- 4. Newburg D. S., Walker W. A. (2007) Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 61, 2–8 [DOI] [PubMed] [Google Scholar]
- 5. Grulee C. G., Sanford H. N., Herron P. H. (1934) Breast and artificial feeding. Influence on morbidity and mortality of twenty thousand infants. JAMA 103, 735–738 [Google Scholar]
- 6. Morrow A. L., Guerrero M. L., Shults J., Calva J. J., Lutter C., Bravo J., Ruiz-Palacios G., Morrow R. C., Butterfoss F. D. (1999) Efficacy of home-based peer counselling to promote exclusive breastfeeding. A randomised controlled trial. Lancet 353, 1226–1231 [DOI] [PubMed] [Google Scholar]
- 7. Jones T. F., Ingram L. A., Fullerton K. E., Marcus R., Anderson B. J., McCarthy P. V., Vugia D., Shiferaw B., Haubert N., Wedel S., Angulo F. J. (2006) A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 118, 2380–2387 [DOI] [PubMed] [Google Scholar]
- 8. Ward P. P., Uribe-Luna S., Conneely O. M. (2002) Lactoferrin and host defense. Biochem. Cell Biol. 80, 95–102 [DOI] [PubMed] [Google Scholar]
- 9. Brandtzaeg P. (2003) Mucosal immunity. Integration between mother and the breast-fed infant. Vaccine 21, 3382–3388 [DOI] [PubMed] [Google Scholar]
- 10. Martin L. J., Woo J. G., Geraghty S. R., Altaye M., Davidson B. S., Banach W., Dolan L. M., Ruiz-Palacios G. M., Morrow A. L. (2006) Adiponectin is present in human milk and is associated with maternal factors. Am. J. Clin. Nutr. 83, 1106–1111 [DOI] [PubMed] [Google Scholar]
- 11. Asakuma S., Hatakeyama E., Urashima T., Yoshida E., Katayama T., Yamamoto K., Kumagai H., Ashida H., Hirose J., Kitaoka M. (2011) Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 286, 34583–34592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcobal A., Barboza M., Sonnenburg E. D., Pudlo N., Martens E. C., Desai P., Lebrilla C. B., Weimer B. C., Mills D. A., German J. B., Sonnenburg J. L. (2011) Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sela D. A., Li Y., Lerno L., Wu S., Marcobal A. M., German J. B., Chen X., Lebrilla C. B., Mills D. A. (2011) An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 286, 11909–11918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu Z.-T., Chen C., Kling D. E., Liu B., McCoy J. M., Merighi M., Heidtman M., Newburg D. S. (2013) The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 23, 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. M'Rabet L., Vos A. P., Boehm G., Garssen J. (2008) Breast-feeding and its role in early development of the immune system in infants. Consequences for health later in life. J. Nutr. 138, 1782S-1790S [DOI] [PubMed] [Google Scholar]
- 16. Newburg D. S. (2009) Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 87, 26–34 [DOI] [PubMed] [Google Scholar]
- 17. Bode L. (2012) Human milk oligosaccharides. Every baby needs a sugar mama. Glycobiology 22, 1147–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz-Palacios G. M., Cervantes L. E., Ramos P., Chavez-Munguia B., Newburg D. S. (2003) Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278, 14112–14120 [DOI] [PubMed] [Google Scholar]
- 19. Morrow A. L., Ruiz-Palacios G. M., Altaye M., Jiang X., Guerrero M. L., Meinzen-Derr J. K., Farkas T., Chaturvedi P., Pickering L. K., Newburg D. S. (2004) Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 145, 297–303 [DOI] [PubMed] [Google Scholar]
- 20. Liu B., Yu Z., Chen C., Kling D. E., Newburg D. S. (2012) Human milk mucin 1 and mucin 4 inhibit Salmonella enterica serovar Typhimurium invasion of human intestinal epithelial cells in vitro. J. Nutr. 142, 1504–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angeloni S., Ridet J. L., Kusy N., Gao H., Crevoisier F., Guinchard S., Kochhar S., Sigrist H., Sprenger N. (2005) Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 15, 31–41 [DOI] [PubMed] [Google Scholar]
- 22. Kuntz S., Rudloff S., Kunz C. (2008) Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br. J. Nutr. 99, 462–471 [DOI] [PubMed] [Google Scholar]
- 23. Kuntz S., Kunz C., Rudloff S. (2009) Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br. J. Nutr. 101, 1306–1315 [DOI] [PubMed] [Google Scholar]
- 24. Cederlund A., Kai-Larsen Y., Printz G., Yoshio H., Alvelius G., Lagercrantz H., Strömberg R., Jörnvall H., Gudmundsson G. H., Agerberth B. (2013) Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 8, e53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stern R., Asari A. A., Sugahara K. N. (2006) Hyaluronan fragments. An information-rich system. Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 26. Coppa G. V., Gabrielli O., Buzzega D., Zampini L., Galeazzi T., Maccari F., Bertino E., Volpi N. (2011) Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology 21, 295–303 [DOI] [PubMed] [Google Scholar]
- 27. Newburg D. S., Linhardt R. J., Ampofo S. A., Yolken R. H. (1995) Human milk glycosaminoglycans inhibit HIV glycoprotein gp120 binding to its host cell CD4 receptor. J. Nutr. 125, 419–424 [DOI] [PubMed] [Google Scholar]
- 28. Laurent T. C., Laurent U. B., Fraser J. R. (1995) Functions of hyaluronan. Ann. Rheum Dis. 54, 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beck-Schimmer B., Oertli B., Pasch T., Wüthrich R. P. (1998) Hyaluronan induces monocyte chemoattractant protein-1 expression in renal tubular epithelial cells. J. Am. Soc. Nephrol. 9, 2283–2290 [DOI] [PubMed] [Google Scholar]
- 30. Chen G. Y., Nuñez G. (2010) Sterile inflammation. Sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 32. Scheibner K. A., Lutz M. A., Boodoo S., Fenton M. J., Powell J. D., Horton M. R. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 [DOI] [PubMed] [Google Scholar]
- 33. Noble P. W., McKee C. M., Cowman M., Shin H. S. (1996) Hyaluronan fragments activate an NF-κB/I-κB α autoregulatory loop in murine macrophages. J. Exp. Med. 183, 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campo G. M., Avenoso A., Campo S., D'Ascola A., Nastasi G., Calatroni A. (2010) Small hyaluronan oligosaccharides induce inflammation by engaging both Toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 80, 480–490 [DOI] [PubMed] [Google Scholar]
- 35. Taylor K. R., Trowbridge J. M., Rudisill J. A., Termeer C. C., Simon J. C., Gallo R. L. (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 279, 17079–17084 [DOI] [PubMed] [Google Scholar]
- 36. O'Neill L. A. (2002) Signal transduction pathways activated by the IL-1 receptor/Toll-like receptor superfamily. Curr. Top Microbiol. Immunol. 270, 47–61 [PubMed] [Google Scholar]
- 37. Zheng L., Riehl T. E., Stenson W. F. (2009) Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 137, 2041–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riehl T. E., Foster L., Stenson W. F. (2012) Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am. J. Physiol. Gastrointest Liver Physiol. 302, G309–G316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hill D. R., Kessler S. P., Rho H. K., Cowman M. K., de la Motte C. A. (2012) Specific-sized hyaluronan fragments promote expression of human β-defensin 2 in intestinal epithelium. J. Biol. Chem. 287, 30610–30624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gariboldi S., Palazzo M., Zanobbio L., Selleri S., Sommariva M., Sfondrini L., Cavicchini S., Balsari A., Rumio C. (2008) Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. J. Immunol. 181, 2103–2110 [DOI] [PubMed] [Google Scholar]
- 41. Dusio G. F., Cardani D., Zanobbio L., Mantovani M., Luchini P., Battini L., Galli V., Diana A., Balsari A., Rumio C. (2011) Stimulation of TLRs by LMW-HA induces self-defense mechanisms in vaginal epithelium. Immunol. Cell Biol. 89, 630–639 [DOI] [PubMed] [Google Scholar]
- 42. Selsted M. E., Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 [DOI] [PubMed] [Google Scholar]
- 43. Garreis F., Schlorf T., Worlitzsch D., Steven P., Bräuer L., Jäger K., Paulsen F. P. (2010) Roles of human β-defensins in innate immune defense at the ocular surface. Arming and alarming corneal and conjunctival epithelial cells. Histochem. Cell Biol. 134, 59–73 [DOI] [PubMed] [Google Scholar]
- 44. Zasloff M. (2002) Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- 45. O'Neil D. A. (2003) Regulation of expression of β-defensins. Endogenous enteric peptide antibiotics. Mol. Immunol. 40, 445–450 [DOI] [PubMed] [Google Scholar]
- 46. Wada A., Mori N., Oishi K., Hojo H., Nakahara Y., Hamanaka Y., Nagashima M., Sekine I., Ogushi K., Niidome T., Nagatake T., Moss J., Hirayama T. (1999) Induction of human β-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 263, 770–774 [DOI] [PubMed] [Google Scholar]
- 47. Ogushi K., Wada A., Niidome T., Mori N., Oishi K., Nagatake T., Takahashi A., Asakura H., Makino S., Hojo H., Nakahara Y., Ohsaki M., Hatakeyama T., Aoyagi H., Kurazono H., Moss J., Hirayama T. (2001) Salmonella enteritidis FliC (flagella filament protein) induces human β-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 276, 30521–30526 [DOI] [PubMed] [Google Scholar]
- 48. Vora P., Youdim A., Thomas L. S., Fukata M., Tesfay S. Y., Lukasek K., Michelsen K. S., Wada A., Hirayama T., Arditi M., Abreu M. T. (2004) β-Defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 173, 5398–5405 [DOI] [PubMed] [Google Scholar]
- 49. Froy O. (2005) Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell. Microbiol. 7, 1387–1397 [DOI] [PubMed] [Google Scholar]
- 50. Coppa G. V., Gabrielli O., Zampini L., Galeazzi T., Maccari F., Buzzega D., Galeotti F., Bertino E., Volpi N. (2012) Glycosaminoglycan content in term and preterm milk during the first month of lactation. Neonatology 101, 74–76 [DOI] [PubMed] [Google Scholar]
- 51. Calabro A., Benavides M., Tammi M., Hascall V. C., Midura R. J. (2000) Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 10, 273–281 [DOI] [PubMed] [Google Scholar]
- 52. Ohya T., Kaneko Y. (1970) Novel hyaluronidase from streptomyces. Biochim. Biophys. Acta 198, 607–609 [DOI] [PubMed] [Google Scholar]
- 53. Shimada E., Matsumura G. (1980) Degradation process of hyaluronic acid by Streptomyces hyaluronidase. J. Biochem. 88, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 54. Aksamitiene E., Hoek J. B., Kholodenko B., Kiyatkin A. (2007) Multistrip Western blotting to increase quantitative data output. Electrophoresis 28, 3163–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid. Polycytidylic acid inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhilocha S., Amin R., Pandya M., Yuan H., Tank M., LoBello J., Shytuhina A., Wang W., Wisniewski H. G., de la Motte C., Cowman M. K. (2011) Agarose and polyacrylamide gel electrophoresis methods for molecular mass analysis of 5–500 kDa hyaluronan. Anal. Biochem. 417, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bals R., Wang X., Meegalla R. L., Wattler S., Weiner D. J., Nehls M. C., Wilson J. M. (1999) Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67, 3542–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 59. Jiang D., Liang J., Noble P. W. (2011) Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meran S., Luo D. D., Simpson R., Martin J., Wells A., Steadman R., Phillips A. O. (2011) Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J. Biol. Chem. 286, 17618–17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Puré E., Assoian R. K. (2009) Rheostatic signaling by CD44 and hyaluronan. Cell. Signal. 21, 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang C., Cao M., Liu H., He Y., Xu J., Du Y., Liu Y., Wang W., Cui L., Hu J., Gao F. (2012) The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 287, 43094–43107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Homer C. R., Richmond A. L., Rebert N. A., Achkar J.-P., McDonald C. (2010) ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology 139, 1630–1641, 1641.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bokor S., Koletzko B., Decsi T. (2007) Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann. Nutr. Metab. 51, 550–556 [DOI] [PubMed] [Google Scholar]
- 65. Balogh L., Polyak A., Mathe D., Kiraly R., Thuroczy J., Terez M., Janoki G., Ting Y., Bucci L. R., Schauss A. G. (2008) Absorption, uptake and tissue affinity of high-molecular weight hyaluronan after oral administration in rats and dogs. J. Agric. Food Chem. 56, 10582–10593 [DOI] [PubMed] [Google Scholar]
- 66. Hisada N., Satsu H., Mori A., Totsuka M., Kamei J., Nozawa T., Shimizu M. (2008) Low-molecular weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci. Biotechnol. Biochem. 72, 1111–1114 [DOI] [PubMed] [Google Scholar]
- 67. Asari A., Kanemitsu T., Kurihara H. (2010) Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J. Biol. Chem. 285, 24751–24758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moon S.-K., Lee H.-Y., Li J.-D., Nagura M., Kang S.-H., Chun Y.-M., Linthicum F. H., Ganz T., Andalibi A., Lim D. J. (2002) Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1α-induced upregulation of β-defensin 2 in human middle ear epithelial cells. Biochim. Biophys. Acta 1590, 41–51 [DOI] [PubMed] [Google Scholar]
- 69. Arnett E., Lehrer R. I., Pratikhya P., Lu W., Seveau S. (2011) Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 13, 635–651 [DOI] [PubMed] [Google Scholar]
- 70. Hunt K. M., Foster J. A., Forney L. J., Schütte U. M., Beck D. L., Abdo Z., Fox L. K., Williams J. E., McGuire M. K., McGuire M. A. (2011) Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 6, e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brandtzaeg P. (2010) The mucosal immune system and its integration with the mammary glands. J. Pediatr. 156, S8–S15 [DOI] [PubMed] [Google Scholar]
- 72. Penders J., Thijs C., Vink C., Stelma F. F., Snijders B., Kummeling I., van den Brandt P. A., Stobberingh E. E. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 [DOI] [PubMed] [Google Scholar]
- 73. Dominguez-Bello M. G., Costello E. K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U.S.A. 107, 11971–11975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Palma G. D., Capilla A., Nova E., Castillejo G., Varea V., Pozo T., Garrote J. A., Polanco I., López A., Ribes-Koninckx C., Marcos A., García-Novo M. D., Calvo C., Ortigosa L., Peña-Quintana L., Palau F., Sanz Y. (2012) Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants. The PROFICEL study. PLoS One 7, e30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Le Huërou-Luron I., Blat S., Boudry G. (2010) Breast- v. formula-feeding. Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 23, 23–36 [DOI] [PubMed] [Google Scholar]
- 76. Owen C. G., Martin R. M., Whincup P. H., Smith G. D., Cook D. G. (2005) Effect of infant feeding on the risk of obesity across the life course. A quantitative review of published evidence. Pediatrics 115, 1367–1377 [DOI] [PubMed] [Google Scholar]
- 77. Oddy W. H. (2012) Infant feeding and obesity risk in the child. Breastfeed Rev. 20, 7–12 [PubMed] [Google Scholar]
- 78. Gdalevich M., Mimouni D., David M., Mimouni M. (2001) Breast-feeding and the onset of atopic dermatitis in childhood. A systematic review and meta-analysis of prospective studies. J. Am. Acad. Dermatol. 45, 520–527 [DOI] [PubMed] [Google Scholar]
- 79. van Odijk J., Kull I., Borres M. P., Brandtzaeg P., Edberg U., Hanson L. A., Høst A., Kuitunen M., Olsen S. F., Skerfving S., Sundell J., Wille S. (2003) Breastfeeding and allergic disease. A multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 58, 833–843 [DOI] [PubMed] [Google Scholar]
- 80. Costello E. K., Stagaman K., Dethlefsen L., Bohannan B. J., Relman D. A. (2012) The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salzman N. H., Hung K., Haribhai D., Chu H., Karlsson-Sjöberg J., Amir E., Teggatz P., Barman M., Hayward M., Eastwood D., Stoel M., Zhou Y., Sodergren E., Weinstock G. M., Bevins C. L., Williams C. B., Bos N. A. (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 11, 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]