Abstract
A number of recent studies have suggested that black carbon (BC), the light-absorbing fraction of soot, is next to CO2 one of the strongest contributors to the global climate change. BC heats the air, darkens the snow and ice surfaces and could contribute to the melting of Arctic ice, snowpacks, and glaciers. Although soot is the oldest known pollutant its importance in climate modification has only been recently recognized. In this article, we trace the historical developments over about three decades that changed the view of the role of BC in the environment, from a pollutant of marginal importance to one of the main climate change agents. We also discuss some of the reasons for the initial lack of interest in BC and the subsequent rigorous research activity on the role of aerosols in climate change.
Keywords: Atmospheric aerosols, History, Black carbon, Climate
Introduction
Combustion of carbon-containing fuels, in addition to being the principal source of CO2, is also the main source of anthropogenic aerosol particles. In general, aerosol particles can affect the radiation balance causing an overall heating or cooling effect with the magnitude and sign of the effect largely dependent on aerosol optical properties, cloud cover, aerosol concentrations, and their vertical extent as well as the underlying surface albedo. Anthropogenic sulfate particles scatter solar radiation back to space and thereby reduce the amount of radiation reaching the ground leading to a cooling effect. Because this effect may somewhat counteract the warming caused by CO2 and because of the existing analysis methods sulfate aerosols have received most attention in climate studies.
Combustion-generated aerosols, however, also have a light-absorbing black carbon (BC) component. BC particles can be transported to remote regions of the earth such as the Arctic and must be considered in the overall effects of aerosols on radiation transfer and climate. Indeed recent studies suggest that BC plays an important role in both regional and global climate (see for example, Jacobson 2002; Hansen and Nazarenko 2004; Ramanathan and Carmichael 2008), especially over snow covered regions like the Arctic where it can lead to enhanced melting of the ice and snowpacks. These studies have been covered widely by the popular press, presented at hearings in the US Congress and have been incorporated in United Nations reports on mitigating manmade climate change (UNEP/GC.26/INF/20/2011).
In this article, we retrace the historical developments that lead to the changing view of BC, from a pollutant considered of marginal importance to one of the main climate change agents. In the following, we first discuss some of the reasons for the initial lack of interest in BC and the subsequent vigorous research activity on the subject.
Historically soot emissions, which have both a BC component and an organic component, were associated with coal burning responsible for the London smog (Brimblecombe 1978). The severity of coal burning pollution on human health and mortality in the early 1950s led to the control of soft coal burning resulting in a dramatic improvement in pollution levels. These developments also prompted the development of the first instrument to measure the concentration of soot in the UK air. This technique measured the darkness of the stain due to smoke particles deposited on a white filter paper, and interpreted as the smoke concentration in air expressed units of British “Black Smoke” (Thomas 1952; Wilkins 1954).
Following the diminishing coal use and increasing consumption of other fuels, principally gasoline a new view of air pollution emerged, especially in the United States and particularly in California. The symptoms of this type of air pollution, known as the Los Angeles Smog, were severe eye irritation and visibility degradation. In 1952, Haagen-Smit proposed an explanation of the formation and properties of the Los Angeles smog. He demonstrated that nitrogen oxides and hydrocarbons from automobile exhaust irradiated by ultraviolet radiation from the sun form ozone and visibility reducing aerosols. The most relevant conclusions were that (a) smog aerosols are formed in the atmosphere entirely by photochemical conversion of gases to particles and (b) primary particles, such as smoke, soot, dust etc. were essentially eliminated or reduced in the Los Angeles County Air Pollution Control District (Haagen-Smit 1952). These views had a decisive influence on the direction of research in aerosol pollution in the Los Angeles and other areas dominated by automobile emissions. As a consequence, the aerosol studies were oriented toward photochemical aerosol formation by gas to particle conversion. The primary particles, such as soot, were not considered as a priority because of the belief that contemporary atmospheres contain no appreciable amounts of these species.
Uncovering BC in Urban Atmospheres
Two multi-investigator field programs, the 1969 Pasadena Smog Experiment (Hidy 1972) and the 1972–1973 Aerosol Characterization Experiment Hidy and Mueller (1980) were important milestones in the development of the thinking of the aerosol research community in the 1970s. Major objectives of these State of California supported programs were (a) to establish the relationships of major aerosol chemical species to specific sources and photochemical formation pathways, and (b) to estimate the contributions from the conversion of organic vapors to carbonaceous aerosols.
Mueller et al. (1972) showed that carbon comprised a substantial fraction of sub-micron Pasadena aerosol particles, a result that these authors stated to be consistent with the above-mentioned expectations. Mueller et al. (1972) and Friedlander (1973) developed the first carbon balance for the Pasadena aerosol. They concluded that directly emitted carbon particles from gasoline powered transportation sources were mostly organic compounds with minimal contribution of elemental carbon. Friedlander (1973) and Gartrell et al. (1980) analyzed the 1972–1973 Aerosol Characterization Experiment data especially to infer the role of aerosol species in visibility degradation and concluding that visibility-degrading aerosols include sulfates, nitrates, organics, and ammonia, with associated water. Because all of these were viewed as essentially secondary (not directly emitted) species, it appeared that improving visibility would require a major reduction in aerosol precursor gases. Gartrell et al. (1980) and Appel et al. (1976) applied a solvent extraction method to Southern California aerosols and concluded that secondary organics dominated at all photochemical smog receptor sites. Elemental carbon and primary organics were of greater importance only at source-dominated sites. Overall, soot or elemental carbon appeared, at the time, to be a minor constituent of Los Angeles smog aerosol.
In the late 1960s and early 1970s, it became obvious that the existing analytical methods were not adequate to address many questions about the nature and sources of the newly recognized Los Angeles smog aerosols. This stimulated applications of some novel analytical techniques such as X-ray and particle excited fluorescence, neutron activation, and X-ray photoelectron spectroscopy (XPS). All of these techniques were capable of quantitatively measuring elemental composition with high sensitivity. XPS, also known as electron spectroscopy for chemical analysis (ESCA), was also capable of determining the chemical states of individual elements even in complex chemical environments.
XPS consists of measuring the kinetic energies of photoelectrons expelled from a sample irradiated by monoenergetic X-rays. The first application of XPS to aerosols was to determine the chemical states of sulfur and nitrogen in size-classified aerosol samples collected during the 1969 Pasadena Aerosol Experiment (Novakov et al. 1972). For all samples the carbon XPS peak was the most intense among all other peaks. This observation and that of Mueller et al. (1972) suggested that carbonaceous material comprised a substantial fraction of sub-micron Pasadena aerosol particles.
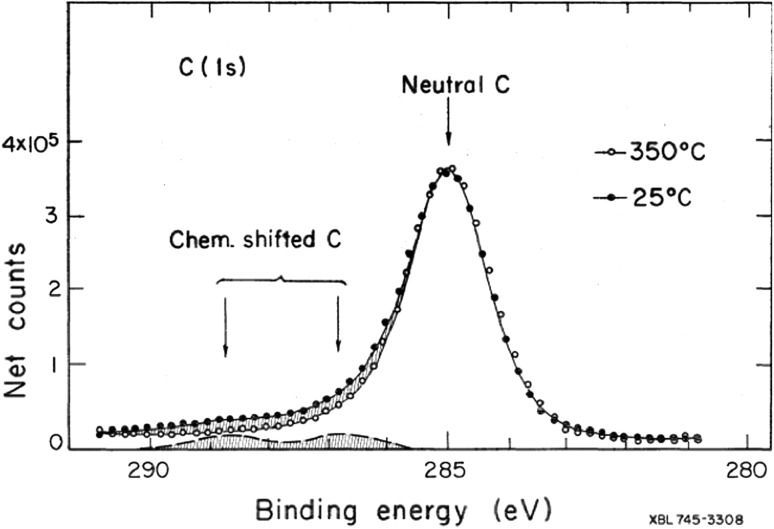
The carbon spectra from XPS analyses of aerosol samples collected at different Southern California sites did not show the pronounced chemical shift that would be expected of postulated oxygenated organic compounds produced by photochemical reactions (Fig. 1). Furthermore, spectra measured at increasing sample temperatures in vacuum up to 450 °C showed that about 80 % of the ambient particulate carbon is nonvolatile and therefore inconsistent with the assumed composition of organic aerosols (Fig. 1). Instead, these studies suggested that ambient aerosol carbon has probably a large elemental or soot fraction (Novakov et al. 1972, 1974, 1976; Novakov 1973; Chang and Novakov 1975).
Fig. 1.
X-ray photoelectron spectrum of a California sample. Up to 80 % of the ambient aerosol carbon is nonvolatile at 350 °C in vacuum
Identification and Properties of BC
Samples used for ESCA analyses have shown different degrees of dark (gray to black) coloration. Sample staining by collected aerosols was used by Hall (1952), in a reflectance-based method, similar to the British smoke instrument, described above, to measure the density of the dark stain (expressed as “Coefficient of Haze” or COH) associated with sooty particles collected on paper filters. The COH values could be converted to either total aerosol mass or its carbon content. The California South-Coast Air-Quality Management District used this method routinely between 1958 and mid-1981 to monitor what was believed to be a measure of particulate matter mass concentration. The COH data were eventually re-interpreted as BC by Cass et al. (1984).
The indications that soot (carbon) may be a significant part of urban aerosols motivated a number of investigations of the properties, effects, sampling, and analysis of carbonaceous aerosols. Lin et al. (1973) devised a method for measurement of aerosol light absorption by measuring the transmission of green light through the aerosol layer collected on a transparent (Nuclepore) filter. The light intensity penetrating the filter was detected by a photomultiplier. The amount of light absorbed by particles on the filter is calculated from the comparison of the transmitted light plus integrated scattered light, with and without particles on the filter. The values of measured absorption coefficients (babs) for New York City aerosols were in the range of 10.4–10.5 m−1. The chemical composition of the absorbing aerosols, however, remained unknown.
A different approach was employed by Rosen et al. (1978), who used Millipore and Quartz filters for particle collection and a He–Ne Laser as the light source. Measured light intensities through a blank and exposed filter I0 and I were used to derive the optical attenuation defined as 100 Log I0/I. Quartz filters materials were used for carbon determination by thermal analysis (see below). Optical attenuation values determined on a number of California samples and aerosol carbon concentrations showed a strong correlation suggesting that the absorbing species are carbonaceous aerosols.
Further inquiry into the nature of these absorbing species involved measurements of wavelength dependence of the absorptivity of ambient and laboratory-generated soot samples. The result demonstrated that absorptivity follows an inverse (1/λ) wavelength dependence over the visible spectral region (4500–7000 Å) (Rosen et al. 1976).
Measurements of attenuation were also performed as a function of sample temperature. These measurements showed that the absorbing species were stable upon heating in air until approximately 400 °C. Further increase in temperature above about 500 °C was accompanied by complete combustion of the light-absorbing material. This observation and the stability of the absorbing species at elevated temperatures show that these species are similar in stability to graphitic carbon. Most organic species should oxidize or vaporize at significantly lower temperatures. Such wavelength dependence is a consequence of the known constancy of imaginary index of refraction of graphite-like structures throughout the visible region (Dalzell and Sarofim 1969).
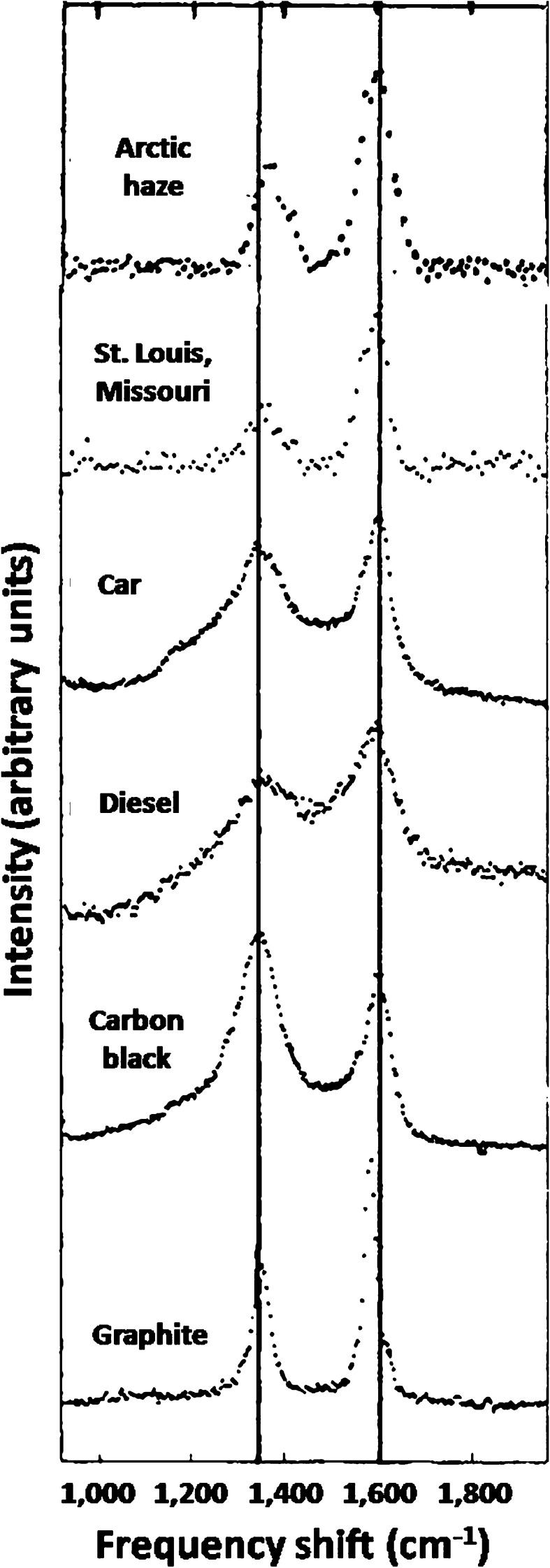
The first application of Raman spectroscopy to aerosol samples resulted in definitive identification of physical structures similar to graphite and activated carbon in both source-enriched and ambient aerosols (Rosen and Novakov 1977). Raman spectroscopy is a highly selective method of analysis that can be used to make unambiguous identifications of different chemical species with characteristic vibrational modes. Examples of Raman spectra (between 920 and 1950 cm-1) of ambient, automobile exhaust, and diesel exhaust particulates are compared with the spectra of activated carbon and polycrystalline graphite in Fig. 2. It is evident that the spectra of activated carbon, diesel exhaust, automobile exhaust, and the ambient samples in urban areas and in the remote Arctic, are very similar, with the positions of the two Raman modes coincident within the estimated experimental error of ±10 cm−1. The same Raman modes were also evident in every urban sample studied, including samples collected in Buffalo (New York) and Berkeley, Fremont, and Anaheim (California) as well as samples collected across the western Arctic, which will be discussed later. A comparison of the integrated intensity of the Raman mode near 1600 cm−1 and the optical attenuation measurements for both ambient and source-enriched samples unmistakably showed that the amount of the absorbing species is directly proportional to the graphitic soot content as defined by Raman spectroscopy (Rosen et al. 1978).
Fig. 2.
Raman spectra of polycrystalline graphite, carbon black, diesel exhaust, and car exhaust compared to spectra of urban and Arctic haze aerosols. The Raman modes near 1350 and 1600 cm−1 are characteristic of “graphitic structures”
Photoacoustic investigations gave an independent demonstration of the light absorption by graphitic carbon. Photoacoustic spectroscopy measures the energy deposited in a sample from light absorption. A comparison of photoacoustic and optical attenuation measurements showed excellent agreement between the results of the two techniques for a variety of ambient and source-enriched particles collected on filters. These results further demonstrated that the optical attenuation measurements were only sensitive to the absorbing or graphitic component of the aerosol (Yasa et al. 1979).
Adams et al. (1989) used a photoacoustic spectrometer for real-time in situ (i.e., without the need to collect aerosol samples) measurements of ambient elemental (i.e., black) carbon. The spectrometer used a photoacoustic cell with the light source at 514.5 nm. Optical absorption of the atmospheric aerosol was converted to BC concentration using an absorption coefficient of 10 m2 g−1. Real-time measurements were validated by correlating photoacoustic BC with concentrations determined by thermal analyses of corresponding filter samples. Regression coefficient of these data was r = 0.926.
Several analytical methods that separate BC (or EC) from organic (OC) and inorganic carbon (carbonate) employ temperature-programmed heating of the sample in an oxidizing or inert atmosphere and detecting the gases evolving from the sample upon heating. The thermal properties of these fractions were used to operationally classify the following components of the carbonaceous aerosol: (a) organic compounds are volatile in N2 or decompose in O2, (b) soot is stable in N2 but combustible in O2, and (c) inorganic carbonates decompose at highest temperatures.
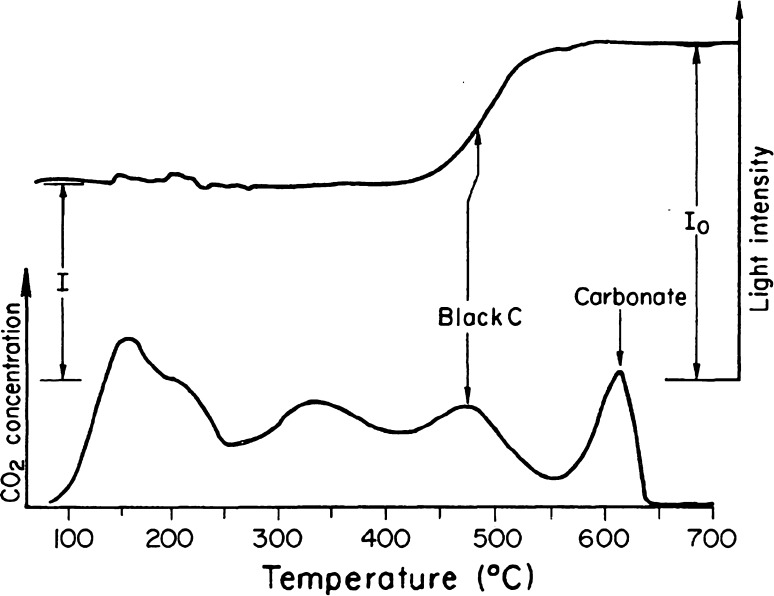
Malissa et al. (1976) described a method that employs progressive heating of a sample in N2 and O2 and coulometric detection of CO2 to separate and quantify carbon components in impactor collected aerosol samples. A related technique known as evolved gas analysis (EGA) also uses heating of a sample on quartz filter at a constant rate in an oxidizing (O2) and neutral (N2) atmosphere (Dod and Novakov 1982). The evolving carbon-containing gases in the O2 mode are converted to CO2 by an oxidizing catalyst and detected by a non-dispersive infrared CO2 analyzer. The rate of carbon evolution plotted as a function of sample temperature gives a carbon “thermogram” as shown in Fig. 3. The area under the thermogram is proportional to total carbon content of the sample. By simultaneously monitoring the optical transmission of the sample, the BC and non-black carbonaceous species are identified. The BC peak corresponds to the increase in optical transmission caused by the removal of BC by combustion. Decomposition of organic and carbonate carbon has no effect on light transmission. As a result of the developments summarized above, BC was defined as the highly absorbing component of soot having a graphitic microstructure (Novakov 1981, 1984).
Fig. 3.
EGA of an ambient sample. Monitoring of light transmission through the filter sample identifies the light-absorbing BC
Huntzicker et al. (1982) developed an instrument based on volatilization of organic carbon (OC) from the filter at temperatures where the elemental carbon remains intact. The volatilized carbon is oxidized to CO2, reduced to CH4 and measured by a flame ionization detector. Elemental carbon is subsequently oxidized to CO2 and measured. The reflectance of a laser beam by the filter is continuously monitored to correct for the charring artifact.
Cachier et al. (1989) developed a two-step thermal method for the determination of soot in atmospheric particles. In the first step, the OC is removed from the collection substrate under a pure oxygen flow at 340 °C. The second step involves combustion of the remaining material (soot) at elevated temperature and its carbon content is measured by coulometric titration of the evolved CO2.
A variant of thermal/optical reflectance apparatus for carbon analysis, originally developed by Huntzicker et al. (1982), was built by Chow et al. (1993) and used to analyze over 27 000 samples from a number of urban and regional air quality studies in the USA. In this variant, a portion of the sample is exposed to a series of temperatures in a pure helium atmosphere, followed by oxidation at several temperature settings in a 2 % oxygen and 98 % helium atmosphere. The carbon content of gases that evolves at each temperature is converted to methane and quantified with a flame ionization detector.
An inherent problem of the thermal techniques is the possibility of an overestimation of the BC content due to pyrolysis (charring) of some organic component during the heating. This analytical artifact depends on the chemical composition of the organic content and on the carrier gas (oxidizing or neutral). These effects can be minimized by pre-extraction with organic solvents or by other procedures for correcting the pyrolysis effects (Gundel et al. 1984; Schmid et al. 2001). These methods analyze samples of aerosols collected during appropriate time periods by filtration or impaction.
Recently, advanced aerosol technologies capable of single particle detection and in situ measurement of aerosol properties with high time resolution have been developed. Methods such as aerosol mass spectrometer (AMS) and SP2 (single particle soot photometer), having the sensitivity to measure individual aerosol particles, have been recently used to characterize the carbonaceous aerosol in situ. For the AMS approach, a hot tungsten filament or laser beam heats the particles and volatile and semi-volatile organics are measured with a time of flight mass spectrometer (Jayne et al. 2000). In SP2, the particles are heated by an intense laser beam. The refractory component, which is assumed to be BC, is determined from the near-infrared radiation emitted by this component (Schwartz et al. 2006).
Both thermal and single particle instruments, besides measuring BC and organic material in the aerosol, have also been used to identify the primary and secondary organic fraction. The thermal methods relayed on measuring BC the tracer of combustion-generated primary aerosol. The secondary organics increase the OC/BC ratio above the values measured, for example, in source-impacted areas where most aerosols should be primary.
Estimated secondary OC fractions (% of total OC) at a number of European urban, rural, and coastal sites ranged from a minimum of 17 %, in Birmingham, UK, during winter, to a maximum of 78 % at a rural coastal area, in Portugal, with air masses transported directly from the ocean (Castro et al. 1999). A seasonal dependence was observed at these sites with minimum secondary OC production during winter. For additional results using similar techniques see, for example, Rosen et al. (1980), Neusüß et al. (2002), Salam et al. (2003).
Jimenez et al. (2009) presented a combination of modeling and measurement study on the organic aerosols. Measurements were conducted by single particle AMS instruments, of the kind described above, at a number of locations worldwide. Measured quantities included aerosol composition, volatility, and oxidation state. According to these investigators ambient oxygenated organic aerosol are secondary species found at concentrations, comparable to those of sulfate aerosol, throughout the Northern Hemisphere.
These conclusions differ from those derived by analyses of filter-collected samples that indicated a far smaller abundance of secondary organics. One reason could be that volatile secondary organic species, detected by AMS, may be lost from the filter during filtration.
Identification and Measurement of BC in the Arctic
Most of the developments mentioned above relate to air quality in urban atmospheres. The first indications of the role of BC in a larger, global context came from studies of the Arctic Haze phenomenon. Analyses of filter-collected samples showed surprisingly large BC concentrations throughout the Arctic troposphere (Rosen et al. 1981, 1984).
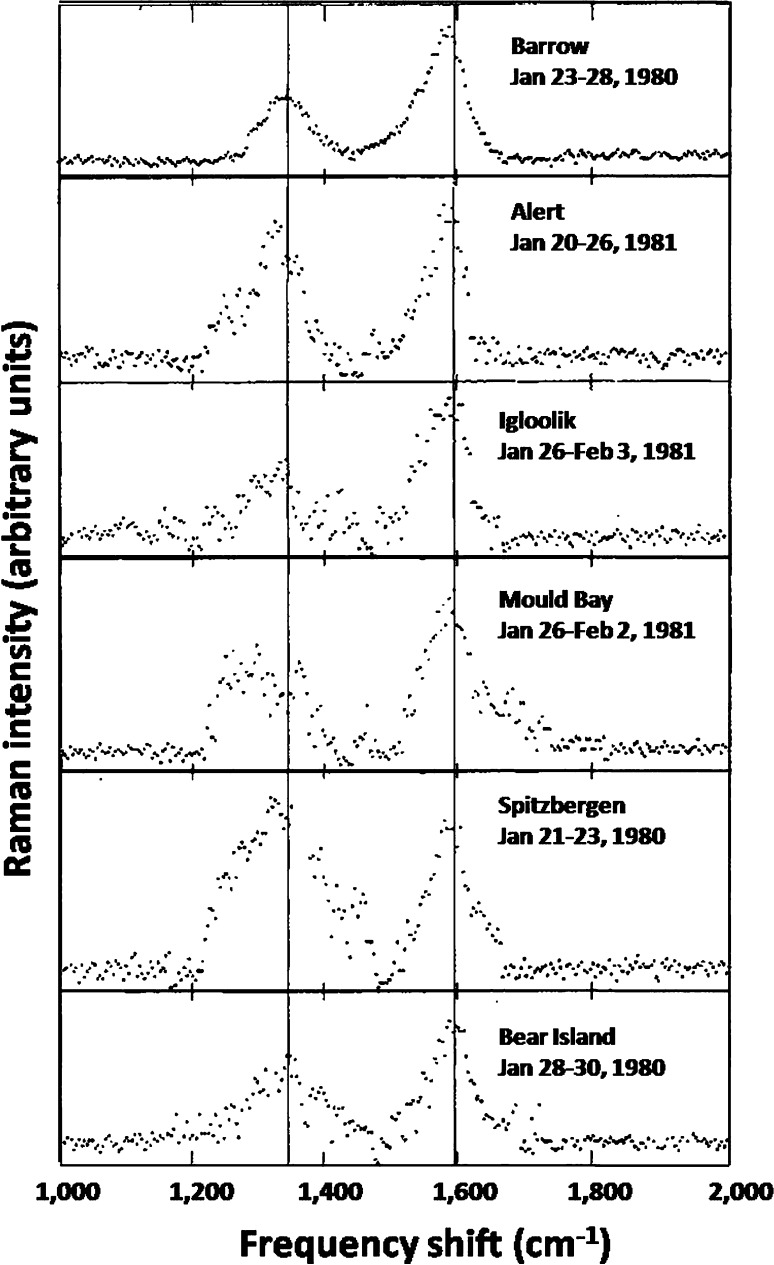
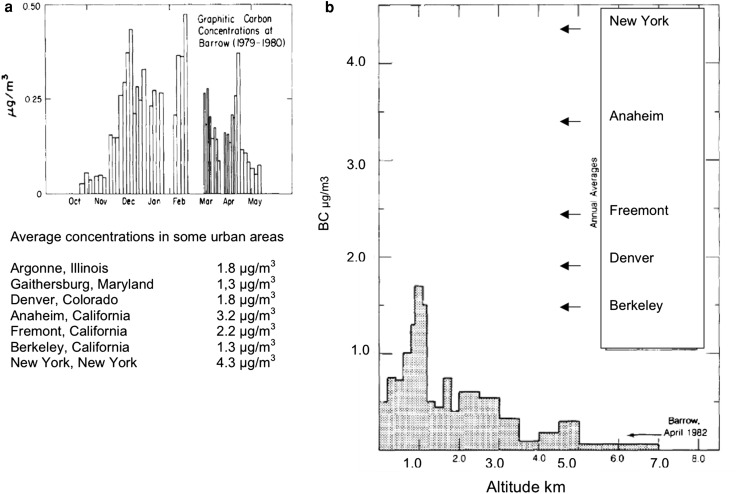
This Arctic Haze phenomenon was studied extensively by a number of investigators including Shaw (1975), Rahn and McCaffrey (1980), Bodhaine et al. (1981), Barrie et al. (1981), and Ottar (1981) by sampling at ground level stations throughout the western Arctic and from aircraft as part of the NOAA AGASP program in the mid 1980s (Schnell 1984). The carbonaceous component of the Arctic Haze was studied by the array of techniques described above and showed very similar characteristics to urban aerosols. As was the case with urban aerosols, the optically absorbing component of the Arctic Haze was identified by Raman Spectroscopy on a molecular level as graphitic or BC at six sampling locations stretching from the Alaskan Arctic to the Canadian and Norwegian Arctic. These spectra, shown in Fig. 4, unambiguously demonstrated that, at this absorbing component of the Arctic Haze, was due to high temperature combustion presumably from anthropogenic sources as the only other contributor could be forest fires, which should be negligible this time of the year. Typical ground level concentrations of BC as a function of time of year are compared to typical values in urban environments in the United States as shown in Fig. 5a. The peak values in winter and spring were smaller than in highly polluted urban environments in the United States but by less than an order of magnitude. Similar variations are observed for the sulfate and organic components of the aerosol (Rahn and McCaffrey 1980; Barrie et al. 1981; Ottar 1981; Rosen et al. 1981). This was a very surprising finding for such a remote location given the view at the time that primary combustion-generated particles would not be transported efficiently on a global scale. Besides carbon, the sub-micron portion of the Arctic Haze consisted primarily of sulfates and nitrates similar in composition to highly polluted urban environments. The sources of these particles were viewed as largely due to long-range transport from combustion sources in Asia, Europe, and North America. BC was also identified in Arctic snow by Clarke and Noone (1985).
Fig. 4.
Raman spectra of particles collected in Alaska, Canadian, and Norwegian Arctic
Fig. 5.
a Seasonal variation of black (graphitic) carbon concentrations at Barrow, Alaska (1979–1980). Similar concentrations are observed for ground level stations across the western Arctic. b Vertical profile of black (graphitic) carbon concentrations in Norwegian Arctic compared to ground level concentrations at Barrow, Alaska and various urban locations
One of the major uncertainties in modeling the effects of the Arctic Haze on the solar radiation balance was limited knowledge of the vertical distributions of BC. The first measurements of such distributions in the Arctic atmosphere were obtained in real-time with an aethalometer (Hansen et al. 1984) as part of the NOAA AGASP program (Schnell 1984). To the best of our knowledge, these represent the first measurements of such distributions anywhere around the globe. The measurements showed substantial concentrations of BC throughout the western Arctic troposphere including the North Pole. The vertical profiles showed either a strongly layered structure or an almost uniform distribution to 8 km (Rosen et al. 1984). In general, the concentrations aloft were higher than ground level. One of the vertical profiles in the Norwegian Arctic with comparisons to concentrations in urban environments is shown in Fig. 5b. Remarkably, the concentrations within layers are as large as those found at ground level in typical mid-latitude urban areas in the United States in the 1980s timeframe.
Impact of BC on Radiative Transfer and Heating of the Arctic
Over a highly reflective surface like polar ice and snow, the solar radiation balance is predominately affected by BC the absorbing component of the aerosol (Cess 1983). The BC profiles shown above can be used to calculate absorption coefficients as a function of altitude and integrating these over the vertical air column, to obtain absorption optical depths. In these calculations, it is important to distinguish between BC particles that are externally mixed with the non-absorbing aerosol components (i.e., separate particles of BC and non-absorbing particles) and those or internally mixed with the other sub-micron non-absorbing components of the aerosol (i.e., each particle contains both BC and non-absorbing components). Such differences, as shown by Ackerman and Toon (1981), can lead to significant changes in aerosol absorption. As an example, we calculated the absorption optical depths for the vertical profile over the Norwegian Arctic shown in Fig. 5b assuming absorption cross-sections 8.3 m2 g−1 for an external mixture and 19 m2 g−1 for an internal mixture using Mie scattering calculations with a core shell morphology as described in Rosen et al. (1984). Other internal mixture configurations are possible with lower absorption cross-sections as suggested by Cappa et al. (2012). The absorption optical depths associated with this vertical profile are quite large with optical depths of 0.023 and 0.052, for external and internal mixtures, respectively. For this flight profile, net flux radiometer measurements as a function of altitude were also measured by Valero et al. (1983). These determined an absorption optical depth of 0.065, which is similar to our results for an internal mixture with core shell morphology.
The average absorption optical depths for all the AGASP flights, which included eight vertical profiles over the Alaskan, Canadian, and Norwegian Arctic, were also large with values, respectively, of 0.013 and 0.030 for an external and internal mixtures (Rosen and Hansen 1985). Optical depths of these magnitudes lead to a substantial change in the solar radiation balance over the highly reflecting Arctic snow surface during the March–April time frame of these measurements (Rosen et al. 1984). This is illustrated by the calculations of Porch and McCracken (1982) and of Cess (1983), who have modeled the Arctic aerosol using, above-mentioned ground level measurement, and an absorption optical depth of 0.021 (which is close to the average of an internal and external mixtures for all the AGASP flights), under cloud-free conditions, and a surface albedo of 0.8. They obtained an increase in the surface-atmosphere energy absorption at the top of the atmosphere (TOA) of ~7 W m−2, averaged over the day for March 15 at 75°N. Cess (1983) has also calculated the TOA as function of month using ground level measurements of BC at Barrow, Alaska (shown in Fig. 5a) to normalize his calculations. He finds annual averages values of TOA of ~2.5 W m−2 from 65°N to 75°N. Comparable changes in the TOA due to BC in the snow were found by Clarke and Noone (1985). These changes in TOA are large and indicated that BC could be an important contributor to Arctic heating and melting of polar ice. These results were viewed in the 1980s as potentially one of the major causes of Arctic warming trends as described in Archives of US Dept. of Energy, Basic Energy Sciences Accomplishments. BC can also cause an additional heating effect when it is deposited in the snow, as calculated by Warren and Wiscombe (1980), due to a reduction in snow albedo. Measurements of spectral albedo of snow in the Cascade Mountains made by Grenfell et al. (1981) supported this theory. Clarke and Noone (1985) measured the BC content of Arctic snow and ice which suggested a reduction of the Arctic snow albedo by one to several percent corresponding to a heating effect of ~3 W m−2 which is comparable to the TOA from the airborne BC.
Recent measurements of BC at ground level stations (Hirdman et al. 2010) and from aircraft (McNaughton et al. 2011; Hoffman et al. 2012) in the Arctic have shown a downward trend compared to the BC values in the 1980s. These downward trends have been attributed to reduced emissions in northern Eurasia. BC in the snow has been recently measured by Doherty et al. (2010). These values are within experimental error similar to those found in the 1980s. As energy use increases dramatically in the next several decades, the future trends of BC in the Arctic and elsewhere will depend on the mix of combustion technologies and the fuel mix used to support this energy growth.
The effects of aerosols on the radiation balance is somewhat simplified in the Arctic and other regions with a high surface albedo, where the underlying surface is covered with snow and ice. For this case, the BC is expected to lead to an overall heating effect (TOA) as the cooling effect of the other non-absorbing aerosol components should be small (Cess 1983). This will be true not only in the Arctic but also on the pathways to the Arctic from mid-latitude combustion sources (snow covered in winter and spring) where even higher concentrations of BC particles are expected, with correspondingly larger absorption optical depths and increased BC in snow. In terms of today’s jargon, these mid-latitude regional effects are described as “brown clouds,” and the Arctic Haze can be viewed as arising from the long-range transport of these urban and industrial pollution clouds to the remote Arctic region. These results set the stage for a number of studies that led to the recognition of the unique role of BC in climate change.
BC and Climate: New Perspectives
From the mid 1980s until the late 1990s the effects of aerosols on global climate primarily concentrated on the role of sulfates, which cool the earth, with minimal attention to the heating effect impact of BC. For example, the modeling studies of Charlson et al. (1992) and Schwartz (1996) assumed purely scattering sulfate aerosols with a single scatter albedo of 1.0. These models provided some insights on the cooling effects of sulfate aerosols but were not a realistic representation of combustion-generated aerosols. These in addition to sulfate, also have a significant absorbing component (BC) and an organic component with comparable sub-micron mass and scattering coefficients as the sulfate aerosol.
These deficiencies were pointed out by Hegg et al. (1997) in the TARFOX study where sun photometer optical depths were combined with airborne measurements of chemical composition and scattering and absorption coefficients of aged aerosols over the mid-Atlantic coast of the eastern United States. The results of these studies did not support the common assumption that sulfate dominates optical depths in polluted regions. A chemical apportionment of the optical depths indicated that the three chemical components, which were condensed water, carbonaceous species, and sulfate. The sub-micron mass of the carbonaceous component was also larger than the sulfate component. Furthermore, the observed average single scatter albedo of 0. 90 indicated a substantial absorbing component in the aerosol due to BC.
Further need for including the carbonaceous component in radiative transfer was demonstrated during an extensive international field project over the Indian Ocean (INDOEX) (Lelieveld et al. 2001). The aerosol mass, chemical composition, and optical depths were measured using airborne and surface platforms from January to March 1999 (Lelieveld et al. 2001). The aerosol mass loadings were comparable to suburban air pollution in North America and Europe. The sub-micron mass of the aerosols was dominated by carbonaceous material, which accounted for 40 % of the mass and sulfates, which accounted for 32 % of the mass. The BC content of the aerosols was high and averaged about 50 % of the total carbon mass. These measurements yielded a single scattering albedo, at ambient relative humidity, between 0.8 and 0.9 and a mean optical depth of 0.2–0.4, heating the boundary layer by ~12 W m−2 and reducing the absorption of solar radiation in the northern Indian Ocean by ~25 W m−2, which the authors suggest would substantially perturb the hydrological cycle and climate. These results are generally consistent with those in the TARFOX study showing somewhat larger concentrations of carbonaceous compared to sulfate aerosols as well as a low single scatter albedo indicating a high absorption due to BC.
Our discussion above has focused on detailed studies of the chemical composition and optical properties of large aged regional air masses. These studies have demonstrated the importance of BC and OC in radiative transfer on regional scales. Sato et al. (2003) have extended BC measurements to a global scale using 322 AERONET sites with a well-calibrated set of sun photometers that can measure aerosol optical depths and absorption optical depths. These studies have concluded that the global levels of BC are about a factor of 2–4 larger than previously calculated from emission estimates and transport models, which had large uncertainties due to the variability of soot emission factors. The inferred global radiative forcing of BC is estimated ~1 W m−2 most of which is anthropogenic in origin.
Chung et al. (2005) have also made a global estimate of the direct effects of anthropogenic aerosols on solar radiation combining satellite measurements with ground-based measurements from AERONET. As expected, large variability in these effects is observed for different regions of the earth from highly polluted source regions to continental and oceanic receptor sites. The annual mean direct global forcing at the TOA was −0.35 W m−2 (±0.25 W m−2), in the atmosphere 3 W m−2 (±0.3 W m−2) and at ground level -3.4 Wm−2 (±0.1 W m−2). These results show a large heating of the atmosphere due to BC and a large reduction of solar radiation at ground level due to the scattering effects of carbon-containing and sulfate aerosols. The forcing at the TOA has a large uncertainty because it is the difference between two large numbers. As expected the sign and magnitude of the TOA changes regionally depending on the surface albedo but the sign of the ground level forcing is always negative and the atmospheric heating always positive. Over snow and ice the TOA forcing is positive while over the oceans it’s negative.
The measurement programs described above have provided a much-improved framework for evaluating the effects of combustion-generated aerosols on global and regional climate using general circulation models. These studies not only consider the cooling effects of the scattering components of the aerosol (sulfates and OC) but also the heating effects of BC in the atmosphere and deposited in the snow and ice. Hansen and Nazarenko (2004) have evaluated the possible climatic impact of BC deposited in snow and ice in the northern hemisphere. They obtain plausible estimates of the effect of BC on snow and ice albedos of 1.5 % in the Arctic and 3 % in northern hemisphere land areas. They suggest that these effects may have contributed to global warming, including the trend toward early springs and the melting of land ice and permafrost. Jacobson (2002) has used the GATOR-GCMM model to evaluate the role of aerosols and greenhouse gases on climate. He concludes that the net global warming trends are due to greenhouse gases, BC and OC, minus substantial cooling by other aerosol particles (e.g., sulfates). Eliminating BC and OC could eliminate 20–40 % of the global warming over a period of 3–5 years. On the other hand, reducing CO2 emissions by a third would have the same effect but only after 50–200 years. Hansen and Nazarenko (2004) have also incorporated both greenhouse gases and aerosols in a climate model. The calculated effects of BC are smaller in this case with the BC global forcing of about 20 % of the greenhouse forcing. In another study of the role of BC in global climate change, Ramanathan and Carmichael (2008) have suggested that the BC forcing is the second most important contributor to global warming, after CO2 emissions. They also suggest that in the Himalayan region BC may be as important as CO2 in the melting of snowpack and glaciers and especially important in the Arctic region.
A recent publication by Bond et al. (2012) contains a comprehensive evaluation of BC climate forcing that includes of all relevant sources, climate related processes and an analysis of potential mitigation strategies including non-science factors, such as technical feasibility, costs, policy design, and implementation feasibility.
Summary
In this paper we have described research over about three decades that changed the view of BC in the environment as a pollutant of marginal importance to one of the main climate change agents. This section summarizes the main research results that shape the contemporary knowledge about the properties and climate effects of BC.
Historically, soot emissions were associated with the burning of coal and were responsible for the London smog. In the early 1970s, in the beginning of modern atmospheric chemistry and physics, soot was considered as a thing of the past and therefore of minimal importance to the researchers at the time. Instead, the emphasis shifted to a new phenomenon, called photochemical smog which was first experienced in Los Angeles, and characterized by periods of severe eye irritation and decreased visibility. The cause of the new smog was speculated to be due to automotive and industrial emissions of nitrogen oxides, hydrocarbons, and SO2 which when exposed to sunshine produce ozone leading to visibility reducing aerosols. These processes were considered to proceed entirely through photochemical reactions in the gas phase and neglected primary soot emissions (BC and OC).
Over the years a variety of modern analytic techniques were applied to gain a better understanding of this type of air pollution and provided a new view of the Los Angeles smog. In this view, both carbon and sulfate particles are largely responsible for visibility degradation in the urban haze. Typically, the concentrations of these particles are similar with similar scattering coefficients.
Many developments mentioned above relate to air quality in urban atmospheres. The first indications of a significant role of BC in a larger, global context came from studies of the Arctic Haze in the early 1980s when large concentrations of combustion-generated particles including carbon and sulfate particles were found throughout the Arctic troposphere. This phenomenon demonstrated that combustion-generated aerosols including primary particles could be transported from mid-latitude sources to remote areas of the earth. In particular, BC concentrations at altitude in the 1980 timeframe were found to be similar to those found in suburban areas of the US. Significant amounts of BC were also found in the snow. Initial modeling calculations showed that the BC in the air and snow could lead to a significant perturbation of the Arctic radiation balance and potentially significant heating effects.
In spite of the Artic haze phenomenon, which was viewed as only a regional anomaly, the effects of aerosols on a global climate from the mid 1980s until the late 1990s primarily concentrated on the role of sulfates, which cools the earth with minimal attention to the impact of BC, which produces heating effects. During the last decade, however, great progress has been made in gaining a better understanding of the global and regional distributions of aerosols, their chemical composition (BC, OC, and Sulfates), their optical properties, and optical depths (scattering and absorption). These observed aerosol properties, although far from complete, have been incorporated into radiative transfer and climate models and their effects have been compared to those of greenhouse gases. These studies suggest that the carbonaceous aerosol, especially the BC component play an important role in radiative transfer and climate modification. Jacobson (2002) and Ramanathan and Carmichael (2008) suggest that aside from CO2, BC is the second most important contributor to present global warming. The most pronounced effects of BC are over snow and ice covered regions where besides heating the atmosphere it can be deposited in the snow reducing the surface albedo. As suggested by Hansen and Nazarenko (2004) and Ramanathan and Carmichael (2008), these effects could be a major contributor to the large increases in Arctic temperatures and reduction in sea ice over the last several decades, as well as, retreating of glaciers in the Himalayas. Finally, it was also suggested that control of BC emissions which have a short atmospheric lifetime and whose emissions can be improved substantially by improved combustion maybe the most viable approach to control the present warming trends especially in the Arctic (Jacobson 2002; Hansen and Nazarenko 2004; Ramanathan and Carmichael 2008). As the melting of the Arctic sea ice represents an irreversible tipping point in climate models, limiting temperature increases in the Arctic and other snow covered regions by controlling BC has been suggested as a high priority item (Hansen and Nazarenko 2004).
An important milestone on the road to the realization of the importance of BC was the 1st International Conference on Carbonaceous Particles in the Atmosphere held at Lawrence Berkeley National Laboratory in 1978. Its organization reflected the view of a number of early participants in the aerosol field that carbonaceous particulate material may be a very important component of atmospheric aerosols, with implications for atmospheric chemistry and physics, climate, air quality, and public health. This conference and a GM-organized symposium were followed by a series of ten international conferences (see Penner and Novakov 1996) on carbonaceous particles in the atmosphere held alternatively in the US and in Austria.
Acknowledgments
The work at Lawrence Berkeley Laboratory summarized above has been supported by the National Science Foundation and by the US Department of Energy.
Biographies
Tica Novakov
a Senior Scientist at the Lawrence Berkeley National Laboratory founded the Atmospheric Aerosol Research group in 1972 focusing on heterogeneous atmospheric reactions and optical properties of black carbon and associated carbonaceous aerosol material. His present activities are mostly on historical aspects of anthropogenic aerosols.
Hal Rosen
was a member of the Atmospheric Aerosol Group at LBNL from 1974 to 1985 when he joined IBM Almaden Research Labs. He is an expert in Aerosol Physics, Optical Spectroscopy, and Storage Technology. He was a Fulbright Scholar and is an APS Fellow and has received many awards including inventor of dual layer DVD. He retired from IBM in 2002 and is now working at Hitachi Research in San Jose.
Contributor Information
Tica Novakov, Phone: 510-486-6928, FAX: 510-486-7303, Email: tnovakov@lbl.gov.
Hal Rosen, Email: hal.rosen@hitachigst.com, Email: haljrosen@gmail.com.
References
- Ackerman T, Toon OB. Absorption of visible radiation in atmosphere containing mixtures of absorbing and nonabsorbing particles. Applied Optics. 1981;20:3661–3668. doi: 10.1364/AO.20.003661. [DOI] [PubMed] [Google Scholar]
- Adams KM, Davis LI, Jr, Japar SM, Pierson WR. Real-time, in situ measurements of atmospheric optical absorption in the visible via photoacoustic spectroscopy-II. Validation for atmospheric elemental carbon aerosol. Atmospheric Environment. 1989;23:693–700. doi: 10.1016/0004-6981(89)90017-6. [DOI] [Google Scholar]
- Appel BR, Colodny P, Wesolowski JJ. Analysis of carbonaceous materials in southern California atmospheric aerosols. Environmental Science and Technology. 1976;10:350–363. doi: 10.1021/es60115a005. [DOI] [Google Scholar]
- Barrie LA, Hoff R, Daggupaty SM. The influence of midlatitudinal pollution sources on haze in the Canadian Arctic. Atmospheric Environment. 1981;15:1407–1419. doi: 10.1016/0004-6981(81)90347-4. [DOI] [Google Scholar]
- Bodhaine BA, Harris JM, Herbert GA. Aerosol light scattering and condensation nuclei measurements at Barrow, Alaska. Atmospheric Environment. 1981;15:1375–1390. doi: 10.1016/0004-6981(81)90344-9. [DOI] [Google Scholar]
- Bond, T.C., S.J. Doherty, D.W. Fahey, P.M. Forster, T. Berntsen, B.J. DeAngelo, M.G. Flanner, S. Ghan, et al. 2012. Bounding the role of black carbon in the climate system: A scientific assessment. Journal of Geophysical Research Atmospheres. doi:10.1002/jgrd.50171.
- Brimblecombe P. Air pollution in industrializing England. Journal of Air Pollution Control Association. 1978;28:115–118. doi: 10.1080/00022470.1978.10470577. [DOI] [Google Scholar]
- Cachier H, Bremond M-P, Buat-Menard A. Determination of atmospheric soot carbon with a simple thermal method. Tellus. 1989;41:379–390. [Google Scholar]
- Cappa CD, Onasch TB, Massoli P, Worsnop DR, Bates TS, Cross EB, Daidovits P, Hakala J, et al. Radiative absorption enhancements due to mixing state of atmospheric black carbon. Science. 2012;337:1078–1081. doi: 10.1126/science.1223447. [DOI] [PubMed] [Google Scholar]
- Cass GR, Conklin MH, Shah JJ, Huntzicker JJ, Macias ES. Elemental carbon concentrations: Estimation of an historical data base. Atmospheric Environment. 1984;18:153–162. doi: 10.1016/0004-6981(84)90238-5. [DOI] [Google Scholar]
- Castro LM, Pio CA, Harrison RM, Smith DJT. Carbonaceous a aerosol in urban and rural European atmospheres: Estimation of secondary organic carbon. Atmospheric Environment. 1999;33:2771–2781. doi: 10.1016/S1352-2310(98)00331-8. [DOI] [Google Scholar]
- Cess RD. Arctic aerosol model estimates of interactive influences upon the surface-atmosphere clear sky radiation budget. Atmospheric Environment. 1983;17:2555–2564. doi: 10.1016/0004-6981(83)90083-5. [DOI] [Google Scholar]
- Chang SG, Novakov T. Formation of pollution particulate nitrogen compounds by NO-soot and NH3-soot gas-particle surface reactions. Atmospheric Environment. 1975;9:495–504. doi: 10.1016/0004-6981(75)90109-2. [DOI] [Google Scholar]
- Charlson RJ, Schwartz SE, Hales JM, Cess RD, Coakley JA, Jr, Hansen JE, Hofmann DJ. Climate forcing by anthropogenic aerosols. Science. 1992;255:423–429. doi: 10.1126/science.255.5043.423. [DOI] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Pritchett LC, Pierson WR, Frazler CA, Purcell RG. The DRI thermal/optical reflectance carbon analysis system: Description, evaluation and applications in US air quality studies. Atmospheric Environment. 1993;27A:1185–1201. [Google Scholar]
- Chung CE, Ramanathan V, Kim D, Podgorny IA. Global anthropogenic aerosol direct forcing derived from satellite and ground-based observations. Journal of Geophysical Research. 2005;110:D24207. doi: 10.1029/2005JD006356. [DOI] [Google Scholar]
- Clarke AD, Noone KJ. Soot in Arctic snowpack: A cause for perturbation in radiative transfer. Atmospheric Environment. 1985;19:2045–2053. doi: 10.1016/0004-6981(85)90113-1. [DOI] [Google Scholar]
- Dalzell WH, Sarofim AF. Optical constants of soot and their application to heat-flux calculations. Journal of Heat Transfer. 1969;91:100–105. doi: 10.1115/1.3580063. [DOI] [Google Scholar]
- Dod, R.L., and T. Novakov. 1982. Application of thermal analysis and photoelectron spectroscopy for the characterization of particulate matter. In Industrial applications of surface analysis, Vol. 199, Chap. 17, eds. L.A. Casper and C.J. Powell, 397–409. American Chemical Society Symposium Series, Washington, DC.
- Doherty SJ, Warren SG, Grenfell TC, Clarke AD, Brandt RE. Light absorbing impurities in Arctic snow. Atmospheric Chemistry and Physics. 2010;10:18807–18878. doi: 10.5194/acp-10-11647-2010. [DOI] [Google Scholar]
- Friedlander SK. Chemical element balances and identification of air pollution sources. Environmental Science and Technology. 1973;7:235–240. doi: 10.1021/es60075a005. [DOI] [PubMed] [Google Scholar]
- Gartrell G, Jr, Heisler SL, Friedlander SK. Relating particulate properties to sources—The results for the California aerosol. Advances in Environmental Science and Technology. 1980;9:665–713. [Google Scholar]
- Grenfell TC, Perovich DK, Ogren JA. Spectral albedos of an alpine snowpack. Cold Regions Science and Technology. 1981;4:121–127. doi: 10.1016/0165-232X(81)90016-1. [DOI] [Google Scholar]
- Gundel LA, Dod RL, Rosen H, Novakov T. The relationship between optical attenuation and black carbon concentration for ambient and source particles. The Science of the Total Environment. 1984;36:197–202. doi: 10.1016/0048-9697(84)90266-3. [DOI] [Google Scholar]
- Haagen-Smit AJ. Chemistry and physiology of Los Angeles smog. Industrial and Engineering Chemistry. 1952;44:1342–1346. doi: 10.1021/ie50510a045. [DOI] [Google Scholar]
- Hall SR. Evaluation of particulate concentrations with collecting apparatus. Analytical Chemistry. 1952;24:996–1000. doi: 10.1021/ac60066a023. [DOI] [Google Scholar]
- Hansen J, Nazarenko L. Soot climate forcing via snow and ice albedos. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:423–428. doi: 10.1073/pnas.2237157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ADA, Rosen H, Novakov T. The Aethalometer: An instrument for real-time measurement of optical absorption by aerosol particles. The Science of the Total Environment. 1984;36:191–196. doi: 10.1016/0048-9697(84)90265-1. [DOI] [Google Scholar]
- Hegg DA, Livinston J, Hobbs PV, Novakov T, Russel P. Chemical apportionment of aerosol column optical depth off the mid-Atlantic coast of the United States. Journal of Geophysical Research. 1997;102:25293–25303. doi: 10.1029/97JD02293. [DOI] [Google Scholar]
- Hidy, G.M. 1972. Aerosols and atmospheric chemistry, 348 pp. New York: Academic Press.
- Hidy, G.M., and P.K. Mueller. 1980. The character and origins of smog aerosols. In Advances in environmental science and technology, eds. J.N. Pitts and R.L. Metcalf, 776 pp. New York: Wiley.
- Hirdman D, Burkhart JF, Sodemann H, Eckhardt S, Jeffereson A, Quinn PK, Sharma S, Strom J, et al. Long-term trends of black carbon and sulphate aerosol in the Arctic, changes in atmospheric transport and source region emissions. Atmospheric Chemistry and Physics. 2010;10:9351–9368. doi: 10.5194/acp-10-9351-2010. [DOI] [Google Scholar]
- Hoffman A, Osterloh L, Stone R, Lampert A, Ritter C, Stock M, Tunved P, Hennig T, et al. Remote sensing and in situ measurements of tropospheric aerosol, a PAMARCMiP case study. Atmospheric Environment. 2012;52:56–66. doi: 10.1016/j.atmosenv.2011.11.027. [DOI] [Google Scholar]
- Huntzicker, J.J., R.L. Johnson, J.J. Shah, and R.A. Cary. 1982. Analysis of organic and elemental carbon in ambient aerosol by a thermal-optical method. In Particulate carbon: Atmospheric life cycle, eds. G.T. Wolff and R.L. Klimisch, 411 pp. New York: Plenum Press.
- Jacobson MZ. Control of fossil-fuel particulate black carbon and organic matter, possibly the most effective method of slowing global warming. Journal of Geophysical Research. 2002;107:4410. doi: 10.1029/2001JD001376. [DOI] [Google Scholar]
- Jayne JT, Leard D, Zhang C, Davidovits P, Smith KA, Kolb CE, Worsnop DR. Development of an aerosol mass spectrometer for size and composition analysis of submicron particles. Aerosol Science and Technology. 2000;33:49–70. doi: 10.1080/027868200410840. [DOI] [Google Scholar]
- Jimenez JL, Canagaratna R, Donahue NM, Prevot ASH, Zhang Q, Kroll JH, DeCarlo PF, Allan JD. Evolution of organic aerosols in the atmosphere. Science. 2009;326:1525–1529. doi: 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Crutzen PJ, Ramanathan V, Andreae MO, Brenninkmeijer CAM, Campos T, Cass GR, Dickerson RR, et al. The Indian Ocean experiment: Widespread air pollution from south and southeast Asia. Science. 2001;291:1031–1036. doi: 10.1126/science.1057103. [DOI] [PubMed] [Google Scholar]
- Lin C-I, Baker M, Charlson RJ. Absorption coefficient of atmospheric aerosol: A method for measurement. Applied Optics. 1973;12:1356–1383. doi: 10.1364/AO.12.001356. [DOI] [PubMed] [Google Scholar]
- Malissa H, Puxbaum H, Pell E. Toward a relative conductometric carbon and sulfur determination in dusts. Fresenius Journal of Analytical Chemistry. 1976;282:109–113. doi: 10.1007/BF00663313. [DOI] [Google Scholar]
- McNaughton CS, Clarke AD, Freitag S, Kapustin VN, Kondo Y, Moteki N, Sahu L, Takegawa N, et al. Absorbing aerosol in the troposphere of the western Arctic during the 2008 ARCTAS/ARCPAC airborne field campaigns. Atmospheric Chemistry and Physics. 2011;11:7561–7582. doi: 10.5194/acp-11-7561-2011. [DOI] [Google Scholar]
- Mueller PK, Mosley RW, Pierce LB. Chemical composition of Pasadena aerosol by particle size and time of day: Carbonate and noncarbonate carbon content. Journal of Colloid and Interface Science. 1972;39:235–239. doi: 10.1016/0021-9797(72)90157-9. [DOI] [Google Scholar]
- Neusüß C, Gnauk T, Plewka A, Herrmann H, Quinn P. Carbonaceous aerosol over the Indian Ocean: OC/EC fractions and selected specifications from size-segregated onboard samples. Journal of Geophysical Research. 2002;107:8031. doi: 10.1029/2001JD000327. [DOI] [Google Scholar]
- Novakov, T. 1973. Chemical characterization of atmospheric pollution particulates by photoelectron spectroscopy. In Proceedings second joint conference on sensing of environmental pollutants, 197–204. Pittsburgh: Instrument Society of America.
- Novakov, T. 1981. Microchemical characterization of aerosols. In Proceedings of the 8th international microchemical symposium, eds. H. Malissa, M. Grasserbauer, and R. Belcher, 141–165. Graz, Austria 1980. Wien: Springer.
- Novakov T. The role of soot in atmospheric chemistry. The Science of the Total Environment. 1984;36:1–10. doi: 10.1016/0048-9697(84)90241-9. [DOI] [Google Scholar]
- Novakov T, Mueller PK, Alcocer AE, Otvos JW. Chemical states of nitrogen and sulfur by photoelectron spectroscopy. Journal of Colloid and Interface Science. 1972;39:225–234. doi: 10.1016/0021-9797(72)90156-7. [DOI] [Google Scholar]
- Novakov T, Chang SG, Harker AB. Sulfates as pollution particulates: Catalytic formation on carbon (soot) particles. Science. 1974;186:259–261. doi: 10.1126/science.186.4160.259. [DOI] [PubMed] [Google Scholar]
- Novakov T, Dod RL, Chang SG. Study of air pollution particulates by photoelectron spectroscopy. Zeitschrift fur Analytische Chemie. 1976;282:287–290. doi: 10.1007/BF00423577. [DOI] [Google Scholar]
- Ottar B. The transfer of airborne pollutants to the Arctic region. Atmospheric Environment. 1981;15:1439–1445. doi: 10.1016/0004-6981(81)90350-4. [DOI] [Google Scholar]
- Penner JE, Novakov T. Carbonaceous particles in the atmosphere: A historical perspective to the fifth international conference on carbonaceous particles in the atmosphere. Journal of Geophysical Research. 1996;101:19373–19378. doi: 10.1029/96JD01175. [DOI] [Google Scholar]
- Porch WM, McCracken MC. Parametric study of the effects of Arctic soot on solar radiation. Atmospheric Environment. 1982;16:1365–1371. doi: 10.1016/0004-6981(82)90057-9. [DOI] [Google Scholar]
- Rahn KA, McCaffrey RJ. On the origin and transport of the winter Arctic aerosol. Annals of the New York Academy of Sciences. 1980;338:486–503. doi: 10.1111/j.1749-6632.1980.tb17142.x. [DOI] [Google Scholar]
- Ramanathan V, Carmichael G. Global and regional climate changes due to black carbon. Nature Geoscience. 2008;1:221–226. doi: 10.1038/ngeo156. [DOI] [Google Scholar]
- Rosen H, Hansen ADA. Estimates of springtime soot and sulfur fluxes entering the Arctic troposphere: Implications to source regions. Atmospheric Environment. 1985;19:2203–2207. doi: 10.1016/0004-6981(85)90129-5. [DOI] [Google Scholar]
- Rosen H, Novakov T. Raman scattering and the characterization of atmospheric aerosol particles. Nature. 1977;266:708–710. doi: 10.1038/266708a0. [DOI] [Google Scholar]
- Rosen H, Hansen ADA, Gundel L, Novakov T. Identification of the optically absorbing component in urban aerosols. Applied Optics. 1978;17:3859–3861. doi: 10.1364/AO.17.003859. [DOI] [PubMed] [Google Scholar]
- Rosen, H., A.D.A. Hansen, R.L. Dod, and T. Novakov. 1976. Characterization of the carbonaceous component of ambient and source particulate samples by an optical absorption technique, 8–18. Lawrence Berkeley Laboratory Report LBL-68l9.
- Rosen H, Hansen ADA, Dod RL, Novakov T. Soot in urban atmospheres: Determination by an optical absorption technique. Science. 1980;208:741–744. doi: 10.1126/science.208.4445.741. [DOI] [PubMed] [Google Scholar]
- Rosen H, Novakov T, Bodhaine B. Soot in the Arctic. Atmospheric Environment. 1981;15:1371–1374. doi: 10.1016/0004-6981(81)90343-7. [DOI] [Google Scholar]
- Rosen H, Hansen ADA, Novakov T. Role of graphitic carbon particles in radiative transfer in the Arctic haze. The Science of the Total Environment. 1984;36:103–110. doi: 10.1016/0048-9697(84)90253-5. [DOI] [Google Scholar]
- Salam A, Baueri H, Kassin K, Ullah SM, Puxbaum H. Aerosol chemical characteristics of an island site in the Bay of Bengal. Journal of Environmental Monitoring. 2003;5:483–490. doi: 10.1039/b212521h. [DOI] [PubMed] [Google Scholar]
- Sato M, Hansen J, Koch D, Lacis A, Ruedy R, Dubovik O, Holben B, Chin M, et al. Global atmospheric black carbon inferred from AERONET. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6319–6324. doi: 10.1073/pnas.0731897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid H, Laskas L, Abraham HJ, Baltensperger U, Lavanchy V, Bizjak M, Burba P, Cachier H, et al. Results of the “carbon conference” international aerosol carbon round robin test stage I. Atmospheric Environment. 2001;35:2111–2121. doi: 10.1016/S1352-2310(00)00493-3. [DOI] [Google Scholar]
- Schnell RC. Arctic haze and the Arctic gas and aerosol sampling program (AGASP) Geophysical Research Letters. 1984;11:361–364. doi: 10.1029/GL011i005p00361. [DOI] [Google Scholar]
- Schwartz SE. The whitehouse effect—shortwave radiative forcing of climate by anthropogenic aerosols: An overview. Journal of Aerosol Science. 1996;27:359–382. doi: 10.1016/0021-8502(95)00533-1. [DOI] [Google Scholar]
- Schwartz JP, Gao RS, Fahey DW, Thomson DS, Watts LA, Wilson JC, Reeves JM, Darbeheshti M, et al. Single-particle measurements of midlatitude black carbon and light scattering aerosols from the boundary layer to the lower stratosphere. Journal of Geophysical Research. 2006;111:D16207. doi: 10.1029/2006JD007076. [DOI] [Google Scholar]
- Shaw GE. The vertical distribution of atmospheric aerosols at Barrow, Alaska. Tellus. 1975;27:39–50. doi: 10.1111/j.2153-3490.1975.tb01653.x. [DOI] [Google Scholar]
- Thomas MD. The present status of the development of instrumentation from the study of air pollution. Proceedings National Air Pollution Symposium. 1952;2:16–23. [Google Scholar]
- Valero PJ, Ackerman TP, Gore WJY. Radiative effects of the Arctic haze. Geophysical Research Letters. 1983;10:1184–1187. doi: 10.1029/GL010i012p01184. [DOI] [Google Scholar]
- Warren SG, Wiscombe WJ. A model for the spectral albedo of snow II. Snow containing atmospheric aerosols. Journal of Atmospheric Science. 1980;37:2734–2745. doi: 10.1175/1520-0469(1980)037<2734:AMFTSA>2.0.CO;2. [DOI] [Google Scholar]
- Wilkins ET. Air pollution and the London fog of December, 1952. Journal Royal Sanitary Institute. 1954;74:1–21. [PubMed] [Google Scholar]
- Yasa Z, Amer NM, Rosen H, Hansen ADA, Novakov T. Photo-acoustic investigations of urban aerosol particles. Applied Optics. 1979;18:2528–2530. doi: 10.1364/AO.18.002528. [DOI] [PubMed] [Google Scholar]