Abstract
Background
Adenosine (AD) elicits cardioprotection through A1-receptor (A1R) activation. Therapy with AD A1R agonists, however, is limited by undesirable actions of full agonism such as bradycardia. This study examined the effects of capadenoson (CAP), a partial AD A1R agonist, on left ventricular (LV) function and remodeling in dogs with heart failure (HF).
Methods and Results
12 dogs with microembolization-induced HF were randomized to 12 weeks oral therapy with CAP (7.5 mg Bid, n=6) or to no therapy (Control, n=6). LV end-diastolic (EDV) and end-systolic (ESV) volumes, ejection fraction (EF), plasma norepinephrine (NE) and n-terminal pro-brain natriuretic peptide (nt-pro BNP) were measured before (PRE) and 1 and 12 weeks after therapy (POST). LV tissue obtained at POST was used to assess volume fraction of interstitial fibrosis (VFIF), SERCA-2a activity, expression of mitochondria uncoupling proteins (UCP) and glucose transporters (GLUT). In controls, EDV and ESV increased and EF decreased significantly from PRE to POST (EF: 30±2 vs. 27±1 %, p<0.05). In CAP-treated dogs, EDV was unchanged; EF increased significantly after one week (36±2 vs. 27±2 %, p<0.05) with a further increase at POST (39±2 %, p<0.05) while ESV decreased. CAP significantly decreased VFIF, normalized SERCA-2a activity and expression of UCP-2 and -3, and GLUT-1 and -2 and significantly decreased NE and nt-pro BNP.
Conclusion
In HF dogs, CAP improves LV function and prevents progressive remodeling. Improvement of LV systolic function occurs early after initiating therapy. The results support development of partial AD A1R agonists for the treatment of chronic HF.
Keywords: Heart failure, Ventricular remodeling, Protein expression, Adenosine receptors
Introduction
Adenosine is a purine nucleoside that exerts a variety of physiological actions by binding to adenosine cell surface receptor subtypes namely A1, A2a, A2b and A3. The cardio-protective effects of adenosine have been extensively studied and are primarily mediated by activation of A1 receptor (A1R) subtype (1–3). Activation of the A1R using “full” agonists, while offering potential therapeutic benefits, is limited by undesirable side effects that include bradycardia, atrio-ventricular (AV) blocks, sedation and anti-diuretic effects. To overcome the side effects of full A1R agonism, efforts were recently placed on tailoring compounds only to the desired pharmacologic efficacy by developing “partial” adenosine A1R agonists that are likely to elicit beneficial therapeutic effects without giving rise to undesirable side effects (4). One such compound is Capadenoson (CAP) (BAY 68-4986), a partial adenosine A1R receptor agonist (4). It has high affinity for the A1R and high selectivity over other adenosine receptors with a favorable pharmacokinetic profile evidenced by long half-life and high bioavailability (5). Previous studies with selective A1R agonists such as tecadenoson (6, 7) and selodenoson (8) showed significant reduction in heart rate up to the effect of causing third degree AV block. Capadenoson, on the other hand, has minimal effect on heart rate (5). Whereas CAP was previously studied in patients with stable angina (9), no studies have emerged to date that describe the effects of CAP or any other partial A1R agonist in heart failure (HF). Despite major advances over the past two decade that led to the development of effective therapeutic modalities for the treatment of HF, the incidence of mortality and morbidity associated with this disease syndrome remains unacceptably high necessitating continued aggressive efforts aimed at developing novel pharmaceuticals and devices to fill an unmet need. The objective of this study was to investigate, for the first time, the effects of selective partial A1R agonism with CAP on LV function and remodeling in dogs with experimentally-induced chronic HF.
Methods
The canine model of intracoronary microembolization-induced chronic HF used in this study was previously described in detail (10). In this study, 12 healthy mongrel dogs, weighing between 19.4 and 24.2 kg, underwent serial coronary microembolizations, 1 to 2 weeks apart, to produce HF. Embolizations were discontinued when LV EF, determined angiographically, was ~30%. Two weeks after the target EF was reached, dogs were randomized to 12 weeks of monotherapy with CAP (7.5 mg twice daily, n=6) or to no therapy at all (Control, n=6). All the procedures were performed during cardiac catheterization under general anesthesia and sterile conditions. Induction of anesthesia was initiated with intravenous hydromorphone (0.22 mg/kg) and diazepam (0.17 mg/kg) and plane of anesthesia was maintained with 1–2% isofluorane. The study was approved by Henry Ford Health System Institutional Animal Care and Use Committee and conformed to the National Institute of Health “Guide and Care for Use of Laboratory Animals”. (NIH publication No. 85–23).
Hemodynamic, Ventriculographic and Electrocardiographic Measurements
Measurements were made at pre-treatment (before initiating therapy) and were repeated at 1 and 12 weeks after initiating therapy. Aortic and LV pressures were measured with catheter-tip micromanometers (Millar Instruments, Houston, TX). LV pressure waveforms were used to calculate LV end-systolic pressure (LVESP) and to derive peak LV +dP/dt and the time constant of isovolumic relaxation, Tau. Left ventriculograms were obtained with the dog placed on its right side and recorded on 35 mm cine film at 30 frame/sec during the injection of 20 ml of contrast material (ISOVU-300, Bracco Diagnostics, Inc., Princeton, NJ). Correction for image magnification was made with a radiopaque calibrated grid placed at the level of the LV. LV end-systolic volume (ESV), end-diastolic volume (EDV) and LV EF were calculated using the area-length method (11). Stroke volume was calculated as the difference between EDV and ESV. Cardiac output (CO) was calculated as the product of stroke volume (SV) and heart rate (HR). Systemic vascular resistance (SVR) was calculated as previously described (11).
In 6 of 12 dogs (3 controls and 3 CAP-treated), LV pressure-volume (P-V) loops were measured using a Millar Instruments MPVS Ultra system pressure-conductance catheter (Houston, TX). Measurements were only made at pre-treatment and at 12 weeks after initiating therapy. P-V loops generated during a transient balloon occlusion of the inferior Vena Cava were used to assess the slope of the end-systolic pressure volume relation (ESPVR), an index of load-independent contractility. All dogs underwent a pre-treatment and a post-treatment 24 hour ambulatory ECG Holter monitoring study. Full Holter disclosures were used to measure maximum, minimum and average heart rate and to evaluate rhythm abnormalities, if any. Peripheral venous blood was used to measure serum electrolytes and plasma levels of n-terminal pro brain natriuretic peptide (nt-pro BNP) and norepinephrine (NE) by ELISA.
Echocardiographic and Doppler Flow Measurements
Echocardiographic and Doppler studies were performed using a General Electric VIVID-7 ultrasound system with a 3.5 MHZ transducer and recorded on a VHS recorder for off-line analysis. LV end-diastolic circumferential wall stress (EDWS) was calculated as previously described (12). Trans-mitral inflow velocity waveforms, measured using pulsed-wave Doppler echocardiography, were used to calculate the time-velocity integral of mitral inflow velocity waveform representing early filling (Ei), the time-velocity integral representing LA contraction (Ai), the ratio Ei/Ai, and deceleration time (DT) of early mitral inflow velocity as previously described (13).
Histomorphometric Measurements
After completion of final hemodynamic study, and while the dog was under general anesthesia, the chest was opened and the heart rapidly removed and LV tissue prepared for histological and biochemical evaluation. From each heart, 3 transverse slices (approximately 3 mm thick) one each from basal, middle and apical thirds of the LV, were obtained. From each slice, transmural tissue blocks were obtained and embedded in paraffin blocks. Tissue blocks were also obtained from the LV free wall, mounted on cork using Tissue-Tek embedding medium, and rapidly frozen in isopentane pre-cooled in liquid nitrogen and stored at −70°C until used. The volume fraction of replacement fibrosis (VFRF), volume fraction of interstitial fibrosis (VFIF), myocyte cross-sectional area (MCSA), a measure of cardiomyocyte hypertrophy, capillary density (CD), and oxygen diffusion distance (ODD) were measured as previously described (14, 15). LV tissue from 6 normal dogs was processed in an identical manner as above and the results used for comparisons.
Biochemical Measurements
To examine the effects of therapy with CAP on myocardial energetics, protein levels of the glucose transporters GLUT-1 and GLUT-4, mitochondria uncoupling proteins UCP-2 and UCP-3, citrate synthase (CS) and muscle carnitine palmitoyl transferase (mCPT-1) were measured in LV tissue as was the expression of calsequestrin (CSQ), a sarcoplasmic reticulum (SR) protein that does not change in HF (internal control) and porin, a mitochondrial protein that is unchanged in HF (internal control). Protein levels were measured in SDS-extract using Western blotting coupled with chemiluminescent method. Band intensity on the gels was quantified in densitometric units (du). To examine the effects of CAP on SR calcium cycling, thapsigargin-sensitive calium-ATPase (SERCA-2a) activity and affinity (Ka) was determined in LV tissue homogenate as was protein level. LV tissue from 6 normal (NL) dogs was used for comparison.
Statistical Analysis
Within group comparisons of hemodynamic, ventriculographic, echocardiographic, and Doppler measures were made using repeated measures analysis of variance (ANOVA) with alpha set at 0.05. If significance was attained, comparisons between measurements made at pre-treatment and those made at 1 week and 12 weeks were tested using the Student-Neuman-Keuls test with p<0.05 considered significant. A t-test for two means was used to compare all measures between the control and treated group at pre-treatment, 1 week, and 12 weeks. For these comparisons, a p-value of <0.05 was considered significant. To assess treatment effect, the change (Δ) in each measure from pre-treatment to post-treatment (12 weeks) was calculated for each of the two study arms. To determine whether significant differences in Δ were present between the control group and the CAP treatment groups, a t-statistic for two means was used with p≤0.05 considered significant. Histomorphometric and biochemical measures between normal, control and CAP-treated dogs were compared using one way ANOVA with alpha set at 0.05. If significance was attained by ANOVA, pairwise comparisons were performed using the Student-Neuman-Kuels test. For all pairwise comparisons, a probability value ≤0.05 was considered significant. All data are reported as mean ± SEM.
Results
None of the study dogs developed acute decompensation or died during the study and none developed ventricular or atrial arrhythmias, bradycardia, AV block, sedation or renal abnormalities. Compared to pre-treatment, there were no differences in serum creatinine or BUN over the course of the study in any of the study groups (Tables 1).
Table 1.
Hemodynamic, ventriculographic, echocardiographic-Doppler and electrolyte measurements obtained at pre-treatment and at 1 week and 12 weeks after initiating follow-up in control group and Capadenoson (CAP)-treated group.
| Pretreatment | 1 weeks | 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | CAP | P- value | Control | CAP | P- value | Control | CAP | P value - | |
| HR (beats/min) | 87±2.9 | 89±4.4 | 0.71 | 88±2.7 | 80±2.4 | 0.05 | 85±3.9 | 84±2.9 | 0.84 |
| mAoP (mmHg) | 72±2.0 | 78±2.0 | 0.06 | 81±3.9 | 84±6.5 | 0.70 | 78±1.9 | 77±5.0 | 0.86 |
| LVEDP (mmHg) | 17±1.2 | 17±1.5 | 1.0 | 19±1.6 | 15±0.9 | 0.05 | 17±1.3 | 14±1.4 | 0.15 |
| LVEDV (ml) | 73±4 | 70±4.2 | 0.62 | 74±3.8 | 70±4.4 | 0.51 | 83±3.1† | 72±6.6 | 0.16 |
| LVESV (ml) | 51±3.5 | 51±3.9 | 1.0 | 52±3.4 | 45±4.1 | 0.22 | 60±2.9† | 44±6.2 | 0.04 |
| LVEF (%) | 30±1.9 | 27±1.8 | 0.28 | 30±2.0 | 36±2.1* | 0.06 | 27±1.4† | 39±2.4* | 0.002 |
| LVESP (mm Hg) | 74±2.1 | 78±2.8 | 0.28 | 77±2.6 | 86±7.7 | 0.29 | 78±3.5 | 79±5.6 | 0.88 |
| LV+dP/dt (mmHg/sec) | 1352±126 | 1589±185 | 0.32 | 1307±37 | 1812±180 | 0.02 | 1357±73 | 1752±281 | 0.20 |
| Tau (msec) | 59±6 | 60±10 | 0.93 | 73±10 | 51±4 | 0.07 | 67±13 | 48±5 | 0.21 |
| SV (ml) | 21±2 | 19±1 | 0.29 | 22±2 | 25±1* | 0.16 | 23±1 | 28±1* | 0.008 |
| CO (L/min) | 1.83±0.11 | 1.69±0.12 | 0.41 | 1.91±0.14 | 1.99±0.10* | 0.65 | 1.93±0.11 | 2.30±0.10* | 0.03 |
| SVR (dynes/sec/cm−5) | 3292±281 | 3776±323 | 0.28 | 3395±145 | 3422±319 | 0.94 | 3486±279 | 2661±114* | 0.02 |
| Ei/Ai | 3.37±0.41 | 3.82±0.49 | 0.50 | 3.66±0.49 | 4.23±0.45 | 0.41 | 3.82±0.3 | 4.55±0.56 | 0.28 |
| DT (msec) | 92±6 | 103±9 | 0.33 | 84±5 | 108±8 | 0.03 | 88±5 | 107±9 | 0.09 |
| LV EDWS (gm/cm2) | 86±15 | 73±9 | 0.47 | 87±11 | 63±7 | 0.09 | 99±18 | 64±14 | 0.16 |
| Slope-ESPVR (mmHg/ml) (n=3 per group) | 2.72 ± 0.62 | 1.54 ± 0.20 | 0.1 | --- | --- | --- | 1.51 ± 0.29 | 1.31 ± 0.18 | 0.57 |
| Creatinine (mg/dL) | 0.92±0.04 | 0.93±0.05 | 0.88 | 0.93±0.03 | 0.92±0.05 | 0.87 | 0.90±0.06 | 1.00±0.07 | 0.30 |
| BUN (mg/dL) | 13±1 | 13±1 | 1.0 | 15±2 | 14±1 | 0.66 | 15±1 | 14±2 | 0.66 |
| Plasma NE (pg/ml) | --- | 908±127 | --- | --- | 520±35* | --- | --- | 210±21* | --- |
Data are shown as Mean ± SEM. HR = heart rate; mAoP = mean aortic pressure; LV = left ventricular; EDP = end-diastolic pressure; EDV = end-diastolic volume; ESV = end-systolic volume; EF = ejection fraction; ESP=end-systolic pressure; +dP/dt=peak rate of change of pressure during isovolumic period; Tau=time constant of isovolumic relaxation; SV = stroke volume; CO = cardiac output; SVR = systemic vascular resistance; Ei = time-velocity integral of the mitral inflow velocity waveform representing early filling; Ai = time-velocity integral representing LA contraction; Ei/Ai = ratio of Ei to Ai; DT = deceleration time of early mitral inflow velocity; EDWS = LV end-diastolic circumferential wall stress; ESPVR=end-systolic pressure-volume relationship; BUN=blood urea nitrogen; NE=norepinephrine. P-value=probability value of control vs. Capadenoson (CAP) at pre-treatment, 1 week and 12 weeks.
p<0.05 vs. pre-treatment in Capadenoson group;
p<0.05 vs. pre-treatment in control group.
Within Group Changes of Hemodynamic, Ventriculographic and Echocardiographic Measures
Hemodynamic, ventriculographic and echocardiographic results obtained at pre-treatment and at 1 and 12 weeks post-treatment in untreated HF controls and CAP-treated HF dogs are shown in Tables 1. There were no significant differences between the 2 study groups in any of the measures obtained at pre-treatment. In untreated controls, HR, mean aortic pressure (mAoP) LV end-diastolic pressure (EDP), peak +dP/dt, LVESP, Tau, SV, CO, SVR, Ei/Ai, DT, EDWS and slope of the ESPVR did not change significantly over the course of 12 weeks of follow-up compared to pre-treatment values (Table 1). In CAP-treated dogs, HR, mAoP, peak +dP/dt, LVESP, Tau and slope of the ESPVR were also not significantly changed over the 12 weeks course of therapy. In this group, LV EDP and EDWS tended to decrease and Ei/Ai and DT tended to increase but none reached statistical significance compared to pre-treatment values (Table 1). Therapy with CAP significantly increased SV and CO and decreased SVR. In untreated control dogs, LV EDV and ESV increased and EF decreased significantly at 12 weeks compared to pre-treatment. In contrast, in CAP-treated dogs, LV EDV was unchanged, ESV tended to decrease and EF increased significantly. Improvements in EF, SV and CO occurred as early as one week after initiating CAP therapy. Treatment with CAP significantly decreased plasma NE at all study time points compared to pre-treatment (Table 1).
Between Groups Changes of Hemodynamic, Ventriculographic and Echocardiographic Measures (Treatment Effect)
Comparisons between controls and CAP-treated dogs at 1 week and 12 weeks are shown in Table 1. Between-group comparisons of the change (Δ) between pre-treatment and post-treatment measurements are shown in Table 2. Compared to control, long-term therapy with CAP had no effect on HR, mAoP, LVESP or slope of the ESPVR but tended to decrease LV EDP, Tau and EDV and tended to increase peak +dP/dt. Treatment with CAP significantly decreased ESV and SVR and increased EF, SV and CO. Measures of LV diastolic function, however, were not significantly altered by long-term treatment with CAP as evidenced by little or no changes in Ei/Ai, DT and EDWS. Compared to control, CAP-therapy also significantly decreased circulating plasma levels of nt-pro BNP (Fig. 1). There were no significant changes between groups with respect to maximum, average and minimum HR derived from ambulatory ECG Holter monitoring studies (Table 2). Significant treatment effects were also present with respect to EF, CO and SV as early as one week after initiating therapy with CAP.
Table 2.
Treatment effect, 3, between pre-treatment and 12 weeks in untreated control and Capadenoson (CAP)-treated dogs.
| Control | Capadenoson | P-value | |
|---|---|---|---|
| ΔHR (beats/min) | −1.67 ± 2.7 | −3.67 ± 5.3 | 0.74 |
| ΔmAoP (mmHg) | 6.3 ± 0.8 | −1 ± 5.4 | 0.2 |
| ΔCO (L/min) | 0.1 ± 0.1 | 0.61 ± 0.2 | 0.02 |
| ΔLV ESP (mm Hg) | 5 ± 2 | 1 ± 7 | 0.57 |
| ΔTau (msec) | 9 ± 10 | −12 ± 7 | 0.11 |
| ΔLV +dP/dt (mmHg/sec) | 4 ± 60 | 163 ± 131 | 0.30 |
| ΔSVR (dynes/sec/cm−5) | 102 ± 146 | −948 ± 195 | 0.002 |
| ΔEi/Ai | 0.4 ± 0.4 | 0.7 ± 0.4 | 0.58 |
| ΔDT (msec) | −2.2 ± 2.2 | 3.0 ± 3.9 | 0.26 |
| ΔLV EDWS (gm/cm^2) | 13.2 ± 7.8 | −8.8 ± 11.4 | 0.14 |
| ΔSlope of ESPVR (mmHg/ml) | −1.2 ± 0.9 (n=3) | −0.23 ± 0.1 (n=3) | 0.34 |
| ECG from Holter | |||
| Max HR (beats/min) | −10 ± 11 | −0.8 ± 10 | 0.48 |
| Avg HR (beats/min) | −8 ± 2 | −8 ± 4 | 0.97 |
| Min HR (beats/min) | −5 ± 3 | −7 ± 3 | 0.67 |
Data are shown as Mean ± SEM. Max = maximum; Avg = average; Min = minimum; ECG = electrocardiogram; Other abbreviations as in Table 1. P-value = probability Control vs. Capadenoson.
Figure 1.

Left: Change 3 (treatment effect) between pre-treatment and 12 weeks post-treatment of left ventricular (LV) end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction (EF) and stroke volume (SV) in untreated control dogs and dogs treated for 3 months with Capadenoson. Right: Change Δ (treatment effect) between pre-treatment and 12 weeks post-treatment in plasma levels of n-terminal pro-brain natriuretic peptide (nt-pro BNP). Data are shown as Mean ± SEM. Probability values are comparisons between untreated control and Capadenoson.
Histomorphometric Findings
Histomorphometric findings are shown in Table 3. Compared to normal dogs, control HF dogs showed a significant increase in VFRF, VFIF, ODD and MCSA and a significant decrease in CD. Compared to untreated control dogs, treatment with CAP resulted in a significant reduction of VFIF, ODD and MCSA and a significant increase in CD. The VFRF also tended to decrease after treatment with CAP, but the change did not reach statistical significance (Table 3).
Table 3.
Histomorphometric Findings at the end of 12 weeks of follow-up or therapy in normal dogs, in untreated control heart failure (HF) dogs and in heart failure dogs treated with Capadenoson (CAP)
| VFRF (%) | VFIF (%) | CD (cap/mm2) | ODD (μm) | MCSA (μm2) | |
|---|---|---|---|---|---|
| Normal | 0.0 | 3.7±0.07 | 2609±79.5 | 8.9±0.17 | 410±10.0 |
| HF-Control | 12.3±1.34* | 15.2±0.63* | 1775±72.9* | 14.5±0.44* | 663±12.3* |
| HF-Capadenoson | 10.8±0.83 | 11.3±1.31† | 2077±46.1† | 11.5±0.51† | 548±24.4† |
Data are shown as Mean ± SEM. VFIF = volume fraction of interstitial fibrosis; VFRF = volume fraction of replacement fibrosis; CD = capillary density; ODD = oxygen diffusion distance; MCSA = myocyte cross-sectional area;
p<0.05 vs. Normal,
p<0.05 vs. HF-Control.
Protein Expression and Other Biochemical Findings
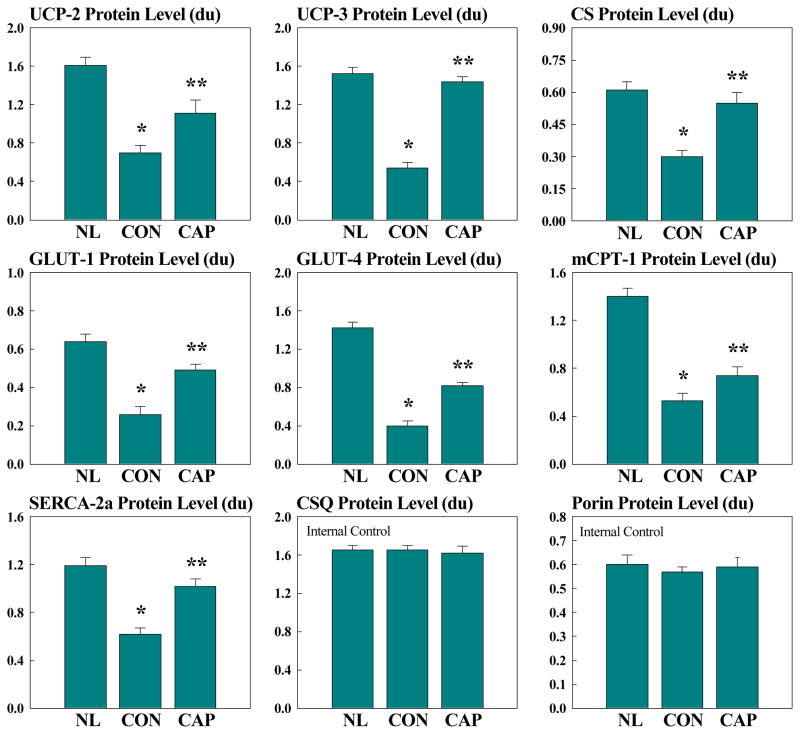
Changes in protein levels in LV myocardium of normal dogs, untreated HF dogs, and CAP-treated HF dogs for GLUTs, UPCs, CS, mCPT-1, porin, and CSQ are shown in figure 2. There were no changes in expression of CSQ and porin between normal dogs, untreated HF dogs and CAP-treated HF dogs. Compared to normal dogs, dogs in the untreated control group showed a significant reduction in the levels of UCP-2, UCP-3, GLUT-1, GLUT-4, CS, mCPT-1 and SERCA-2a. Compared to untreated controls, treatment with CAP was associated with a significant increase in the expression of all these proteins to near normal levels (Fig. 2). Compared to normal dogs, the Vmax for Ca2+-ATPase activity (measured as nmol/mg protein) decreased significantly in the untreated control group and treatment with capadenoson prevented this decline (Fig. 3). These changes were reflected in the affinity (Ka) as well which showed significant increase in the control group that was normalized when treatment with CAP was instituted (Fig. 3).
Figure 2.
Bar graphs depicting changes in left ventricular myocardium protein levels of various metabolic and sarcoplasmic reticulum proteins in normal (NL) dogs, untreated heart failure control dogs (CON) and dogs with heart failure treated with Capadenoson (CAP). UCP = uncoupling protein; CS = citrate synthase; GLUT= glucose transporter; mCPT-1 = muscle carnitine palmitoyl transferase-1; SERCA-2a = calcium ATPase; CSQ = calsequestrin. Data are shown as Mean ± SEM. * = p<0.05 vs. NL; ** = p<0.05 vs. CON.
Figure 3.
Left: Bar graphs depicting changes in Ca2+-ATPase activity (Vmax) in LV myocardium. Right: Bar graph depicting changes in affinity for calcium (Ka) in LV myocardium. Changes are for normal (NL) dogs, untreated heart failure control dogs (CON) and dogs with heart failure treated with Capadenoson (CAP). Data are shown as Mean ± SEM. * = p<0.05 vs. NL; ** = p<0.05 vs. CON.
Discussion
This is the first study to evaluate the short (1 week) and long-term (12 weeks) efficacy of a selective partial A1R agonist in HF dogs. The results indicate that 12 weeks monotherapy with CAP improves LV systolic function and prevents progressive LV enlargement as evidence by a significant improvement in LV EF and significant reduction of end-systolic volume and no significant increase in LV end-diastolic volume compared to untreated controls. Of interest is the observation of increased LV EF early (1 week) in the course of therapy. This observation suggests that unlike the delayed therapeutic benefits encountered with prototypical drugs used in the treatment of HF such as beta-blockers and angiotensin-converting enzyme (ACE) inhibitors the benefits derived from A1R agonism are manifested early in the course of therapy. The improvement of LV systolic function was accompanied by significant increases of SV and CO with a significant decrease of SVR. In the absence of a change in mAoP and LVESP the decrease in SVR can be attributed to increased CO. It should be noted, however, that a possible reduction of AoP may have been masked by an increase in CO. While vasodilation cannot be completely excluded as a contributing factor in improvement of LV systolic function, most, if not all, pharmacologic agents that elicit their benefits through vasodilation lower systemic blood pressure, a hemodynamic alteration not seen in the present study after therapy with CAP. Consistent with its partial agonism properties, and unlike side effects seen with full A1R agonist, CAP had no effects on HR and did not at any time during the study trigger AV block, sedation or anti-diuretic effects. There was no evidence of worsening renal function as evidenced by a lack of an increase of either creatinine or blood urea nitrogen (BUN) with CAP therapy. The concept that HF patients with renal dysfunction can benefit from selective antagonism of the A1R failed to materialize in large, randomized, placebo-controlled clinical trials (16).
The improvement of systolic function seen in the present study cannot be explained on the basis of reduced HR, reduced systemic blood pressure or an intrinsic improvement in contractility. Therapy with CAP did not induce bradycardia, did not lower systemic pressure and did not significantly alter the slope of the ESPVR, a load-independent measure of contractility or peak LV +dP/dt, a load-dependent measure of isovolumic tension. Because the slope of the ESPVR was based on a small sample size, and because +dP/dt tended to increase with CAP therapy, one cannot exclude a “contractility” increase as a contributing factor to the overall benefits on LV function elicited by CAP therapy. Nonetheless, these observations suggest that CAP does not act through negative dromotropic or chronotropic effects.
While the literature is replete with studies describing the effects of adenosine and its receptors in myocardial ischemia and infarction (17–19), little is known, of the effects of adenosine and its receptors in chronic HF. Adenosine and adenosine analogs have been long recognized as in-vivo “cardioprotective” agents and mediators of anti-ischemic preconditioning (20). These cardioprotective effects are believed to result from activation of downstream effectors such as protein kinase C (PKC), KATP channel and some isoforms of mitogen activated protein kinase (MAPK) (20) and partly by inhibition of adenylate cyclase activation and reduction of cAMP levels. Given that regional myocardial ischemia and/or hypoxia frequently exist in the failing heart, it is likely that the benefits seen in the present study with CAP reflect reduced cellular injury resulting from the antiischemic cardioprotective effects of adenosine A1R activation. Studies have shown that adenosine levels in the heart rise with adrenergic stimulation (21). Adenosine has been shown to inhibit β-adrenoceptor-induced enhancement of contractile activity (22). These observations support the concept that adenosine serves an important anti-adrenergic role in the heart to protect it from over-responding both mechanically and metabolically to excessive catecholamine stimulation. Adenosine acting through its A1 receptors has also been shown to inhibit norepinephrine release viewed as a protective mechanism in myocardial ischemia (23, 24). In the present study, CAP significantly decreased plasma levels of norepinephrine early and late during the course of therapy. The above observations suggest that partial A1R agonists in the setting of HF might act partly through similar signalling cascades such as adenylate cyclase inhibition as β-blockers. Long-term (3 months) monotherapy with metoprolol, a selective β1-receptor blocker, in the same animal model of HF used in the present study, was also shown to significantly increase LV EF (25) although to a lesser extent compared to CAP. The hemodynamic response to β-blockade, however, differs from that seen with the partial A1R agonist. It is well known that in patients with HF, β-blockers reduce HR and induce a negative inotropic effect early in the course of therapy evidenced by reduced LV systolic function before improvement takes place later in the course of therapy. In contrast, results from the present study show that the partial A1R agonist CAP does not lower HR and, contrary to β-blockers, elicits a marked and significant improvement in LV EF as early as one week after initiating therapy. These differences argue in favor of additional mechanisms unique to partial A1R agonism that partly drive the observed improvement in LV systolic performance.
Improvement of LV systolic function with CAP may also be the result of improved myocardial energetics elicited through selective activation of the adenosine A1R. In ischemia and infarction, adenosine, acting through its A1R, is known to slow ATP depletion through stimulation of glycolysis, increasing glucose uptake, inhibiting adrenergic stimulation and neutrophil activation and reducing the generation of free oxygen radicals (8, 19, 26–28). The failing myocardium is often described as being “energy starved” and/or “oxygen deprived”, suggesting that poor availability of ATP and lack of oxygen may be partly responsible for the characteristic poor LV performance; a signature of the failing heart. The beneficial effects of selective A1R agonism in HF can act to improve energy metabolism of the failing heart via improvement of mitochondrial function and/or energy substrate utilization. We previously showed that the failing myocardium is characterized by mitochondrial dysfunction evidenced by 1) poor mitochondrial respiration, 2) low mitochondria membrane potential, and 3) abnormal mitochondria membrane permeability transition all of which can lead to poor electron flux through electron transport chain and subsequent reduction of ATP synthesis (29–32). Mitochondrial UCPs, in particular, UCP-2 and UCP-3, are transport proteins located in the inner mitochondrial membrane and control the mitochondrial membrane potential and consequently regulate mitochondrial ATP synthesis and the production of reactive oxygen species by the mitochondria (33, 34). Several studies have shown marked down-regulation of UCP-2 and UCP-3 in the failing myocardium (33, 35–38). In the present study, we also showed a significant down-regulation of UCP-2 and UCP-3 in LV myocardium of untreated HF control dogs. In failing myocardium caused by doxorubicin toxicity, UCP-2 and UCP-3 were significantly decreased and was associated with a significant reduction in mitochondria state-3 and state -4 respiration and ATP synthesis (39). Several studies have shown that increased expression of UCPs decrease reactive oxygen species production, improve cardiomyocyte survival and improve contractile function in the setting of ischemia/reperfusion injury (37, 40, 41). In the present study, long-term therapy with CAP was associated with a near normalization of UCP-2 and UCP-3 protein levels in the LV myocardium.
Selective adenosine A1R agonist can also impact positively on mitochondrial function in HF by modulating the mitochondrial permeability transition pore (mPTP). In addition to providing energy for biological reactions, mitochondria directly regulate cell necrosis and apoptosis through opening of mPTP. We have shown that HF is associated with opening of the mPTP with an attendant increase in the levels of cytochrome c in the cytosol and consequently an increase in cardiomyocyte apoptosis (31, 32, 42, 43). We also showed that prevention of mPTP opening with cyclosporine A can have marked beneficial effects on mitochondrial respiration and ATP synthesis (32). Studies by Xiang et al. in isolated cardiomyocytes showed that exposure of cardiomyocytes to hypoxia increased both mPTP opening and production of reactive oxygen species while decreasing cell viability and mitochondrial membrane potential (44). In their study, exposure of the hypoxic cardiomyocytes to the adenosine A1R agonist 2-cholor-N6-cyclopentyladenosine (CCPA), blocked the increase in mPTP opening and the production of reactive oxygen species and maintained cell viability and mitochondrial membrane potential under hypoxic conditions (44). These observations of improved mitochondrial function following long-term therapy with an adenosine A1R agonist are consistent with our finding in the present study of normalization of citrate synthase levels in LV myocardium of dogs treated with CAP compared to untreated controls. Citrate synthase, the enzyme responsible for catalyzing the first reaction of the citric acid cycle, is known to be down-regulated in HF and is often used as a marker of intact mitochondria (36). Improved ATP synthesis by mitochondria can also account for our observation of increased SR Ca2+-ATPase activity and affinity after treatment with CAP, a finding consistent with improved SR calcium cycling and a desired reduction in calcium overload, long recognized as a key maladaptation in HF. The observations made in this study along with observations made by others as outlined above, support the concept that in HF, adenosine A1R agonism favorably modulates mitochondrial function and, in doing so, restores availability of ATP to the working myocardium and limit cell injury and loss that may result from excess reactive oxygen species production and programmed cell death. A limitation of the present study is the lack of direct assessment of mitochondrial function in the form of respiration, membrane potential and opening of permeability transition pores as well as measurements of ATP synthesis. These measurements must be performed in fresh tissue and are being contemplated for future studies of the effects of A1R agonists in HF.
Consistent with observation by others (36, 37), results from the present study point to a significant decrease in key metabolic proteins in untreated HF dogs compared to normal dogs specifically, a reduction in the expression of the glucose transporters GLUT-1 and GLUT-4 and the regulator of fatty acid oxidation, mCPT-1. These observations are in-line with the concept of reversion of the failing heart to a fetal metabolic phenotype by down-regulating adult gene transcripts rather than up-regulating fetal gene transcripts (36). In the normal heart, recruitment of the glucose transport proteins GLUT-1 and GLUT-4 is a cellular mechanism by which the heart increases glucose transport for metabolism in response to increased energy demands. The observed down-regulation of GLUT-1 and GLUT-4 is consistent with impaired glucose uptake in HF; a maladaptation that can result in worsening of the HF state (45). In the present study, treatment with a partial adenosine A1R agonist restored protein levels of GLUT-1 and GLUT-4 to near normal levels. Observation in this study also pointed to significant down-regulation of mCPT-1 in LV myocardium of untreated HF dogs. Expression of mCPT-1 has been shown to correlate positively with palmitate oxidation suggesting that decreased mCPT-1 protein levels can lead to reduced fatty acid oxidation (46). In the present study, long-term therapy with CAP normalized protein expression of mCPT-1. Restoration of expression of these key metabolic proteins can result in better balance of substrate utilization in the failing heart and, subsequently, improved energy metabolism leading to improved LV pump function.
Global remodeling of the failing LV is invariably accompanied by structural changes at the cellular level characterized by cardiomyocyte hypertrophy, accumulation of collagen in the interstitium termed “reactive interstitial fibrosis”, reduced capillary density and increased oxygen diffusion distance. Myocardium of failing heart is associated with structural changes in the form of cardiac hypertrophy and collagen accumulation in the interstitium (14, 15, 47). These abnormalities favor increased LV stiffness and the development of hypoxia that can lead to progressive worsening of LV function (47). A reduction in interstitial fibrosis and cardiomyocyte hypertrophy, therefore, is likely to reduce LV stiffness and, in doing so, improve “passive” LV filling. In the present study, CAP treatment prevented the increase in VFIF, ODD and decrease in CD. CAP also decreased MCSA, are markers of cardiac hypertrophy. We have shown that hypoxia of the failing myocardium can be an important mediator of apoptosis through activation of key pro-apoptotic proteins (48–50). As discussed earlier, hypoxia can also have an adverse impact on mitochondrial function and, in turn, ATP synthesis (44). The long-term use of a partial adenosine A1R agonist in the present study resulted in amelioration of all of the cellular markers of structural remodeling suggesting that this approach to therapy for HF can directly or indirectly modulate LV remodeling favourably in addition to improving LV systolic function.
In conclusion, the results of this study support the continued development of selective and partial adenosine A1R agonists for the chronic treatment of HF. Use of CAP in this study was devoid of adverse effects often seen with full A1R agonist. The benefits of this targeted approach to the treatment of HF lies in the ability of partial A1R agonism to afford protection to the failing myocardium by limiting triggers of cell injury and death and by providing the necessary energy to the working myocardium through better energy substrate utilization and improved mitochondrial function.
Acknowledgments
Funding Sources
This study was supported in part by research grant from Bayer Pharma AG and by National Heart, Lung, and Blood Institute PO1 HL074237-09.
Footnotes
Conflicts of Interest Disclosures
Dr. Sabbah has received research grants from Bayer Pharma AG and is a consultant for Bayer Pharma AG. Drs. Gupta, Rastogi, Kohli, Wang and Zhang have no disclosures. Drs. Zimmermann, Diedrichs and Albrecht-Küpper are full time employees of Bayer Pharma AG.
References
- 1.Hussain T, Mustafa SJ. Binding of A1 adenosine receptor ligand [3H]8-cyclopentyl-1,3-dipropylxanthine in coronary smooth muscle. Circulation research. 1995;77:194–198. doi: 10.1161/01.res.77.1.194. [DOI] [PubMed] [Google Scholar]
- 2.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handbook of experimental pharmacology. 2009;163:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musser B, Morgan ME, Leid M, Murray TF, Linden J, Vestal RE. Species comparison of adenosine and beta-adrenoceptors in mammalian atrial and ventricular myocardium. European journal of pharmacology. 1993;246:105–111. doi: 10.1016/0922-4106(93)90086-o. [DOI] [PubMed] [Google Scholar]
- 4.Nell PG, Albrecht-Kupper B. The adenosine A1 receptor and its ligands. Progress in medicinal chemistry. 2009;47:163–201. doi: 10.1016/S0079-6468(08)00204-X. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht-Kupper BE, Leineweber K, Nell PG. Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic signalling. 2012;8(Suppl 1):91–99. doi: 10.1007/s11302-011-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellenbogen KA, O’Neill G, Prystowsky EN, Camm JA, Meng L, Lieu HD, Jerling M, Shreeniwas R, Belardinelli L, Wolff AA. Trial to evaluate the management of paroxysmal supraventricular tachycardia during an electrophysiology study with tecadenoson. Circulation. 2005;111:3202–3208. doi: 10.1161/CIRCULATIONAHA.104.510982. [DOI] [PubMed] [Google Scholar]
- 7.Peterman C, Sanoski CA. Tecadenoson: a novel, selective A1 adenosine receptor agonist. Cardiology in review. 2005;13:315–321. doi: 10.1097/01.crd.0000181621.84565.9d. [DOI] [PubMed] [Google Scholar]
- 8.Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handbook of experimental pharmacology. 2009;193:25–58. doi: 10.1007/978-3-540-89615-9_2. [DOI] [PubMed] [Google Scholar]
- 9.Tendera M, Gaszewska-Zurek E, Parma Z, Ponikowski P, Jankowska E, Kawecka-Jaszcz K, Czarnecka D, Krzeminska-Pakula M, Bednarkiewicz Z, Sosnowski M, Ochan Kilama M, Agrawal R. The new oral adenosine A1 receptor agonist capadenoson in male patients with stable angina. Clinical research in cardiology: official journal of the German Cardiac Society. 2012;101:585–591. doi: 10.1007/s00392-012-0430-8. [DOI] [PubMed] [Google Scholar]
- 10.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. The American journal of physiology. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 11.Dodge HT, Sandler H, Baxley WA, Hawley RR. Usefulness and limitations of radiographic methods for determining left ventricular volume. The American journal of cardiology. 1966;18:10–24. doi: 10.1016/0002-9149(66)90191-3. [DOI] [PubMed] [Google Scholar]
- 12.Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M. Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. The American journal of cardiology. 2007;99:41A–46A. doi: 10.1016/j.amjcard.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi S, Guerrero M, Wang M, Ilsar I, Sabbah MS, Gupta RC, Sabbah HN. Myocardial transfection with naked DNA plasmid encoding hepatocyte growth factor prevents the progression of heart failure in dogs. American journal of physiology Heart and circulatory physiology. 2011;300:H1501–H1509. doi: 10.1152/ajpheart.00636.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. The Journal of clinical investigation. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation. 2000;102:1990–1995. doi: 10.1161/01.cir.102.16.1990. [DOI] [PubMed] [Google Scholar]
- 16.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 17.Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992;85:893–904. doi: 10.1161/01.cir.85.3.893. [DOI] [PubMed] [Google Scholar]
- 18.Sommerschild HT, Kirkeboen KA. Adenosine and cardioprotection during ischaemia and reperfusion--an overview. Acta anaesthesiologica Scandinavica. 2000;44:1038–1055. doi: 10.1034/j.1399-6576.2000.440903.x. [DOI] [PubMed] [Google Scholar]
- 19.Olafsson B, Forman MB, Puett DW, Pou A, Cates CU, Friesinger GC, Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation. 1987;76:1135–1145. doi: 10.1161/01.cir.76.5.1135. [DOI] [PubMed] [Google Scholar]
- 20.Liang B, Stewart D, Jacobson K. Adenosine A1 and A3 receptors: distinct cardioprotection. Drug Development Research. 2001;52:366–378. [Google Scholar]
- 21.DeWitt DF, Wangler RD, Thompson CI, Sparks HV., Jr Phasic release of adenosine during steady state metabolic stimulation in the isolated guinea pig heart. Circulation research. 1983;53:636–643. doi: 10.1161/01.res.53.5.636. [DOI] [PubMed] [Google Scholar]
- 22.Dobson JG., Jr Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circulation research. 1983;52:151–160. doi: 10.1161/01.res.52.2.151. [DOI] [PubMed] [Google Scholar]
- 23.Richardt G, Waas W, Kranzhofer R, Mayer E, Schomig A. Adenosine inhibits exocytotic release of endogenous noradrenaline in rat heart: a protective mechanism in early myocardial ischemia. Circulation research. 1987;61:117–123. doi: 10.1161/01.res.61.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Bott-Flugel L, Bernshausen A, Schneider H, Luppa P, Zimmermann K, Albrecht-Kupper B, Kast R, Laugwitz KL, Ehmke H, Knorr A, Seyfarth M. Selective attenuation of norepinephrine release and stress-induced heart rate increase by partial adenosine A1 agonism. PloS one. 2011;6:e18048. doi: 10.1371/journal.pone.0018048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation. 1994;89:2852–2859. doi: 10.1161/01.cir.89.6.2852. [DOI] [PubMed] [Google Scholar]
- 26.Mauser M, Hoffmeister HM, Nienaber C, Schaper W. Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circulation research. 1985;56:220–230. doi: 10.1161/01.res.56.2.220. [DOI] [PubMed] [Google Scholar]
- 27.Reibel DK, Rovetto MJ. Myocardial adenosine salvage rates and restoration of ATP content following ischemia. The American journal of physiology. 1979;237:H247–H252. doi: 10.1152/ajpheart.1979.237.2.H247. [DOI] [PubMed] [Google Scholar]
- 28.Schwabe U, Schonhofer PS, Ebert R. Facilitation by adenosine of the action of insulin on the accumulation of adenosine 3′:5′-monophosphate, lipolysis, and glucose oxidation in isolated fat cells. European journal of biochemistry/FEBS. 1974;46:537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- 29.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. Journal of molecular and cellular cardiology. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 30.Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. Journal of molecular and cellular cardiology. 1998;30:1757–1762. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 31.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. Journal of molecular and cellular cardiology. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. Journal of molecular and cellular cardiology. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 33.Laskowski KR, Russell RR., 3rd Uncoupling proteins in heart failure. Current heart failure reports. 2008;5:75–79. doi: 10.1007/s11897-008-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Bioscience reports. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 35.Noma T, Nishiyama A, Mizushige K, Murakami K, Tsuji T, Kohno M, Rahman M, Fukui T, Abe Y, Kimura S. Possible role of uncoupling protein in regulation of myocardial energy metabolism in aortic regurgitation model rats. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15:1206–1208. doi: 10.1096/fj.000569fje. [DOI] [PubMed] [Google Scholar]
- 36.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 37.Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravec CS, Davies PJ, Frazier OH, Taegtmeyer H. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–209. doi: 10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 38.Young ME, Patil S, Ying J, Depre C, Ahuja HS, Shipley GL, Stepkowski SM, Davies PJ, Taegtmeyer H. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15:833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 39.Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR., 3rd Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer chemotherapy and pharmacology. 2011;67:1381–1388. doi: 10.1007/s00280-010-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodyak N, Rigor DL, Chen YS, Han Y, Bisping E, Pu WT, Kang PM. Uncoupling protein 2 modulates cell viability in adult rat cardiomyocytes. American journal of physiology Heart and circulatory physiology. 2007;293:H829–835. doi: 10.1152/ajpheart.01409.2006. [DOI] [PubMed] [Google Scholar]
- 41.Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovascular research. 2006;72:210–219. doi: 10.1016/j.cardiores.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Sabbah HN. Apoptotic cell death in heart failure. Cardiovascular research. 2000;45:704–712. doi: 10.1016/s0008-6363(99)00348-x. [DOI] [PubMed] [Google Scholar]
- 43.Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. The American journal of pathology. 1996;148:141–149. [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang F, Huang YS, Zhang DX, Chu ZG, Zhang JP, Zhang Q. Adenosine A1 receptor activation reduces opening of mitochondrial permeability transition pores in hypoxic cardiomyocytes. Clinical and experimental pharmacology & physiology. 2010;37:343–349. doi: 10.1111/j.1440-1681.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 45.Sack MN, Kelly DP. The energy substrate switch during development of heart failure: gene regulatory mechanisms (Review) International journal of molecular medicine. 1998;1:17–24. doi: 10.3892/ijmm.1.1.17. [DOI] [PubMed] [Google Scholar]
- 46.Yu GAM, Makkinje A, Yi-Chun L, Tod G. CPT-1 gene expression and fatty acid oxidation are regulated by p38 MAP kinase through MEF2 factors. Circulation. 2000;102 (II):7–8. [Google Scholar]
- 47.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Molecular and cellular biochemistry. 1995;147:29–34. doi: 10.1007/BF00944780. [DOI] [PubMed] [Google Scholar]
- 48.Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart failure reviews. 2000;5:131–138. doi: 10.1023/A:1009880720032. [DOI] [PubMed] [Google Scholar]
- 49.Sharov VG, Todor A, Suzuki G, Morita H, Tanhehco EJ, Sabbah HN. Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1. European journal of heart failure. 2003;5:121–129. doi: 10.1016/s1388-9842(02)00254-4. [DOI] [PubMed] [Google Scholar]
- 50.Todor A, Sharov VG, Tanhehco EJ, Silverman N, Bernabei A, Sabbah HN. Hypoxia-induced cleavage of caspase-3 and DFF45/ICAD in human failed cardiomyocytes. American journal of physiology Heart and circulatory physiology. 2002;283:H990–H995. doi: 10.1152/ajpheart.01003.2001. [DOI] [PubMed] [Google Scholar]