Abstract
Purpose
This study investigates whether certain embryos considered unsuitable for cryopreservation on day 3 might nevertheless have the potential to develop into worthwhile blastocysts that could be vitrified in the same cycle.
Methods
Retrospective study: between 2010 and 2011, embryo transfers and cryopreservation took place mainly on day 3 in our centre. Supernumerary embryos of intermediate to poor quality were reassessed on days 5/6 and any good quality blastocysts were vitrified.
Results
Out of 914 cleavage stage (day 3) embryos left in culture, 16 % were vitrified on days 5/6. Fifty blastocyst warming cycles resulted in a 76 % survival rate, 44 % clinical pregnancy rate and 39 % implantation rate. During the same time period, 213 warming cycles of good quality cleavage stage embryos rendered survival rates, clinical pregnancy and implantation rates of 97 %, 23 % and 16 % respectively.
Conclusions
Supernumerary average quality day 3 embryos should be given a second chance to be selected for cryopreservation. If blastocysts are obtained and survive vitrification, there is a good chance of implantation thus reducing embryo waste.
Keywords: Blastocyst, Day 3 embryo, Vitrification, Embryo wastage, Embryo quality
Introduction
With the development of efficient culture systems, it is becoming more reliable to obtain blastocysts in vitro. Certain laboratories opt for a fresh blastocyst transfer since uterine contractility is decreased [10] and embryo-endometrial synchronicity is enhanced [41]. Moreover the risk of multiple pregnancies can be reduced without compromising pregnancy rates by improving selection and transferring fewer embryos [14, 34, 39]. However, the population attending IVF in most centers is not a majority of young and good prognosis patients, therefore a possible drawback of extended culture could be a transfer cancellation [4, 19]. The policy at the CHU St Pierre is a fresh day 3 transfer combined with cryopreservation of good quality embryos the same day. Since 2010, we have switched from slow freezing supernumerary embryos to vitrification [5, 38, 40]. Indeed, many studies comparing slow freezing and vitrification have demonstrated superior survival rates for vitrification of day 3 embryos [2, 20, 22]. The emergence of vitrification technology has also allowed for the possibility of cryopreserving blastocysts with high pregnancy and implantation rates [18, 21, 28, 43, 44]. Cryopreservation increases the cumulative success rates [23] which in turn could help reduce patient drop out. Indeed, several reports have concluded to a discontinuation of IVF treatments due to psychological stress and emotional burden [15, 30].
Selection of cleavage stage (day 3) embryos for cryopreservation varies from one centre to another. It is possible that laboratories with strict inclusion criteria might be discarding competent reproductive material resulting in embryo wastage. Certain studies have shown that some poor quality cleavage stage embryos are capable nevertheless of reaching the blastocyst stage, implanting [1, 32] and producing healthy babies [14].
The aim of this study was to evaluate whether the extended culture of embryos considered unsuitable for cryopreservation on day 3 could produce worthwhile blastocysts that could be successfully vitrified in the same cycle.
Results of these blastocyst as well as cleavage stage embryo warming cycles performed during the same time period are presented. A small group of patients had both day 3 and day 5 embryos cryopreserved in the same fresh cycle. The results of their warming cycles were equally analyzed.
Material and methods
Ovarian stimulation and oocyte retrieval
Patients selected for IVF were monitored and managed according to standardized clinical protocols as previously reported [6]. Briefly, ovarian stimulation was performed with hMG, recombinant FSH or corifollitropine Alfa (long acting FSH). The dose of gonadotropins were determined on an individual basis according to the woman’s age, day 3 serum FSH value and antral follicle count. Pituitary inhibition was obtained by GnRH analogue (long or short protocol) or GnRH antagonist. When three or more leading follicles reached 17–18 mm, 5000 UI of hCG were administered.
Oocyte retrieval was performed transvaginally and ultrasound-guided 34–36 h after hCG injection.
Embryo culture and selection
17–20 h after ICSI/IVF, fertilization was monitored and zygotes were cultured individually in G1 (Vitrolife, Sweden) or CLM (Cook, Australia) media under 6 % CO2, 37 °C until day 3. Embryos with extended culture were transferred to fresh G2 or BLM under the same conditions until days 5/6.
On the mornings of days 2 and 3 embryo, morphology was assessed by an embryologist under an inverted microscope. The best quality embryos (number according to the Belgian law) were transferred on day 3 and supernumerary embryos of good quality were vitrified. The following embryos considered unsuitable for vitrification on day 3 were left in culture for 2 to 3 additional days:—embryos with 20 % or more fragmentation [29, 47];—slow cleaving embryos (<6 cells) [24];—embryos presenting strong granularity, vacuolization [7], zona anomalies or a combination of these features;—fast cleaving day 2 embryos (>6 cells) [24];—day 2 embryos presenting three regular cells.
Zona thickness was not taken into account, only large pouches or elongated embryos [8] were considered abnormal.
The decision to discard or extend an embryo was left to the appreciation of the trained biologist doing the morphological examination. Special care was taken to standardize the procedure as well as the microscope settings. Very poor quality cleavage stage embryos including; arrested, multi-nucleated, poly-fragmented embryos or embryos with several negative characteristics were generally discarded.
The others were left in culture and re-evaluated on days 5/6. Blastocysts with an Inner Cell Mass and trophoectoderm mainly of grades A/B [12] were vitrified.
Vitrification cooling protocol
The Irvine Scientific Freeze Kit (Irvine, USA) combined with CBS-VIT High Security straws from CryoBioSystem were used for vitrification. All basic solutions contained HEPES-buffered Medium-199, gentamicin sulphate 35 μg/mL, and 20 % v/v Dextran Serum Supplement (DSS). One to two embryos were progressively brought to room temperature and then incubated 8 min (cleavage stage embryos) to 10 min (blastocysts) in a 20 μl ES drop (Equilibration Solution: 7.5 % v/v of each DMSO and ethylene glycol) followed by 2 times 5 s and 1 time 10 s in 20 μl VS drops (Vitrification Solution: 15 % v/v of each DMSO and ethylene glycol, 0.5 M sucrose). The smallest possible volume of VS containing the embryo(s) was loaded into the gutter of the straw, which in turn was inserted into an external sheath; heat sealed and plunged horizontally into liquid nitrogen (LN2). The embryos were in contact with the VS between 60 and 90 s. The whole procedure was carried out at room temperature.
Vitrification warming protocol
The Irvine Scientific Thaw Kit (Irvine, USA) was used for warming. Again, all basic solutions contained HEPES-buffered Medium-199, gentamicin sulphate 35 μg/mL, and 20 % v/v Dextran Serum Supplement (DSS). Straws to be warmed were transferred into a small recipient containing LN2. The external sheath was cut; the inner straw removed from LN2 and plunged directly in a large droplet (200 μl) of TS media (Thawing Solution: 1 M sucrose) preheated to 37 °C. The embryo(s) were left in this media for 1 min on a non heated stage and then transferred into 20 μl of DS media (Dilution Solution: 0.5 M sucrose) twice for 2 min, followed by 3 times 3 min incubation in WS media (Washing Solution: HEPES-buffered solution of Medium-199 containing gentamicin sulphate 35 μg/mL HEPES and 20 % DSS). During the last incubation step, embryos were brought progressively back to 37 °C, cultured for 1 h in G2 or BLM containing 20 % HSA and then in media with 10 % HSA until transfer. Survival of embryos was monitored straight after the warming procedure and before transfer. Blastocysts were transferred end of the morning. Day 3 embryos were cultured overnight in order to assess development recovery. Warmed cleavage stage embryos were submitted to assisted laser hatching.
Outcome parameters
Embryos vitrified during the years 2010, 2011 and thereafter warmed before August 2012, were taken into account in this study.
Embryological outcome
Embryo survival was assessed immediately after warming and was defined for cleavage stage embryos as the loss of less than 50 % of the blastomeres. Blastocysts with partial or no damage were considered to have survived and were transferred even if re-expansion had not always occurred at the time of transfer.
Clinical outcome
Serum βhCG levels were measured 14 days after oocyte retrieval. The Implantation Rate (IR) was defined as the number of gestational sacs (intra uterine and extra uterine) divided by the number of transferred embryos. A Clinical Pregnancy (CP) was defined as a pregnancy with a gestational sac. Ongoing pregnancies were defined as pregnancies that had progressed beyond 22 weeks but had yet not resulted in a birth at the time of article submission. Results of live births were recorded.
Statistical analysis
Since this is a retrospective analysis, we used exploratory statistics. Differences in terms of vitrification were assessed between groups of extended embryo’s (Fig. 2) using Chi Square test (significance set at p < 0.05). For a small group of 14 patients who had an embryo cryopreservation on days 3 and on days 5/6 in the same fresh cycle, pregnancy outcomes for warming cycles were also assessed (Table 3) using Chi Square test (considering that all events are independent occurrences which is not the case). The conclusions drawn from the exploratory statistics used should be done with caution [3].
Fig. 2.
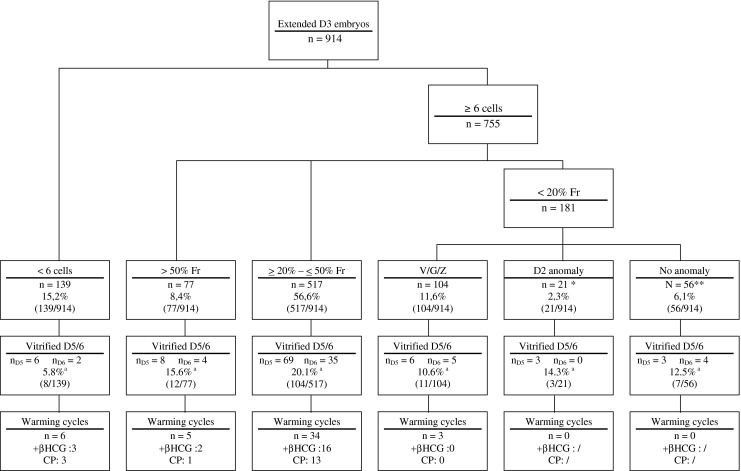
Day 3 embryo classification regarding main negative features, percentage of vitrified/warmed embryos and resulting pregnancies. D3 = Day 3; D2 = Day 2; D5/6 = Days 5/6; Fr = Fragmented; V = Vacuolization; G = Granularity; Z = Zona anomaly; CP = Clinical Pregnancy. * ≥ 6 cells day 2 or 3 regular cells day 2 **some good quality day 3 embryos were extended for various reasons. Two warming cycles are not presented due to mixed transfers of 2 embryos from different groups a (chi square: 20.8; p < 0.001)
Table 3.
Results of day 3 and day 5 warming cycles for embryos cryopreserved in the same fresh IVF cycle for 14 patients
| Blastocyst (D5/6) | Cleavage stage (D3) | |||
|---|---|---|---|---|
| Warming cycles: | 16 | 21 | ||
| Transfers: | 15 | 21 | ||
| Warmed embryos: | 24 | 40 | ||
| Positive βHCG/TF: | 10/15 a | 66.7 % | 4/21 a | 19.0 % |
| IR: | 8/20 b | 40.0 % | 2/38 b | 5.3 % |
| Clinical Pregnancy/TF: | 7/15 c | 46.7 % | 2/21 c | 9.5 % |
| Pregnancies: | 10 | 4 | ||
| Babies born: | 5* | 1 | ||
| Early miscarriage: | 2 | 1 | ||
| Extra uterine: | 1 | 0 | ||
| Biochemical: | 3 | 2 | ||
D5/6 days 5/6; D3 day 3; TF transfer. IR implantation rate
*1 pregnancy produced twins
a(chi square: 8.3; p < 0.004)
b(chi square: 6.7; p < 0.05)
c(chi square: 6.4; p < 0.02)
Ethical statement
All our protocols have been approved by the local Ethics Committee.
Results
Characteristics of stimulated IVF cycles
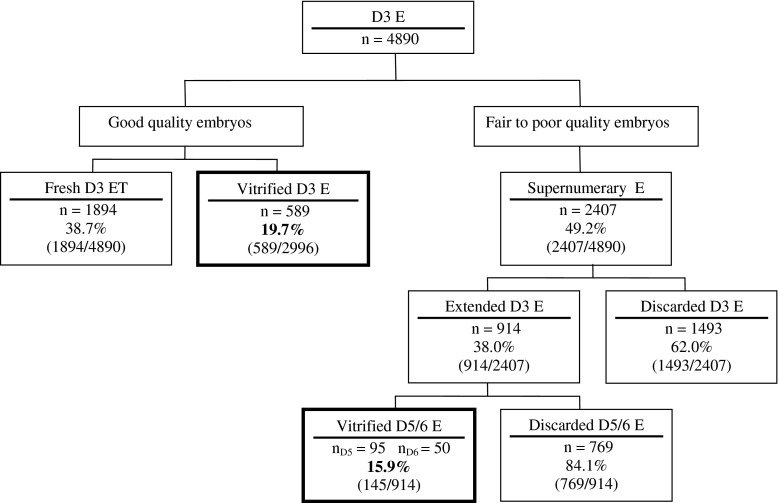
During 2010 and 2011, 1142 stimulated IVF cycles including a fresh day 3 transfer took place. Characteristics of these cycles are presented in Table 1. On day 3, out of a total of 4890 embryos, the best quality ones were selected for embryo transfer (n = 1894) or vitrified the same day (n = 589) (Fig. 1). Out of a total of 2407 embryos considered unsuitable for vitrification on day 3, 914 were cultured to day 5 or 6 or directly discarded generally due to very poor quality (n = 1493). 145 good quality blastocysts on days 5/6 were vitrified, thus increasing the total number of cryopreserved supernumerary embryos by 4.8 %
Table 1.
Characteristics of stimulated IVF cycles with a fresh day 3 embryo transfer
| Stimulated cycles with a transfer (n): | 1142 |
| Mean patients age ± SD (years): | 35.1 ± 5.2 |
| Positive βHCG/cycle: | 35.3 % (403/1142) |
| IR: | 20.1 % (380/1894) |
| CP/Transfer: | 29.8 % (341/1142) |
| Mean embryos/cycle ± SD: | 4.3 ± 2.7 |
| Mean embryos TF/cycle ± SD: | 1.7 ± 0.7 |
| Proportion of cycles with embryo cryopreservation: | 27.8 % (318/1142) |
IR implantation rate; CP clinical pregnancy; TF transferred; SD standard deviation
Fig. 1.
Fate of day 3 embryos. D3 = Day 3; E = Embryo; T = Transfer; D5/6 = Days 5/6
Extended embryo culture
914 embryos were left in culture and further assessed on days 5/6. We classified them into groups regarding their main negative feature (Fig. 2). The proportions of vitrified embryos as well as the pregnancy rates obtained after warming cycles were calculated for each group (Fig. 2).
The percentage of vitrified embryos was found to be statistically different between the different groups (Fig. 2).
Day 3 and day 5 warming cycles
The outcome for blastocyst and cleavage stage embryo warming cycles is presented in Table 2. Fifty blastocyst warming cycles resulted in a 75.8 % survival rate, 43.6 % clinical pregnancy rate and 39.1 % implantation rate. 213 warming cycles of good quality cleavage stage embryos rendered survival rates, clinical pregnancy and implantation rates of 96.6 %, 23.0 % and 15.5 % respectively (Table 2). Although, 84.3 % of cleavage stage embryos were intact after warming, only 63.9 % showed signs of compaction or mitosis overnight (gain of ≥ 2 cells). The clinical pregnancy rate for transfers involving non evolving embryos was 19.0 %.
Table 2.
Outcome of blastocyst and cleavage stage warming cycles
| Blastocyst (D5/6) | Cleavage stage (D3) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Warming cycles: | 50 | 213 | ||
| Patients: | 46 | 151 | ||
| Mean patients age ± SD (years): | 33.7 ± 4.0 | 33.2 ± 5.0 | ||
| Warmed embryos: | 63 | 373 | ||
| Transferred embryos | 46 | 336 | ||
| Mean embryos TF/cycle ± SD: | 1.2 ± 0.4 | 1.6 ± 0.6 | ||
| Lost embryos: | 1 | 1.6 % | 16 | 4.3 % |
| Survival: | 47 | 75.8 % | 345 | 96.6 % |
| Transfers: | 39 | 78.0 % | 204 | 96.0 % |
| Positive βHCG/Transfer: | 22 | 56.0 % | 62 | 30.4 % |
| CP/Transfer: | 17 | 43.6 % | 47 | 23.0 % |
| IR: | 18 | 39.1 % | 52 | 15.5 % |
| TFs with live births: | 10 | 25.6 % | 30 | 13.7 % |
| Cycles with live births: | 10 | 20.0 % | 30 | 13.1 % |
| Babies born: | 10 | 30a | ||
| Ongoing pregnancies: | 1 | 2 | ||
D3 day 3; D5/6 days 5/6; TF transferred; TFs transfers; CP clinical pregnancy; IR implantation rate; SD standard deviation
aFour babies were born from two twin pregnancies
Day 3 and day 5 warming cycles for the same patient
For a small group of 14 patients who had an embryo cryopreservation on days 3 and on days 5/6 in the same fresh cycle, pregnancy outcomes for warming cycles were compared (Table 3). The CP and implantation rates were 46.7 % and 40.0 % for blastocyst warming cycles versus 9.5 % and 5.3 % for cleavage stage embryo cycles. Priority was given to warming day 3 embryos first and some patients had several day 3 and/or day 5 warming cycles. Exploratory statistics showed that the proportion of positive βHCG’s, clinical pregnancies and implantation rates were statistically different between day 3 and day 5 cycles (Table 3).
Discussion
The findings in this study show that some fair and even poor quality embryos that would have been discarded following our centre’s previous guidelines can develop to the blastocyst stage and provide good results after warming. A total of 145 blastocysts (15.9 %) were vitrified and to date, 63 have been warmed, enabling the birth of ten healthy babies and one ongoing pregnancy. Clearly, the extended culture allowed us to reduce embryo wastage, since the percentage of cryopreserved embryos was raised from 19.7 % on day 3 to 24.5 % when blastocysts were taken into account.
In a similar study, 6.6 % blastocysts were vitrified after the extended culture of poor quality embryos. The embryo utilization rate was increased from 30.8 % to 32.6 % and after warming IR and CP rates were 32.8 % and 40.9 % respectively [31]. In 2011, Guerif et al., studied a population of young patients without top quality cleavage stage embryos. Patients with the poorest overall embryo quality achieved a blastocyst transfer in 78 % of cases. Implantation rates were significantly higher at the blastocyst stage with a single embryo transfer compared with a double embryo transfer on day 2 (40.9 % vs. 7.8 %) [14]. In an earlier study, Balaban and colleagues concluded that a blastocyst transfer originating from poor quality day 3 embryos is feasible and results in significantly higher implantation rates than a day 3 transfer (15 % vs. 5.9 %) [1]. These results confirm the knowledge that cleavage stage embryo morphology alone has its limits for embryo selection [13, 27, 32].
New techniques, including non-invasive metabolomic, proteomic or transcriptomic profiling [16, 33, 36] are under evaluation to help select an embryo capable of implanting. A recent and interesting publication by Wong and co-workers demonstrated that a combination of cytokinetic and mitotic parameters in the first two cleavage divisions, before embryonic genome activation can predict blastocyst formation at a high rate. By correlating time-lapse image analysis and gene expression profiling from zygotes to blastocysts, they showed that embryos follow a strict developmental timeline that is correlated with gene expression patterns [45]. Future clinical studies will evaluate the possibility of predicting blastocyst formation at day 2.
Although in our study 15.9 % good quality blastocysts could be cryopreserved, it is well documented, that extended culture does not guarantee that all genetic anomalies will be screened out [11, 17, 35]. This underlines the importance of following up pregnancy outcomes.
The largest group amongst the extended embryos (56.6 %) consisted of embryos presenting grade B fragmentation (≥20 % – ≤50 % fragments), and 20 % of these embryos could be vitrified at the blastocyst stage. The majority of pregnancies obtained to date after blastocyst warming cycles, were from these grade B fragmented embryos. Our cut off for cryopreservation on day 3 is less than 20 % fragments. There could be a difference between embryos displaying 25 %, 35 % or 45 % fragments as well as for embryos with a dispersed or grouped distribution of fragments. For example, a scattered appearance was found to be correlated with an increased incidence of chromosome abnormalities [24]. In our study, we did not consider these different features.
Morphokinetic analysis for the different groups revealed that most of the blastocysts were vitrified on day 5 (66 %). Slow cleaving embryos produced the least good quality blastocysts (5.8 %). Indeed, 62 % extended embryos were either arrested or degenerated and less than one percent were expanded blastocysts on day 5 (data not shown).
Vitrification was implemented in our centre end of 2009. As expected from the literature, survival rates for cleavage stage embryos were just below 100 % and thus an important improvement compared to our previous results for slow freezing (survival rate 57.9 % CP rate 28.8 %, IR 19.4 % in 2008). In a recent review, Edgar and Gook concluded that cleavage stage embryos and blastocysts which survive cryopreservation by vitrification or slow freezing can implant at the same rate as equivalent fresh embryos [9].
Success of vitrification is dependant on many factors including embryo stage, embryo selection, warming and cooling protocols, as well as patient and stimulation characteristics. Good quality cleavage stage embryos were vitrified in this study. Blastocysts on the other hand, were obtained from lesser quality day 3 embryos but nevertheless resulted in good clinical pregnancy and implantation rates.
It is possible that patients who had a blastocyst warming cycle had a better prognosis than those who had a cleavage stage warming cycle. However, for a small group of patients who had both day 3 and day 5 warming cycles for embryos cryopreserved in the same fresh cycle, a trend in favor of blastocyst transfers was also observed.
Mesut et al. [26] reported better outcome measures, when comparing vitrified blastocysts to vitrified cleavage embryos. The same advantage was also observed if day 3 cryopreserved embryos were allowed to develop to blastocysts after thawing [26].
Our data do not allow us to distinguish between the effects associated with improved embryo selection after extended culture from those possibly linked to better adapted cooling/warming protocols to blastocysts versus cleavage embryos. Blastocysts have the advantage of a higher cell number which can help compensate for partial cryo-damage. They also have a higher membrane/cytoplasmic ratio which should theoretically be another advantage during vitrification [42].
Several healthy babies and ongoing pregnancies were obtained thanks to the extended culture of fair to poor quality embryos.
The main goal in ART is to obtain a healthy baby but in a reasonable lapse of time. Patients who have several fresh and cryopreserved cycles without a positive outcome are at a higher risk of cancelling their treatment for reasons other than financial. Patient drop-out due to stress, depression and anxiety is well described in the literature.
In view of our results, changing our strategy from fresh day 3 transfers to mainly day 5 transfers as well as cryopreserving embryos at the blastocyst stage seems a logical strategy.
Fresh blastocyst transfers have been shown to be beneficial for good prognosis patients with several good quality embryos on day 3 [4]. Combining a transfer and cryopreservation at the blastocyst stage could help achieve a pregnancy quicker. Probably less embryos would be cryopreserved but with a higher implantation potential. On the other hand, Zhu et al., reported that it might be time for a new embryo transfer strategy, indeed several groups have observed better results after warmed blastocyst transfers compared to fresh [37, 46]. In the study by Zhu for example, clinical pregnancy rates were 36.4 % for fresh and 55.1 % for warmed embryo cycles despite a survival rate of 85.7 %. Endometrial receptivity could be adversely affected by controlled ovarian stimulation and additionally the vitrification/warming procedure may weed out blastocysts with poor developmental competence. Only warmed blastocysts that had expanded in 14–16 h were considered to have survived.
A certain reluctance to carry out blastocyst transfers is related in part to the current concern assigned to epigenetic effects. In mice, extended culture has been associated with epigenetic modifications, but not yet in humans [25].
Poor prognosis patients with few or poor quality embryos should maybe benefit from a day 3 transfer. Indeed, transfer cancellations are extremely disappointing and are also known to contribute to patient drop-out.
This study has several limitations, on the one hand, data was analyzed retrospectively and day 3 and 5 warming cycles were not randomized. On the other hand, good quality day 3 embryos were vitrified in contrast to blastocysts which were obtained from fair to poor quality day 3 embryos.
Despite these limitations, we observed that supernumerary average quality day 3 embryos should be given a second chance to be selected for cryopreservation. If blastocysts are obtained and survive vitrification, there is a good chance of implantation thus reducing embryo wastage.
Acknowledgments
We thank the rest of our IVF team who participated in the clinical work of this study.
Footnotes
Capsule
We extended the culture of cleavage stage embryos considered unsuitable for cryopreservation and found that good quality blastocysts could be produced with high pregnancy rates after warming.
References
- 1.Balaban B, Urman B, Alatas C, Mercan R, Aksoy S, Isiklar A. Blastocyst-stage transfer of poor-quality cleavage-stage embryos results in higher implantation rates. Fertil Steril. 2001;75(3):514–518. doi: 10.1016/S0015-0282(00)01756-8. [DOI] [PubMed] [Google Scholar]
- 2.Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23(9):1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 3.Begley CG. Six red flags for suspect work. Nature. 2013;497(7450):433–434. doi: 10.1038/497433a. [DOI] [PubMed] [Google Scholar]
- 4.Blake DA, Farquhar CM, Johnson N, Proctor M (2011) Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. Oct 17;(4):CD002118. Review. Update in: Cochrane Database Syst Rev. 2012;7:CD002118. [DOI] [PubMed]
- 5.Capalbo A, Rienzi L, Buccheri M, Maggiulli R, Sapienza F, Romano S, et al. The worldwide frozen embryo reservoir: methodologies to achieve optimal results. Ann N Y Acad Sci. 2011;1221:32–39. doi: 10.1111/j.1749-6632.2010.05931.x. [DOI] [PubMed] [Google Scholar]
- 6.Delvigne A, Kostyla K, Murillo D, Van Hoeck J, Rozenberg S. Oocyte quality and IVF outcome after coasting to prevent ovarian hyper stimulation syndrome. Int J Fertil Womens Med. 2003;48(1):25–31. [PubMed] [Google Scholar]
- 7.Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83(6):1635–1640. doi: 10.1016/j.fertnstert.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23(1):62–66. doi: 10.1093/humrep/dem280. [DOI] [PubMed] [Google Scholar]
- 9.Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–554. doi: 10.1093/humupd/dms016. [DOI] [PubMed] [Google Scholar]
- 10.Fanchin R, Ayoubi JM, Righini C, Olivennes F, Schonauer LM, Frydman R. Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod. 2001;16(6):1115–1119. doi: 10.1093/humrep/16.6.1115. [DOI] [PubMed] [Google Scholar]
- 11.Fragouli E, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133(2–4):149–159. doi: 10.1159/000323500. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthernon Press; 1999. pp. 378–388. [Google Scholar]
- 13.Graham J, Han T, Porter R, Levy M, Stillman R, Tucker MJ. Day 3 morphology is a poor predictor of blastocyst quality in extended culture. Fertil Steril. 2000;74(3):495–497. doi: 10.1016/S0015-0282(00)00689-0. [DOI] [PubMed] [Google Scholar]
- 14.Guerif F, Frapsauce C, Chavez C, Cadoret V, Royere D. Treating women under 36 years old without top-quality embryos on day 2: a prospective study comparing double embryo transfer with single blastocyst transfer. Hum Reprod. 2011;26(4):775–781. doi: 10.1093/humrep/der020. [DOI] [PubMed] [Google Scholar]
- 15.Hammarberg K, Astbury J, Baker H. Women’s experience of IVF: a follow-up study. Hum Reprod. 2001;16(2):374–383. doi: 10.1093/humrep/16.2.374. [DOI] [PubMed] [Google Scholar]
- 16.Hardarson T, Ahlström A, Rogberg L, Botros L, Hillensjö T, Westlander G, et al. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27(1):89–96. doi: 10.1093/humrep/der373. [DOI] [PubMed] [Google Scholar]
- 17.Hardarson T, Caisander G, Sjögren A, Hanson C, Hamberger L, Lundin K. A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human pre-embryos compared with control blastocysts. Hum Reprod. 2003;18(2):399–407. doi: 10.1093/humrep/deg092. [DOI] [PubMed] [Google Scholar]
- 18.Kader AA, Choi A, Orief Y, Agarwal A. Factors affecting the outcome of human blastocyst vitrification. Reprod Biol Endocrinol. 2009;7:99. doi: 10.1186/1477-7827-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolibianikis EM, Zikopoulos K, Verpoest W, Camus M, Joris H, Van Steirteghem AC. Should we advise patients undergoing IVF to start a cycle leading to a day 3 or a day 5 transfer? Hum Reprod. 2004;19(11):2550–2554. doi: 10.1093/humrep/deh447. [DOI] [PubMed] [Google Scholar]
- 20.Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Current Opinion Obstetric Gynecology. 2009;21(3):270–274. doi: 10.1097/GCO.0b013e3283297dd6. [DOI] [PubMed] [Google Scholar]
- 21.Liebermann J. Vitrification of human blastocysts: an update. Reprod Biomed Online. 2009;19(Suppl 4):4328. [PubMed] [Google Scholar]
- 22.Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–193. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Lundin K, Bergh C. Cumulative impact of adding frozen-thawed cycles to single versus double fresh embryo transfers. Reprod Biomed Online. 2007;15(1):76–82. doi: 10.1016/S1472-6483(10)60695-5. [DOI] [PubMed] [Google Scholar]
- 24.Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87(3):534–541. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- 25.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91(2):305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesut N, Ciray HN, Mesut A, Aksoy T, Bahceci M. Cryopreservation of blastocysts is the most feasible strategy in good responder patients. Fertil Steril. 2011;96(5):1121–1125. doi: 10.1016/j.fertnstert.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril. 2002;77(6):1191–1195. doi: 10.1016/S0015-0282(02)03104-7. [DOI] [PubMed] [Google Scholar]
- 28.Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoels using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006;21(12):3246–3252. doi: 10.1093/humrep/del285. [DOI] [PubMed] [Google Scholar]
- 29.Munné S. Chromosome abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online. 2006;12(2):234–253. doi: 10.1016/S1472-6483(10)60866-8. [DOI] [PubMed] [Google Scholar]
- 30.Olivius C, Friden B, Borg G, Bergh C. Why do couples discontinue in vitro fertilization treatment? A cohort study. Fertil Steril. 2004;81(2):258–261. doi: 10.1016/j.fertnstert.2003.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Ren X, Liu Q, Chen W, Zhu G, Li Y, Jin L, et al. Selection and vitrification of embryos with a poor morphological score: a proposal to avoid embryo wastage. J Huazhong Univ Sci Technolog Med Sci. 2012;32(3):405–409. doi: 10.1007/s11596-012-0070-2. [DOI] [PubMed] [Google Scholar]
- 32.Rijnders M, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13(10):2869–2873. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 33.Racowsky C, Machtinger R. Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26(3):210–221. doi: 10.1016/j.rbmo.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Ryan GL, Sparks AE, Sipe CS, Syrop CH, Dokras A, Van Voorhis BJ. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril. 2007;88(2):354–360. doi: 10.1016/j.fertnstert.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Sandalinas M, Sadowy S, Alikani M, Calderon G, Cohen J, Munné S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum Reprod. 2001;16(9):1954–1958. doi: 10.1093/humrep/16.9.1954. [DOI] [PubMed] [Google Scholar]
- 36.Scott RT, Jr, Treff NR. Assessing the reproductive competence of individual embryos: a proposal for the validation of new “-omics” technologies. Fertil Steril. 2010;94(3):791–794. doi: 10.1016/j.fertnstert.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 38.Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9(6):583–605. doi: 10.1093/humupd/dmg041. [DOI] [PubMed] [Google Scholar]
- 39.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89(6):1702–1708. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Vajta G, Nagy ZP, Cobo A, Conceicao J, Yovich J. Vitrification in assisted reproduction: myths, mistakes, disbeliefs and confusion. Reprod Biomed Online. 2009;19(Suppl 3):1–7. doi: 10.1016/S1472-6483(10)60278-7. [DOI] [PubMed] [Google Scholar]
- 41.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–968. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 42.Vanderzwalmen P, Zech N, Greindl AJ, Ectors F, Lejeune B. Cryopreservation of human embryos by vitrification. Gynecol Obstet Fertil. 2006;34(9):760–769. doi: 10.1016/j.gyobfe.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Vanderzwalmen P, Ectors F, Grobet L, Prapas Y, Panagiotidis Y, Vanderzwalmen S, et al. Aseptic vitrification of blastocysts from infertile patients, egg donors and after IVM. Reprod Biomed Online. 2009;19(5):700–707. doi: 10.1016/j.rbmo.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Van Landuyt L, Stoop D, Verheyen G, Verpoest W, Camus M, Van de Velde H, et al. Outcome of closed blastocyst vitrification in relation to blastocyst quality: evaluation of 759 warming cycles in a single-embryo transfer policy. Hum Reprod. 2011;26(3):527–534. doi: 10.1093/humrep/deq374. [DOI] [PubMed] [Google Scholar]
- 45.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 46.Zhu D, Zhang J, Cao S, Zhang J, Heng BC, Huang M, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles–time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–1695. doi: 10.1016/j.fertnstert.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Ziebe S, Lundin K, Loft A, Bergh C, Nyboe Andersen A, Selleskog U, et al. FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum Reprod. 2003;18(12):2575–2581. doi: 10.1093/humrep/deg489. [DOI] [PubMed] [Google Scholar]