Abstract
Background:
Epigenetic silencing by promoter methylation and chromatin remodelling affects hundreds of genes and is a causal event for lung cancer. Treatment of patients with low doses of the demethylating agent 5-azacytidine in combination with the histone deacetylase inhibitor entinostat has yielded clinical responses. The subcutaneous dosing route for consecutive days and reduced bioavailability of 5-azacytidine because of inactivation by cytidine deaminase may limit the expansion of epigenetic therapy into Phase III trials. To mitigate these barriers, an aerosol of 5-azacytidine was generated and characterised.
Methods:
The effect of aerosol vs systemic delivery of 5-azacytidine on tumour burden and molecular response of engrafted lung tumours in the nude rat was compared.
Results:
Pharmacokinetics revealed major improvement in the half-life of 5-azacytidine in lung tissue with aerosol delivery. Aerosolised 5-azacytidine significantly reduced lung tumour burden and induced global demethylation of the epigenome at one-third of the comparable effective systemic dose. High commonality for demethylation of genes was seen in tumours sampled throughout lung lobes and across treated animals receiving the aerosolised drug.
Conclusion:
Collectively, these findings show that aerosolised 5-azacytidine targets the lung, effectively reprogrammes the epigenome of tumours, and is a promising approach to combine with other drugs for treating lung cancer.
Keywords: gene methylation, 5-azacytidine, aerosol delivery, lung cancer, epigenome
Epigenetic-mediated silencing of genes by cytosine DNA methylation in CpG islands localised in promoter regions and chromatin remodelling involving the recruitment of transcriptional corepressors and modification of histone tails is a causal event for the initiation and progression of lung cancer (Jones and Baylin, 2002; Herman and Baylin, 2003). Silencing of hundreds of genes affecting all aspects of cell regulation has been detected through candidate and whole-genome approaches (Belinsky, 2005; Brena et al, 2007; Tessema et al, 2009). The minimal efficacy of conventional chemotherapy for advanced stage lung cancer and the reversibility of epigenetic-mediated gene silencing by pharmacologic agents has stimulated clinical efforts to evaluate the efficacy of epigenetic therapy. Cytosine methylation appears dominant in transcriptional repression, and inhibitors of the cytosine DNA methyltransferases, vidaza (5-azacytidine (5-Aza)) and decitabine (5-aza-2′-deoxyazacytidine) can induce in vitro re-expression of genes silenced through promoter hypermethylation (Jones and Baylin, 2002; Herman and Baylin, 2003). Importantly, although inhibitors of histone deacetylation (HDAC) are not very effective in inducing re-expression of genes silenced by promoter hypermethylation, such inhibitors can synergise with demethylating agents to relieve transcriptional repression (Cameron et al, 1999). The initial use of demethylating agents suggested that they were too toxic and not efficacious as cancer cytotoxic agents. However, laboratory findings revealed that the concentration of these drugs needed to induce gene demethylation and re-expression was much lower than that required to produce maximal cytotoxicity (Cameron et al, 1999). In fact, cell cycle arrest in response to cytotoxicity will reduce gene demethylation owing to the requirement of incorporation of these cytidine analogs into replicating DNA. Thus, these drugs were reassessed at doses much lower than the maximum-tolerated dose and proved to be a potent therapy for myelodysplasia (MDS), a precursor state to acute myelogenous leukaemia, leading to their approval by the FDA for treatment of these diseases (Silverman et al, 2002; Yang et al, 2006). Clinical trials with DNA-demethylating agents combined with HDAC inhibitors are also showing promising responses in the treatment of myeloid malignancies (Gore et al, 2006).
This low-dose strategy was extended in a Phase I/II trial in which patients with refractory advanced non-small-cell lung cancer were treated with 5-Aza and the HDAC inhibitor entinostat. For the Phase II component, patients received 40 mg m−2 per day 5-Aza subcutaneously on days 1 to 6 and 8 to 10 with entinostat taken orally on days 3 and 10 of each 28-day cycle. This therapy was well tolerated, and importantly, objective (complete or partial) responses were observed in two patients. Furthermore, 4 of 19 patients had major objective responses to subsequent anticancer therapies given immediately after epigenetic therapy. Although pretreatment tumour tissue was not available, the methylation state of a four-gene panel previously associated with rapid tumour recurrence was studied in serial plasma specimens (Juergens et al, 2011). Demethylation of these genes was associated with improved progression-free survival.
The expansion of epigenetic therapy into Phase III trials or to adjuvant therapy may be constrained by the required continuous daily subcutaneous dosing schedule that is also associated with some low-grade toxicity at the injection site (Juergens et al, 2011). Oral formulations are problematic because of hydrolysis and catabolism by cytidine deaminase; however, a recent film-coated formulation of 5-Aza has shown some bioavailability and response in a Phase I trial of MDS (Garcia-Manero et al, 2011). Cytidine deaminase expression is also highest in the liver where conversion of 5-Aza to its uridine counterpart occurs with subcutaneous administration as well and this reduces the concentration of active drug seen by the lung (Ho and Frei, 1971). Furthermore, sequence variants within the promoter region of cytidine deaminase have been associated with a threefold variation in enzymatic activity (Fitzgerald et al, 2006). Finally, increased activity of cytidine deaminase was associated with reduced half-life of 5-Aza and worse outcome for male patients with MDS (Mahfouz et al, 2013).
These barriers to expanding the use and improving the response to epigenetic therapy could be mitigated by aerosolised delivery that deposits drugs directly to the airways of the lungs by gravitational sedimentation and inertial impaction and to the peripheral region by diffusion. The absorption of the aerosol into the pulmonary vasculature also avoids hepatic first-pass metabolism. Thus, individual differences in cytidine deaminase activity would be eliminated and lower doses of drug could be delivered directly to the lungs to achieve equivalent clinical effects.
The development and testing of different exposure routes for therapy requires an animal model that best mimics human lung cancer and barriers for treatment. We have developed an orthotopic lung cancer model in which xenografts of human lung cancer-derived cell lines are efficiently engrafted throughout the lungs of the Rowett nude rat (March et al, 2001; Belinsky et al, 2011). Our first studies demonstrated that combination therapy with systemic delivery of 5-Aza and entinostat at doses and schedule similar to the Phase II clinical trial were synergistic in suppressing tumour growth and induced reprogramming of the epigenome as detected by gene demethylation and re-expression. The current study extends this work by generating and characterising an aerosol of 5-Aza and then comparing the effect of aerosol vs systemic delivery of 5-Aza on tumour burden and molecular response of engrafted lung tumours in the nude rat.
Materials and methods
Treatment protocols and tumour cell implantation
Male Rowett nude rats (Cr:NIH-ru), 8–10 weeks old were obtained from Frederick Cancer Research and Development (Frederick, MD, USA). For pharmacokinetic studies, rats (three per group per time point) were treated with a single dose of 5-Aza (Sigma, St Louis, MO, USA) dissolved in saline administered systemically by intraperitoneal injection (2 mg kg−1) or as an aerosol (0.6 mg kg−1 inhaled deposited dose) for 90 min via a nose-only nebuliser. The aerosol dose delivered to the rat lungs was calculated based on the aerosol concentration times the respiratory minute volume times the exposure period times the deposition fraction divided by body weight (Bide et al, 2000). Animals were serially killed for collection of plasma and lung tissue at 11 time points over 96 h after exposure. Calu-6 cells (containing K-ras and p53 mutations) obtained from American Type Culture Collection (Manassas, VA, USA) were cultured and instilled via orotracheal intubation as described (March et al, 2001; Belinsky et al, 2011). Three weeks following instillation of tumour cells, rats (n=20 per group) were treated with vehicle (saline), 5-Aza by intraperitoneal injection (2 mg kg−1), or aerosol (0.6 mg kg−1) for 4 consecutive days over 4 consecutive weeks as described (Belinsky et al, 2011). An additional six rats that did not receive tumour cells were included to determine tumour-free lung weights for comparison with treatment groups.
Inhalation exposure, aerosol characterisation, and pharmacokinetics
All animal procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee at Lovelace Respiratory Research Institute, which is accredited by the Association for Assessments and Accreditation of Laboratory Animal Care International. Rodents were housed in nose-only tubes during the exposures. Chamber oxygen content was collected directly from an enclosed nose-only exposure port. Temperature readings were obtained from a monitor placed within the secondary containment that enclosed the exposure chamber. All flow rates and system pressures (e.g., pressure in the sample, exhaust, and lines) were monitored by a rotameter (flow) and magnahelic (pressure) enclosed in a control panel. Flow rates were point-calibrated (calibrated at a specific flow rate) with a flow meter.
The aerosol of the solution nebuliser formulation (5 mg ml−1) was generated with three Pari LC Plus nebulisers (Pari Respiratory Equipment, Midlothian, VA, USA). The Pari LC Plus nebulisers were operated with clean, regulated inlet air at a pressure of 20 psi, which resulted in an output of ∼15 l min−1. The 5-Aza aerosol was directed through ∼24 in of a 1.58-cm diameter stainless-steel tube into a 52 port flow-past exposure chamber. The chamber exhaust was maintained at ∼14 l min−1.
Exposure monitoring was conducted by collecting material onto Pallflex 47-mm membrane filters (Pall Corporation, Port Washington, NY, USA). After sample collection, the filters were analysed gravimetrically by differential weights. Particle size (mean mass aerodynamic diameter (MMAD)) of the nebulised formulation was measured using a Mercer-style impactor (In-Tox Products Inc., Moriarty, NM, USA) operated at approximately 2.0 l min−1. The impactor sample was collected directly from the breathing zone of the nose-only exposure system. The analysis of impactor data and the calculation of MMAD were performed as described by Thiel (2002).
Plasma and lung samples were prepared via a solid-phase extraction before LC/MS/MS analysis. Chromatographic separation was conducted with an Agilent 1200 HPLC fitted with a C18 column held at 25 °C. The shallow gradient used buffered water and methanol to elute 5-Aza in ∼2 min (Kissinger and Stemm, 1986). Detection was performed with MS/MS analysis using an electrospray ionisation source in the positive mode.
Tissue collection and estimation of tumour burden
Before being killed, four animals from each treatment group and vehicle were randomly selected for collection of tumours (n=5 per animal) for molecular assays. After weighing the remaining lungs, they were inflated with 10% neutral-buffered formalin at a constant hydrostatic pressure of 25 cm for 6 h. Paraffin-embedded lungs were sectioned at 5-μm thickness and stained with haematoxylin and eosin. Our previous study demonstrated that treatment-related reduction in tumour burden was highly correlated with estimates of tumour volume (Belinsky et al, 2011). Therefore, tumour burden was used to assess the response to systemic vs aerosol delivery of 5-Aza. Tumour burden was quantitated 7 weeks following instillation of Calu-6 cells as the change in normal lung weight compared with tumour-bearing lung weights in the vehicle and 5-Aza treatment groups.
Gene expression
RNA was isolated from lung tumours (five per animal) collected from four rats per treatment group and vehicle following TRI-reagent (Sigma, St Louis, MO, USA) instructions. Total RNA (1 μg) was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's protocol. RT-qPCR was performed with the ABI PRISM 7900HT and inventoried Taqman assays (Applied Biosystems). Experiments were normalised to GAPDH or β-actin. Results were repeated in three separate experiments. Data were analysed with respect to a calibrator sample using the 2−ΔΔCt method and reported as relative quantity.
Gene methylation profiling
Bisulphite-modified DNA isolated from vehicle, 5-Aza intraperitoneally, or 5-Aza aerosol-treated Calu-6 tumours (four animals per group with three tumours per animal) were hybridised to the Illumina Methylation450 (HM450K) Beadchip (Illumina, San Diego, CA, USA). Bisulphite-modified DNA from normal bronchial epithelial cell lines obtained from three, cancer-free never smokers was included to identify genes methylated in normal cells.
Statistical analyses
Pharmacokinetic analysis was performed with WinNonLin (Pharsight Corp., Cary, NC, USA; Version 5.0.1) using non-compartmental analysis of plasma and lung samples that were above the limit of quantitation for the analytical assay. This analysis averages the amount of 5-Aza from the three animals at each time point to define the area under the curve, half-life, and concentration of the drug for systemic and aerosol delivery. Thus, no error values are given with the values presented in Table 1. The two-sample t-test and analysis of variance were used to compare the two treatment groups and the two groups with the vehicle, respectively. To check the appropriateness of the statistical methods, analyses were re-run with transformed data if appropriate or using the Kruskal–Wallis test.
Table 1. Pharmacokinetic parameters for aerosol and systemic delivery of 5-Aza.
| |
AUC (h × ng ml−1) |
Half-life (h) |
Concentrationmax
(ng ml−1) |
|||
|---|---|---|---|---|---|---|
| Delivery route | Plasma | Lung | Plasma | Lung | Plasma | Lung |
| Systemic | 19 128 | 1082 | 0.42 | 0.14 | 15 492 | 2147 |
| Aerosol | 1887 | 8460 | 1.24 | 1.84 | 893 | 7883 |
Abbreviations: 5-Aza=5-azacytidine; AUC=area under the curve; Max=maximum.
Owing to the strong association between methylation of CpGs around the transcriptional start site (TSS) and gene silencing, our analytic strategy for the HM450K arrays focused on this region to assess the methylation status of 84 735 CpG oligonucleotide probes within 200 bp 5′ of the TSS and extending through the 5′-untranslated region (X and Y chromosome and imprinted genes excluded). The output for the methylation arrays was preprocessed in GenomeStudio (San Diego, CA, USA), and the background was corrected using the normal exponential method in R. Average signal intensity between the methylated and unmethylated probes was determined, and a β-value from 0–1 (fully methylated) was calculated. Genes whose average β-values were ⩾0.2 across the interrogated region in normal bronchial epithelial cells were excluded from further analysis. Average β-values ⩾0.45 for genes were scored as positive for methylation in vehicle-treated tumours and a reduction in β-value of ⩾20% for a methylated gene was scored as demethylation. Analyses were carried out with SAS9.2 (Cary, NC, USA), unless otherwise indicated.
Results
Characterisation of the 5-Aza aerosol and pharmacokinetics
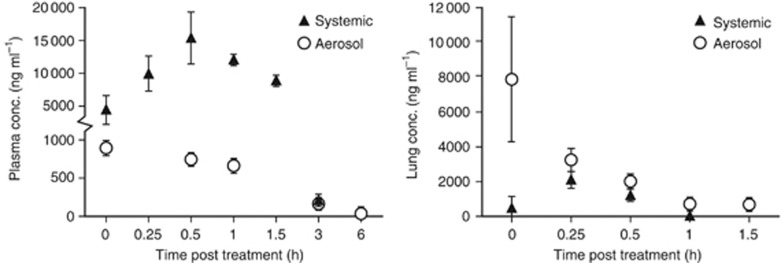
Because aerosol delivery should be more potent per unit dose, our target dose for 5-Aza was 75% less than the effective systemic dose of 2 mg kg−1. The actual calculated delivered dose of 5-Aza was 0.6 mg kg−1 based on an average aerosol concentration of 0.1 mg l−1 delivered over 90 min and pulmonary deposition assumed to be approximately 10% (Kuehl et al, 2012). Particle size analysis revealed an average MMAD of 0.71 μm with a geometric standard deviation of 1.96, values optimal for achieving good pulmonary distribution to the airways and parenchyma. The pharmacokinetic profile was determined in the blood and lung tissue following a single dose of 5-Aza delivered systemically (2 mg kg−1) vs via the nebulised aerosol (0.6 mg kg−1). As expected, the area under the curve for overall drug retention in blood was significantly greater for systemic than aerosol delivery, whereas the opposite was seen for delivery to the lungs (Figure 1 and Table 1). However, the half-life for 5-Aza in blood and lung tissue was increased 2.9- and 13-fold by aerosol delivery compared with systemic exposure (Table 1).
Figure 1.
Pharmacokinetics of 5-Aza delivered as an aerosol vs systemically. Plasma and lung tissue concentration as a function of time for the two delivery routes are depicted. Values are mean±s.e.m. from three animals per time point.
Aerosol delivery of 5-Aza reduces tumour burden and markedly increases the expression of the proapoptotic gene Bik
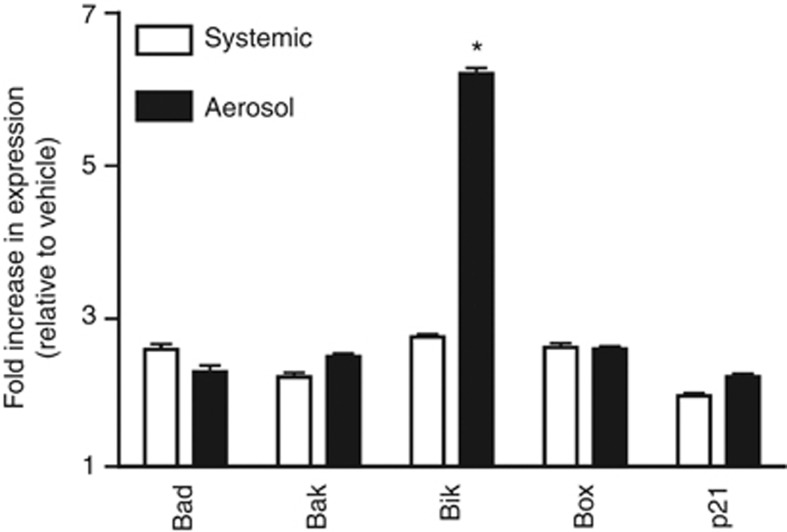
The delivery of 5-Aza systemically or via aerosol over 4 weeks resulted in an equivalent (34% vs 29%), significant reduction in tumour burden (Table 2). Rats also showed similar weight gain of 15–20%, irrespective of treatment route. Previous histological characterisation of Calu-6 tumours in the nude rat revealed two morphologically distinct cell populations and this was recapitulated in the current study (Belinsky et al, 2011). One population was composed of well-differentiated mucus-secreting adenocarcinoma cells and rare mitotic figures. A second interspersed cell population was composed of solid cords and nests of pleomorphic cells that occupied up to 75% of the tumour mass. These cells displayed high mitotic activity with up to 10 mitotic figures per × 40 field. Previously, the reduction in tumour burden seen with systemic delivery of 5-Aza was largely mediated through ablation of the pleomorphic cell population (<5% of the residual tumour) with a portion of the well-differentiated tumours remaining (Belinsky et al, 2011). Inhaled delivery of 5-Aza was equally effective as systemic delivery in removing the pleomorphic cell population (not shown). Expression of the proapoptotic genes studied previously (Belinsky et al, 2011), Bad, Bak, and Bok, were increased approximately threefold, irrespective of the exposure route in tumours when normalised to GAPDH or β-actin (Figure 2; not shown). Aerosol delivery of 5-Aza caused a more profound effect on the expression of Bik, a major proapoptotic gene in lung epithelial cells (Mebratu et al, 2008) than systemic delivery (Figure 2). The cyclin-dependent kinase inhibitor p21 (WAF1) functions as a regulator of cell cycle progression and senescence and its expression was also increased to a similar extent following systemic or aerosol delivery of 5-Aza (Figure 2).
Table 2. Epigenetic therapy delivered by aerosol reduces lung tumour burden.
| Treatment groupa | N | Delivery route | Lung weight (g), mean±s.d. | Adjustment for control, weight (±s.d.) |
|---|---|---|---|---|
| Controlb |
6 |
NA |
1.74±0.21 |
NA |
| Vehicle |
16 |
NA |
4.52±1.82 |
2.78±1.57 |
| 5-Aza |
18 |
Systemic |
3.58±0.95 |
1.84±0.82* |
| 5-Aza | 17 | Aerosol | 3.72±0.82 | 1.98±0.77* |
Abbreviations: 5-Aza=5-azacytidine; NA, not applicable.
*P<0.05 compared with vehicle.
Size of treatment groups differ because of engraftment. Instillation through the orotracheal intubation sometimes misses the trachea, resulting in placement into the oesophagus.
Control animals received no tumour cells.
Figure 2.
Delivery of 5-Aza by aerosol or systemically increases the expression of proapoptotic genes and p21. TaqMan PCR was used to quantify the change in expression of the proapoptotic genes Bad, Bak, Bok, and Bik and p21 in tumours from animals treated with 5-Aza delivered as an aerosol or systemically. The fold increase in expression is relative to expression seen in vehicle tumours (set to 1) and normalised to glyceraldehyde dehydrogenase (GAPDH). Values are the mean±s.e.m. from 20 tumours per group (four rats per group). All individual assays were conducted in triplicate. *P<0.001 compared with systemic delivery.
Treatment with 5-Aza induces global demethylation of the epigenome
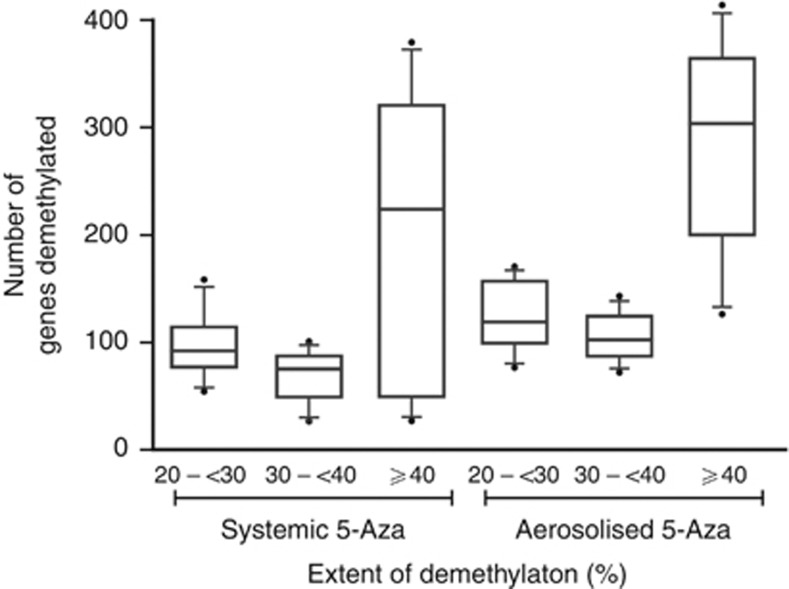
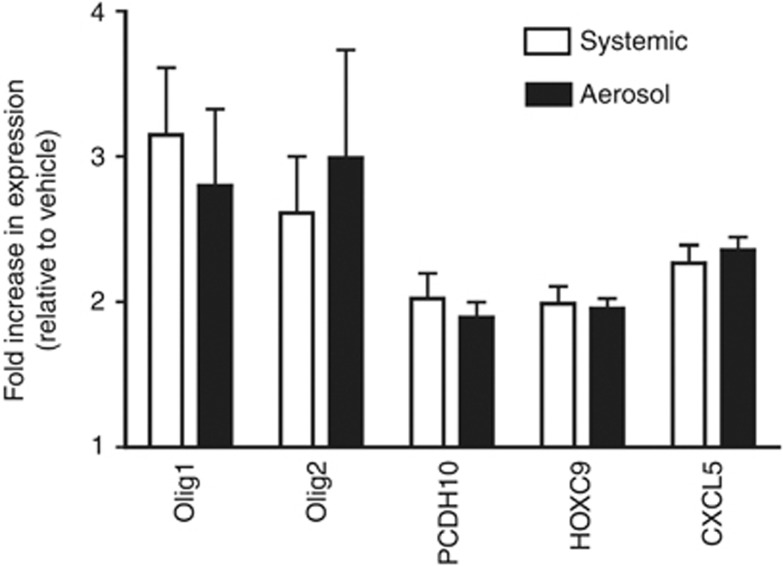
The HM450K array was used to compare the effectiveness of aerosol vs systemic delivery of 5-Aza for inducing demethylation of the epigenome. After excluding genes that were methylated in normal bronchial epithelial cell lines, the methylation state of 14 100 gene promoters defined by 200 bp 5′ of the TSS and extending through the 5′-untranslated region was determined in vehicle Calu-6 tumours. The number of methylated genes in vehicle-treated tumours varied by <1%, with 1019 genes methylated in all tumours. Classifying the extent of demethylation as 20–<30, 30–<40, or ⩾40% revealed a similar number of genes in each category independent of exposure route, although the average number was always greater in the tumours receiving 5-Aza as an aerosol (Figure 3). As seen in our previous study using the Illumina Methylation27 Beadchip (Illumina), the number of methylated genes and the extent of demethylation varied between tumours within and across animals, with the variance being largest for the number of genes showing ⩾40% demethylation. However, our previous study showed through bisulphite sequencing of the p16 CpG island a significant correlation between the extent of demethylation and increased gene expression (Belinsky et al, 2011). Overall, an average of 352 and 521 genes showed ⩾20% demethylation with systemic vs aerosol delivery of drug. Most striking, however, was the fact that there were 300 genes in common across all tumours from aerosol-treated rats that were demethylated ⩾20%, whereas only 20 commonly demethylated genes were seen with systemic treatment in all tumours. Relaxing the criteria to commonly demethylated in 75% of tumours identified 501 and 275 genes commonly associated with aerosol vs systemic exposure. Demethylation of all 275 genes following systemic exposure was also seen in tumours treated by aerosol delivery. Genes showing demethylation were involved in key cell functions (see Supplementary Table S1 for a complete list) that included adhesion and invasion (e.g., cadherins, laminins, keratins), migration (e.g., SLIT family), apoptosis (e.g., Elmo3), and cell trafficking (e.g., CXCL chemokines). Moreover, genes encoding for transcription factors (e.g., FOX, HOX, GATA families) were demethylated. Five genes of diverse function and exhibiting average demethylation of ⩾40% were selected to assess quantitatively the resulting change in expression compared with vehicle-treated tumours. Expression increases of 2- to 3.3-fold were seen when normalised to GAPDH or β-actin (Figure 4; not shown).
Figure 3.
5-Azacytidine therapy induces reprogramming of the epigenome. Boxplots depict the total number of genes with 20–<30, 30–<40, and ⩾40% demethylation following treatment with 5-Aza (systemic vs aerosol delivery) compared with vehicle-treated animals. The horizontal line in the boxes reflects the median number of genes demethylated, the lower boundary is the 25th quartile, and the upper boundary is the 75th quartile. The lines extend 1.2 times the upper or lower boundary and the asterisk signifies outliers. Four animals per treatment group and three tumours per animal (n=12 per group) were compared with four sham tumours using the HM450K array.
Figure 4.
5-Azacytidine increases gene expression. TaqMan PCR was used to quantify the change in expression of the CXCL5, OLIG1, OLIG2, HOXC9, and PCDH10 genes in tumours from animals treated with 5-Aza delivered as an aerosol or systemically. The fold increase in expression is relative to expression seen in vehicle tumours (set to 1) and normalised to glyceraldehyde dehydrogenase (GAPDH). Values are the mean±s.e.m. from 20 tumours per group (four rats per group). All individual assays were conducted in triplicate.
Discussion
These studies show that 5-Aza delivered as an aerosol can effectively reduce lung tumour burden and induce global demethylation of the epigenome at more than one-third of the comparable effective systemic dose. The improved pharmacokinetic profile seen in the lung, as manifested by a substantial increase in area under curve and drug half-life for aerosol compared with systemic delivery, is likely the major contributor to the increased inhaled target activity of this drug. Of great importance was the finding that the inhalation treatment resulted in a biochemically effective dose of 5-Aza that is distributed to the airways and parenchyma. This conclusion is based on the finding that although there was variation in the level of gene demethylation, tremendous commonality for genes demethylated ⩾20% was seen in the tumours collected throughout the lung lobes of rats receiving the aerosolised 5-Aza. Our studies did not address survival as an outcome because the aim was to focus first on improving the pharmacokinetic profile for this drug. However, Mahesh et al (2010) has shown that intratracheal compared with intravenous delivery of 5-Aza at equivalent doses increased the survival of mice bearing orthotopic H460 and H358 xenografts. Our future studies will include an HDAC inhibitor such as entinostat, as this combination is being used clinically and has shown superior efficacy to a demethylating agent alone in our murine and rat models (Belinsky et al, 2003, 2011).
On average, approximately 50% of the genes classified by the HM450K array as methylated in the Calu-6 tumours responded to the aerosol delivery. These genes as exemplified by our re-expression studies participate in many of the critical cell regulatory processes. CXCL5, a chemokine important in regulating cell migration and tumour metastasis, was demethylated and re-expressed following aerosolised 5-Aza. This chemokine along with two other family members, CXCL12 and CXCL14 (also demethylated following therapy), are silenced in more than 85% of primary lung adenocarcinomas (Tessema et al, 2010). Moreover, our previous work showed that forced expression of CXCL14 increased the expression of cell cycle inhibitor and proapoptosis genes and markedly abrogated growth of tumour xenografts (Tessema et al, 2010). Oligodendrocyte transcription factor 1 (OLIG1), whose silencing is significantly associated with poor prognosis for lung cancer patients, is demethylated by >70% and re-expressed with treatment (Brena et al, 2007). Finally, protocadherin-20, a homophilic cell adhesion member of the protocadherin family that is important in calcium-dependent cell–cell adhesion and signal transduction and silenced in 50% of tumours, was re-expressed by epigenetic therapy (Tang et al, 2012). These examples highlight the robust response seen with aerosol delivery of 5-Aza with regard to awakening key tumour suppressor genes to impact the growth of the Calu-6 line that also harbours mutations in the K-ras and p53 genes.
The promise of aerosol delivery for treatment of lung cancer is also substantiated through Phase I clinical trials with cisplatin and gemcitabine, two drugs used as first-line therapy for non-small-cell lung cancer. Cisplatin was encapsulated in lipid vesicles that are dispersed throughout the aqueous phase and delivered via nebuliser to patients with advanced and/or metastatic lung cancer who had progressed after multiple other therapies (Wittgen et al, 2007). Pharmacokinetic results showed very low plasma levels of cisplatin and no nephrotoxicity that is associated with intravenous delivery, whereas dose-limiting toxicity of the aerosolised cisplatin was not reached. Importantly, 12 of 16 patients had stable disease for up to eight, 21-day cycles of treatment, whereas the remaining patients progressed on therapy. Gemcitabine was reconstituted in saline and delivered by nebuliser in a dose escalation study to lung cancer patients 1 day per week for 9 consecutive weeks (Lemarie et al, 2011). The initial dose of drug included 99mTc-diethylene triamino pentaacetic acid to facilitate pharmacokinetic assessment of delivery to the lungs. On average, the total dose of gemcitabine delivered to the lungs was 42±16%, low plasma levels were observed, and no haematologic toxicity, nephrotoxicity, or neurotoxicity were observed. A minor response was seen in one patient, stable disease in four patients, and progressive disease in four patients. These studies demonstrate the feasibility and safety of aerosolised delivery of chemotherapeutics and support additional assessment in patients with less severe and extensive disease.
The rapid improvement in aerosol formulation and delivery devices could greatly change the landscape for therapy against lung cancer (Nanjwade et al, 2001; Gagnadoux et al, 2008). Clearly, the delivery of cytotoxins, drugs, and small molecules targeting genes or pathways to the primary tumour with minimal systemic toxicity will allow the testing of new combinatorial approaches and more intensive dose schedules. Dry powder inhalers offer improved stability and bioavailability for some drugs and the opportunity to combine drugs into a single formulation. For example, nanoparticles of paclitaxel have been assembled to form low-density microparticles in the presence of cisplatin in solution, a process that actually accelerated the dissolution of the paclitaxel (El-Gendy and Berkland, 2009). Delivering drugs in microparticles and nanoparticles can also overcome solubility constraints, facilitate sustained release, and achieve relatively uniform distribution of drug dose within the lung parenchyma for peripheral tumours (Willis et al, 2012; Meenach et al, 2013). Combination therapy with systemically delivered 5-Aza and the histone deacetylase inhibitor entinostat were synergistic for reducing tumour burden and further effecting global gene expression of Calu-6, A549 (K-ras mutant), and H1975 (EGFr mutant) tumours in our orthotopic lung cancer model (Belinsky et al, 2011). Moreover, hundreds of genes were demethylated highlighted by the re-expression of polycomb-regulated genes coding for transcription factor binding proteins. In addition, highly significant gene expression changes were seen in key regulatory pathways involved in cell cycle, DNA damage, apoptosis, and tissue remodelling.
Similar studies combining entinostat with aerosolised delivery of 5-Aza can now be pursued to see if the response to this drug combination is magnified further, given the improved pharmacokinetic profile for this drug with respect to lung half-life and commonality for demethylation between tumours. The target population for translating this work into the clinic is likely those patients with cancer largely confined to the lungs (Stages II and III), whereas aerosol delivery of 5-Aza could also benefit Stage I patients after resection as an adjuvant therapy to prevent a second primary cancer. Our studies cannot address another important question, whether the systemic exposure following aerosol delivery, albeit at a lower doses than subcutaneous delivery, but avoiding first-pass metabolism through the liver, will effect occult metastases likely present in Stage III lung cancer patients. This question will necessitate clinical trials in this patient population. Finally, it is attractive to propose chemoprevention targeting the epigenome for smokers identified to be at high risk for lung cancer through the detection of methylated genes in their sputum (Belinsky et al, 2006; Leng et al, 2012). Although 5-Aza cannot be used in cancer prevention because of its mutagenic properties, the development of aerosols for dietary polyphenols (whose inherent low bioavailability may limit effectiveness) that show in vitro activity toward inhibiting cytosine DNA methyltransferases may be a promising alternative (Fang et al, 2007).
Acknowledgments
This work was supported largely by the National Cancer Institute Specialised Program of Research Excellence P50 CA58184 and, in part, by the American Association for Cancer Research/Stand Up to Cancer Dream Team Translational Cancer Research Grant, grant number SU2C-AACR-DT0109.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Belinsky SA. Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer. Carcinogenesis. 2005;26:1481–1487. doi: 10.1093/carcin/bgi020. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y, Channell MM, Liu Y, Casero RA, Jr, Baylin SB, Reed MD, Tellez CS, March TH. Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res. 2011;71:454–462. doi: 10.1158/0008-5472.CAN-10-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, Baylin SB. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, Haney J, Kennedy TC, Hirsch FR, Miller Y, Franklin WA, Herman JG, Baylin SB, Bunn PA, Byers T. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 2000;20:273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::aid-jat657>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brena RM, Morrison C, Liyanarachchi S, Jarjoura D, Davuluri RV, Otterson GA, Reisman D, Glaros S, Rush LJ, Plass C. Aberrant DNA methylation of OLIG1, a novel prognostic factor in non-small cell lung cancer. PLoS Med. 2007;4:e108. doi: 10.1371/journal.pmed.0040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- El-Gendy N, Berkland C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm Res. 2009;26:1752–1763. doi: 10.1007/s11095-009-9886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- Fitzgerald SM, Goyal RK, Osborne WR, Roy JD, Wilson JW, Ferrell RE. Identification of functional single nucleotide polymorphism haplotypes in the cytidine deaminase promoter. Hum Genet. 2006;119:276–283. doi: 10.1007/s00439-006-0142-0. [DOI] [PubMed] [Google Scholar]
- Gagnadoux F, Hureaux J, Vecellio L, Urban T, Le Pape A, Valo I, Montharu J, Leblond V, Boisdron-Celle M, Lerondel S, Majoral C, Diot P, Racineux JL, Lemarie E. Aerosolized chemotherapy. J Aerosol Med Pulm Drug Deliv. 2008;21:61–70. doi: 10.1089/jamp.2007.0656. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ, Laille E, Giordano H, Sakoian S, Jabbour E, Kantarjian H, Skikne B. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29:2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, Rudek MA, Zhao M, Smith BD, Manning J, Jiemjit A, Dover G, Mays A, Zwiebel J, Murgo A, Weng LJ, Herman JG. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Ho DH, Frei E., III Clinical pharmacology of 1-beta-D-arabinofuranosyl cytosine. Clin Pharmacol Ther. 1971;12:944–954. doi: 10.1002/cpt1971126944. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, Sebree R, Rodgers K, Hooker CM, Franco N, Lee B, Tsai S, Delgado IE, Rudek MA, Belinsky SA, Herman JG, Baylin SB, Brock MV, Rudin CM. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger LD, Stemm NL. Determination of the antileukemia agents cytarabine and azacitidine and their respective degradation products by high-performance liquid chromatography. J Chromatogr. 1986;353:309–318. doi: 10.1016/s0021-9673(01)87101-6. [DOI] [PubMed] [Google Scholar]
- Kuehl PJ, Anderson TL, Candelaria G, Gershman B, Harlin K, Hesterman JY, Holmes T, Hoppin J, Lackas C, Norenberg JP, Yu H, McDonald JD. Regional particle size dependent deposition of inhaled aerosols in rats and mice. Inhal Toxicol. 2012;24:27–35. doi: 10.3109/08958378.2011.632787. [DOI] [PubMed] [Google Scholar]
- Lemarie E, Vecellio L, Hureaux J, Prunier C, Valat C, Grimbert D, Boidron-Celle M, Giraudeau B, le Pape A, Pichon E, Diot P, el Houfia A, Gagnadoux F. Aerosolized gemcitabine in patients with carcinoma of the lung: feasibility and safety study. J Aerosol Med Pulm Drug Deliv. 2011;24:261–270. doi: 10.1089/jamp.2010.0872. [DOI] [PubMed] [Google Scholar]
- Leng S, Do K, Yingling CM, Picchi MA, Wolf HJ, Kennedy TC, Feser WJ, Baron AE, Franklin WA, Brock MV, Herman JG, Baylin SB, Byers T, Stidley CA, Belinsky SA. Defining a gene promoter methylation signature in sputum for lung cancer risk assessment. Clin Cancer Res. 2012;18:3387–3395. doi: 10.1158/1078-0432.CCR-11-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh S, Saxena A, Qiu X, Perez-Soler R, Zou Y. Intratracheally administered 5-azacytidine is effective against orthotopic human lung cancer xenograft models and devoid of important systemic toxicity. Clin Lung Cancer. 2010;11:405–411. doi: 10.3816/CLC.2010.n.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz RZ, Jankowska A, Ebrahem Q, Gu X, Visconte V, Tabarroki A, Terse P, Covey J, Chan K, Ling Y, Engelke KJ, Sekeres MA, Tiu R, Maciejewski J, Radivoyevitch T, Saunthararajah Y. Increased CDA expression/activity in males contributes to decreased cytidine analog half-life and likely contributes to worse outcomes with 5-azacytidine or decitabine therapy. Clin Cancer Res. 2013;19:938–948. doi: 10.1158/1078-0432.CCR-12-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March TH, Marron-Terada PG, Belinsky SA. Refinement of an orthotopic lung cancer model in the nude rat. Vet Pathol. 2001;38:483–490. doi: 10.1354/vp.38-5-483. [DOI] [PubMed] [Google Scholar]
- Mebratu YA, Dickey BF, Evans C, Tesfaigzi Y. The BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of activated ERK1/2 to mediate IFNgamma-induced cell death. J Cell Biol. 2008;183:429–439. doi: 10.1083/jcb.200801186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenach SA, Vogt FG, Anderson KW, Hilt JZ, McGarry RC, Mansour HM. Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols. Int J Nanomed. 2013;8:275–293. doi: 10.2147/IJN.S30724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjwade BK, Adichwal SA, Gaikwad KR, Parikh KA, Manvi FV. Pulmonary drug delivery: novel pharmaceutical technologies breathe new life into the lungs. PDA J Pharm Sci Technol. 2001;65:513–534. doi: 10.5731/pdajpst.2011.00704. [DOI] [PubMed] [Google Scholar]
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- Tang X, Yin X, Xiang T, Li H, Li F, Chen L, Ren G. Protocadherin 10 is frequently downregulated by promoter methylation and functions as a tumor suppressor gene in non-small cell lung cancer. Cancer Biomark. 2012;12:11–19. doi: 10.3233/CBM-2012-00280. [DOI] [PubMed] [Google Scholar]
- Tessema M, Klinge DM, Yingling CM, Do K, Van Neste L, Belinsky SA. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene. 2010;29:5159–5170. doi: 10.1038/onc.2010.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessema M, Yu YY, Stidley CA, Machida EO, Schuebel KE, Baylin SB, Belinsky SA. Concomitant promoter methylation of multiple genes in lung adenocarcinomas from current, former and never smokers. Carcinogenesis. 2009;30:1132–1138. doi: 10.1093/carcin/bgp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CG. Cascade impactor data and the lognormal distribution: nonlinear regression for a better fit. J Aerosol Med. 2002;15:369–378. doi: 10.1089/08942680260473443. [DOI] [PubMed] [Google Scholar]
- Willis L, Hayes D, Jr, Mansour HM. Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery. Lung. 2012;190:251–262. doi: 10.1007/s00408-011-9360-x. [DOI] [PubMed] [Google Scholar]
- Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ, Postmus PE. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–2421. doi: 10.1158/1078-0432.CCR-06-1480. [DOI] [PubMed] [Google Scholar]
- Yang AS, Doshi KD, Choi SW, Mason JB, Mannari RK, Gharybian V, Luna R, Rashid A, Shen L, Estecio MR, Kantarjian HM, Garcia-Manero G, Issa JP. DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res. 2006;66:5495–5503. doi: 10.1158/0008-5472.CAN-05-2385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.