Abstract
Purpose
Previous studies reported that patients with endometriosis had excess nitric oxide (NO) in the reproductive tract and poor embryo development in IVF cycles. This study aims to elucidate the effects of NO on early embryo development.
Methods
Zygotes from superovulated B6CBF1 mice were cultured to blastocysts in a variety of media. Sodium nitroprusside (SNP) and NG-nitro-L-arginine (LNA) were added to the culture medium as a NO donor and a NO synthase inhibitor, respectively. The localization and fluorescence intensity of S-nitrosylated (SNO) proteins within 2-cell stage embryos were analyzed with confocal microscopy. Apoptosis and ATP production in the blastocysts were measured.
Result(s)
Subsequent to NO exposure, the SNO proteins mainly colocalized with the mitochondria and endoplasmic reticulum and the intensity of SNO proteins increased. The addition of a quanylate cyclase inhibitor and a cyclic GMP mimic agent induced nonsignificant changes in SNO proteins, whereas addition of a superoxide scavenger or a reduced form of glutathione rescued the embryos from the effects of NO. However, superoxide scavenger supplementation resulted in decreased blastocyst ATP production.
Conclusion(s)
Elevated NO exerts deleterious effects on embryo development, possibly through protein S-nitrosylation in the mitochondria and endoplasmic reticulum. Including glutathione as a component in the culture medium might counteract this effect.
Keywords: Nitric oxide, S-nitrosylation, Mitochondria, Apoptosis, Embryo development
Introduction
The detrimental effect of a minimal and mild stage of endometriosis on fertility is controversial [11]. Most reports indicate that the poor oocyte/ embryo quality may be responsible for infertility in patients with endometriosis [11]. Previous studies have reported elevation of nitric oxide (NO) and/or NO synthase (NOS) levels in the endometrial tissue, follicular fluid, and peritoneal fluid of patients with endometriosis [22, 24, 41]. Furthermore, high levels of serum and follicular NO in patients with endometriosis were associated with poor embryo quality and pregnancy outcome in assisted reproduction technology cycles [22]. Other previous studies have indentified the association of a polymorphism of endothelial NOS with advanced stage endometriosis [20]. Overall, increased microenviromental NO (in follicular fluid, tubal secretions, and endometrial tissue) might contribute to the pathologic effects of endometriosis on the development potential of embryos.
NO is a highly diffusible molecule that plays an important role in mammalian reproductive function, including folliculogenesis [14], fertilization [21], and implantation [7]. Endogenous NO is produced following the conversion of oxygen and L-arginine to NO and L-citrulline, catalyzed by three isoforms of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) [12]. All three isoforms are expressed in preimplantation mouse embryos and function as regulators of embryo development, which ceases following the inhibition of NOS activity [5, 27, 37]. Endogenous NO is, therefore, essential for pre-implantation embryo development.
NO is, however, a free radical and an important bioregulator of apoptosis [6]. Excess NO has been implicated in multiple types of cell death. The intracellular targets of NO can vary depending on the type and timing of an apoptotic stimulus, the source of NO, and the redox chemistry within a specific cell type [34]. NO is also recognized as a major regulator of cell respiration and mitochondrial metabolism in several somatic cell lines [26], and targets complex I and complex IV (cytochrome c oxidase) of the respiratory electron transport chain in mitochondria [26].
Results from previous studies have suggested that NO might induce apoptosis through a mitochondria- dependent pathway during preimplantation embryo development in vitro [5, 22]. In mammalian species, mitochondria are exclusively maternally derived. Oocyte mitochondria display some unique features, such as sphericalness, a high density matrix, a low number of cristae, and , possibly, low metabolic activity [18]. Although the “immature” mitochondria in mammalian fertilized eggs differ from the “mature” mitochondria in somatic cells, the “immature” mitochondria are also involved in the early phase of oxidative stress-induced apoptosis [23]. In mouse oocytes and embryos, constant levels of ATP are maintained by active mitochondrial oxidative phosphorylation [9, 38].
The study by Nisoli et al. established that the activation of guanylate cyclase (GC) and subsequently increased cyclic guanosine monophosphate (cGMP) mediates the effect of NO intracellularly [28]. The NO/GC/cGMP pathway is also involved in cell survival [32, 35]. Previous studies have associated NO in apoptosis with the formation of highly reactive oxidants, such as peroxynitrite (ONOO-) or hydroxyl peroxide [26, 30]. In addition, Hess et al. recognized that NO-based protein thiol modification, such as S-nitrosylation, is the prototype of mechanisms that transport redox-based cellular signal s [15]. S-Nitrosylation modifies the function of molecular chaperones and plays a central role in the regulation of apoptosis [2]. However, the involvement of protein S-nitrosylation in the effects of NO on mammalian embryos has yet to be elucidated.
The oviducts and uterus are the major microenvironment for preimplantation embryo development in vivo. In addition to NOS, the tubal epithelium synthesizes or expresses several types of antioxidants and enzymes, such as glutathione, superoxide dismutase, and glutathione reductase [13]. Glutathione peroxidase activity has been implicated in endometriosis-related infertility [29]. Furthermore, carriage of the GSTT1 (glutathione-S-transferase) null deletion is associated with moderate risk of endometriosis [36]. Antioxidant agents that can eliminate reactive oxygen species might negate the deleterious effects of NO and have beneficial effects on embryo development in vivo and ,possibly, the natural fertility of patients with minimal to mild endometriosis. Therefore, high levels of NO and low levels of antioxidants could induce nitrative or oxidative stress in patients with endometriosis, which may be responsible for impaired embryo quality and infertility of those patients.
In this study, we investigated the effects of microenvironmental NO on preimplantation mouse embryo development, and aimed to elucidate the mechanism of apoptosis modulated by NO in these embryos. We evaluated the cGMP- independent pathway (protein S-nitrosylation) in NO signaling , and further aimed to elucidate the effect of antioxidants that counteract the apoptotic effects of NO during preimplantation development.
Materials and methods
One-cell embryo collection and in vitro culture
The use of the mice in this study obeyed the guideline approved by the Institutional Animal Care and Use Committee of Chung Shan Medical University and Lee Women’s Hospital, Taichung, Taiwan. Six- to eight-week-old B6CBF1 female mice were superovulated with intraperitoneal injection of 5 IU pregnant mare’s serum gonadotropin (PMSG; Sigma–Aldrich, St Louis, MO, US), followed by 5 IU human chorionic gonadotropin (hCG; Serono, Bari, Italy) 48 h later. The mice were placed into the same cage overnight with males of the same strain. We checked for vaginal plugs the next morning as the evidence of mating. Mated mice were sacrificed by cervical dislocation 24 h later following the administration of hCG.
One-cell embryos (zygotes) were released from excised oviducts into modified human tubal fluid medium (mHTF), which was prepared according to a previous report [31]. Cumulus cells were removed by exposure to 80 IU/mL hyaluronidase (Sigma-Aldrich). The embryos were cultured under oil in groups of 10–15 embryos for 96 h. The media was freshly prepared and utilized for 96 h at 37 °C in a humidified atmosphere containing 5 % CO2. Embryo development was monitored at an interval of 24 h. Specifically, embryos at 2-cell, 4-cell, morula, and blastocyst stages were observed after 18 h to 22 h, 44 h to 46 h, 66 h to 71 h, and 92 h to 96 h of culture, respectively.
In the experiment of NO effect and signaling, one-cell embryos were cultured in 1 ml of mHTF medium supplemented with sodium nitroprusside (SNP; Sigma-Aldrich), NG-nitro-L-arginine (LNA; Sigma-Aldrich), 8-bromo-guanosine 3′ ,5′-cyclic monophosphate (BrGMP; Sigma-Aldrich), 1H-(1,2,4) oxadiaxlol-(4,3-a) quinoxalin-1-one (ODQ; Sigma-Aldrich) or combinations of those materials. The medium was freshly prepared for each culture experiment and utilized till the development of blastocysts. The 2-cell block was defined as the development of embryos arrested at the 2-cell stage without apparent degeneration for 48 h. The osmolarity and pH levels of culture media were evaluated after chemical supplementation and prior to usage to assure appropriate osmotic pressure and pH for embryo development.
Assay of apoptosis
The terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labeling (TUNEL) was utilized to label DNA strand breaks, which is an apoptotic event subsequent to cleavage of genomic DNA. A commercial kit, In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Penzberg, Germany) was applied to detect blastomere apoptosis within the embryos. The procedure was modified from our previous report [16].
Analysis of ATP content
The ATP content was determined by measurement of the luminenscence (Tropix TR717 luminometer) generated in an ATP-dependent luciferin-luciferase bioluminescence assay [39]. Briefly, the mouse embryos were rapidly frozen in 200 μl ultrapure water and stored at −80 °C. A mixture of 160 μg/ml luciferin with 60 μg/ml luciferase from a stock solution of a commercial kit (ATP Bioluminescent somatic cell assay kit; Sigma-Aldrich) was diluted 25 fold with ATP assay buffer (100 mM Tris acetate, pH 5.75, 5 mM magnesium acetate, 0.1 mM EDTA, 5 % bovine serum albumin). A standard curve containing 14 ATP concentrations from 10 fmol up to 5 pmol was generated for each series of analysis. To reduce potential variability between samples, embryos from several experimental and control groups were pooled and analyzed simultaneously.
S-nitrosylation proteins in embryos
We modified the method for detecting S-nitrosoproteins by immunofluorescence staining as described previously [17]. This method depends on first blocking thiols with a rapidly acting thiol-reactive agent S-methyl methanethiosulfonate (MMTS; Sigma-Aldrich), followed by reducing the S-nitrosothiols with ascorbate, after which the thiols generated by ascorbate reduction were labeled with a fluorescent derivative of methanethiosulfonate (MTSEA-Texas red; Toronto research Chemicals, North York, Ontario, Canada). Specifically, cells were first fixed in methanol at −20 °C for 10 min. Thiol groups were then blocked with 200 mM MMTS (chosen for its rapid reaction kinetics) in 80 % methanol containing 100 mM Hepes (pH 7.7), 1 mM EDTA, and 0.2 mM neocuproine (named HEN_methanol), at 50 °C for 30 min. The cells were then washed four times with HEN_methanol, after which they were incubated with 0.2 mM ascorbate and 0.2 mM MTSEA-Texas red in HEN_methanol at room temperature for 1 h. Excess dye was removed by washing the cells repeatedly with methanol.
The mitochondria were stained with 20 μM MitoTracker Green FM (Invitrogen , Carlsbad, CA, US) for 20 min then washed four times with Dulbecco’s phosphate buffered saline (DPBS; Invitrogen). In another group of embryos, the endoplasmic reticulum was stained with fluorescent conjugates of concanavalin A (Con A; Invitrogen) for 50 min then washed four times with DPBS. Fluorescent images were taken with a Leica TCSNT confocal microscope. Eight fields magnified x400 were analyzed per embryo. Fluorescence intensity was quantified by subtracting background fluorescence, then integrating the image with the ImageJ software 1.46r. Subcellular colocalization analysis under the ImageJ software was performed with the plugin JACoP [3].
The addition of antioxidants and analysis of blastocyst development
To counteract the apoptotic action of NO on the development of mouse embryos, the culture medium was further supplemented with the following antioxidants: First, Mn(III) tetrakis(4-benzoic acid)porphyrin chloride (TBAP; Cayman Chemical, Ann Arbor, MI, US) is a superoxide dismutase (SOD) mimetic agent and is able to dismute the superoxide radical (O2−) [10]. Second, glutathione methyl ester (GSHM; Sigma-Aldrich) at 1 mM was applied to the culture medium to reduce the oxidative stress and to prevent the S-nitrosylation action of NO on potential enzymes [1].
The number of embryos developing to the expanded blastocyst stage (blastocyst formation rate) was assessed after 96 h of culture. In order to evaluate the afterward development potential of the blastocysts derived from the above culture conditions, the total cell numbers and apoptotic cell numbers were calculated after DAPI (Abbot, Des Plaines, IL, US) and TUNEL assay for nuclei and apoptotic DNA fragmentation, respectively. In addition, the amount of ATP for individual blastocysts was also measured.
Statistical analysis
Pearson’s coefficients were chosen to demonstrate the results of subcellular colocalization analysis with the ImageJ software and the plugin JACoP [3]. The rate of blastocyst formation was tested for significance using the χ2-test or Fisher’s exact test determined by the condition. Intensity of S-nitrosylation staining and ATP amount of blastocyst were subjected to Student’s t test or Mann-Whitney U test to evaluate the significance of the results compared to the control group. A confidence level of p < 0.05 was considered to constitute the limit of statistical significance.
Results
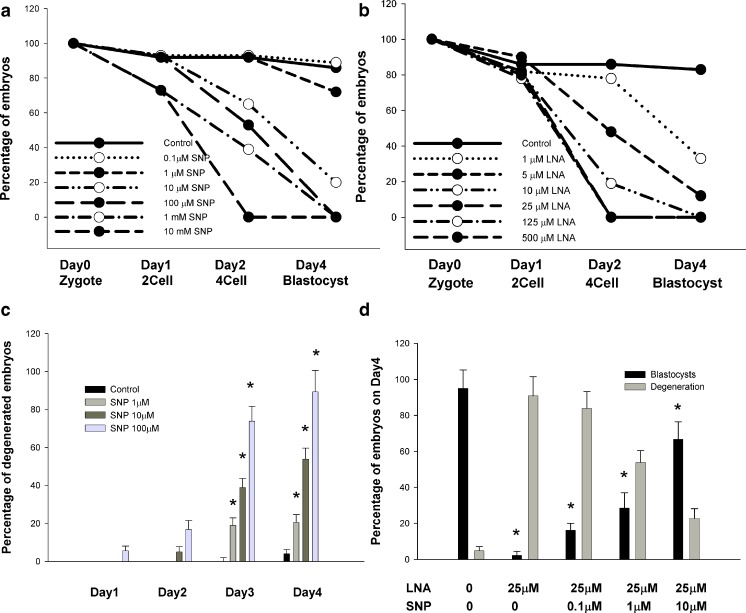
Figure 1 shows the development of preimplantation mouse zygotes postexposure to various concentrations of SNP and LNA during in vitro culture. We used these curves to identify protecting and damaging conditions. In the presence of a SNP concentration greater than or equal to 100 μM, blastocysts failed to form (Fig. 1a). The addition of SNP to the culture medium inhibited blastocyst formation in a dose-dependent manner. The addition of LNA to the culture medium also inhibited mouse embryo development in vitro. Most embryo development (83.3 %) was arrested at a 2-cell stage, with 16.7 % developing to a blastocyst in the presence of an LNA concentration of 25 μM (Fig. 1b).
Fig. 1.
Mouse zygotes cultured with various concentrations of a NO donor (SNP) and a NOS inhibitor (LNA) in vitro. Mouse embryos at 2-cell, 4-cell, morula, and blastocyst stages were observed using inverted microscopy after 18 h to22 h (Day 1), 44 h to 46 h (Day 2), 66 h to 71 h (Day 3), and 92 h to 96 h (Day4) of culture, respectively. a Zygotes (n = 84) and b zygotes (n = 91) were divided into 7 groups for in vitro culture. Blastocyst formation rates decreased with increasing SNP or LNA concentration. High levels of LNA did not influence the percentage of 2-cell embryos on Day 1. c Mouse embryo degeneration increased with elevating SNP concentration. d At an inhibiting LNA concentration, SNP supplementation rescued mouse embryo development. * p < 0.05 compared to the control group according to χ2 test. Vertical bars and bars of error represent the mean and standard error of at least 3 different experiments, respectively. Each group contained at least ten 2-cell embryos
During the in vitro culture of mouse embryos, the addition of SNP induced embryo degeneration in a concentration-dependent manner (Fig. 1c). However, LNA did not induce mouse embryo degeneration until 48 h after its addition. The addition of SNP to LNA-exposed embryos rescued a proportion of the embryos, which then developed into blastocysts (Fig. 1d). We used 10 μM as the working SNP concentration for all experiments unless indicated otherwise.
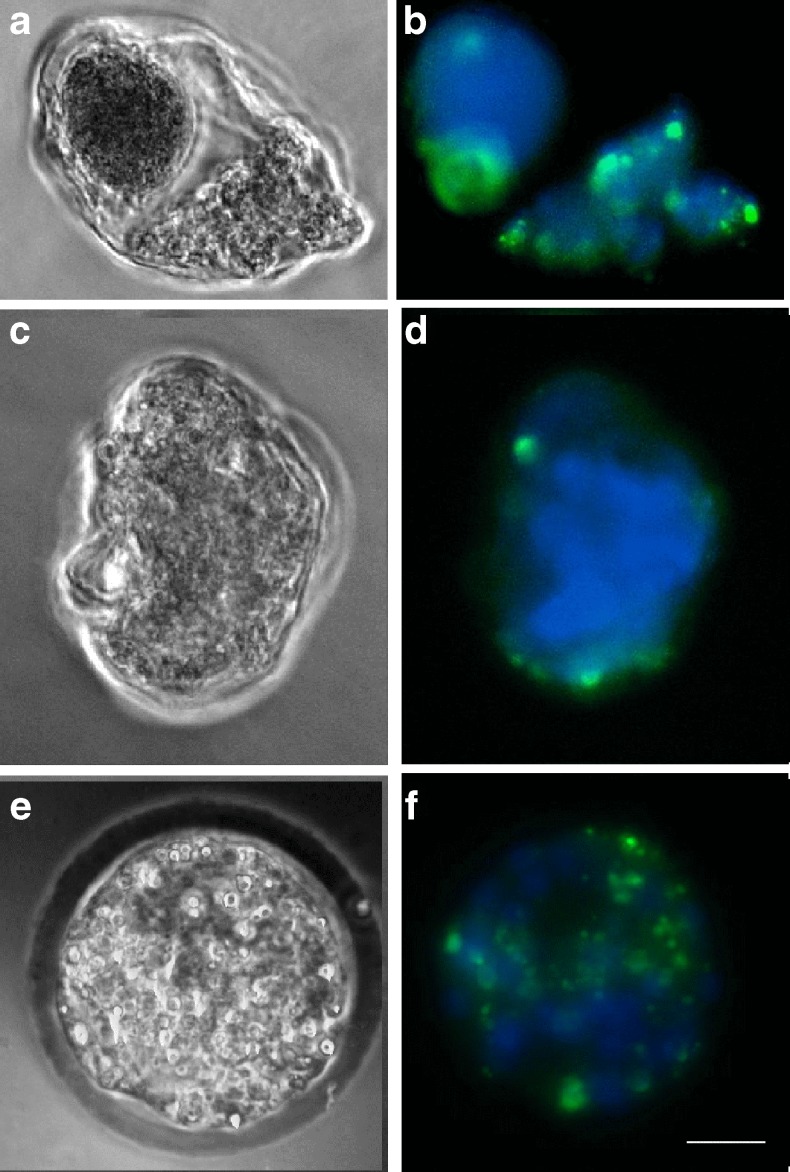
At each stage, embryo degeneration was associated with apoptosis, as confirmed using the TUNEL assay (Fig. 2). The addition of SNP to the culture medium led to apoptosis of preimplantation mouse embryos. However, LNA did not induce apoptosis until 48 h after its addition. According to the results from the TUNEL assay, approximately 78 % of the degenerated embryos were apoptotic.
Fig. 2.
Identification of apoptosis (green) in degenerated mouse embryos exposed to aNO donor using TUNEL stain. Chromatin (nuclei) was stained using DAPI (blue). a and b Day1: 2-cell stage exposed to100 μM SNP; c and d Day3: morula stage exposed to 10 μM SNP; e and f Day4: blastocyst stage exposed to 10 μM SNP. The bar in f indicated 20 μm. The microscope used was a Leica TCS NT with a 40× objective and NA 0.55
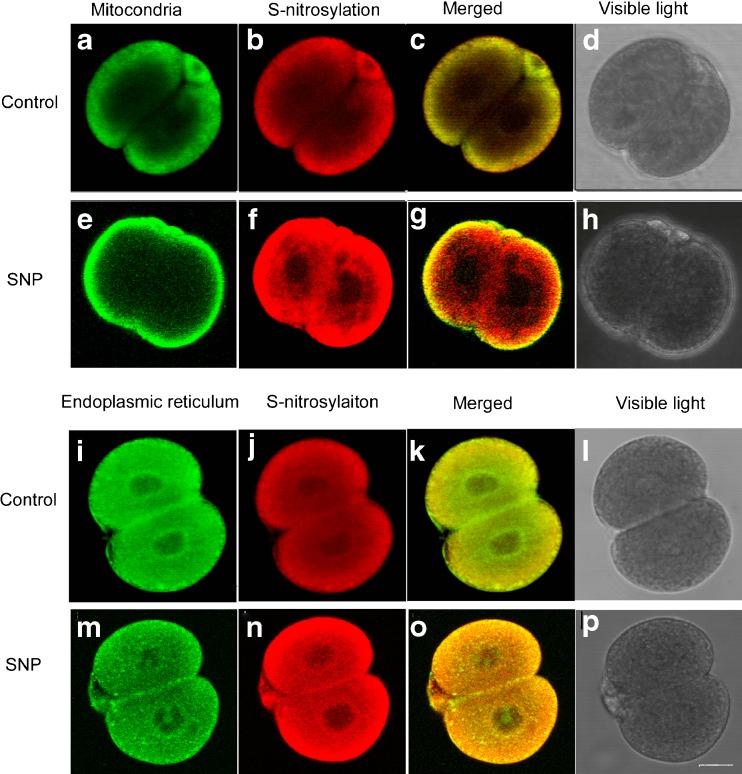
To elucidate the effects of microenvironmental NO on preimplantation embryo’development and their possible underlying mechanisms, we investigated protein S-nitrosylation in 2-cell stage embryos. We evaluated S-nitrosylated protein localization using mitochondria and endoplasmic reticulum (ER) markers. Confocal microscopy examination revealed that the S-nitrosylated proteins occurred mainly in the cortical area of the cytoplasm in the control group and colocalized with mitochondria (Fig. 3a, b, c, d; Pearson’s coefficient 0.984). The addition of a NO donor increased cytoplasmic protein S-nitrosylation in peripheral and peri-nuclear areas, whereas the mitochondria remained mainly in the peripheral cortical area (Fig. 3e, f, g, h; Pearson’s coefficient 0.742). We further identified that the S-nitrosylated proteins were more closely co-localized with the ER in the SNP-treated group (Fig. 3m, n, o, p; Pearson’s coefficient 0.953) compared to the control group (Fig. 3i, j, k, l; Pearson’s coefficient 0.798). In addition to shifting location of S-nitrosylated proteins from mitochondria dominant to ER dominant, another major difference between the two groups was the elevated intensity of S-nitrosylated proteins in the presence of a NO donor (Fig. 3f vs. b; and n vs. j).
Fig. 3.
Patterns of protein S-nitrosylation in 2-cell mouse embryos following NO donor (10 μM SNP) exposure were observed using confocal microscopy. a and e Mitochondria were stained with MitoTracker Green FM and the mitochondria of 2-cell mouse embryos were mostly located in the cortical area of the cytoplasm both in control and NO exposure group; i and m The endoplasmic reticulum (ER) was stained with Con-A; b, f, j and n S-nitrosylated proteins were stained using the biotin switch method and MTSEA-Texas red. f vs. b and n vs. j Protein S-nitrosylation increased post-NO exposure, and extended from the cortical area toward the nucleus. c and g S-nitrosylated proteins were more colocalized with mitochondria in the control group compared to SNP-treated group. k and o S-nitrosylated proteins closely colocalized with ER, prominently post-NO exposure. The bar in p indicates 20 μm. Images were captured using a Leica TCS NT microscope with a 40× objective and NA 0.55
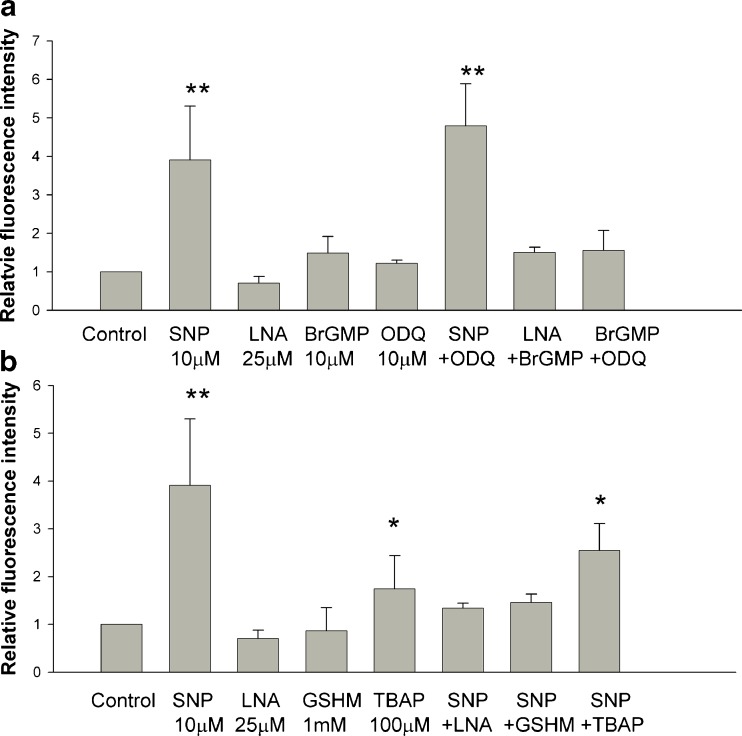
We assigned the fluorescence intensity of S-nitrosylation in the control group as the reference level 1.0 (Fig. 4). Addition of SNP to the culture medium increased S-nitrosylation, whereas, the addition of LNA did not reduce the levels of S-nitrosylation (Fig. 4a, b). Furthermore, embryos in the media supplemented with BrGMP and ODQ displayed nonsignificant changes in S-nitrosylation compared to the control (Fig. 4a). The addition of ODQ together with SNP did not reduce the levels of S-nitrosylation compared to the SNP-supplemented group (Fig. 4a). By contrast, the addition of LNA, GSHM, and TBAP to the culture medium counteracted the effect of SNP -induced S-nitrosylation. However, the addition of TBAP alone or SNP+TBAP still resulted in a significant increase in S-nitrosylation (Fig. 4b).
Fig. 4.
Assessment of the intensity of protein S-nitrosylation in 2-cell mouse embryos using the biotin switch method. a The NO donor (SNP) significantly increased protein S-nitrosylation, whereas the NOS inhibitor (LNA) did not significantly reduce S-nitrosylation. The addition of the cGMP mimic BrGMP and the GC inhibitor ODQ had nonsignificant effects on S-nitrosylation. b Addition of LNA or GSHM to NO-exposed embryos reduced S-nitrosylation significantly. However, TBAP supplementation alone or SNP+TBAP induced an increase in protein S-nitrosylation. * p < 0.05 and **p < 0.01 compared to the control group according to Student’s t-test. The vertical bars and bars of error represent the mean and standard error of at least 3 different experiments, respectively. Each group contained at least ten 2-cell embryos
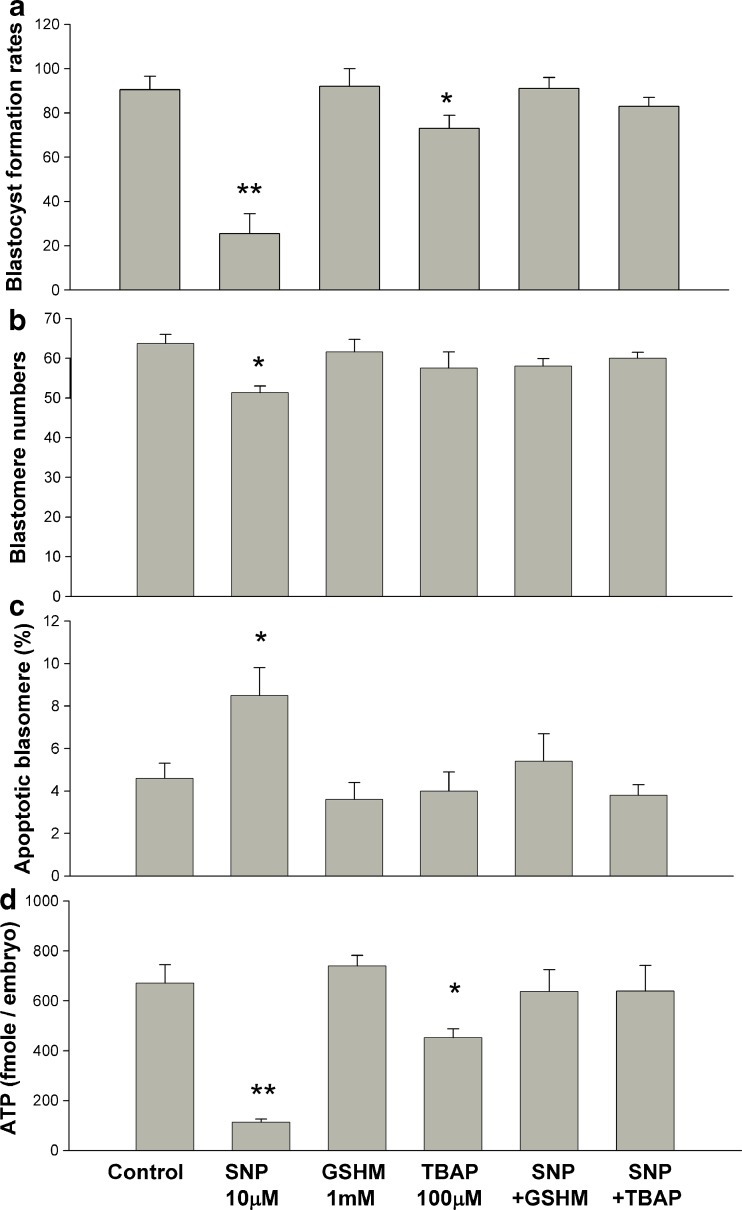
We then evaluated the effects of antioxidants (100 μM TBAP and 1 mM GSHM) and the possible reversal of the detrimental effects of SNP on mouse zygote development (Fig. 5). In each experiment, the rate of blastocyst formation in the control group was approximately 90 %. The addition of 10 μM SNP to the culture medium reduced the rate of blastocyst formation to approximately 20 %, and the addition of TBAP and GSHM reversed the adverse effects of SNP on embryo development (Fig. 5a). However, the addition of TBAP alone reduced the rate of blastocyst formation compared to that of the control group (Fig. 5a). We observed that GSHM did not exert any harmful effects on mouse embryo development based on the blastocyst formation rates (Fig. 5a).
Fig. 5.
Blastocyst formation rate following the addition of SNP (NO donor) and antioxidants (TBAP and GSHM). a Approximately 77 % of the blastocysts developed following the culture of mouse zygotes with TBAP in a mHTF medium. NO-exposed zygotes continuously developed to blastocysts following the addition of TBAP. The addition of GSHM into the mHTF medium did not influence the rate of blastocyst formation and was able to reverse the detrimental effects of SNP on mouse zygotes. b The number of blastomeres in blastocysts with normal morphology decreased post-SNP supplementation, and returned to the level of the control group following the addition of antioxidants (GSHM or TBAP). c The proportion of apoptotic cells in blastocysts with normal morphology increased in response to NO donor exposure. d Blastocyst ATP content decreased post- SNP or -TBAP supplementation. The vertical bars and error bars represent the mean and standard error of at least 3 different experiments, respectively. Each group in (a) contained 60 to 69 embryos. Each group in (b), (c), and (d) contained at least 10 blastocysts. * p < 0.05, ** p < 0.01 compared to the control group according to χ2 test in (a) and Mann-Whitney U test in (b), (c), (d)
Each formed blastocyst contained a similar number of cells except those exposed to SNP, as indicated by DAPI stain (Fig. 5b). We identified the presence of apoptotic cells in individual blastomeres using the TUNEL assay. Blastocysts from those zygotes treated with a NO donor had a higher percentage of apoptotic cells than those cultured with TBAP, GSHM, SNP+TBAP, or SNP+GSHM (Fig. 5c). We further assayed the blastocyst ATP content to determine the effects of the NO donor on mitochondrial activity, observing that the ATP content was reduced significantly lower in blastocysts treated with SNP or TBAP, but not in blastocysts exposed to SNP+TBAP or SNP+GSHM. The addition of TBAP or GSHM not only reversed the blastocyst formation rates but also maintained ATP production activity of NO-exposed embryos (Fig. 5d).
Discussion
Our results indicated that direct inhibition of NOS activity and NO production had detrimental effects on the development of embryos and confirmed the requirement for a NO signaling pathway during early embryo development. During the culturing process of our experiments, embryos exposed to LNA predominately arrested at the 2-cell stage but did not immediately induce embryo degeneration or apoptosis. This indicated that the 2-cell block phenomenon occurred post-addition of NOS inhibitors. Nevertheless, embryos developed continuously following the addition of the NO donor to the culture medium. These observed results were consistent with those reported previously [27, 37].
In the present study, we confirmed the occurrence of apoptosis at the presence of a NO donor in degenerated cleavage-stage embryos and developed blastocysts using the TUNEL assay. These results are similar to a previous report that NO is a regulator of apoptosis during preimplantation development of mouse embryos [5]. One previous study also revealed the detrimental effects of elevating follicular NO on human embryo development (fragmentation) [22]. Taken together, a high microenvironmental level of NO would induce apoptosis during preimplantation development of mammalian embryos.
We further indentified that NO exerts its effects on preimplantation embryos through the S-nitrosylation of proteins. In this study, the NO donor SNP had direct effects on levels and location of S-nitrosylated proteins, which shifted from mitochondria- to ER-dominant proteins. Previous studies have suggested that NO might exert its effects on cells through a cGMP- dependent or cGMP- independent pathway [42]. Zhang and Hogg proposed that one of the major cGMP- independent pathways can be attributed to posttranslation protein modification (S-nitrosylation and/or nitrotyrosine) [42]. Our study is the first to demonstrate that protein S-nitrosylation occurs in the absence of exogenous stimulation during in vitro culture (the control group) and is intimately colocalized with mitochondria in the cytoplasm of 2-cell stage embryos. Results on the distribution of mitochondria within 2-cell embryos were consistent with those reported previously [40], which further indicated the reliability of our data. In the study by Nishikimi et al., NOS activities increased in the one-cell and 2-cell stages of mouse embryos [27]. Overall, our results suggested that the S-nitrosylation of mitochondrial proteins in 2-cell stage embryos might be a physiological signal related to mitochondrial activity.
The S-nitrosylation process involves specific and critical Cys residues of the target proteins and oxidative products of NO [42]. NO is reportedly a poor nitrosating agent and hardly reaches, by itself, the intracellular proteins to S-nitrosate them [43]. In the present study, the NO donor SNP increased protein S-nitrosylation in 2-cell stage embryos. Specifically, the S-nitrosylated proteins closely colocalized with mitochondria and ER. The results might be attributed to the fact that both mitochondria and ER contain a high concentration of lipid membranes, which increase the rate of formation of oxidative products of NO, such as nitrogen dioxide [33].
Our data clearly demonstrated that NO can induce S-nitrosylation in lipid membrane-rich organelles in an intact cell system and might exert its effects through protein S-nitrosylation within such organelles. Bustamante observed sequential NO production by mitochondria and the ER during apoptosis of rat thymocytes and recognized that NOS activity was associated with the ER membrane [4]. Our results further emphasized the role of NO in regulation of protein S-nitrosylation and indicated an association between S-nitrosylation of ER proteins and the pathological effect of exogenous NO.
In this study, the addition of TBAP to the culture medium increased S-nitrosylation levels, reduced blastocyst ATP content, and resulted in a minor reduction in the blastocyst formation rate. Janssen-Heininger et al. suggested that SOD (the TBAP mimic Mn-SOD) supplementation would decrease superoxide and peroxynitrite, prolong the local action of NO, and consequently, increase protein S-nitrosylation [19]. The increase of S-nitrosylated proteins by TBAP in this study was associated with decreasing mitochondrial activity instead of increasing apoptosis. The concentrations of S-nitrothiols (S-nitrosylated Cys residues) and H2O2 are kept under tight control to ensure that NO- and H2O2− induced signaling events occur in spatially and temporally controlled settings [19]. The moderate increase of S-nitrosylation by TBAP might lead to reduced mitochondrial activity and a decreased rate of blastocyst formation; however, the set of S-nitrosylated proteins under such condition did not lead to apoptosis.
In our experiments, TBAP supplementation reversed the detrimental effects of NO on early embryo development. One of the possible mechanisms underlying the effects of TBAP is the elimination of toxic peroxynitrite production by efficient reduction of excess superoxide. Protein S-nitrosylation levels were lower in embryos exposed to SNP plus TBAP than in those exposed to SNP alone. This indicated that TBAP supplementation reduced NO toxicity in an oxidative environment; however, the addition of TBAP alone might not benefit embryo development in a normal or reductive status. Nevertheless, the interaction between NO- and H2O2- signaling events during mammalian embryo development needs further investigation.
Zhang and Hogg suggested that glutathione provides the major protection against intracellular S-nitrosylation by excess NO and described that only after significant depletion of glutathione levels, the level of intracellular S-nitrothiols increased to concentrations higher than 10 nM/ mg cellular protein [42]. Our study results indicated that the reduced form of glutathione is able to reverse the toxic effects of the NO donor by preventing excess protein S-nitrosylation. The results indicated that glutathione might be the major antioxidant to balance the toxic effect of a high NO microenvironment, ex., ectopic endometrial tissues in patients with endometriosis.
The metabolism of SNP results in the liberation of NO and cyanide (CN). The latter may bind with cytochrome oxidase and thus interfere with oxidative phosphorylation [25]. The detoxification of CN needs the presence of thiosulfate by the rhodanese system in mitochondria. The reaction also involves the thiol group on Cysteine-247 of rhodanese [8]. The results of the present study may also result from the toxicity of CN. Further investigation with another type of NO source might be needed to prove the specificity of the NO effect. Nevertheless, the effect of TBAP and the amount of S-nitrosylation proteins related to SNP suggest the toxicity of SNP mainly depends on NO instead of CN in this type of experiment.
The concentration of SNP used in this study was selected to simulate an elevated NO microenvironment, such as peritoneal fluid or tubal secretion from patients with endometriosis. In a previous study, 10 % or 30 % peritoneal fluid from minimal to mild endometriosis patients decreased the cleavage rate of 2-cell mouse embryos [24]. In the previous report, the effect of 10 % and 30 % peritoneal fluid is similar to the effect of 0.1 ~10 μM and 1 mM SNP concentration, respectively [24]. Therefore, the concentration of 10 μM SNP used in the present study was able to reflect the microenvironment effect of NO in the patients with endometriosis.
In previous studies, excess oxidation and high levels of NO within endometrial tissue, follicular fluid, and peritoneal fluid were identified in patients with endometriosis [22, 24, 41]. Furthermore, studies of single nucleotide polymorphism s reveal that GSTT1 (glutathione-S- transferase) null deletion [36] and eNOS [20] are associated with moderate risk of endometriosis. In addition, endometriosis-related infertility is associated with glutathione peroxidase activity [29]. Taken together, the oviduct in patients with endometriosis might be a microenvironment featuring excess NO and insufficient glutathione. According to our study results, we recognized that such an environment would induce protein S-nitrosylation in mitochondria /ER and impair embryo growth. Further investigation focused on re-dox status of patients with endometriosis is indicated.
In conclusion, the apoptotic effects of excess NO on embryo development are closely related to protein S-nitrosylation within lipid-membrane rich organelles, such as the mitochondria and ER. These effects might not be dependent on NO/GC/cGMP signaling. Using an NOS inhibitor to eliminate NO production had deleterious effects on embryo development. The addition of a reduced form of glutathione to NO-exposed embryos maintained their development, and ensured adequate proliferation of blastomeres, and blastocyst ATP production.
Acknowledgments
We would like to thank Mei-Chun Liu of the Instrument Center of Taichung Veterans General Hospital for assistance with confocal microscopy imaging and analysis. Our research grants include NSC 95-2314-B-039-031 (MS Lee) and NSC 98-2314-B-040-012 (TH Lee) from the National Science Council, Taiwan.
Authorship
TH Lee, MS Lee, and YS Yang contributed to conception and design. CC Huang, HM Taso, PM Lin did acquisition of data, analysis and interpretation of data. TH Lee and MS Lee drafted the article. JY Shew, HN Ho and Yang YS revised the article critically for important intellectual content. JY Shew and YS Yang approved the final version of manuscript.
Conflict of interest
All the authors have no conflicts of interest to declare.
Footnotes
Capsule
Excessive environmental nitric oxide is detrimental to embryo development through protein S-nitrosylation in the mitochondria and endoplasmic reticulum. Glutathione supplementation could counteract such effect.
References
- 1.Beltran B, Orsi A, Clementi E, Moncada S. Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol. 2000;129:953–60. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benhar M, Stamler JS. A central role for S-nitrosylation in apoptosis. Nat Cell Biol. 2005;7:645–6. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 3.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–32. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante J, Bersier G, Badin RA, Cymeryng C, Parodi A, Boveris A. Sequential NO production by mitochondria and endoplasmic reticulum during induced apoptosis. Nitric Oxide. 2002;6:333–41. doi: 10.1006/niox.2001.0420. [DOI] [PubMed] [Google Scholar]
- 5.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75:1163–71. doi: 10.1016/S0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]
- 6.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–9. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- 7.Chwalisz K, Garfield RE. Role of nitric oxide in implantation and menstruation. Hum Reprod. 2000;15(Suppl 3):96–111. doi: 10.1093/humrep/15.suppl_3.96. [DOI] [PubMed] [Google Scholar]
- 8.Cipollone R, Ascenzi P, Tomao P, Imperi F, Visca P. Enzymatic detoxification of cyanide: clues from Pseudomonas aeruginosa Rhodanese. J Mol Microbiol Biotechnol. 2008;15:199–211. doi: 10.1159/000121331. [DOI] [PubMed] [Google Scholar]
- 9.Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–67. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–6. [PubMed] [Google Scholar]
- 11.Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8:95–103. doi: 10.1093/humupd/8.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–36. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 13.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 14.Hefler LA, Gregg AR. Inducible and endothelial nitric oxide synthase: genetic background affects ovulation in mice. Fertil Steril. 2002;77:147–51. doi: 10.1016/S0015-0282(01)02952-1. [DOI] [PubMed] [Google Scholar]
- 15.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 16.Huang CC, Lin DP, Tsao HM, Cheng TC, Liu CH, Lee MS. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril. 2005;84:130–40. doi: 10.1016/j.fertnstert.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001:l1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 18.Jansen RP, de Boer K. The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998;145:81–8. doi: 10.1016/S0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 19.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Ku SY, Kim SH, Lee GH, Choi YM, Kim JM, et al. Endothelial nitric oxide synthase gene Glu298Asp polymorphism is associated with advanced stage endometriosis. Hum Reprod. 2009;24:2656–9. doi: 10.1093/humrep/dep212. [DOI] [PubMed] [Google Scholar]
- 21.Kuo RC, Baxter GT, Thompson SH, Stricker SA, Patton C, Bonaventura J, et al. NO is necessary and sufficient for egg activation at fertilization. Nature. 2000;406:633–6. doi: 10.1038/35020577. [DOI] [PubMed] [Google Scholar]
- 22.Lee TH, Wu MY, Chen MJ, Chao KH, Ho HN, Yang YS. Nitric oxide is associated with poor embryo quality and pregnancy outcome in in vitro fertilization cycles. Fertil Steril. 2004;82:126–31. doi: 10.1016/j.fertnstert.2004.02.097. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod. 2000;62:1745–53. doi: 10.1095/biolreprod62.6.1745. [DOI] [PubMed] [Google Scholar]
- 24.Luo Q, Chen XJ, Ding GL, Dong MY, Huang HF. Downregulative effects of nitric oxide on oocyte fertilization and embryo development: possible roles of nitric oxide in the pathogenesis of endometriosis-associated infertility. Cell Physiol Biochem. 2010;26:1023–8. doi: 10.1159/000323977. [DOI] [PubMed] [Google Scholar]
- 25.McDowall DG, Keaney NP, Turner JM, Lane JR, Okuda Y. The toxicity of sodium nitroprusside. Br J Anaesth. 1974;46:327–32. doi: 10.1093/bja/46.5.327. [DOI] [PubMed] [Google Scholar]
- 26.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–20. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 27.Nishikimi A, Matsukawa T, Hoshino K, Ikeda S, Kira Y, Sato EF, et al. Localization of nitric oxide synthase activity in unfertilized oocytes and fertilized embryos during preimplantation development in mice. Reproduction. 2001;122:957–63. doi: 10.1530/rep.0.1220957. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 29.Ota H, Igarashi S, Kato N, Tanaka T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril. 2000;74:313–8. doi: 10.1016/S0015-0282(00)00638-5. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn P. Enhanced results in mouse and human embryo culture using a modified human tubal fluid medium lacking glucose and phosphate. J Assist Reprod Genet. 1995;12:97–105. doi: 10.1007/BF02211377. [DOI] [PubMed] [Google Scholar]
- 32.Seya K, Motomura S, Furukawa K. Cardiac mitochondrial cGMP stimulates cytochrome c release. Clin Sci (Lond) 2007;112:113–21. doi: 10.1042/CS20060144. [DOI] [PubMed] [Google Scholar]
- 33.Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci U S A. 2001;98:7212–7. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 35.Takuma K, Phuagphong P, Lee E, Mori K, Baba A, Matsuda T. Anti-apoptotic effect of cGMP in cultured astrocytes: inhibition by cGMP-dependent protein kinase of mitochondrial permeable transition pore. J Biol Chem. 2001;276:48093–9. doi: 10.1074/jbc.M108622200. [DOI] [PubMed] [Google Scholar]
- 36.Tempfer CB, Simoni M, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: part II–endometriosis. Hum Reprod Update. 2009;15:97–118. doi: 10.1093/humupd/dmn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tranguch S, Steuerwald N, Huet-Hudson YM. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol Reprod. 2003;68:1538–44. doi: 10.1095/biolreprod.102.009282. [DOI] [PubMed] [Google Scholar]
- 38.Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–74. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- 39.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 40.Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- 41.Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003;18:2668–71. doi: 10.1093/humrep/deg484. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Hogg N. S-Nitrosothiols: cellular formation and transport. Free Radic Biol Med. 2005;38:831–8. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Jin B, Li L, Block ER, Patel JM. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol. 2005;288:C840–9. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]