Abstract
Background:
It is unknown whether comorbidity interacts with colorectal cancer (CRC) to increase the rate of mortality beyond that explained by the independent effects of CRC and comorbid conditions.
Methods:
We conducted a cohort study (1995–2010) of all Danish CRC patients (n=56 963), and five times as many persons from the general population (n=271 670) matched by age, gender, and specific comorbidities. To analyse comorbidity, we used the Charlson Comorbidity Index (CCI) scores. We estimated standardised mortality rates per 1000 person-years, and calculated interaction contrasts as a measure of the excess mortality rate not explained by the independent effects of CRC or comorbidities.
Results:
Among CRC patients with a CCI score=1, the 0–1 year mortality rate was 415 out of 1000 person-years (95% confidence interval (CI): 401, 430) and the interaction accounted for 9.3% of this rate (interaction contrast=39 out of 1000 person-years, 95% CI: 22, 55). For patients with a CCI score of 4 or more, the interaction accounted for 34% of the mortality (interaction contrast=262 out of 1000 person-years, 95% CI: 215, 310). The interaction between CRC and comorbidities had limited influence on mortality beyond 1 year after diagnosis.
Conclusion:
Successful treatment of the comorbidity is pivotal and may reduce the mortality attributable to comorbidity itself, and also the mortality attributable to the interaction.
Keywords: biological interaction, colorectal neoplasm, epidemiology, survival, synergy
With a lifetime risk of ∼5%, colorectal cancer (CRC) is the second most common malignancy in the western world (Ingle and Limburg, 2007; Ferlay et al, 2010). As the disease primarily occurs in patients over the age of 65 years, who are likely to suffer from other chronic diseases, as many as one-third of newly diagnosed CRC patients are burdened by severe coexisting diseases (i.e., comorbidities), some of which are associated with increased CRC risk (e.g., diabetes; Yancik et al, 1998; Iversen et al, 2009; Jorgensen et al, 2012).
Even in the absence of comorbidities, CRC is associated with 5-year survival of only 40–50%. However, in the presence of a high comorbidity burden, defined for instance as a Charlson Comorbidity Index (CCI) score ⩾3, 5-year survival is as low as 20% (Iversen et al, 2009). In a study of nearly 30 000 American CRC patients over the age of 67 years at diagnosis investigating population-attributable risks (and therefore specific for this US population), ∼9% of deaths were attributable to congestive heart failure, 5% to chronic obstructive pulmonary disease, and nearly 4% to diabetes (Gross et al, 2006a). The study also confirmed that multimorbidity was common and had a substantial effect on CRC survival. Several other studies have shown that CRC patients burdened by comorbidity have higher mortality than CRC patients without coexisting disease (De Marco et al, 2000; Rieker et al, 2002; Ouellette et al, 2004; Read et al, 2004; Janssen-Heijnen et al, 2005; Lemmens et al, 2005; Gross et al, 2006b; Janssen-Heijnen et al, 2007; Iversen et al, 2009; Sarfati et al, 2009; Panis et al, 2011; Sarfati et al, 2011; Jorgensen et al, 2012).
To our knowledge, however, no study of CRC mortality has (1) included a comparison cohort free of CRC and (2) accounted for comorbidity. Therefore, it is not known whether comorbidity interacts with CRC to increase the rate of mortality beyond that explained by CRC and comorbidity acting independently. Such information is needed to improve our biological understanding of the influence of comorbidity on CRC mortality, may be helpful in clinical practice, and would contribute to improving outcomes after CRC. On this basis, we conducted a nationwide cohort study of all Danish CRC patients diagnosed during a recent 16-year period and a matched population-based comparison cohort free of CRC, in order to study the interaction between comorbidity and CRC, and subsequent risk of death.
Materials and methods
We conducted this cohort study in the setting of the entire Danish population (accumulated 6.9 million people during the 1995–2010 study period). The Danish healthcare system provides tax-supported healthcare to all Danish residents. The unique civil registration number assigned to all Danes at birth or upon immigration by the Civil Registration System allows unambiguous linkage between databases (Frank, 2000; Pedersen, 2011). The Civil Registration System also tracks vital status and the residence of all Danish citizens, and is updated daily.
The study was approved by the Danish Data Protection Agency.
The CRC cohort
We used the Danish Cancer Registry to identify all patients diagnosed with incident CRC between 1 January 1995 and 31 December 2010 (Storm et al, 1997; Gjerstorff, 2011). The Danish Cancer Registry maintains records on all incident malignant neoplasms diagnosed in Denmark since 1943, including patients' civil registration number, month and year of cancer diagnosis, cancer type according to the International Classification of Disease (ICD), 10th revision (ICD-10), and tumour spread at diagnosis (see Appendix for ICD-10 codes for CRC).
Population comparison cohort
We used the Danish National Registry of Patients and Civil Registration System to match each CRC patient with five persons from the general population, who were alive and were without a CRC diagnosis as of the CRC patient's diagnosis date (index date). Matching criteria were age (5-year intervals), gender, and history of the comorbid diseases included in the CCI (see below and Appendix) (Andersen et al, 1999; Pedersen, 2011). The Danish National Registry of Patients has recorded all non-psychiatric hospitalisations in Denmark since 1977 and hospital outpatient contacts since 1995. It recorded dates of admission and discharge, treatment and procedure codes, and up to 20 diagnoses coded by physicians according to ICD-8 from 1977 to 1993 and ICD-10 since 1994. In an event in which an individual from the comparison cohort developed CRC during the study period, follow-up time was terminated and the individual joined the CRC cohort. In total, 4895 (1.8%) subjects from the comparison cohort were later diagnosed with CRC.
Comorbidity
On the basis of the Danish National Registry of Patients records dating to 1977, we defined comorbidities according to diagnoses of the conditions in the CCI, excluding CRC. The CCI's scoring system assigns weights between one and six to a range of diseases (see Appendix). In addition to the original conditions in the CCI, we also included a prior diagnosis of atrial fibrillation/flutter and obesity (both assigned a weight of one). The CCI disease groups were not only considered individually for matching and analysis, but also as the components of a summed, aggregate score that we classified as follows: score of 0 (no comorbidity), score of 1 (low comorbidity), score of 2–3 (moderate comorbidity), and score of 4 or more (high comorbidity; Thygesen et al, 2011).
Statistical analysis
We calculated the frequency and proportion of persons in the CRC and the matched comparison cohorts within categories of demographic variables and comorbidities. CRC patients and persons matched to them were followed from the index date to the date of death from any cause, emigration, or end of follow-up (31 December 2011), whichever came first. We calculated mortality rates by dividing the number of deaths by total follow-up time for the CRC and matched comparison cohorts. To evaluate short-term and long-term mortality separately, we computed mortality rates between the index date and 365 days (0–1 year) and from 366 days to 5 years (2–5 years). The analysis within strata of follow-up period required that we dissolve the matching, as the age and gender distribution was different among 1-year survivors than among all participants. For all analyses, we standardised the mortality rates to the age and gender distribution of the CRC inception cohort. Furthermore, as a measure of mortality rate ratios, we calculated hazard ratios (HRs) using Cox regression analysis comparing CRC patients with matched persons from the general population, adjusting for age (as a continuous variable), gender, year of index date (1995–1999 vs 2005–2010, 2000–2004 vs 2005–2010), and, in the overall analysis, comorbidity scores.
We computed interaction contrasts to estimate the excess mortality rate in patients with both CRC and comorbid diseases, beyond that expected from the independent effects of these diseases (Greenland et al, 2008). We used standardised rates for this analysis, using the persons without comorbidity from the general population as the reference. Positive interaction contrasts describe the excess mortality rate caused by the interaction (i.e., the synergy between comorbidity and CRC that increases the mortality hazard); a negative interaction contrast would indicate a protective or antagonistic interaction. We also calculated the proportion of the mortality rate that could be explained by the interaction as the interaction contrast divided by the standardised mortality rate of the relevant comorbidity strata.
Analyses were stratified by CRC stage (non-metastatic vs metastatic, see Appendix), age group (0–69, 70–79, and 80+ years), gender, and cancer site (i.e., colon and rectal cancer). We also calculated standardised mortality rates and interaction contrasts restricted to CRC patients without metastatic disease undergoing colorectal surgery, as defined by relevant procedure codes (see Appendix), within 60 days before and 180 days after the diagnosis date, and persons matched to them from the general population. These patients were followed for 30 days after the date of first surgery/index to evaluate the influence of interaction on post-operative mortality. Finally, we calculated standardised mortality rates and interaction contrasts for each individual disease included in the CCI (with the reference group of persons free of any comorbidity, as above).
Results
Characteristics
We identified 56 963 CRC patients and 271 670 persons from the general population, who were matched by age (median age=72 years), gender (men=51%), year of index date, and comorbidity (Table 1). As we were unable to match five persons from the general population to all CRC patients, small differences occurred between the characteristics of the CRC patients and general population comparison cohort. At the aggregated CCI level, however, it was mainly among CRC patients with a high comorbidity burden that these differences were noticeable (CCI score of 4 or more: 4.9% vs 2.8%).
Table 1. Characteristics of CRC patients and a population-based comparison cohort matched by gender, age, year of diagnosis, and comorbidity, Denmark 1995–2010.
| |
CRC cohort |
Population-based comparison cohorta |
||
|---|---|---|---|---|
| Number | % | Number | % | |
|
Sex | ||||
| Female | 27 665 | 49 | 132 537 | 49 |
| Male |
29 298 |
51 |
139 133 |
51 |
|
Age at diagnosis/index (years) | ||||
| 0–59 | 10 285 | 18 | 51 467 | 19 |
| 60–69 | 14 541 | 26 | 70 613 | 26 |
| 70–79 | 18 547 | 33 | 87 444 | 32 |
| 80+ |
13 590 |
24 |
62 146 |
23 |
|
Year of diagnosis/index date | ||||
| 1995–1999 | 16 230 | 29 | 78 136 | 29 |
| 2000–2004 | 17 359 | 31 | 83 088 | 31 |
| 2005–2010 |
23 374 |
41 |
110 446 |
41 |
|
Stage of CRC | ||||
| Non-metastatic | 37 381 | 66 | NA | — |
| Metastatic | 12 687 | 22 | NA | — |
| Unknown |
6895 |
12 |
NA |
— |
|
Cancer location | ||||
| Colon | 37 859 | 67 | NA | — |
| Rectal | 19 014 | 33 | NA | — |
| Colon and rectal |
90 |
0.2 |
NA |
— |
|
Comorbidities included in the CCI | ||||
| Myocardial infarction | 3270 | 5.7 | 13 825 | 5.1 |
| Congestive heart failure | 2783 | 4.9 | 10 652 | 3.9 |
| Peripheral vascular disease | 2322 | 4.1 | 9299 | 3.4 |
| Cerebrovascular disease | 5014 | 8.8 | 21 852 | 8.0 |
| Dementia | 594 | 1.0 | 2297 | 0.8 |
| Chronic pulmonary disease | 4009 | 7.0 | 17 061 | 6.3 |
| Connective tissue disease | 1567 | 2.8 | 6293 | 2.3 |
| Ulcer disease | 3026 | 5.3 | 12 711 | 4.7 |
| Mild liver disease | 478 | 0.8 | 1670 | 0.6 |
| Diabetes type I and II | 3007 | 5.3 | 11 945 | 4.4 |
| Haemiplegia | 100 | 0.2 | 252 | 0.1 |
| Moderate to severe renal disease | 811 | 1.4 | 2557 | 0.9 |
| Diabetes with end-organ failure | 1384 | 2.4 | 4901 | 1.8 |
| Any tumour (excluding CRC) | 5037 | 8.8 | 22 517 | 8.3 |
| Leukemia | 158 | 0.3 | 494 | 0.2 |
| Lymphoma | 295 | 0.5 | 1010 | 0.4 |
| Moderate to severe liver disease | 113 | 0.2 | 311 | 0.1 |
| Metastatic solid tumour | 519 | 0.9 | 1944 | 0.7 |
| AIDS |
10 |
0.0 |
25 |
0.0 |
|
Diseases not originally included in the CCI | ||||
| Atrial fibrillation/flutter | 1164 | 2.0 | 4213 | 1.6 |
| Obesity |
1197 |
2.1 |
4320 |
1.6 |
|
CCI scoresb | ||||
| 0 (No comorbidity) | 34 918 | 61 | 172 041 | 63 |
| 1 (Low comorbidity) | 9747 | 17 | 47 139 | 17 |
| 2–3 (Moderate comorbidity) | 9522 | 17 | 44 788 | 17 |
| 4+ (High comorbidity) | 2776 | 4.9 | 7702 | 2.8 |
Abbreviations: CCI=Charlson Comorbidity Index; CRC=colorectal cancer; NA=not applicable.
Matched on age, gender, year of CRC diagnosis, and the presence of individual comorbidities listed in this table.
The CCI included the 19 diseases from the original index with the addition of atrial fibrillation/flutter and obesity (both assigned one point).
Short-term mortality
CRC patients had a 0–1 year standardised mortality rates of 400 (95% confidence interval (CI): 394, 406) per 1000 person-years, compared with 48 (95% CI: 47, 48) per 1000 person-years in the population comparison cohort, confirming overall higher mortality among patients with CRC (adjusted mortality rate ratio=8.3, 95% CI: 8.1, 8.5). Our findings indicated substantial synergy/interaction between CRC and comorbidities, and the synergy seemed to increase with increasing level of comorbidity (Table 2). For instance, the difference in mortality rates between CRC patients and comparison cohort members was 586 deaths (761−175) per 1000 person-years for those with the highest comorbidity burden (CCI=4). This difference in mortality rates can be assigned to CRC, because the CRC patients and the comparison cohort members are matched on comorbidity. In the group of people without comorbidity (CCI=0), the difference in mortality rates was 324 deaths (351 minus 27) per 1000 person-years. The interaction contrast equals the difference in these two rate differences, 262 deaths (586−324) per 1000 person-years, and represents the excess mortality in individuals with both CRC and severe comorbidity attributable to CRC and the comorbidities affecting mortality synergistically. As the mortality rate for CRC patients with the highest comorbidity burden (CCI=4) was 761 deaths per 1000 person-years, 34% of this rate was due to the synergy.
Table 2. Mortality, MRR, and interaction contrasts for CRC patients compared with persons in a matched population-based comparison cohort, overall and by CCI score.
| CCI score | Cohort | No. of persons | No. of deaths | Person-years | Standardised mortality rates per 1000 person-years (95% CI) | Adjusted MRRsa (95% CI) | Interaction contrast (95% CI) | Proportion of the mortality explained by interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
0–1 Year of follow-up | ||||||||||
| All | CRC | 56 963 | 17 089 | 45 559 | 400 | (394, 406) | 8.3 (8.1, 8.5) | NA | — | — |
| All | Comparison | 271 670 | 11 962 | 265 223 | 48 | (47, 48) | Ref. | NA | — | — |
| 0 | CRC | 34 918 | 8881 | 29 245 | 351 | (343, 359) | 15 (14, 15) | Ref. | — | — |
| 0 | Comparison | 172 041 | 3652 | 170 008 | 27 | (26, 28) | Ref. | — | — | — |
| 1 | CRC | 9747 | 3254 | 7509 | 415 | (401, 430) | 7.4 (7.0, 7.7) | 39 | (22, 55) | 9.4% |
| 1 | Comparison | 47 139 | 2755 | 45 723 | 53 | (51, 55) | Ref. | — | — | — |
| 2–3 | CRC | 9522 | 3548 | 7035 | 489 | (447, 530) | 5.1 (4.9, 5.3) | 79 | (36, 121) | 16% |
| 2–3 | Comparison | 44 788 | 4234 | 42 521 | 86 | (83, 89) | Ref. | — | — | — |
| 4+ | CRC | 2776 | 1406 | 1771 | 761 | (715, 807) | 3.9 (3.7, 4.3) | 262 | (215, 310) | 34% |
| 4+ |
Comparison |
7702 |
1321 |
6971 |
175 |
(165, 185) |
Ref. |
— |
— |
— |
|
Two to 5 years of follow-up | ||||||||||
| All | CRC | 39 862 | 14 274 | 102 813 | 143 | (141, 146) | 3.0 (2.9, 3.0) | NA | — | — |
| All | Comparison | 258 729 | 40 310 | 808 019 | 50 | (49, 50) | Ref. | NA | — | — |
| 0 | CRC | 26 029 | 8606 | 69 909 | 131 | (128 134) | 4.2 (4.1, 4.3) | Ref. | — | — |
| 0 | Comparison | 167 766 | 17 549 | 549 904 | 36 | (36, 37) | Ref. | — | — | — |
| 1 | CRC | 6490 | 2482 | 16 193 | 146 | (140, 152) | 2.3 (2.2, 2.4) | −9.8 | (−17, −3.1) | NA |
| 1 | Comparison | 44 215 | 9585 | 131 313 | 61 | (60, 62) | Ref. | — | — | — |
| 2–3 | CRC | 5973 | 2491 | 13 917 | 172 | (165, 179) | 2.0 (1.9, 2.0) | −3.0 | (−11, 4.9) | NA |
| 2–3 | Comparison | 40 390 | 10 902 | 111 329 | 80 | (79, 82) | Ref. | — | — | — |
| 4+ | CRC | 1370 | 695 | 2793 | 261 | (231, 290) | 1.7 (1.6, 1.8) | 37 | (7.0, 68) | 14% |
| 4+ | Comparison | 6358 | 2274 | 15 473 | 129 | (123, 134) | Ref. | — | — | — |
Abbreviations: CCI=Charlson Comorbidity Index; CI=confidence interval; CRC=colorectal cancer; MRR=mortality rate ratios; NA=not applicable; Ref.=reference.
Adjusted for age, gender, and CRC/index year. For the overall analysis, we also adjusted for CCI scores.
Long-term mortality
Although to a lesser extent than during the first year after diagnosis, CRC remained associated with increased subsequent mortality (overall adjusted 2- to 5-year mortality rate ratio=3.0, 95% CI: 2.9, 3.0). The 2- to 5-year standardised mortality rates increased with higher CCI scores. However, only among CRC patients with a CCI score of 4+ did the mortality increase more than among matched persons in the population comparison cohort, with the interaction accounting for 14% of the mortality rate (Table 2).
Stratified and restricted analyses
Tables 3 and 4 present the standardised mortality rates and interaction contrasts by CRC stage at diagnosis. These results confirm that CRC mortality during the period 0–1 year after diagnosis interacted with comorbidity among CRC patients with both metastatic and non-metastatic disease. For example, among patients with a CCI score of 4+, the interaction accounted for 28% of the mortality rates in patients without metastases and 24% in patients with metastatic spread. Consistent with the overall results, interaction between CRC and comorbidity had less impact on mortality among CRC patients, regardless of non-metastatic and metastatic disease, during the period 2–5 years after the CRC diagnosis (Tables 3 and 4).
Table 3. Mortality, MRR, and interaction contrasts for patients with non-metastatic CRC compared with persons in a matched population-based comparison cohort, by CCI scores.
| CCI score | Cohort | No. of persons | No. of deaths | Person-years | Standardised mortality rates per 1000 person-years (95% CI) | Adjusted MRRsa (95% CI) | Interaction contrast (95% CI) | Proportion of the mortality explained by interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
0–1 Year of follow-up | ||||||||||
| 0 | CRC | 23 428 | 3211 | 21 405 | 177 | (171, 183) | 7.5 (7.1, 7.9) | Ref. | — | — |
| 0 | Comparison | 115 423 | 2382 | 114 121 | 26 | (25, 27) | Ref. | — | — | — |
| 1 | CRC | 6420 | 1407 | 5448 | 241 | (228, 254) | 4.7 (4.4, 5.1) | 40 | (26, 55) | 17% |
| 1 | Comparison | 31 049 | 1728 | 30 161 | 49 | (47, 52) | Ref. | — | — | — |
| 2–3 | CRC | 6004 | 1481 | 4983 | 288 | (251, 326) | 3.1 (2.9, 3.3) | 52 | (14, 90) | 18% |
| 2–3 | Comparison | 28 299 | 2620 | 26 892 | 85 | (82, 88) | Ref. | — | — | — |
| 4+ | CRC | 1529 | 551 | 1145 | 441 | (401, 481) | 2.6 (2.3, 2.9) | 125 | (83, 168) | 28% |
| 4+ |
Comparison |
4237 |
700 |
3850 |
164 |
(151, 177) |
Ref. |
— |
— |
— |
|
Two to 5 years of follow-up | ||||||||||
| 0 | CRC | 20 211 | 5487 | 59 101 | 101 | (98, 104) | 3.2 (3.1, 3.3) | Ref. | — | — |
| 0 | Comparison | 112 638 | 11 875 | 376 876 | 37 | (36, 37) | Ref. | — | — | — |
| 1 | CRC | 5012 | 1673 | 13 586 | 116 | (110, 122) | 1.9 (1.8, 2.0) | −8.4 | (−15, −1.8) | NA |
| 1 | Comparison | 29 219 | 6285 | 88 895 | 60 | (59, 62) | Ref. | — | — | — |
| 2–3 | CRC | 4522 | 1667 | 11 481 | 139 | (132, 146) | 1.6 (1.5, 1.7) | −5.3 | (−13, 2.7) | NA |
| 2–3 | Comparison | 25 581 | 6807 | 72 780 | 80 | (78, 82) | Ref. | — | — | — |
| 4+ | CRC | 978 | 456 | 2190 | 202 | (179, 226) | 1.4 (1.3, 1.6) | 10 | (−14, 35) | 5.0% |
| 4+ | Comparison | 3525 | 1309 | 8708 | 128 | (121, 135) | Ref. | — | — | — |
Abbreviations: CCI=Charlson Comorbidity Index; CI=confidence interval; CRC=colorectal cancer; MRR=mortality rate ratios; NA=not applicable; Ref.=reference.
Adjusted for age, gender, and CRC/index year.
Table 4. Mortality, MRRs, and interaction contrasts for patients with metastatic CRC compared with persons in a matched population-based comparison cohort, by CCI scores.
| CCI score | Cohort | No. of persons | No. of deaths | Person-years | Standardised Mortality rates per 1000 person-years (95% CI) | Adjusted MRRsa (95% CI) | Interaction contrast (95% CI) | Proportion of the mortality explained by interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
0–1 Year of follow-up | ||||||||||
| 0 | CRC | 7956 | 4476 | 5138 | 1023 | (990, 1055) | 53 (49, 58) | Ref. | — | — |
| 0 | Comparison | 39 232 | 681 | 38 819 | 22 | (20, 24) | Ref. | — | — | — |
| 1 | CRC | 2030 | 1313 | 1148 | 1166 | (1102, 1231) | 22 (20, 25) | 116 | (44, 189) | 9.9% |
| 1 | Comparison | 9839 | 528 | 9570 | 49 | (45, 54) | Ref. | — | — | — |
| 2–3 | CRC | 2005 | 1314 | 1093 | 1244 | (1062, 1426) | 13 (12, 15) | 169 | (−16, 354) | 14% |
| 2–3 | Comparison | 9442 | 815 | 9009 | 74 | (69, 80) | Ref. | — | — | — |
| 4+ | CRC | 696 | 515 | 332 | 1548 | (1403, 1693) | 7.9 (6.9, 9.1) | 373 | (224, 523) | 24% |
| 4+ |
Comparison |
2051 |
338 |
1858 |
174 |
(154, 193) |
Ref. |
— |
— |
— |
|
Two to 5 years of follow-up | ||||||||||
| 0 | CRC | 3479 | 2409 | 5035 | 490 | (470, 511) | 22 (21, 23) | Ref. | — | — |
| 0 | Comparison | 38 400 | 3425 | 123 082 | 26 | (25, 27) | Ref. | — | — | — |
| 1 | CRC | 716 | 495 | 1020 | 496 | (450, 542) | 10 (9.2, 11) | −16 | (−67, 35) | NA |
| 1 | Comparison | 9272 | 1792 | 27 385 | 48 | (45, 50) | Ref. | — | — | — |
| 2–3 | CRC | 691 | 478 | 924 | 492 | (444, 540) | 6.3 (5.7, 7.0) | −40 | (−92, 13) | NA |
| 2–3 | Comparison | 8595 | 2241 | 23 084 | 67 | (63, 70) | Ref. | — | — | — |
| 4+ | CRC | 181 | 133 | 233 | 590 | (449, 730) | 4.9 (4.0, 5.9) | 15 | (−127, 158) | 2.5% |
| 4+ | Comparison | 1704 | 529 | 4278 | 110 | (99, 121) | Ref. | — | — | — |
Abbreviations: CCI=Charlson Comorbidity Index; CI=confidence interval; CRC=colorectal cancer; MRR=mortality rate ratios; NA=not applicable; Ref.=reference.
Adjusted for age, gender, and CRC/index year.
For mortality within 0–1 year after the index date, the interaction between CRC and comorbidity was particularly important for the younger age groups (0–69 years). For example, the interaction contrast was 257 per 1000 person-years (95% CI: 176, 338) for CRC patients with a CCI score of 4+, accounting for 45% of the total mortality rate. The interaction contrasts for the older age groups were closer to the overall estimates (Supplementary Table S1). For mortality within 2–5 years, the interaction between CRC and comorbidity was only evident for the age group 0–69 years with a CCI score of 4+, where it accounted for 30% (interaction contrast=74, 95% CI: 19, 128). We observed nearly identical patterns of interaction for colon and rectal cancers (Supplementary Table S2)
Whereas there was no material difference in interaction for men and women in the first 0–1 year, the overall interaction observed for the 2- to 5-year mortality among CRC patients with a CCI score of 4 or more was mainly found in women (interaction contrast=49 per 1000 person-years, 95% CI: 9.4, 89) and to a lesser extent in men (interaction contrast=27 per 1000 person-years, 95% CI: −19, 72).
In patients with non-metastatic CRC undergoing colorectal resection, interaction accounted for 32% of the 30-day post-operative mortality among those with a CCI score of 1 (interaction contrast=310 per 1000 person-years, 95% CI: 210, 410), 34% among those with a CCI score of 2–3 (interaction contrast=369 per 1000 person-years, 95% CI: 261, 478), and 47% among those with a CCI score of 4+ (interaction contrast=745 per 1000 person-years, 95% CI: 486, 1004; Supplementary Table S3).
Individual comorbidities
Table 5 presents standardised mortality rates for CRC patients according to the presence of individual comorbidities. For CRC patients, a variety of comorbidities interacted with CRC to increase mortality during the first 0–1 year following diagnosis, in particular dementia, liver disease, haemiplegia, renal diseases, and leukemia. In contrast, the interactions had limited influence on mortality during the subsequent 2–5 years.
Table 5. Standardised mortality rates (per 1000 person-years) and interaction contrasts for CRC patients compared with persons in a matched population-based comparison cohort, according to the presence of selected comorbid diseases included in the CCI.
| |
0–1 Year of follow-up |
Two to 5 years of follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standardised mortality rates, CRC cohort (95% CI) | Interaction contrasts (95% CI) | %a | Standardised mortality rates, CRC cohort (95% CI) | Interaction contrasts (95% CI) | %a | |||||
| Myocardial infarction |
470 |
(439, 500) |
73 |
(40, 105) |
16% |
155 |
(142, 169) |
−11 |
(−26, 3.1) |
NA |
| Congestive heart failure |
630 |
(588, 672) |
188 |
(144, 231) |
30% |
205 |
(184, 225) |
−2.8 |
(−24, 19) |
NA |
| Peripheral vascular disease |
542 |
(503, 581) |
130 |
(90, 171) |
24% |
195 |
(176, 214) |
10 |
(−9.6, 30) |
5.1% |
| Cerebrovascular disease |
512 |
(487, 537) |
105 |
(78, 131) |
21% |
160 |
(149, 171) |
−17 |
(−29, −5.7) |
NA |
| Dementia |
1010 |
(851, 1169) |
538 |
(378, 698) |
53% |
318 |
(225, 412) |
72 |
(−23, 166) |
23% |
| Chronic pulmonary disease |
566 |
(537, 595) |
145 |
(114, 175) |
26% |
183 |
(169, 197) |
−7.2 |
(−22, 7.4) |
NA |
| Connective tissue disease |
466 |
(424, 508) |
85 |
(42, 127) |
18% |
201 |
(165, 238) |
47 |
(9.8, 84) |
23% |
| Ulcer disease |
490 |
(461, 520) |
94 |
(63, 125) |
19% |
160 |
(144, 177) |
−7.5 |
(−24, 9.4) |
NA |
| Mild liver disease |
767 |
(638, 896) |
277 |
(94, 459) |
36% |
209 |
(168, 250) |
−0.1 |
(−45, 44) |
NA |
| Diabetes type I and II |
505 |
(474, 536) |
105 |
(73, 138) |
21% |
177 |
(163, 191) |
1.3 |
(−13, 16) |
0.7% |
| Haemiplegia |
997 |
(571, 1423) |
617 |
(190, 1044) |
62% |
351 |
(138, 564) |
162 |
(−52, 377) |
46% |
| Moderate to severe renal disease |
644 |
(575, 714) |
208 |
(137, 279) |
32% |
183 |
(155, 211) |
−4.8 |
(−34, 25) |
NA |
| Diabetes with end-organ failure |
546 |
(495, 597) |
134 |
(81, 187) |
25% |
185 |
(164, 206) |
−5.6 |
(−28, 17) |
NA |
| Any tumour (excluding CRC) |
484 |
(454, 515) |
60 |
(28, 91) |
12% |
182 |
(171, 193) |
13 |
(1.1, 25) |
7.1% |
| Leukemia |
778 |
(490, 1065) |
372 |
(84, 661) |
48% |
331 |
(101, 562) |
147 |
(−84, 378) |
44% |
| Lymphoma |
529 |
(417, 641) |
108 |
(−6.2, 222) |
20% |
274 |
(207, 341) |
92 |
(24, 160) |
34% |
| Moderate to severe liver disease |
1266 |
(656, 1877) |
818 |
(205, 1430) |
65% |
207 |
(113, 300) |
−9.9 |
(−108, 89) |
NA |
| Metastatic solid tumour |
945 |
(828, 1062) |
358 |
(237, 478) |
38% |
625 |
(4.0, 1246) |
415 |
(−206, 1036) |
66% |
| AIDS |
3334 |
(−3201, 9869) |
2998 |
(−3537, 9533) |
90% |
202 |
(−143, 548) |
98 |
(−248, 443) |
49% |
|
Diseases not originally included in the CCI | ||||||||||
| Atrial fibrillation/flutter | 450 | (405, 494) | 53 | (6.7, 99) | 12% | 175 | (152, 197) | 5.2 | (−18, 29) | 3.0% |
| Obesity | 485 | (425, 545) | 102 | (41, 163) | 21% | 177 | (153, 202) | 11 | (−16, 37) | 6.2% |
Abbreviations: CCI=Charlson Comorbidity Index; CI=confidence interval; CRC=colorectal cancer; NA=not applicable.
Proportion of the mortality explained by interaction.
Discussion
In this large, nationwide, population-based matched cohort study, we found that comorbidity interacted with CRC to increase mortality, particularly in the first year after diagnosis. The interaction accounted for 9% of the total mortality in patients with low comorbidity (CCI score of 1), but as much as 34% in those with high comorbidity burdens (CCI score of 4+). Nearly the same results were found for men and women, both when CRC patients with either non-metastatic or metastatic disease were evaluated, and for colon and rectal cancers. The interaction seemed particularly important for patients aged 69 years or younger, and was evident for a wide variety of comorbidities in CRC patients. Except for the interaction between CRC and a high comorbidity burden (CCI score of 4+) accounting for 14% of mortality 2–5 years after diagnosis, mortality in this period was not higher than that explained by CRC and comorbidity acting independently. Finally, the interaction between comorbidity and CRC also had substantial impact on the 30-day post-operative mortality.
Our study extends the existing literature by including a population comparison cohort free of CRC, thereby allowing evaluation of the excess mortality caused by the interaction between comorbidity and CRC. Our findings strongly suggest that treatment of comorbidities should be considered an integral part of clinical care for newly diagnosed CRC patients. Successful treatment of comorbidity would reduce the mortality attributable to comorbidity itself, and also the mortality attributable to the synergy (i.e., interaction) between comorbidity and CRC.
No earlier study has evaluated the independent effects of CRC and comorbidity, or their synergistic effect on mortality, although they have generally demonstrated that CRC patients with comorbidities have poorer survival than CRC patients without comorbidities; a pattern also observed in our study. Impaired survival has been demonstrated over the short-term (Panis et al, 2011; Sarfati et al, 2011; Jorgensen et al, 2012) and long-term (Iversen et al, 2009; Sarfati et al, 2011; Jorgensen et al, 2012), and in population-based studies (Janssen-Heijnen et al, 2005; Iversen et al, 2009; Panis et al, 2011) and single-centre studies (Ouellette et al, 2004; Read et al, 2004). Impaired survival has been also found when comorbidities were evaluated using indices, such as CCI or Adult Comorbidity Index (ACE-27; Ouellette et al, 2004; Read et al, 2004; Iversen et al, 2009) and for individual diseases, including cardiovascular disease, pulmonary disease, diabetes, previous malignancy, and renal disease (Lemmens et al, 2005; Gross et al, 2006a, 2006b; Janssen-Heijnen et al, 2007; Sarfati et al, 2009). In addition, impaired survival of CRC patients with comorbidities has been demonstrated regardless of treatment received, anatomical site of CRC, gender, and age (Ouellette et al, 2004; Janssen-Heijnen et al, 2005; Iversen et al, 2009). Nonetheless, at least two studies have indicated that comorbidity does not have as important a role in mortality among patients with late-stage CRC. A recent study from North America (exploratory analysis of the CO.17 clinical trial), including 572 patients with metastatic CRC, found that patients with more comorbidity had improved survival compared with patients with less comorbidity (HR=0.8, 95% CI 0.65, 1.00; Asmis et al, 2011). A single-centre German study of 233 CRC patients with metastatic disease undergoing non-curative elective surgery found no association between comorbidities (measured by number of affected organs) and the 30-day mortality (Kleespies et al, 2009). These findings suggest that among patients severely ill with CRC, the coexistence of other often less-aggressive diseases has little effect on their poor prognosis. In our study, however, we found that the synergy between comorbidity and CRC had a substantial role for mortality in CRC patients with metastatic disease, although primarily during the first year of follow-up and among those with high comorbidity burdens. The same pattern is likely to be observed with increasing age, which our study also confirmed.
Although it was beyond the scope of our study to evaluate underlying reasons for excess mortality among CRC patients with comorbidities, there are several possibilities. First, it has been shown that diseases, such as diabetes and inflammatory bowel disease, increase CRC risk, and it has been speculated that this association might result in particularly aggressive CRC with poor survival (Ording et al, 2013). Had this been true, we would have expected to observe interaction between CRC and comorbidities also after the first year of follow-up, which we did not. Second, severe comorbidities might impair or delay cancer diagnosis or interfere with diagnostic follow-up, leading to more advanced spread (Bjerager et al, 2006), although some studies have shown decreased delays among comorbid CRC patients (Mitchell et al, 2008). Our results also do not support any difference in interaction between CRC stages. Third, physician behaviour and patient compliance may be affected by the presence of other diseases. Finally, treatment and post-treatment care might be suboptimal in the presence of comorbidities, and comorbidity can result in a higher frequency of complications eventually leading to death (Lemmens et al, 2005, 2007; Sarfati et al, 2009; Koroukian et al, 2010). This potential mechanism is supported by our findings of the interaction being restricted primarily to the first year of follow-up. However, all potential explanations remain speculative and need to be confirmed in future investigations.
The strengths of our study include its population-based cohort design and a setting providing free access to healthcare, which virtually eliminates referral bias. We were able to study a large, well-defined population with complete follow-up owing to computerised nationwide registries, thus making selection biases negligible. Because of the large number of CRC patients and matched persons from the general population without CRC, we were able to estimate the independent effects of cancer and other conditions, and how their co-occurrence affects mortality. Earlier research has called for such an investigation (Gross et al, 2006b).
Our study also had several limitations. Inaccurate coding in the nationwide registries is an important concern in registry-based analyses such as ours. Fortunately, the completeness and positive predictive values of diagnoses in the Danish Cancer Registry have been found to be 95–98% (Storm et al, 1997; Gjerstorff, 2011). The positive predictive value of the coding of comorbidities also has been shown to be high, whereas the completeness of coding is likely to be lower (Thygesen et al, 2011). In addition, even though we included comorbidities in the CCI, with the addition of atrial fibrillation/flutter and obesity, we may have missed other diseases affecting mortality. These factors could have led us to underestimate comorbidity burdens and to classify patients with comorbidities in the group without comorbidity, resulting in more uniform mortality rates and mortality rate ratios approaching 1.0. Furthermore, although we attempted to deal with potential confounding caused by age and gender by matching, standardisation, and adjustment, our results may have been affected by confounding by unmeasured factors, such as alcohol consumption, smoking, and medication use. Nevertheless, given the strength of the associations, we find it unlikely that these unmeasured factors could explain our results completely. Finally, we did not include data on causes of death and were therefore unable to compare causes of death between CRC patients and the population comparison cohort. However, as we would not expect comparison cohort members to die of CRC, and because deaths due to CRC in the CRC cohort would affect the distribution of deaths from other causes, such a comparison would be of limited value.
In conclusion, our population-based matched cohort study showed that comorbidities interacted with CRC to increase mortality beyond that explained by CRC and comorbidities acting independently, particularly in the first year after CRC diagnosis. Successful treatment of the comorbidity is pivotal, as it would delay death from the comorbidity and death caused by the interaction.
Acknowledgments
The work was supported by a grant from the Danish Agency for Science Technology and Innovation, a grant from the Danish Cancer Society (R73-A4284-13-S17), and from the Karen Elise Jensen Foundation. RE was supported by a scholarship from Aarhus University.
Appendix
Codes used in the present study
Colorectal cancer: Colon: ICD-10: C18
Rectal: ICD-10: C19-20
Surgery codes for colorectal resection: KJFB20-97, KJFH00-33, KJFH96, KJGB00-50, and KJGB96-97
Charlson's Comorbidity Index
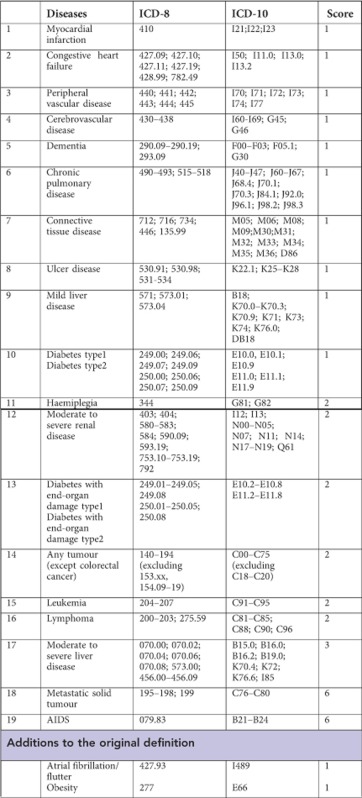
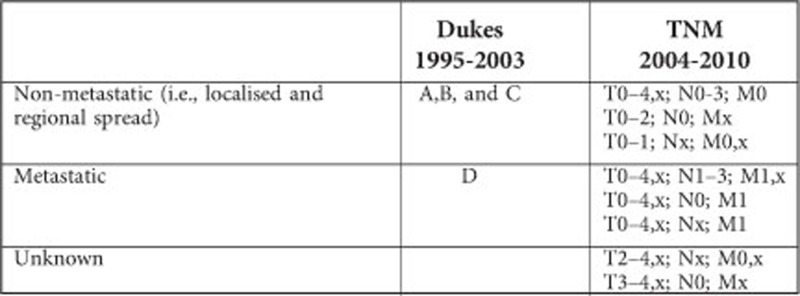
Definition of colorectal cancer stage in the Danish Cancer Registry
The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study. The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- Asmis TR, Powell E, Karapetis CS, Jonker DJ, Tu D, Jeffery M, Pavlakis N, Gibbs P, Zhu L, Dueck DA, Whittom R, Langer C, O'Callaghan CJ. Comorbidity, age and overall survival in cetuximab-treated patients with advanced colorectal cancer (ACRC)—results from NCIC CTG CO.17: a phase III trial of cetuximab versus best supportive care. Ann Oncol. 2011;22 (1:118–126. doi: 10.1093/annonc/mdq309. [DOI] [PubMed] [Google Scholar]
- Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56 (532:863–868. [PMC free article] [PubMed] [Google Scholar]
- De Marco MF, Janssen-Heijnen ML, van der Heijden LH, Coebergh JW. Comorbidity and colorectal cancer according to subsite and stage: a population-based study. Eur J Cancer. 2000;36 (1:95–99. doi: 10.1016/s0959-8049(99)00221-x. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127 (12:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39 (7 Suppl:42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- Greenland S, Lash T, Rothman K.2008Concepts of interaction Modern Epidemiology3rd edn71–83.Lippincott Williams & Wilkins [Google Scholar]
- Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006a;54 (12:1898–1904. doi: 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006b;145 (9:646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- Ingle SB, Limburg P.2007Colorectal carcinoma GI Epidemiology(1st edn) Talley NJ, Locke GR, Saito JA, (eds). pp170–175.Blackwell Publishing: MA, USA [Google Scholar]
- Iversen LH, Norgaard M, Jacobsen J, Laurberg S, Sorensen HT. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006—a population-based cohort study. Dis Colon Rectum. 2009;52 (1:71–78. doi: 10.1007/DCR.0b013e3181974384. [DOI] [PubMed] [Google Scholar]
- Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55 (3:231–240. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Janssen-Heijnen ML, Maas HA, Houterman S, Lemmens VE, Rutten HJ, Coebergh JW. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43 (15:2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Jorgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106 (7:1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleespies A, Fuessl KE, Seeliger H, Eichhorn ME, Muller MH, Rentsch M, Thasler WE, Angele MK, Kreis ME, Jauch KW. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis. 2009;24 (9:1097–1109. doi: 10.1007/s00384-009-0734-y. [DOI] [PubMed] [Google Scholar]
- Koroukian SM, Xu F, Bakaki PM, Diaz-Insua M, Towe TP, Owusu C. Comorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancer. J Gerontol A Biol Sci Med Sci. 2010;65 (3:322–329. doi: 10.1093/gerona/glp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens VE, Janssen-Heijnen ML, Houterman S, Verheij KD, Martijn H, Poll-Franse L, Coebergh JW. Which comorbid conditions predict complications after surgery for colorectal cancer. World J Surg. 2007;31 (1:192–199. doi: 10.1007/s00268-005-0711-8. [DOI] [PubMed] [Google Scholar]
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, Houterman S, Repelaer van Driel OJ, Coebergh JW. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92 (5:615–623. doi: 10.1002/bjs.4913. [DOI] [PubMed] [Google Scholar]
- Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98 (1:60–70. doi: 10.1038/sj.bjc.6604096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ording AG, Horvath-Puho E, Erichsen R, Long MD, Baron JA, Lash TL, Sorensen HT. Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn's disease: a nationwide population-based cohort study. Inflamm Bowel Dis. 2013;19 (4:800–805. doi: 10.1097/MIB.0b013e3182802af7. [DOI] [PubMed] [Google Scholar]
- Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8 (8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Panis Y, Maggiori L, Caranhac G, Bretagnol F, Vicaut E. Mortality after colorectal cancer surgery: a French survey of more than 84,000 patients. Ann Surg. 2011;254 (5:738–743. doi: 10.1097/SLA.0b013e31823604ac. [DOI] [PubMed] [Google Scholar]
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39 (7 Suppl:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, Piccirillo JF. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22 (15:3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Rieker RJ, Hammer E, Eisele R, Schmid E, Hogel J. The impact of comorbidity on the overall survival and the cause of death in patients after colorectal cancer resection. Langenbecks Arch Surg. 2002;387 (2:72–76. doi: 10.1007/s00423-002-0291-0. [DOI] [PubMed] [Google Scholar]
- Sarfati D, Hill S, Blakely T, Robson B, Purdie G, Dennett E, Cormack D, Dew K. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study. BMC Cancer. 2009;9:116. doi: 10.1186/1471-2407-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati D, Tan L, Blakely T, Pearce N. Comorbidity among patients with colon cancer in New Zealand. NZ Med J. 2011;124 (1338:76–88. [PubMed] [Google Scholar]
- Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull. 1997;44:535–539. [PubMed] [Google Scholar]
- Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, Yates JW. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82 (11:2123–2134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


