Abstract
Background:
Cancer incidence in the Mayak Production Association (PA) cohort was analysed to investigate for the first time whether external gamma-ray and internal plutonium exposure are associated with raised incidence of solid cancers other than lung, liver and bone (other solid cancers).
Methods:
The cohort includes 22 366 workers of both sexes who were first employed between 1948 and 1982. A total of 1447 cases of other solid cancers were registered in the follow-up period until 2004. The Poisson regression was used to estimate the excess relative risk (ERR) per unit of cumulative exposure to plutonium and external gamma-ray.
Results:
A weak association was found between cumulative exposure to external gamma-ray and the incidence of other solid cancers (ERR/Gy=0.07; 95% confidence intervals (CIs): 0.01–0.15), but this association lost its significance after adjusting for internal plutonium exposure. There was no indication of any association with plutonium exposure for other solid cancers. Among 16 individual cancer sites, there was a statistically significant association with external exposure for lip cancer (ERR/Gy=1.74; 95% CI: 0.37; 6.71) and with plutonium exposure for pancreatic cancer (ERR/Gy=1.58; 95% CI; 0.17; 4.77).
Conclusion:
This study of Mayak workers does not provide evidence of an increased risk of other solid cancers. The observed increase in the risk of cancer of the lip and pancreas should be treated with caution because of the limited amount of relevant data and because the observations may be simply due to chance.
Keywords: cancer incidence, external gamma radiation, internal plutonium exposure, Mayak radiation workers
Much of what is known about the long-term carcinogenic effects of radiation exposure come from the Life Span Study cohort of the A-bomb survivors in Japan (Preston et al, 2007; UNSCEAR, 2008; Ozasa et al, 2012). The radiation dose received by these survivors was delivered acutely, primarily gamma-ray with a small neutron contribution. Long-term follow-up of the health of radiation workers in the nuclear industry is important to determine whether risk estimates derived from the A-bomb survivor studies are directly applicable to the lower protracted external and internal exposures experienced by workers. There are important statistical limitations within such studies, the number of people in the studies, the period of observations (follow-up) and the sizes of radiation doses received. If the number of people in a study is small and the radiation doses are low, then a study will have low power to detect and estimate raised cancer risk. Furthermore, there are also other potential uncertainties arising from confounding and other sources of bias in some studies. Despite such difficulties, studies on cohorts of radiation workers have provided important information on cancer risks (Cardis et al, 2007; Muirhead et al, 2009a). A study of the UK radiation workers has shown an association between exposure to external gamma rays and the incidence of solid cancer and leukaemia, consistent with risk estimates derived from the A-bomb survivor data (Muirhead et al, 2009a).
Studies of the radiation workers at the Russian Mayak Production Association (Mayak PA) have provided information on risks from external exposures and internal exposures from plutonium (Shilnikova et al, 2003; Sokolnikov et al, 2008; Azizova et al, 2012; Gilbert et al, 2013). These studies have demonstrated significant association between cancer and non-cancer diseases and exposure to internal plutonium and external gamma ray. Risks of morbidity from cancers of the lung, liver and bone – the organs of primary plutonium deposition – have been reported previously (Labutina et al, 2013). The focus of this paper is to present results for incidence of solid cancers other than those of the lung, liver and bone (here after referred to as ‘other solid cancers'), in relation to external and internal plutonium exposure among radiation workers in the Mayak PA cohort.
Materials and methods
The Mayak PA is the first and largest nuclear weapon-grade plutonium (239Pu) production plant in Russia and started operation in 1948. A substantial number of workers during the early period of operation were exposed to high levels of radiation from both external gamma-ray and internal alpha-particle irradiation from incorporated plutonium (239Pu) in body tissues.
Definition of cohort and follow-up
The cohort design, data collection and methods used to determine the vital status have been described in detail previously (Koshurnikova et al, 1999; Labutina et al, 2013). We summarise here some of the main aspects. In brief, the Mayak PA study cohort included 22 373 workers first employed in one of the main facilities (nuclear reactors, radiochemical production and plutonium production) during 1948–1982 and followed up to the end of 2004. Of these, seven workers were excluded from the study: two were diagnosed before the start of the follow-up and five were diagnosed with two first solid malignant neoplasms on the same date. Thus, the final study population comprised 22 366 workers.
The cancer registry was established by the SUBI Epidemiological laboratory (which continues to maintain it) jointly with Federal State Health Institution of the Central Medical sanitary Department of the Federal Medical-Biological Agency of Russia. Information was obtained from the medical records of oncology, postmortem services, as well as the archival data of the Mayak workers' polyclinic for the period from 1948 to 2004. The diagnoses were coded in accordance with the Ninth Revision of International Classification of Diseases, ICD-9 (WHO, 1977). Cancer cases were identified for workers who lived in the city at the time of diagnosis. The data completeness is restricted by the lack of diagnosed cases after workers migrate from Ozyorsk (this date terminates the follow-up period for a worker). The analyses of other solid cancer incidence data are based on first diagnosis but excluded non-melanoma skin cancers because of incomplete registration. Overall, 1447 other solid cancer cases were identified; among these, 0.7% of cancers (10 cases) reported were obtained from death certificates, whereas 5.5% of cases were diagnosed through autopsy findings. The majority of cases had morphological diagnosis (91.2%). Autopsies were carried out for about 30% of workers who had been diagnosed with cancer. In the first decades of Mayak PA operation, about 60% of cancer cases had postmortem or medico legal reports completed, but the current autopsy rate is 15% with such examinations more likely to be performed on workers who had higher levels of plutonium exposure.
Information on exposure to external gamma-ray and to internal plutonium
The analysis was performed using external and internal dose estimates from an updated dosimetry system, Mayak Doses-2008 (MWDS 2008) (Vasilenko et al, 2007; Khokhryakov et al, 2013). External radiation exposure to Mayak workers was mainly by gamma-ray from the reactor plant and monitored by the Mayak PA Radiation Protection Department using individual film badges with adjustment for energy and angular dependence of the detector. The measurements for cumulative external exposure in workers are expressed as the recorded operational quantity [Hp(10)], which is the personal equivalent dose at a tissue penetrating to a depth of 10 mm (ICRU, 1988), expressed as in Gy. Individual doses from external gamma-ray were available for the whole study cohort.
The main sources of internal plutonium exposure were from the Radiochemical and Plutonium production plants. All workers employed at the radiochemical and plutonium production plants were potentially exposed to internally deposited 239Pu and external gamma radiation. A small number of workers at the reactor plant have also been monitored for plutonium exposure but the rest of the reactor workers were assumed to have zero internal plutonium doses. However, information on internal exposures was available for only about 30% of the workers in the cohort because routine urine measurements did not begin until about 1970. Among workers monitored for plutonium exposure, absorbed dose to the liver was used as a proxy dose estimate, recorded in Gy. The internal doses from inhalation of 239Pu were estimated from plutonium concentration in urine samples using biokinetic models of the behaviour of plutonium in the body. The calculations took account of occupational history, solubility of inhaled plutonium aerosols and the smoking status of each worker (Khokhryakov et al, 2013). For those workers who were potentially exposed to plutonium, but had no direct measurements, six categories of potential exposure have been developed (Shilnikova et al, 2003), referred to as plutonium-surrogate indices, based on the workers' employment: (1) auxiliary and reactor plant workers hired between 1948 and 1982; (2) radiochemical plant workers hired between 1954 and 1982, main plutonium department workers hired between 1964 and 1982 and plutonium auxiliary department workers hired between 1959 and 1982; (3) plutonium auxiliary department workers hired between 1950 and 1958, radiochemical plant workers hired between 1948 and 1953 and main plutonium department workers hired between 1959 and 1963; (4) Plutonium auxiliary department workers hired between 1948 and 1949 and main plutonium department workers hired 1954–1958; (5) main plutonium department workers hired between 1950 and 1953; and (6) main plutonium department workers hired between 1948 and 1949. For this analysis, plutonium exposures of unmonitored workers were designated as low (plutonium-surrogate indices 2, 3 and 4) and high, to include workers employed in the main plutonium department between 1948 and 1958 (indices 5 and 6).
Auxiliary plant workers were excluded here because of data quality problems and the likely small impact on the risk estimates that their inclusion would provide.
Statistical methods
The data were analysed using the same methods as in previous studies of this cohort (Shilnikova et al, 2003; Sokolnikov et al, 2008; Labutina et al, 2013). The Poisson regression was used to test for an association between incidence from other solid cancers and exposure to both external gamma-ray using Hp(10) dose (Gy) and plutonium using internal liver dose (Gy). For each worker, person-years at risk were accumulated over time from the date of first employment at one of the main plants of the reactor, radiochemical or plutonium production plants of Mayak PA between 1948 and 1982 entry into the study to the date of exit from the study, which ended on the date of first cancer registration, date of death, date of leaving Ozyorsk, or the 31 December 2004, whichever was earliest. Tabulations of person-years at risk and other solid cancer incidence cases were created with the DATAB module of the EPICURE software (Preston et al, 1998). Data were cross-classified by gender, attained age (14 categories by 5-year age intervals), calendar year in 12 categories, age at first plutonium exposure in 4 categories, smoking status in 3 categories (unknown, nonsmoker and smoker), plant in 3 categories (nuclear reactors, radiochemical production and plutonium production), alcohol consumption in 6 categories, estimated cumulative external exposure in 8 categories (0, 0.05, 0.2, 0.5, 1, 2, 3 and 4 Gy) and estimated cumulative internal exposure in 7 categories (0, 0.0001, 0.025, 0.05, 0.1, 0.25 and 1 Gy) for internal cumulative dose. To allow for a latent period in a radiation effect, analyses were also performed using the cumulative external dose and internal liver dose lagged by 0, 5, 10, 15 and 20 years. Because of indications that some workers were monitored for plutonium as a result of suspected diseases, person-years were classified as unmonitored until 2 years following the first monitoring date (Sokolnikov et al, 2008; Gilbert et al, 2013). The data were fitted to the following model:
where λ0 is the background cancer incidence rate in the absence of radiation exposure (dose=0) and depends on attained age (a), gender (g) and smoking status (sm); excess relative risk is the ERR modelled by combining separately ERRed due to external gamma-rays and ERRid due to internal alpha-radiation for monitored workers; ERRsur is the excess relative risk (RR) due to internal alpha-radiation for unmonitored workers in the radiochemical and plutonium production plants. More specifically the ERR was modelled as:
ERR=ERRed+ERRid+ERRsur
Allowance was made in the analyses to control for background factors affecting cancer risk using various parametric models by including covariates in the model as well as non-parametric models through stratification. The results from all of these models showed that attained age, gender and smoking status were the most important factors in modelling the background rates for other solid cancer incidence. However, the parametric approach produced a slightly better description of the background rates compared with the non-parametric approach, and this approach has also been used in previous analyses of Mayak cohort studies (Skolnikov et al, 2008; Gilbert et al, 2013). The final model for the baseline risks included a function of the gender-specific logarithm of attained age, the logarithm of attained age squared and gender-specific smoking status. Additional adjustment of the baseline model for year of birth cohort and calendar time, alcohol consumption, period of first employment or plant did not have any significant effect for the model.
Deviations from the linear model (Equation 1) are evaluated by fitting alternative model, for example, the linear-quadratic model is a dose–response model that measures the concavity of the dose–response relationship. Analyses were also conducted to evaluate factors that may modify the dose–response trend such as attained age, gender, age at first employment and so on. All the analyses here were carried out using the AMFIT module in the EPICURE (Preston et al, 1998). Likelihood ratio tests and likelihood-based confidence intervals (CIs) were reported. All P-values quoted were two-sided and a statistical significance level of 5% was used.
Results
The study cohort included 22 366 workers, of whom 5687 (25%) were females, first employed at one of the main plants during 1948 to 1982 and with a total of 535 932 person-years follow-up to the end of 2004. Table 1 summarises the characteristics of the study cohort. About half of the Mayak PA workers (55%) were first employed during the period of first production (1948–1958). At the end of the follow-up period, vital status was known for almost all cohort members (95.2%). About half of the workers had died (48%) and 41% of the workers had migrated from the city Ozyorsk at the end of 2004. Information on alcohol consumption was available for 78% of the workers. Most importantly, information on smoking status was collected for about 89% of the Mayak PA cohort members (Table 1).
Table 1. Characteristics of the Mayak PA worker's cohort first employed from 1948 to 1982 and followed up to 2004.
| Exposed to plutonium (monitored)a | No plutonium exposureb | Potential plutonium exposure (unmonitored)c | Total | |
|---|---|---|---|---|
| Number of workers | 6699d | 5154 | 10 513 | 22 366 |
| Females (%) |
30 |
22 |
24 |
25 |
| Number of person-years |
256 866 |
1 179 698 |
161 097 |
535 932 |
|
Year of employment | ||||
| 1948–1958 | 3331 | 2979 | 5979 | 12 289 |
| 1959–1982 |
3368 |
2175 |
4534 |
10 077 |
|
Vital status (%) | ||||
| Known | 99.9 | 95.1 | 92.7 | 95.2 |
| Alive | 62.6 | 50.4 | 46.5 | 51.9 |
| Dead | 37.4 | 49.6 | 53.5 | 48.1 |
| Untraced | 0.1 | 4.9 | 7.3 | 4.8 |
| Migrated |
3.9 |
43.1 |
60.5 |
41.3 |
|
Plant | ||||
| Reactor | 260 | 5154 | — | 5414 |
| Radiochemical | 3537 | — | 5654 | 9191 |
| Pu production |
2902 |
— |
4859 |
7761 |
|
Attained age | ||||
| <55 | 1656 | 3258 | 8301 | 13 215 |
| 55–64 | 1754 | 754 | 1165 | 3673 |
| 65–74 | 2214 | 745 | 737 | 3696 |
| 75+ |
1075 |
397 |
310 |
1782 |
|
Age at hire | ||||
| <20 | 2013 | 1346 | 3581 | 6640 |
| 20–24 | 2294 | 2030 | 3386 | 7710 |
| 25–29 | 1046 | 841 | 1412 | 3299 |
| 30+ |
1346 |
937 |
2134 |
4417 |
|
Smoking status | ||||
| Nonsmoker | 2911 | 1832 | 3767 | 8510 |
| Smoker | 3260 | 2761 | 5373 | 11 394 |
| Unknown |
528 |
561 |
1373 |
2462 |
|
Alcohol consumption status | ||||
| Never drinkers | 1448 | 1057 | 1945 | 4450 |
| Drinkers | 3912 | 3089 | 5919 | 12 920 |
| Unknown |
1339 |
1008 |
2549 |
4896 |
| Mean external gamma Hp(10) dose in Gy (range) |
0.62 (0–5.5) |
0.44 (0–6.8) |
0.38 (0–6.7) |
0.51 (0–6.8) |
| Mean internal liver dose in Gy (range)e |
0.31 (0–36) |
— |
— |
0.31 (0–36) |
| Solid cancers other than the lung, liver and bone | 759 | 290 | 398 | 1447 |
Abbreviation: PA=Production Association.
Workers with internal dose estimates.
Reactor workers exposed only gamma.
Radiochemical and plutonium plant workers who were unmonitored for internal dose.
Of those, 260 workers are from the reactor plant.
Among exposed workers.
The mean cumulative external gamma Hp(10) dose was 0.51 Gy (0.54 Gy for men and 0.44 Gy for women), but the mean dose was substantially higher among cohort members who started to work between 1948 and 1958 (0.81 Gy), compared with those who started to work between 1959 and 1982 (0.15 Gy). A total of 3862 workers were exposed to more than 1 Gy, and 95 workers to more than 4 Gy. Among the plutonium-exposed cohort members, the mean cumulative liver plutonium dose was 0.31 Gy (0.24 Gy for men and 0.47 Gy for women). There were a total of 1447 cases (33% in females) of other solid cancers.
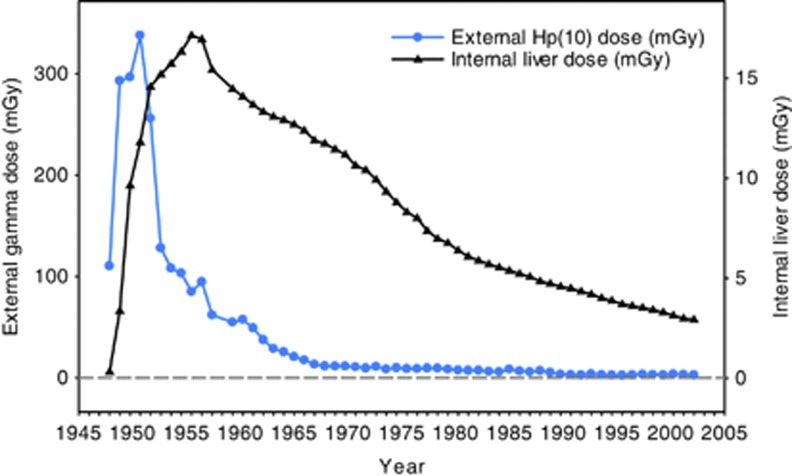
Figure 1 shows the mean annual exposure values for external Hp(10) dose and for liver dose from plutonium exposure. The gamma-ray dose decreased sharply after 1951 because of improvements in radiation protection, whereas the internal liver dose showed a slightly different pattern because of the long-term retention of plutonium in body organs. As explained previously, internal dose values were estimated on the basis of occupational history and urine measurements where available.
Figure 1.
The mean annual external Hp(10) dose from gamma-ray exposure (mGy), the internal liver dose from plutonium exposure (mGy) among workers by calendar year.
Risks from external gamma-ray and internal plutonium exposure
A total of 1447 other solid cancers were recorded for the 22 366 workers in the Mayak PA cohort. A statistically borderline significant relationship with cumulative external gamma dose was observed for other solid cancers as a group on the basis of a zero-year lag (ERR/Gy=0.07; 95% CI: 0.01; 0.15; P=0.06) after adjusting for age, gender and smoking status. Alternative lag periods of 5, 10, 15 or 20 years were used, but the resulting risk estimates did not change substantially. There was no statistically significant association for other solid cancers with internal liver dose for monitored workers (ERR/Gy=0.10; 95% CI: −0.02; 0.26, P=0.15) or plutonium-surrogate indices for unmonitored workers (P>0.5). The evidence for a trend with external dose became weaker and was not statistically significant after adjusting for internal dose for monitored workers (ERR/Gy=0.06, 95% CI: −0.01; 0.14, P=0.12), whereas the external dose findings were little changed after adjusting for unmonitored plutonium exposed among workers using the surrogate indices (ERR/Gy=0.07, 95% CI: −0.005; 0.15, P=0.07).
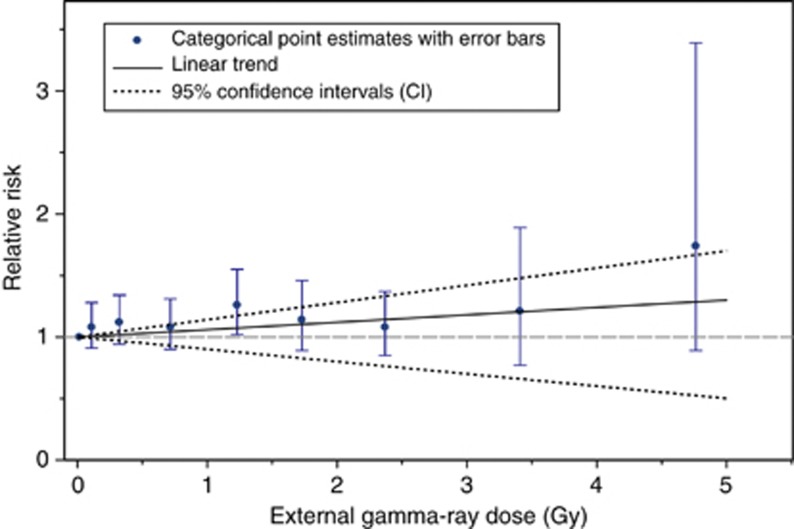
Figure 2 shows the external dose-category-specific estimates and the linear dose–response trend for other solid cancers. Having adjusted for internal exposure, the point estimates increase with dose category, although the RRs do not differ significantly from those at zero dose. Figure 2 also shows reasonable good agreement between a linear trend and exposure dose categories, but the external dose trend was not statistically significant. There was also no evidence of nonlinearity in the external dose response based on the linear-quadratic model (P>0.5) or when the data used were restricted to cohort members with external doses under 3 Gy (P>0.5).
Figure 2.
Relative risks of other solid cancer incidence in relation to external exposure categories and the linear trend (and 95% CI), having adjusted for internal exposure (based on 0-year lag).
Table 2 presents the ERR/Gy estimates for the modifying factors of interest for external dose response after adjusted non-radiation factors. The ERR/Gy increased with attained age and the greatest risk was observed in workers for ages 70 and older, but the differences were not statistically significant. There was also no indication of strong effect modification by gender, age at first employment, gender smoking status or plant.
Table 2. The ERR/Gy across categories of modifying factors of interest and relative risks (RR) by external dose categories for other solid cancers with adjustment for internal dose.
| Cases | Person-years | ERR/Gya (95% CI) | P-valueb | |
|---|---|---|---|---|
|
Gender: | ||||
| Male | 968 | 379 932 | 0.06 (−0.03; 0.16) | |
| Female |
479 |
156 838 |
0.11 (−0.03; 0.30) |
0.41 |
|
Smoking status | ||||
| Never smokers | 604 | 223 846 | 0.04 (−0.07; 0.18) | |
| Current smokers | 766 | 263 613 | 0.06 (−0.03; 0.16) | 0.18 |
| Unknown |
77 |
48 473 |
0.64 (0.03; 1.75) |
|
|
Plant | ||||
| Reactor | 327 | 128 369 | 0.03 (−0.10; 0.17) | |
| Radiochemical | 650 | 221 595 | 0.07 (−0.01; 0.17) | >0.5 |
| Plutonium plant |
470 |
186 968 |
0.14 (−0.04; 0.37) |
|
|
Attained age (years) | ||||
| <50 | 296 | 370 393 | −0.05(<0; 0.09) | |
| 50–54 | 383 | 91 269 | 0.02 (−0.1; 0.15) | |
| 60–64 | 467 | 54 145 | 0.10 (−0.05; 0.18) | 0.08 |
| 70+ |
301 |
20 125 |
0.22 (0.08; 0.38) |
|
|
Age at first hired (years) | ||||
| <20 | 242 | 164 348 | 0.04 (−0.09; 0.19) | |
| 20–24 | 456 | 184 604 | 0.10 (0.01; 0.22) | >0.5 |
| 25–34 | 434 | 125 218 | 0.02 (−0.08; 0.14) | |
| 35+ | 315 | 61 761 | 0.07 (−0.06; 0.25) | |
Abbreviations: ERR=excess relative risk; CI=confidence interval.
Background rates adjusted for age, gender and smoking status and based on 0-year lag period.
Test of homogeneity of the ERR/Gy across categories.
Table 3 gives RR estimates for various external gamma-ray dose categories (<0.2 Gy, 0.2–0.5 Gy, 0.5–1.0 Gy and >1 Gy) for other solid cancers as a group and the selected 16 individual cancer sites and the corresponding gender averaged ERR/Gy with 95% CIs. For the analysis of each specific cancer site, adjustments were made for attained age, gender and smoking unless otherwise indicated. The majority showed a positive dose–response relationship (11 out of 16), although the relationship was only significant for cancer of the lip (ERR=1.74, 95% CI: 0.37; 6.71, P=0.002) and borderline statistical significance for oesophagus (ERR=0.89, 95% CI: <0; 5.29, P=0.06) and stomach (ERR/Gy=0.15, 95% CI: −0.01; 0.39, P=0.07) with external dose, irrespective of whether adjustment was made for internal exposure or any of the plutonium-surrogate indices for unmonitored workers. However, no association was found for the other 12 sites with external exposures.
Table 3. Relative risk (RR), ERR/Sv and 95% confidence interval (CI) for selected cancer sites by cumulative external exposure from gamma-ray (based on 0-year lag period and background rates were adjusted for attained age, gender and smoking).
| Cancer site (ICD-9 code) | <0.2 Sv | 0.2–0.5 Sv | 0.5–1.0 Sv | ⩾1 Sv | Total (no. of female) | ERR/Sv (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Solid cancers other than lung, liver, bone (140–154,156–161, 163–169, 171, 172, 174–199) |
Cases RR (95% CI) |
536 1.00 |
299 1.08 (0.94; 1.25) |
227 1.06 (0.90; 1.24) |
385 1.16 (1.01; 1.33)b |
1447 (47) |
0.07 (0.01; 0.15) |
0.06a |
| Person-years at risk | — | 272 834 | 101 958 | 70 004 | 91 134 | 535 932 (156,838) | — | — |
| Mean external gamma exposure |
— |
0.068 |
0.32 |
0.72 |
1.84 |
0.51 (0.44) |
— |
— |
| Lip (140) | Cases | 6 | 7 | 4 | 18 | 35 (2) | 1.74 (0.37; 6.71) | 0.002b |
| |
RR (95% CI) |
1.00 |
2.13 (0.71; 6.39) |
1.63 (0.45; 5.89) |
4.93 (1.89; 12.9)b |
|
|
|
| Mouth, tongue, salivary gland and pharynx (141–149) |
Cases RR (95% CI) |
23 1.00 |
10 0.75 (0.36; 1.59) |
7 0.67 (0.29; 1.58) |
10 0.60 (0.28; 1.28) |
85 (11) |
−0.14 (NA) |
0.24 |
| Oesophagus (150) | Cases | 6 | 5 | 4 | 15 | 30 (5) | 0.89 (NA; 5.29)c | 0.06a |
| |
RR (95% CI) |
1.00 |
1.43 (0.41; 5.02) |
1.37 (0.34; 5.58) |
2.80 (0.81; 9.59) |
|
|
|
| Stomach (151) | Cases | 97 | 68 | 53 | 83 | 301 (56) | 0.15 (−0.01; 0.39)d | 0.07a |
| |
RR (95% CI) |
1.00 |
1.31 (0.96; 1.80) |
1.33 (0.94; 1.87) |
1.34 (0.98; 1.82)a |
|
|
|
| Colon (153) | Cases | 63 | 27 | 22 | 32 | 144 (50) | −0.14 (NA) | 0.23 |
| |
RR (95% CI) |
1.00 |
0.83 (0.52; 1.30) |
0.88 (0.54; 1.43) |
0.81 (0.52; 1.25) |
|
|
|
| Rectum (154) | Cases | 36 | 25 | 21 | 27 | 109 (37) | 0.03 (−0.18; 0.38) | >0.50 |
| |
RR (95% CI) |
1.00 |
1.36 (0.81; 2.27) |
1.45 (0.84; 2.51) |
1.16 (0.69; 1.94) |
|
|
|
| Gallbladder (156) | Cases | 10 | 5 | 5 | 9 | 29 (12) | 0.15 (−0.48; 1.72) | >0.50 |
| |
RR (95% CI) |
1.00 |
1.05 (0.36; 3.11) |
1.39 (0.47; 4.14) |
1.64 (0.64; 4.18) |
|
|
|
| Pancreas (157) | Cases | 21 | 14 | 15 | 21 | 71 (11) | 0.14 (−0.16; 0.70) | 0.25 |
| |
RR (95% CI) |
1.00 |
1.13 (0.57; 2.23) |
1.53 (0.78; 3.00) |
1.32 (0.71; 2.45) |
|
|
|
| Larynx (161) | Cases | 18 | 8 | 8 | 11 | 45 (4) | −0.12 (NA) | >0.50 |
| |
RR (95% CI) |
1.00 |
0.72 (0.31; 1.67) |
0.92 (0.40; 2.13) |
0.78 (0.36; 1.68) |
|
|
|
| Female breast (174)w | Cases | 54 | 21 | 14 | 18 | 107 | 0.21 (−0.08; 0.69) | 0.24 |
| |
RR (95% CI) |
1.00 |
1.23 (0.74; 2.05) |
1.18 (0.65; 2.13) |
1.19 (0.69; 2.04) |
|
|
|
| Uterus (179–182)w | Cases | 33 | 12 | 4 | 18 | 67 | 0.35 (−0.07; 1.11) | 0.12 |
| |
RR (95% CI) |
1.00 |
1.10 (0.57; 2.14) |
0.52 (0.18; 1.47) |
1.81 (1.01; 3.23) |
|
|
|
| Prostate (185)m | Cases | 13 | 17 | 9 | 31 | 70 | 0.16 (−0.12; 0.73) | 0.27 |
| |
RR (95% CI) |
1.00 |
1.82 (0.88; 3.75) |
1.12 (0.48; 2.63) |
2.04 (1.06; 3.91) |
|
|
|
| Bladder (188) | Cases | 20 | 7 | 15 | 16 | 58 (4) | 0.004 (NA) | >0.50 |
| |
RR (95% CI) |
1.00 |
0.53 (0.22; 1.26) |
1.41 (0.72; 2.76) |
0.87 (0.45; 1.71) |
|
|
|
| Kidney (189) | Cases | 33 | 16 | 16 | 20 | 85 (21) | −0.12 (NA) | 0.49 |
| |
RR (95% CI) |
1.00 |
0.86 (0.47; 1.57) |
1.11 (0.60; 2.02) |
0.86 (0.48; 1.52) |
|
|
|
| Brain and CNS (191-192) | Cases | 19 | 12 | 8 | 9 | 48 (7) | −0.15 (NA) | 0.19 |
| |
RR (95% CI) |
1.00 |
1.17 (0.57; 2.43) |
1.05 (0.46; 2.43) |
0.78 (0.35; 1.76) |
|
|
|
| Thyroid (193) | Cases | 10 | 8 | 2 | 9 | 29 (13) | 0.40 (−0.24; 1.84) | 0.22 |
| RR (95% CI) | 1.00 | 1.93 (0.75; 4.94) | 0.67 (0.15; 3.10) | 2.16 (0.86; 5.47) |
Abbreviations: ERR=excess relative risk; ICD=International Classification of Diseases; NA=not applicable; RR=relative risk, confidence intervals cannot be calculated because the model did not converge to a maximum likelihood solution; w=women only; m=men only.
Statistically borderline significant.
Statistically significant (P<0.05).
Additional adjustment period of first employment and plant.
Adjusted for age, gender and alcohol.
Except for pancreatic cancer, there was no statistically significant trend for the other cancer sites with either internal liver dose among monitored workers or plutonium-surrogate indices for unmonitored workers. Of the 60 cases of pancreatic cancer among the male workers, 32 and 15 cases were among monitored and unmonitored plutonium workers, respectively. A significant positive association between internal plutonium exposure and pancreatic cancer was observed (ERR/Gy=1.35, 95% CI: 0.14; 3.74) after adjusting for age and gender. Additional adjustment for smoking status had little effect. The RR increased with increasing dose; the RR compared with the reference category of <0.001 Gy (38 cases) in categories 0.001–0.049 Gy (9 cases), 0.05–0.49 Gy (19 cases) and ⩾0.5 Gy (5 cases) were 1.19 (95% CI: 0.57; 2.46), 1.79 (95% CI: 1.01; 3.18) and 2.11 (95% CI: 0.81–5.47), respectively. The RR among workers with liver doses of above 0.05 Gy was twice that of workers with cumulative liver doses of <0.001 Gy. There was no evidence of any effect of the surrogate indices (based on 19 cases diagnosed among the unmonitored workers). Additional adjustment for surrogate indices for unmonitored workers or for external dose showed no substantial modifying effects on the overall ERR/Gy for the pancreatic cancer.
Discussion
Considering solid cancers other than lung, liver and bone cancers, a strong association has been reported previously between external dose and other solid cancers in mortality analyses of the Mayak cohort (Shilnikova et al, 2003). The current study of incidence data supports this finding from the mortality data, although the association with external dose was shown to be weak when adjusted for smoking status. Unlike the mortality analyses, the incidence analysis did not show a statistically significant effect for external dose after adjusting for internal liver dose, although the estimates from this study and the mortality data were nearly identical (Table 4). There was also no evidence of nonlinearity for the external dose response. In terms of plutonium exposure, the incidence data did not show any effect of internal exposures for other solid cancers as a group. This finding is not surprising as plutonium doses are delivered largely to the lung, liver and bone, with much lower doses to other organs. However, the mortality study of the Mayak cohort suggested a smaller but still statistically significant effect of internal exposure on the risk of death for these cancers (Shilnikova et al, 2003). The differences between the results of this study and those obtained from the mortality study (Shilnikova et al, 2003) can be attributed to the use of the revised dosimetry system MWDS-2008 in place of earlier systems using plutonium body burden and the inclusion of smoking adjustment and the extended follow-up period. It should also be noted that the previous mortality analyses were based on ‘archive' doses, whereas MWDS 2008 included corrections for energy and angular dependence for external gamma-radiation doses. In contrast to the mortality analyses, the incidence analyses excluded cancers that were diagnosed after migration.
Table 4. Comparison of estimates of the gender-averaged ERR/Gy (90% CI) for exposure to external dose for cancers in the Mayak cohort, NRRW, the 15-country nuclear worker study and the Japanese A-bomb survivors.
| Study period | No. of study population | Range of doses in Gy (mean) | No. of deaths or cases | ERR/Gy (90% CI) | |
|---|---|---|---|---|---|
|
Mayak PA workers studya | |||||
| Incidence (0-year lag) (this study) | 1948–2004 | 22 366 | 0–7 (0.5) | 1447 | 0.06 (−0.001; 0.13) |
| Mortality (5-year lag) (Shilnikova et al, 2003) |
1948–1972 |
21 500 |
0–>10 (0.8) |
1062 |
0.08 (0.02; 0.12) |
|
Third NRRW study (10-year lag) | |||||
| Mortality
Incidence
(Muirhead et al, 2009a) |
1955–2001 |
174 541 |
0–0.5 or more (0.025) |
5118
8443 |
0.32 (0.02; 0.67)b
0.31 (0.05; 0.58)b |
|
15-country nuclear worker study (10-year lag) | |||||
| Mortality
(Cardis et al, 2007) |
1943–2000 |
407 391 |
0–0.5 or more (0.019) |
3528 |
0.59 (−0.16; 1.51)b |
|
Japanese A-bomb survivorsc (5-year lag) | |||||
| Mortality (Ozasa et al, 2012) | 1950–2003 | 86 611 | 0–4 (0.20) | 10 929 | 0.42 (0.32; 0.53) |
| Incidence (Preston et al, 2007) | 1958–1998 | 105 427 | 17 448 | 0.47 (0.40; 0.54) | |
Abbreviations: CI=confidence interval; ERR=excess relative risk; NRRW=National Registry for Radiation Workers.
Based on all solid cancers other than lung, liver, bone and non-melonama skin cancers.
Based on all solid cancers excluding lung and pleura cancer.
Based on solid cancers and survivors exposed at ages of 30 years or more, at an attained age of 70 years, 5 year. c: 95% confidence interval.
The International Agency for Research on Cancer (IARC) has carried out a combined analysis of mortality among nuclear industry workforces in 15 countries to provide greater precision in direct estimates of cancer risk (Cardis et al, 2007). In the UK, the third analysis of the National Registry for Radiation Workers (NRRW) examined mortality and incidence data among radiation workers (Muirhead et al, 2009a). These groups of radiation workers were exposed primarily to a protracted low-dose exposure from X-rays or gamma rays. In addition, in a study of 14 319 workers employed at the Sellafield plant of British Nuclear Fuels in the UK, cancer mortality and incidence were examined in relation to exposures to plutonium and external radiation (Omar et al, 1999). Table 4 shows estimates of the ERR/Gy from the current study and other radiation worker studies, together with results from analysis of the Japanese A-bomb survivors data. The risk estimates from the present study and the previous mortality analysis of other solid cancers are lower than the tabulated values from other studies, although the CIs from the Mayak studies overlap with those for the UK workers (Muirhead et al, 2009a) and the studies of 15-country nuclear workers (Cardis et al, 2007). However, the Mayak results are inconsistent with the Japanese A-bomb study; surprisingly, a lower estimate was obtained for this study as compared with that derived from the A-bomb survivors. The differences between these two studies are likely due to the small number of cases in the present study, the magnitude of radiation doses received (higher in the Mayak cohort) and/or the use of Hp(10) doses for the Mayak analysis, whereas the A-bomb data analyses involve estimates of organ dose to colon. The study of plutonium workers in the UK (Omar et al, 1999) did not find any association between cancers of any specific site, or all cancers combined, and cumulative plutonium and external radiation doses.
Sixteen individual cancer sites were evaluated in this study, and, as a consequence, there is an 80% chance of obtaining at least one statistically significant result even if no real effects exist. The site-specific results in this study showed a statistically significant association with radiation for cancer of the lip and pancreas for which no association with radiation was reported in previous studies. The paper by Preston et al (2010) also presents preliminary site-specific estimates on the basis of Mayak mortality data and also discusses general difficulties in evaluating site-specific risks including the problem of multiple testing.
Lip
This study provides evidence of a relationship with increasing cumulative exposure to external dose (ERR/Gy=1.74; 95% CI: 0.37; 6.71, P=0.002), but there have not been any previous reports of such an association. Additional adjustment for internal dose among monitored and also for unmonitored workers led to no change to the ERR/Gy. The RR increased with increasing external dose; the highest exposure category (1⩾Gy) was associated with a five-fold significantly higher risk of incidence compared with the reference category <0.2. However, we cannot rule out the possibility that this may reflect chance finding due to the small number of cases (35 cases). Although measured and unmeasured confounding factors may have influenced association of external exposure with lip cancer risk, we were able to control, in particular, cigarette smoking, but for UV-radiation exposure, it was difficult to obtain the information among workers.
Oesophagus
Having adjusted for age, gender, smoking, period of first employment and plant, this study suggested a weak association with external exposure (ERR/Gy=0.89, 95% CI: 0.001; 5.29, P=0.06) based on 30 cases (among these 16 and 13 cases of oesophageal cancer diagnosed among the monitored and unmonitored plutonium workers, respectively). This is consistent with the findings from the study of the Japanese A-bomb survivors (Preston et al, 2007), which found statistically significant elevated risks (ERR/Gy=0.52, 90% CI: 0.15; 1.0) based on 352 cases. However, the IARC study of nuclear workers and the third analysis for the UK radiation workers found no evidence of a significant association with external dose for oesophageal cancer (Cardis et al, 2007; Muirhead et al, 2009a). Additional adjustment for internal liver dose and plutonium-surrogate indices led to a small increase of the ERR/Gy for external dose and the result remains the same.
Stomach
There was some indication of an increased risk from external exposure (ERR/Gy=0.15, 95% CI: −0.01; 0.39, P=0.07), supporting the finding of a significant increased risk for the Japanese A-bomb survivors (ERR/Gy=0.34, 90% CI: 0.22; 0.47) (Preston et al, 2007). Neither the IARC 15 country study nor the UK third analysis reports an association between stomach cancer and external exposure of radiation workers (Cardis et al, 2007, Muirhead et al, 2009a). The findings for external dose remained the same after adjusting for internal liver dose as well as additional adjustment for the plutonium-surrogate categorical indices for unmonitored workers. There was no statistically significant trend in stomach cancer either with internal liver dose (P>0.5) or any of the plutonium-surrogate indices (P=0.33), based on 138 and 92 cases of stomach cancers diagnosed among the monitored and unmonitored plutonium workers, respectively.
Pancreas
There was a significant association of pancreatic cancer incidence with internal plutonium dose, both with and without adjusting for external gamma dose (ERR/Gy=1.58; 95% CI; 0.17; 4.77, P=0.02). Tobacco use is the main identified risk factor for cancer of pancreas. For this analysis, the background model was adjusted for age and gender, but additional adjustment for smoking status had little effect on the dose–response analysis for internal dose. This finding is surprising and not fully understood as plutonium deposition leads to relatively little exposure (or dose) to tissues other than those primary deposition organs, lung, liver and bone. Thus, the observed increase in the risk of this cancer should be treated with caution because of the lack of an established associated between pancreatic cancer and either external or internal radiation exposure (Omar et al, 1999; UNSCEAR, 2006; Cardis et al, 2007; Preston et al, 2007; UNSCEAR, 2008; Muirhead et al, 2009a) or may occur simply by chance because of multiple testing.
Strengths and limitations
The major strengths of this study are the large number of workers including both male and female individuals who were monitored for internal plutonium exposure as well as external gamma radiation, the long follow-up period, the wide range of radiation exposures, the cancer registry and particularly the information available on confounding factors such as smoking and alcohol use. A limitation in this study include the proportion of missing plutonium dose estimates in the early years, uncertainties in organ dose estimates for plutonium and unknown incidence data particular among the migrants. In addition, the small number of observed cancers to certain sites limits the power of the study to show significant associations with radiation exposure and the problem of multiple testing.
Acknowledgments
This work was supported in part by the European Commission of the 7th Framework Programme under EC-SOLO project (CP-IP: 249675). This study was performed using data from the Mayak Medical Dosimetry Registry established at the Epidemiological Department of SUBI. The analyses were performed using data from the Mayak Worker Dosimetry System, MWDS-2008, developed in the framework of Russian-American Program under the auspices of JCCRER funded by US Department of Energy (Program coordinator: Barry Fountos). Special thanks are also to Dale Preston (Hirosoft USA), ME Sokolnikov (SUBI), Richard Haylock (PHE, UK) and Michael Gillies (PHE, UK) for many valuable discussions.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Azizova TV, Muirhead CR, Moseeva MB, Grigoryeva ES, Vlasenko EV, Hunter N, Haylock RG, O'Hagan JA. Ishemic heart disease in nuclear workers first employed at the Mayak PA in 1948-1972. Health Phys. 2012;103:3–14. doi: 10.1097/HP.0b013e3182243a62. [DOI] [PubMed] [Google Scholar]
- Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, Yoshimura T, Bermann F, Cowper G, Fix J, Hacker C, Heinmiller B, Marshall M, Thierry-Chef I, Utterback D, Ahn YO, Amoros E, Ashmore P, Auvinen A, Bae JM, Bernar J, Biau A, Combalot E, Deboodt P, Diez Sacristan A, Eklof M, Engels H, Engholm G, Gulis G, Habib RR, Holan K, Hyvonen H, Kerekes A, Kurtinaitis J, Malker H, Martuzzi M, Mastauskas A, Monnet A, Moser M, Pearce MS, Richardson DB, Rodriguez-Artalejo F, Rogel A, Tardy H, Telle-Lamberton M, Turai I, Usel M, Veress K. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- Gilbert ES, Sokolnikov ME, Preston DL, Schonfeld SJ, Schadilov AE, Vasilenko EK, Koshurnikova NA. Lung cancer risks from plutonium: An update analysis of data from the Mayak worker cohort in Mayak workers. Radiat Res. 2013;179:332–342. doi: 10.1667/RR3054.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICRU: International Commission on Radiation Units and Measurements Determination of Dose Equivalents From External Radiation SourcesPart 2. Report 43Bethesda, MD, USA; 1988 [Google Scholar]
- Khokhryakov VV, Khokhryakov VF, Suslova KG, Vostrotin VV, Vvedensky VE, Sokolova AB, Krahenbuhl MP, Birchall A, Miller SC, Schadilov AE, Ephimov AV. Mayak Worker Dosimetry System 2008 (MWDS-2008): assessment of internal dose from measurement results of plutonium activity in urine. Health Phys. 2013;104:366–378. doi: 10.1097/HP.0b013e31827dbf60. [DOI] [PubMed] [Google Scholar]
- Koshurnikova NA, Shilnikova NS, Okatenko PV, Kreslov VV, Bolotnikova MG, Sokolnikov ME, Khokhryakov VF, Suslova KG, Vassilenko EK, Romanov SA. Characteristics of the cohort of workers at the Mayak Nuclear Complex. Radiat Res. 1999;152:352–363. [PubMed] [Google Scholar]
- Labutina EV, Kuznetsova IS, Hunter N, Harrison J, Koshurnikova NA. Radiation risk of malignant neoplasms in organs of main deposition for plutonium in the cohort of Mayak workers with regard to histological types. Health Phys. 2013;105:165–176. doi: 10.1097/HP.0b013e31828f57df. [DOI] [PubMed] [Google Scholar]
- Muirhead CR, O'Hagan JA, Haylock RGE, Phillipson MA, Willcock T, Berridge GLC, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009a;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead CR, O'Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, Zhang W. Health Protection Agency, HPA-RPD-062, Chilton Oxfordshire-UK; 2009b. Third Analysis of the National Registry for Radiation Workers: Occupational Exposure to Ionising Radiation in Relation to Mortality and Cancer Incidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar RZ, Barber JA, Smith PJ. Cancer mortality and morbidity among plutonium workers at the Sellefield plant of British Nuclear Fuels. Br J Cancer. 1999;79:1288–1301. doi: 10.1038/sj.bjc.6690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the Mortality of Atomic Bomb Survivors, Report 14, 1950-2003; An overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–243. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- Preston DL, Krestinina YuL, Sokolnikov ME, Ron E, Davis FG, Ostroumova EV, Gilbert ES. How much can we say about site-specific cancer radiation risk. Radiat Res. 2010;174:816–824. doi: 10.1667/RR2024.1. [DOI] [PubMed] [Google Scholar]
- Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure, release 2.10. HiroSoft: Seattle, WA, USA; 1998. [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, Koshurnikova NA. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159:787–798. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sokolnikov ME, Gilbert ES, Preston DL, Ron E, Shilnikova NS, Khokhryakov VV, Vasilenko EK, Koshurnikova NA. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer. 2008;123:905–911. doi: 10.1002/ijc.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko EK, Khokhryakov VF, Miller SC, Fix JJ, Eckerman K, Choe DO, Gorelov M, Khokhryakov VV, Knyasev V, Krahenbuhl MP, Scherpelz RI, Smetanin M, Suslova K, Vostrotin V. Mayak worker dosimetry study: an overview. Health Phys. 2007;93:190–206. doi: 10.1097/01.HP.0000266071.43137.0e. [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008. Effects of Ionizing Radiation.
- UNSCEAR 2006. Report to the General Assembly, with scientific annexes United NationsNew York [Google Scholar]
- World Health Organisation (WHO) 1977International Classification of Diseases, Injuries and Causes of Death9th revisionWHO: Geneva, Switzerland [Google Scholar]