Abstract
Background:
An increased body mass index (BMI) is significantly associated with favourable prognosis in renal cell carcinoma (RCC). This study investigated the associations among sex, BMI, and prognosis in clear cell RCC patients.
Methods:
We retrospectively analysed 435 patients with clear cell RCC who underwent a nephrectomy. The associations among sex, BMI, clinicopathologic factors, and cancer-specific survival (CSS) were analysed.
Results:
As a continuous variable, increased BMI was associated with higher CSS rate by univariate analysis in the whole population (hazard ratio, 0.888 per kg m–2; 95% confidence interval, 0.803–0.982; P=0.021). A sub-population analysis by sex demonstrated that BMI was significantly associated with CSS in men (P=0.004) but not in women (P=0.725). Multivariate analysis revealed BMI to be an independent predictor of CSS in only men.
Conclusion:
Body mass index was significantly associated with clear cell RCC prognosis. However, the clinical value of BMI may be different between men and women.
Keywords: body mass index, cancer-specific survival, clear cell carcinoma, renal cell carcinoma
Renal cell carcinoma (RCC) is the most common malignancy of the kidney, accounting for 2%–3% of all adult malignancies (Siegel et al, 2011). The risk factors for the development of RCC include obesity, hypertension, and cigarette smoking (Chow et al, 2010). Increased body mass index (BMI), a commonly used index of obesity, is strongly associated with the development of RCC (Renehan et al, 2008). Paradoxically, increased BMI has also been associated with a favourable prognosis in patients with RCC (Donat et al, 2006; Parker et al, 2006; Jeon et al, 2010; Choi et al, 2013).
Although several studies have indicated that women with RCC have a significantly lower BMI than men with RCC (Donat et al, 2006; Parker et al, 2006; Jeon et al, 2010), few studies have investigated the sex-related differences for the prognostic implications of BMI. In addition, several studies have demonstrated that women with RCC have better prognoses than men with RCC (Aron et al, 2008; Woldrich et al, 2008; May et al, 2013). However, the better prognosis in women seems to be inconsistent with the relatively lower BMI in women with RCC than men with RCC. Thus, we assumed that there might be a sex-related difference in the prognostic value of BMI in patients with RCC. The aim of this study was to investigate the associations among sex, BMI, and prognosis in patients with RCC. As recent studies have reported obesity as a stronger risk factor for the clear cell subtype of RCC, compared with other subtypes (Purdue et al, 2013), only patients with clear cell RCC were examined.
Materials and methods
Patients
This study was conducted according to the ethics guidelines for clinical studies of the Ministry of Health, Labour and Welfare in Japan and was approved by the ethics committee of our facility. The medical charts of 482 consecutive patients who had undergone nephrectomy at Tokyo Medical University Hospital between 1990 and 2009 were retrospectively reviewed; 435 patients, identified as having clear cell RCC using the seventh edition of the tumour-node-metastasis staging system (Edge et al, 2010), were included in the study. Sixty patients underwent partial nephrectomy and 375 underwent total nephrectomy. At our hospital, extended lymphadenectomy is generally not included in routine nephrectomies, but regional lymph node dissection was performed when enlarged or palpable lymph nodes were recognised. The patients were weighed and measured by medical staff at the time of their hospitalisation, before nephrectomy. Body mass index was calculated as weight (kg) divided by the square of the height (m2), and expressed as kg m–2. All the patients underwent a physical examination, blood evaluation, and chest radiography 3 months, postoperatively, and were examined by computed tomography at 6 months, postoperatively; other radiologic studies were conducted when necessary. If the disease recurred, most patients, before March 2008, received cytokine therapy (interferon alpha or interleukin-2). After the approval of the first targeted molecular agents in April 2008, patients received either cytokine therapy or targeted molecular therapy. Typically, the patients underwent their follow-up examinations at our hospital. Follow-up assessment ended in September 2012. The mean follow-up period was 71 months (range, 1–285 months). Over the course of the investigation, 73 patients had recurrence and 53 died of RCC.
Statistical analyses
The end point of this study was cancer-specific survival (CSS). The CSS period was calculated from the date of the nephrectomy to the date of death from the cancer.
The patients were classified using Asian-specific BMI cutoff values (underweight to normal weight, BMI<23 kg m–2; overweight, BMI⩾23 to <25 kg m–2; obese, BMI⩾25 kg m–2; Choi et al, 2013). The variables for the different BMI groups were compared using Pearson's χ2 test and analysis of variance; trends were analysed using the Cochran–Armitage test. The correlations among the continuous variables were assessed using Spearman's rank analysis. A Cox proportional hazards model was developed to assess the association between CSS and the clinicopathologic variables. Body mass index was included as both a continuous and categorical variable in the Cox regression analyses. Cox multivariate analyses were performed using a forward stepwise variable selection procedure, and survival curves were constructed using the Kaplan–Meier method with the log-rank test. All P-values were two-tailed, and P-values<0.05 were considered statistically significant. All statistical analyses were performed using Stata software, version 11 (StataCorp, College Station, TX, USA).
Results
Associations among sex, BMI, and clinicopathologic factors
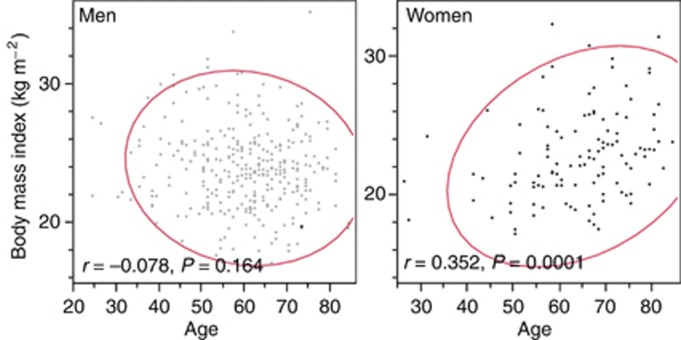
The incidence of RCC demonstrated a male-to-female ratio of 2.7 : 1 for the whole cohort. However, this ratio decreased as the number of women with RCC significantly increased with advancing age (Cochran–Armitage test, P=0.010). Men with RCC were found to have a significantly higher mean preoperative BMI (24.0±2.9 kg m–2) than women with RCC (22.9±3.3 kg m–2; P<0.001; Supplementary Table 1). Interestingly, whereas BMI decreased, although not significantly, with increasing age in men (r=−0.078, P=0.164), it significantly increased in women (r=0.352, P<0.001; Figure 1). The incidence of incidentally detected tumours, low nuclear-grade tumours, and low C-reactive protein (CRP) levels, which are frequently observed in obese patients (Supplementary Table 2), were examined by sub-analyses by sex. These sub-analyses identified a significant, positive association between BMI and the incidence of both incidentally detected and low nuclear-grade tumours in obese men, only (Supplementary Tables 3).
Figure 1.
Correlation between age and body mass index (BMI). BMI decreased, although not significantly, with increasing age in men (r=−0.078, P=0.164), but significantly increased with advancing age in women (r=0.352, P=0.0001).
Survival analyses
Whole population analyses
A significant difference in the CSS, by sex, was not observed. The 5-year CSS rate in obese patients was significantly higher than in patients classified into the overweight and underweight to normal weight categories (Supplementary Figure 1). The univariate analyses showed that age; presentation mode; tumour size, pT, pN, and M stage; nuclear grade; microscopic venous invasion; Eastern Cooperative Oncology Group Performance Status (ECOG-PS); CRP level; and BMI were significantly associated with CSS (Table 1). Increased BMI was associated with a higher CSS rate (hazard ratio (HR), 0.888 per kg m–2; 95% confidence interval (95% CI), 0.803–0.982; P=0.021).
Table 1. The results of univariate and multivariate analyses.
| |
|
Whole population |
Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
||||||
| HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value | ||
| Age |
Continuous |
1.034 |
0.012 |
|
|
1.034 |
0.040 |
|
|
1.033 |
0.193 |
|
|
| Gender |
M vs F |
1.404 |
0.242 |
|
|
|
|
|
|
|
|
|
|
| Presentation |
Incidental vs symptomatic |
4.241 |
<0.001 |
|
|
4.597 |
<0.001 |
|
|
3.211 |
0.020 |
|
|
| Tumour size |
Continuous |
1.259 |
<0.001 |
|
|
1.299 |
<0.001 |
|
|
1.198 |
0.001 |
|
|
| pT stage |
1 vs 2 vs 3 vs 4 |
3.285 |
<0.001 |
2.261 (1.652–3.096) |
<0.001 |
3.485 |
<0.001 |
2.418 (1.644–3.556) |
<0.001 |
2.560 |
0.001 |
2.718 (1.342–5.507) |
0.005 |
| pN stage |
0 or x vs positive |
9.885 |
<0.001 |
|
|
9.783 |
<0.001 |
|
|
8.894 |
0.004 |
|
|
| M stage |
No vs yes |
26.431 |
<0.001 |
8.037 (3.729–17.325) |
<0.001 |
25.113 |
<0.001 |
11.100 (5.100–24.161) |
<0.001 |
26.694 |
<0.001 |
23.767 (5.288–106.819) |
<0.001 |
| Nephrectomy |
Partial vs total |
7.331 |
0.005 |
|
|
565543.080 |
0.001 |
|
|
1.644 |
0.605 |
|
|
| Nuclear grade |
⩽2 vs ⩾3 |
12.066 |
<0.001 |
5.051 (2.601–9.808) |
<0.001 |
14.830 |
<0.001 |
6.687 (2.929–15.265) |
<0.001 |
8.039 |
<0.001 |
5.162 (1.445–18.437) |
0.012 |
| MVI |
No vs yes |
6.005 |
<0.001 |
|
|
6.415 |
<0.001 |
|
|
4.648 |
0.002 |
|
|
| ECOG-PS |
0 vs ⩾1 |
14.046 |
<0.001 |
2.911 (1.432–5.917) |
<0.001 |
13.408 |
<0.001 |
|
|
14.082 |
<0.001 |
10.908 (3.327–35.762) |
<0.001 |
| CRP (mg dl–1) |
<0.3 vs ⩾0.3 |
5.743 |
<0.001 |
|
|
6.323 |
<0.001 |
|
|
4.301 |
<0.001 |
|
|
| BMI | Continuous | 0.888 | 0.021 | 0.822 | 0.004 | 0.804 (0.677–0.955) | 0.013 | 1.028 | 0.725 | ||||
Abbreviations: BMI-body mass index; CI=confidence interval; CRP, C-reactive protein; ECOG-PS=Eastern Cooperative Oncology Group Performance Status; F=female; HR=Hazard ratio; M=male; MVI=microscopic venous invasion.
Sub-population analyses by sex
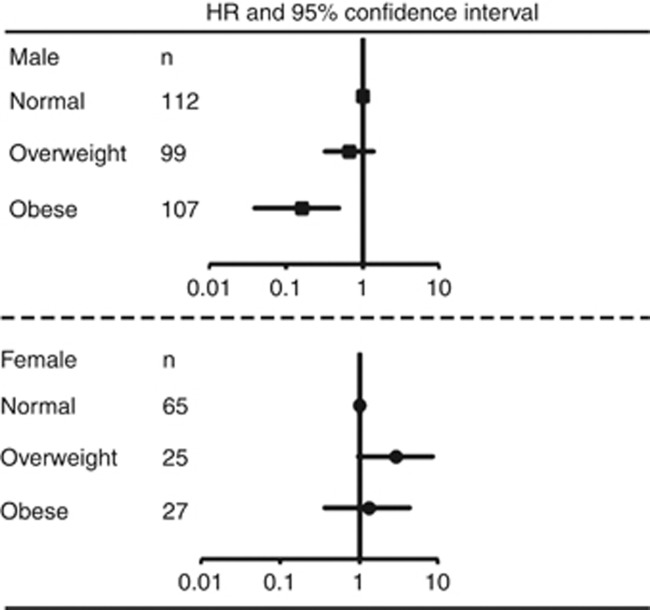
In men with RCC, the univariate analyses showed that all variables were significantly associated with CSS. The multivariate analysis indicated that BMI as well as T stage, M stage, and nuclear grade were independent predictors of CSS in men with RCC. Conversely, there was no significant association between BMI and CSS in women with RCC (P=0.725; Table 1). The multivariate analysis indicated that pT stage, M stage, nuclear grade, and ECOG-PS were independent predictors of CSS in women with RCC. Further multivariate analyses, including using BMI as a categorical variable instead of a continuous variable, demonstrated that BMI was an independent predictor of CSS only in men with RCC (Supplementary Table 4). The HRs and 95% CIs for CSS, by BMI category, for men and women are shown in Figure 2. There was an important sex-related difference in the prognostic value of the patients' BMI category.
Figure 2.
Hazard ratios (HRs) and 95% confidence intervals (95% CIs) for cancer-specific survival by body mass index category. Patients with a BMI<23 kg m–2 were used as a reference; the HR and 95% CI of overweight (BMI, 23–25 kg m–2) and obesity (BMI⩾25 kg m–2) are plotted.
Discussion
This study demonstrated that increased BMI was significantly associated with a favourable prognosis in patients with clear cell RCC and that the mean BMI was significantly lower in women than in men with RCC. In addition, BMI was significantly associated with cancer prognosis in men, but not in women, with RCC.
To date, only two studies have included a description of the associations among sex, BMI, and RCC prognosis. In one study, Donat et al (2006) retrospectively reviewed the records of 1137 patients with RCC and reported that there was a trend toward better survival with increasing BMI. They also reported that there was no significant survival difference between sexes, based on BMI. Conversely, Reeves et al (2007) reported the results of the Million Women Study, which was a large cohort study conducted in the United Kingdom, investigating cancer incidence and mortality in relation to BMI. Their study demonstrated that increased BMI was associated with increased incidence and mortality associated with kidney cancer in women.
This study demonstrated that increased BMI was associated with incidental and low nuclear-grade tumours in men. Previous studies that have demonstrated an association of increased BMI with a favourable cancer prognosis have also reported an association between increased BMI and low-stage and low-grade tumours (Parker et al, 2006; Jeon et al, 2010); however, the underlying mechanism of the association remains unclear. In this study, there was no significant association between BMI and clinicopathologic factors, other than the CRP level in women. This lack of a significant association may be a reason for the sex-related difference in the association of BMI with RCC prognosis.
We also demonstrated that BMI increased with advancing age in women with RCC. In addition, the proportion of female patients increased with advancing age, as reported previously (Guðmundsson et al, 2011; Hew et al, 2012). It has been reported that menopause is associated with a progressive gain in body weight and an increased tendency for central adiposity with advancing age (Astrup, 1999). The change in hormones after menopause may affect the increased incidence of RCC with increasing age in women. These features, specific to women, may also contribute to the difference in the prognostic value of BMI between the sexes. Meanwhile, two previous studies by Donat et al (2006) and Reeves et al (2007) and this study showed a different result in relation to the association of sex with prognostic value of BMI in kidney cancer patients. Thus, further studies are needed to clarify the nature of this association.
Although this study provides important insights into the clinical implications of BMI in clear cell RCC prognosis, it has several limitations. First, this is a retrospective analysis of data collected from a single institution; hence, the number of cases is relatively small and the ability to extrapolate to a more general population is limited. Second, although the male-to-female ratio in this study is comparable to those in other Japanese studies (Iimura et al, 2009; Kanao et al, 2009), a male predominance appears to be more prominent in Japan than in Western countries (Donat et al, 2006; Hew et al, 2012). In addition, as shown in this study, the number of patients with metastatic disease at the time of diagnosis is relatively small among Japanese patients compared with Western patients (Iimura et al, 2009; Kanao et al, 2009). Thus, there may be racial or sociogeographic differences in the baseline characteristics between countries or regions. Third, body weight loss and preoperative nutritional status are also reported to be important prognostic factors in RCC (Brookman-May et al, 2011; Morgan et al, 2011). These factors have also been reported to be more important than BMI for determining the prognosis of RCC. However, this study could not include these factors because this information was not available for part of the cohort. Fourth, the Asian-specific BMI cutoff value is different from that for European populations. In addition, the proportion of RCC patients with a BMI⩾25 kg m–2 differs between European and Asian studies (Donat et al, 2006; Parker et al, 2006). Further, prospective studies examining the effects of racial and sociogeographic differences are needed to clarify the association of BMI with a sex-based RCC prognosis. On the other hand, to our knowledge, this is the first study to demonstrate sex differences in the prognostic value of BMI for RCC patients. Therefore, we believe that this study provides important insights into the study of BMI in patients with clear cell RCC.
Conclusions
Body mass index was significantly associated with the prognosis for patients with clear cell RCC; however, a sub-population analysis by sex demonstrated that BMI was significantly associated with CSS in men, but not in women. The clinical value of BMI appears to differ between men and women with clear cell RCC.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–140. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Astrup A. Physical activity and weight gain and fat distribution changes with menopause: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S564–S567. doi: 10.1097/00005768-199911001-00013. [DOI] [PubMed] [Google Scholar]
- Brookman-May S, Kendel F, Hoschke B, Wieland WF, Burger M, Rossler W, May M. Impact of body mass index and weight loss on cancer-specific and overall survival in patients with surgically resected renal cell carcinoma. Scand J Urol Nephrol. 2011;45:5–14. doi: 10.3109/00365599.2010.515610. [DOI] [PubMed] [Google Scholar]
- Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–634. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat SM, Salzhauer EW, Mitra N, Yanke BV, Snyder ME, Russo P. Impact of body mass index on survival of patients with surgically treated renal cell carcinoma. J Urol. 2006;175:46–52. doi: 10.1016/S0022-5347(05)00054-6. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A.editors. (2010AJCC Cancer Staging Manual7th ed.Springer-Verlag: New York, NY, USA [Google Scholar]
- Guðmundsson E, Hellborg H, Lundstam S, Erikson S, Ljungberg B. Metastatic potential in renal cell carcinomas ⩽7 cm: Swedish Kidney Cancer Quality Register data. Eur Urol. 2011;60:975–982. doi: 10.1016/j.eururo.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Hew MN, Zonneveld R, Kümmerlin IP, Opondo D, de la Rosette JJ. Age and gender related differences in renal cell carcinoma in a European cohort. J Urol. 2012;188:33–38. doi: 10.1016/j.juro.2012.02.2573. [DOI] [PubMed] [Google Scholar]
- Iimura Y, Saito K, Fujii Y, Kumagai J, Kawakami S, Komai Y, Yonese J, Fukui I, Kihara K. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C-reactive protein and TNM classification: the TNM-C score. J Urol. 2009;181:1004–1012. doi: 10.1016/j.juro.2008.10.156. [DOI] [PubMed] [Google Scholar]
- Jeon HG, Jeong IG, Lee JH, Lee CJ, Kwak C, Kim HH, Lee SE, Lee E. Prognostic value of body mass index in Korean patients with renal cell carcinoma. J Urol. 2010;183:448–454. doi: 10.1016/j.juro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Kanao K, Mizuno R, Kikuchi E, Miyajima A, Nakagawa K, Ohigashi T, Nakashima J, Oya M. Preoperative prognostic nomogram (probability table) for renal cell carcinoma based on TNM classification. J Urol. 2009;181:480–485. doi: 10.1016/j.juro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- May M, Aziz A, Zigeuner R, Chromecki t, Cindolo L, Schips L, De Cobelli O, Rocco B, De Nuizio C, Tubaro A, Coman I, Truss M, Dalpiaz O, Hoschke B, Gilfrich C, Feciche B, Stoltze A, Fenske F, Fritsche HM, Figenshau RS, Madison K, Sanchez-Chapado M, Martin MD, Salzano L, Lotrecchiano G, Joniau S, Waidelich R, Stief C, Brookman-May S, Members of the CORONA project the Young Academic Urologists Renal Cancer Group 2013Gender differences in clinicopathological features and survival in surgically treated patients with renal cell carcinoma: an analysis of the multicenter CORONA database World J UrolEpub ahead of print. [DOI] [PubMed]
- Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JA, Jr, Clark PE. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923–928. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AS, Lohse CM, Cheville JC, Thiel DD, Leibovich BC, Blute ML. Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma. Urology. 2006;68:741–746. doi: 10.1016/j.urology.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Moore LE, Merino MJ, Boffetta P, Colt JS, Schwartz KL, Bencko V, Davis FG, Graubard BI, Janout V, Ruterbusch JJ, Beebe-Dimmer J, Cote ML, Shuch B, Mates D, Hofmann JN, Foretova L, Rothman N, Szeszenia-Dabrowska N, Matveev V, Wacholder S, Zaridze D, Linehan WM, Prennan P, Chow WH. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer. 2013;132:2640–2647. doi: 10.1002/ijc.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ. Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993–2004. J Urol. 2008;179:1709–1713. doi: 10.1016/j.juro.2008.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.