Abstract
The health concerns of women in their mid-adult years – when the prime age of reproduction has passed – have been traditionally given little or no attention by health systems and donors, despite the heavy burden that diseases such as breast and cervical cancer impose on women and their families. The risk of sexually transmitted infections that accompanies sexual relations and the risk of death and morbidity associated with pregnancy have long been recognized and have stimulated major control efforts that are finally yielding positive results. Much less attention has been focused, however, on how experiences in early life can affect women’s health in adulthood.
Breast and cervical cancers kill more women than any other types of cancer in all parts of the developing world. In most of Asia and Latin America and some African countries, deaths from these two forms of cancer now outnumber pregnancy-related deaths. There are five compelling reasons for focusing on these cancers now to try to reverse these epidemiologic trends: (i) the burden of breast and cervical cancer is large and is growing; (ii) effective screening and treatment are available; (iii) research is generating new knowledge; (iv) there are opportunities for synergy with other health programmes; and (v) noncommunicable diseases are the focus of much current interest.
Résumé
Les problèmes de santé des femmes au milieu de l'âge adulte (une fois passé le premier âge de la reproduction) ont traditionnellement bénéficié de peu d'attention ou ont été ignorés par les systèmes de santé et les donateurs, malgré le lourd fardeau que constituent les maladies, comme le cancer du sein et du col de l'utérus pour les femmes et leurs familles. Le risque d'infections sexuellement transmissibles qui accompagne les relations sexuelles, et le risque de mortalité et de morbidité associé à la grossesse sont reconnus depuis longtemps et ont guidé d'importants efforts de lutte, qui finissent par donner des résultats positifs. Beaucoup moins d'attention a cependant été accordée à la façon dont les expériences en début de vie peuvent affecter la santé des femmes à l'âge adulte.
Les cancers du sein et du col de l'utérus tuent plus de femmes que tout autre type de cancer dans tous les pays du monde en voie de développement. Dans la plupart des pays d'Asie, d'Amérique latine et dans certains pays d'Afrique, les décès résultant de ces deux formes de cancer sont maintenant plus nombreux que les décès liés à la grossesse. Il y a cinq raisons impérieuses de se concentrer maintenant sur ces cancers afin d'essayer d'inverser ces tendances épidémiologiques: (i) le fardeau du cancer du sein et du cancer du col de l'utérus est toujours plus lourd; (ii) un dépistage et des traitements efficaces sont disponibles; (iii) la recherche génère de nouvelles connaissances; (iv) il existe des possibilités de synergie avec d'autres programmes de santé; et (v) les maladies non transmissibles génèrent beaucoup d'intérêt actuellement.
Resumen
Los problemas de salud de las mujeres de mediana edad, una vez han pasado la plenitud de la edad reproductora, han recibido tradicionalmente poca o ninguna atención por parte de los sistemas sanitarios y donantes, a pesar de la gran carga que enfermedades como el cáncer de mama o de cuello uterino representan para las mujeres y sus familias. El riesgo de enfermedades de transmisión sexual a través de relaciones sexuales, y el riesgo de muerte y enfermedad asociados con el embarazo han sido reconocidos y se han impulsado intentos de control a gran escala que al fin están dando resultados positivos. Sin embargo, se ha prestado mucha menos atención a cómo las experiencias en las primeras etapas de la vida pueden afectar la salud de las mujeres en la vida adulta.
En el mundo desarrollado, los cánceres de mama y de cuello uterino matan a más mujeres que ningún otro tipo de cáncer. En la mayor parte de Asia y América Latina y algunos países africanos, las muertes por estos dos tipos de cáncer superan en número a las muertes relacionadas con el embarazo. Hay cinco razones de peso para centrar la atención en estos tipos de cáncer con objeto de intentar revertir estas tendencias epidemiológicas: (i) la carga de los cánceres de mama y de cuello uterino es muy elevada, y sigue creciendo; (ii) existen controles y tratamientos eficaces; (iii) la investigación está proporcionando conocimientos nuevos; (iv) existen oportunidades de sinergia con otros programas sanitarios y (v) las enfermedades no transmisibles reciben gran parte de la atención actual.
ملخص
عادة ما تولي النظم الصحية والجهات المانحة اهتماماً قليلاً أو لا تولي اهتماماً بالمخاوف الصحية للنساء في مرحلة أواسط العمر، عند فوات أنسب سن للإنجاب، على الرغم من العبء الثقيل الذي تفرضه أمراض مثل سرطان الثدي وعنق الرحم على النساء وأسرهم. وتم الإقرار منذ فترة طويلة بخطورة العدوى المنقولة جنسياً التي تصاحب العلاقات الجنسية وخطورة الوفاة ومعدلات المراضة المرتبطة بالحمل وأدى ذلك إلى تحفيز جهود كبرى للمكافحة مما أسفر عن نتائج إيجابية في النهاية. ومع ذلك، تم إيلاء القليل من الاهتمام بكيفية تأثير الخبرات في المراحل المبكرة من الحياة على صحة المرأة في مرحلة البلوغ.
يقتل سرطان الثدي وسرطان عنق الرحم عدداً أكبر من النساء مقارنة بأنواع السرطان الأخرى في جميع مناطق العالم النامي. وفي معظم أسيا وأمريكا اللاتينية وبعض البلدان الأفريقية، يزيد عدد الوفيات الناجمة عن هذين الشكلين من أشكال السرطان عن عدد الوفيات ذات الصلة بالحمل. وتوجد خمسة أسباب دامغة للتركيز على هذين المرضين الآن لمحاولة حسر هذه الاتجاهات الوبائية: (1) عبء سرطان الثدي وسرطان عنق الرحم كبير ومتنام؛ (2) توافر الفحص والعلاج الفعالان؛ (3) توفر الأبحاث معرفة جديدة؛ (4) توجد فرص للتآزر مع البرامج الصحية الأخرى؛ (5) إيلاء قدر كبير من الاهتمام في الوقت الراهن بالأمراض غير السارية.
摘要
尽管乳腺癌和宫颈癌等疾病对妇女及其家庭带来沉重的负担,传统上卫生系统和捐助者对中年女性(已过了生殖黄金年龄)的健康问题关注甚少或毫无关注。性关系伴生的性病感染风险以及与妊娠相关的死亡风险和发病率早已被确认,并刺激主要的控制工作,最终取得积极成果。但是,对早期生活如何影响妇女成年健康的关注度远远不够。
在所有发展中国家,死于乳腺癌和宫颈癌的妇女比其他类癌症都要多。在大多数亚洲和拉丁美洲国家以及一些美洲国家,这两种癌症的死亡数已经超过与妊娠相关的死亡数。现在需要集中精力尝试扭转这些流行病学趋势有五个令人信服的理由:(i) 乳腺癌和宫颈癌的负担很大,且在不断增加;(ii) 有可用的有效筛查和治疗;(iii) 研究正在产生新的知识;(iv) 有机会与其他卫生计划协同;以及 (v) 非传染性疾病是当前许多关注的焦点。
Резюме
Системами здравоохранения и организациями-донорами уделяется традиционно мало внимания охране здоровья женщин средних лет, уже миновавших основной репродуктивный возраст, несмотря на тяжелое бремя заболеваний, таких как рак молочной железы и рак шейки матки, накладываемое на женщин и их семьи. Риск инфекций, передаваемых половым путем, и риск смерти и осложнений, связанных с беременностью, являются давно признанными проблемами, которые стимулировали основные усилия, направленные на их решение и дающие, наконец, положительные результаты. Гораздо меньше внимания уделяется, однако, тому, как опыт в начале жизни женщин может повлиять на их здоровье в зрелом возрасте.
Рак молочной железы и шейки матки убивает больше женщин, чем любые другие типы рака во всех частях развивающегося мира. В большинстве стран Азии, Латинской Америки и некоторых африканских странах смертность от этих двух форм рака в настоящее время превышает число случаев смертности, связанных с беременностью. Ниже перечислены пять веских причин для активизации борьбы с этими видами рака, которая поможет переломить текущие тенденции эпидемиологического характера: (I) бремя рака молочной железы и рака шейки матки значительно и продолжает расти; (II) имеются методы эффективного обследования и лечения; (III) современные научные исследования приводят к получению новых знаний; (IV) есть возможности для взаимодействия с другими программами здравоохранения; (V) неинфекционные болезни сегодня находятся в центре внимания общества.
Introduction
It has been recognized for decades that women in low-resource settings suffer a crushing burden of morbidity and mortality associated with the universal life experience of reproduction. Since the clarion call from Allan Rosenfield and Deborah Maine in 19851 and the first Safe Motherhood meeting, which was held in Nairobi in 1987, there has been a dedicated movement to reduce this toll – an effort that is finally showing good results.2,3 Much attention has been paid to women in their teens and twenties because of the risks associated with sexuality and pregnancy – namely, infection with the human immunodeficiency virus (HIV) and other sexually transmitted infections, unwanted pregnancy and associated unsafe abortion, and obstetric complications. Much less attention has been focused by governments and donors on how these earlier life experiences affect the health of women when they reach their thirties, forties and fifties – those ill-defined middle years between youth and old age.
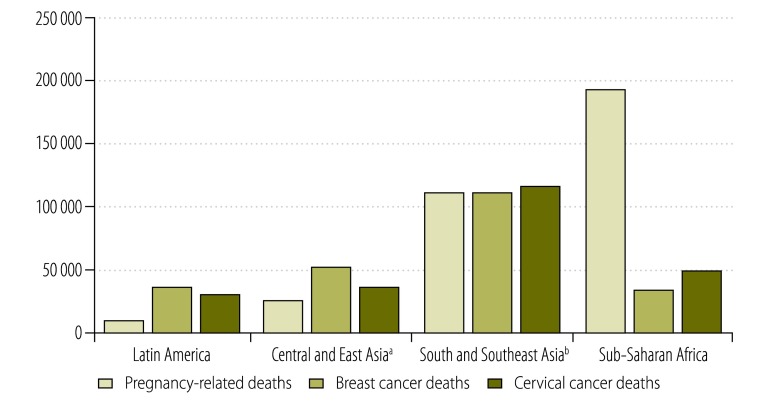
Cancers of the breast and cervix kill more women than any other forms of cancer in all parts of the developing world. While in the past maternal deaths dominated reproductive mortality in low and middle-income countries, in most countries of Asia and Latin America and some countries of Africa, deaths due to the complications of pregnancy are now outnumbered by deaths from breast or cervical cancer (Fig. 1). The causes of breast and cervical cancer are related, at least in part, to a woman’s sexual and reproductive choices and other exposures in early life – i.e. history of infection with the human papillomavirus (HPV), age at first pregnancy and number of pregnancies, breastfeeding history, diet and physical activity. However, the same reproductive factors that protect against one form of cancer increase the risk of the other form. Women who have early and frequent pregnancies and who breastfeed their children have a lower risk of getting breast cancer but are at increased risk of developing cervical cancer.5 In low- and middle-income countries, where acute, infectious diseases and pregnancy-related morbidity and death are common, health care has understandably been designed primarily around these areas. However, there is a growing recognition in these countries that new epidemiologic patterns are emerging because of lifestyle changes and gains in life expectancy and that noncommunicable diseases, including cancer, are becoming an increasingly important part of the health landscape.
Fig. 1.
Deaths from cancers of the breast and cervix compared with pregnancy-related deaths in low- and middle-income countries in four geographical regions, 2008
a Central and East Asia includes: Afghanistan, China, Democratic People’s Republic of Korea, Kazakhstan, Kyrgyzstan, Mongolia, Tajikistan, Turkmenistan and Uzbekistan.
b South and South-east Asia includes: Bangladesh, Bhutan, Cambodia, India, Indonesia, Lao People's Democratic Republic, Malaysia, Myanmar, Nepal, Pakistan, Papua New Guinea, Philippines, Sri Lanka, Thailand and Viet Nam.
The time is right to focus on breast and cervical cancer and to support critical interventions for reducing the incidence of these two diseases and their case-fatality rates. Thanks to a concerted global effort, in many places maternal mortality is no longer the leading cause of death among adult females. With similar effort, comparable strides can be made towards reducing the morbidity and mortality linked to breast and cervical cancer. This paper presents five compelling reasons for marshalling our resources and taking action now, a time during which need and opportunity are converging.
Burden is high, growing and inequitable
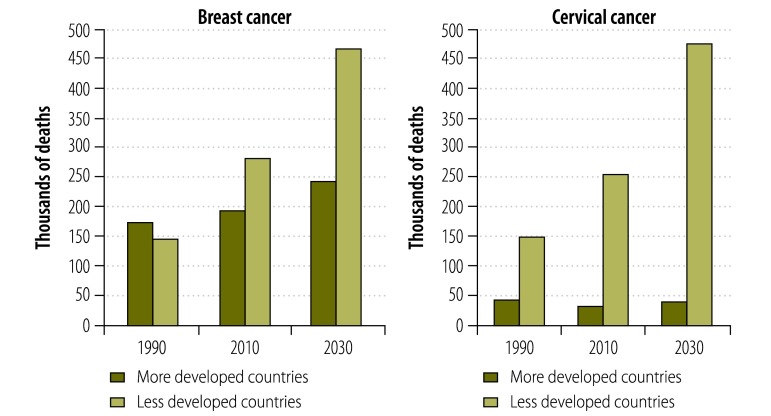
In “less developed countries”, as defined by GLOBOCAN,4 690 000 new cases of breast cancer and 450 000 new cases of cervical cancer occurred in 2008. Unless action is taken to reverse epidemiologic trends, by 2030 incident cases of breast and cervical cancer will have increased to 1.1 million and 730 000, respectively – figures that represent an increase of more than 60% in disease incidence over a period of just over 20 years. The gap between more and less developed regions is expected to widen (Fig. 2) as the proportion of global deaths from cervical cancer and breast cancer that occur in less developed parts of the world rises from the current 88% to 99% and from 59% to 63%, respectively. Despite the common misconception that breast cancer is primarily a problem of high-income countries, in 2010 the majority of the world’s 425 000 deaths from breast cancer occurred in developing countries (as defined by Forouzanfar et al.).6 Of the breast cancer deaths that occurred in developing countries, 68 000 were in women less than 50 years old.6
Fig. 2.
Estimated global deaths from breast and cervical cancer, by country level of development as defined by GLOBOCAN,a 1990, 2010 and 2030
a The GLOBOCAN project, which presents epidemiologic data on all forms of cancer as provided by the International Agency for Research on Cancer in Lyon, France, classifies North America, Europe, Australia/New Zealand and Japan as “more developed” and the rest of the world as “less developed”.
Disparities in morbidity and mortality rates reflect the influence not only of biological and environmental factors, but also of social and cultural determinants linked to the question of fairness and social justice. Equity is an important aspect to consider in control efforts, which should be guided by special consideration for those who are more vulnerable to illness or less able to access health-care services because of social, economic or demographic factors beyond their control.7 The “cancer divide” between rich and poor countries is further exacerbated by gender discrimination.8 Because of a combination of gender-related social and economic factors, in some countries women have traditionally had less access to health-care services than men.9,10 Cancers of the breast and cervix most often strike women in their forties and fifties, when they are still raising families. In addition to the suffering this brings to women, the accompanying illness and death are detrimental to their children and other dependants in the family and deprive communities of their contribution to society as experienced health workers, teachers and food producers.11
Cervical cancer is largely preventable with effective screening and treatment of precancerous lesions, and breast cancer survival rates are greatly reduced through early detection and treatment. Hence, much of the disparity in disease burden is rooted in inequitable access to care. This becomes even more evident when the number of deaths is divided by the number of incident cases to obtain an approximation of case-fatality rates. Many more women die of their cancers in African countries than in industrialized countries. In the United States of America, the ratio of the number of cervical cancer deaths to the number of new cases was 0.27 in 2008, whereas in less developed regions it was 0.53 and in sub-Saharan Africa it was 0.67. Similarly, the ratio of breast cancer deaths to new cases was just 0.22 in the United States, whereas in less developed regions it was 0.39 and in sub-Saharan Africa it was 0.54.4 Rates of screening for breast and cervical cancer are very low in low-income countries. According to data from the World Health Survey of 2003, 4.1% of women between the ages of 18 and 69 years had been screened for cervical cancer in the previous three years and 2.2% of women between the ages of 40 and 69 years had had a mammogram in the previous five years.12 Living in a poor household, in a rural area and in a country with low government expenditure on health were the primary determinants of reported low rates of cervical cancer screening.12
Availability of proven interventions
Two myths that deter women from getting screened for cervical and breast cancer are that effective interventions against these diseases are not available or not affordable.13 For cervical cancer and, to a lesser extent, breast cancer, effective control measures are available and affordable.
Cervical cancer
Cervical cancer can be largely prevented through vaccination against HPV infection and by screening for and treating precancerous lesions. The two HPV vaccines in use are extremely safe and efficacious.14 In addition, experience has shown that well-designed immunization programmes can achieve high coverage among young adolescent girls in low- and middle-income countries.15–17 High vaccine costs, an important barrier for many governments, have come down steadily in low- and middle-income countries and in 2012 the GAVI Alliance accepted applications from eligible countries for subsidized HPV vaccine.18 Although the vaccine has only been in use for a few years and any impact on cervical cancer rates will not be appreciable for another 25 to 30 years, there is already preliminary evidence of an effect on the prevalence of HPV infection19,20 and cervical abnormalities.21
Advances in screening have also been made. New screening tests have been developed and new screening programme models have been validated. Visual inspection with acetic acid (VIA) and new tests for the detection of HPV DNA, which became commercially available in China and India in 2013, provide low- and middle-income countries with cheaper and less cumbersome alternatives to the traditional Papanicolaou (Pap) smear. Although screening based on the Pap smear ushered in the decline in cervical cancer rates in the second half of the 20th century in industrial countries, it has proved difficult to establish and sustain in low- and middle-income countries.22,23 As of December 2012, 24 countries had selected VIA as their national screening strategy and 29 others had piloted VIA screening.24 “Screen and treat” approaches, in which VIA or HPV testing is followed by treatment of precancerous lesions with cryotherapy without further confirmatory diagnostic testing, have led to reductions in cervical cancer incidence and mortality.25–27 Tests for the detection of HPV DNA have the added advantage that a woman can obtain her own vaginal sample and take it for testing to a nearby laboratory. Recent studies in India, Nicaragua and Uganda, which have strikingly different cultures, have shown that self-collection of vaginal specimens for testing with a low-cost HPV DNA test designed for low-resource settings was highly acceptable to women. In addition, the test showed higher sensitivity in detecting high-grade cervical lesions than Pap smears or VIA, despite good specificity.28
Breast cancer
In the case of breast cancer, evidence surrounding the effectiveness of primary prevention is less straightforward. Nonetheless, low- and middle-income countries present opportunities for action. Interventions that promote changes in lifestyle, including reduced alcohol consumption, reduced fat intake and the practice of regular physical activity, can lower the risk of developing the disease and will have additional benefits by also lowering the risk of developing other noncommunicable diseases.29 The highest impact on mortality, though, will come from earlier detection, accurate diagnosis and more widely available basic treatment. Although screening mammography has been shown to reduce breast cancer mortality in high-income countries,30 it is generally neither affordable nor appropriate for detecting tumours in the advanced stages usually seen in low-resource settings, where women often present with tumours that are easily palpable, visible or ulcerated through the skin.31 Interventions that promote clinical breast examination and increase community awareness of the symptoms of breast cancer and of the importance of screening can greatly increase the fraction of tumours that are detected at an earlier stage, before they become readily palpable or visible, as shown by Mittra et al. and Sankaranarayanan et al.32,33
The role of screening mammography is still being debated. Opinions differ on the age when it should be initiated and how often it should be performed. In most high- and upper-middle-income countries in Europe, Latin America and North America where mammography screening is practised, the established age of initiation is generally 50 years and subsequent mammograms are performed every two years, as recommended in 2009 by the United States Preventive Services Task Force.34 As important as these debates may be for wealthy countries, they are largely irrelevant for low-and lower-middle-income countries, where the per capita cost of mammographic screening surpasses what the government can afford to pay. However, women in these same countries usually present to health services with advanced disease and poor quality of life. In such cases, the cure rate is low and case management, except for palliative care, becomes resource-intensive and costly. Educational interventions and basic clinical tools, such as regular breast examinations by a well-trained health-care professional, can result in early detection and lead more women to receive basic treatment at an affordable cost.
The optimal age to start screening with clinical breast examination has not been determined because data from randomized clinical trials are not yet available regarding the method’s impact on stage at diagnosis and on breast cancer mortality in low- and middle-income countries. In high-income countries, population-based screening mammography is not routinely performed before the age of 40 – or in many cases 50 years – but in the United States clinical breast examination is recommended instead of mammography every one to three years during routine wellness visits in women between the ages of 20 and 40 years.35 This generates many false–positive results that need to be verified by means of expensive and invasive tests and has a relatively low yield in terms of the number of cancers detected, given the low rate of cancer in this age group. In low- and middle-income countries, the average age in which breast cancer is diagnosed is in the early forties. Since clinical breast examination does not require any technology, routine screening with this method can be initiated at the age of 35 years, as in Mumbai, India, or at the age of 40 years in settings with more limited resources with which to perform diagnostic evaluations.32
In Peru, an innovative, community-based breast cancer screening model is being tested. It spans the continuum from community education and clinical breast examination at the primary care level to diagnostic triage at the community hospital level and referral of women with breast cancer to a regional cancer centre for treatment. PATH, an international non-profit organization, is working with Peru’s National Cancer Institute and Ministry of Health and with the Regional Cancer Institute in the northern region of La Libertad to offer screening services to rural women. Community health promoters hold educational sessions with women to explain to them about breast cancer awareness and the eight key signs and symptoms of the disease and to encourage women between the ages of 40 and 64 years to undergo annual clinical breast examinations at their local health centres. Midwives have been trained to perform such examinations and to counsel women and refer them to appropriate services, and local physicians have been trained to obtain a fine needle aspiration biopsy and examine the specimen for adequacy before sending it for analysis by a pathologist trained in breast cytology. Women with positive biopsies are referred for full evaluation and treatment. More than 3000 women were screened in the first 18 months of the programme and six cases of cancer were diagnosed. As a next step, two components will be added to the care model: (i) to perform an ultrasound for more thorough evaluation before obtaining a biopsy, and (ii) to offer women community support during and after treatment. The competency-based curricula for promoters, midwives and physicians have been coordinated to ensure consistent messages at all levels.36 In Mexico, breast cancer screening and treatment has been incorporated into the national health insurance scheme. In Brazil, a group has developed an urban mammography-based screening programme.37,38
An important tool for the detection and management of breast cancer is a set of guidelines that cover the whole spectrum of breast care and that are stratified by resource level. The Breast Health Global Initiative (BHGI), established in 2002, applied a consensus panel process to develop evidence-based, resource-sensitive guidelines for breast cancer early detection, diagnosis and treatment and for improving health-care delivery systems for women with breast cancer in low- and middle-income countries.39 Under the BHGI framework, a four-tiered system of resource allotment is used to establish prioritization schemes based on the level of existing resources (basic, limited, enhanced and maximal) and the stage of disease at diagnosis. In the past, health ministries and other decision-making bodies have lacked the tools needed to perform internal analyses to determine the suitability of the existing infrastructure for breast cancer screening, diagnosis and treatment, the areas that would need to be improved and the cost of improving them at the basic level. The BHGI framework provides a useful tool for objective and rational decision-making based upon verifiable local and global evidence.
Where available, basic surgery, low-cost generic drugs and radiation therapy are the cornerstones of breast cancer treatment.40 Modified radical mastectomy is the mainstay of locoregional treatment at the basic level. Endocrine therapy with generic (low-cost) drugs such as tamoxifen provides effective post-surgical treatment for tumours that are positive for estrogen receptors (ERs), or binding sites. Unlike systemic chemotherapy, which requires complex systems for dose determination, infusion and monitoring, endocrine therapy is oral and can be dispensed from a pharmacy. However, a tumour’s ER status must be known before the drug can be used. For estrogen-receptor-negative cancers, systemic cytotoxic chemotherapy is effective but needs to be administered in a safe, sterile environment and requires monitoring for drug toxicity in the form of periodic blood chemistry profiles and complete blood counts. Some older cytotoxic drugs are effective and affordable and efforts are under way to include them in essential medicine lists. Radiation therapy allows for breast-sparing surgery and is used for chest wall irradiation after mastectomy and for the palliation of painful or symptomatic metastases. It is not sufficiently available yet, but the International Atomic Energy Agency is working with countries to increase its availability.41
Partnerships between industrialized and developing countries to build specialist capacity or to provide access to specialist care while local staff are in training have been quite successful. A collaborative training programme between a pathology department in Tromsø, Norway, and the Komfo Anokye Teaching Hospital (KATH) in Kumasi, Ghana, provides an example of how pathology diagnostic services can be made available to patients in low-income settings.42 Problems observed in the Ghana laboratory, such as poor specimen quality and inadequate descriptions of macroscopic specimens, led to the development of new onsite guidelines for tissue fixation procedures, macroscopic examination and tissue block selection. Telepathology can also enhance training in some settings and has been used by doctors in the United Republic of Tanzania and other countries to consult with North American and European colleagues on challenging cases.43
Research to fill knowledge gaps
Cryotherapy is generally used to treat precancerous cervical lesions because it can be easily taught to mid-level care providers, the initial equipment purchase price is lower than for alternative therapeutic methods, and the level of infrastructure needed, such as electricity, is less. However, it requires a reliable supply of gas (nitrous oxide or carbon dioxide), which has proven difficult to secure in some rural settings. The International Agency for Research on Cancer is evaluating the feasibility and effectiveness of cold coagulation as an alternative treatment and several private companies are also developing alternative treatment modalities, including pharmacological methods.
Appropriate breast cancer treatment depends on an accurate pathology diagnosis, which in turn requires the availability of tissue sampling procedures. Fine-needle aspiration biopsy is the most cost-effective procedure and has a short turnaround time. However, the choice of sampling procedure – fine needle aspiration biopsy, core needle biopsy or excisional biopsy – should be based on the relative availability of cytologists or pathologists in each medical community and on the availability and cost of the required equipment and supplies. In Peru, for example, the cost of large-bore needles for core biopsy (about 60 United States dollars per unit) was prohibitive. One of the authors (JJ) is working with PATH’s technology development group to investigate less expensive sources and alternative designs for core-needle devices and components.
The accurate determination of a tumour’s ER status spares women with ER-negative breast cancer from the side-effects and expense of endocrine treatment. The use of immunohistochemistry to determine a tumour’s ER status requires substantial resources. The development of a low-cost technology for assessing ER status in the field would reduce this problem.44 PATH’s diagnostic development group is exploring alternative approaches for rapid, point-of-care tests based on nucleic acid amplification.
Two models of care that have long been studied in India may prove to be viable and effective. Mittra et al. are conducting joint breast and cervical cancer screening in Mumbai using VIA and clinical breast examination.32 In Trivandrum, where clinical breast examination and community education about breast cancer’s warning signs and symptoms are being taught, as recommended by the World Health Organization, long-term follow-up will reveal whether education without screening but linked to diagnosis and adequate treatment can lead to the detection of breast cancer at an earlier stage.33
Opportunities for synergy
With a coordinated approach to breast and cervical cancer screening, opportunities for synergy exist at several levels. At the primary care level, midwives and nurses frequently have skills that enable them to conduct cervical examination. Both midwives and nurses are trusted by women in the community, who often consult them when they develop problems with their breasts in the postnatal period. More formal training about normal and abnormal breast tissue would improve these health workers’ ability to detect breast cancer early.
The prevention and early detection of breast and cervical cancer have some features in common and involve a similar audience. For example, women need to get screened even if they feel well. Some age stratification is needed since cervical screening should start when women are in their thirties and breast cancer screening when they are in their forties. The frequency of screening may also differ, with clinical breast exams performed annually, usually starting at the age of 40 years,34 and cervical cancer screening at least once or twice in a woman’s lifetime (and more often only after high coverage with a single screening has been attained).45
Although a relatively small proportion of cervical abnormalities need specialty care, all breast cancers detected through screening or case-finding have to be treated by physicians with specialized training. Regional and national referral services are needed for both breast and cervical cancer and will also benefit patients with other types of cancer. Strengthening pathology services, ensuring the capacity to perform basic surgery and administer chemotherapy, establishing at least one source of radiotherapy in the country and developing appropriate palliative care policies and services can start with breast and cervical cancer and then become the foundation for other cancer care. Success with these two forms of cancer, which are amenable to prevention or early detection, can help reverse the prevailing myth that cancer is uniformly fatal in low- and middle-income countries.
There is momentum now
The 2011 high-level meeting of the United Nations General Assembly on the prevention and control of NCDs led to heightened awareness of the importance of leading killers such as cancer, heart disease, diabetes and chronic respiratory disease. The ensuing political declaration by the United Nations explicitly mentions cervical cancer and promotes “increased access to cost-effective cancer screening programmes, as determined by national situations”.46 After a consultation with its Member States, WHO recommended the inclusion of indicators related to palliative care, cervical cancer screening and HPV vaccine in the monitoring framework. This is indicative of the growing recognition of the importance of cervical cancer, but breast cancer is not mentioned in the political declaration.47 In fairness, it is difficult at this time to select a single specific breast cancer metric to recommend universally to countries, given the limited availability of baseline data and the difficulty of collecting reliable routine data in resource-constrained settings. A useful initial metric would be, for example, a marker of disease stage at diagnosis, such as tumour size, since the frequency of late-stage presentation and diagnosis will drive outcomes.
The synergies and momentum created by the recent international focus on noncommunicable diseases will have varying impact depending on the region, local resources and competing demands. By themselves they are not enough to create the opportunity for change, but they can enhance such an opportunity when combined with the availability of proven interventions and the results from ongoing research.
Conclusion
The health concerns of women in their mid-adult years have long been given little or no attention in most low-resource settings, despite the heavy burden of suffering that diseases such as breast and cervical cancer impose on women and their families.
The time has come to tackle these two cancers. There are numerous opportunities to prevent cervical cancer and to improve survival in women with cancer of the breast or cervix. It will take time to build the necessary human capacity, establish programmes, change community attitudes of fatalism and stigma and see the benefits of these measures become apparent. Further delay in taking up the opportunities that are now available will harm another generation of women.
Competing interests:
None declared.
References
- 1.Rosenfield A, Maine D. Maternal mortality–a neglected tragedy. Where is the M in MCH? Lancet. 1985;326:83–5. doi: 10.1016/S0140-6736(85)90188-6. [DOI] [PubMed] [Google Scholar]
- 2.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–23. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 3.Trends in maternal mortality: 1990 to 2008 Geneva: World Health Organization, United Nations Children’s Fund, United Nations Population Fund & The World Bank; 2010. [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, editors. GLOBOCAN 2008 v1.2. Cancer incidence and mortality worldwide: IARC CancerBase No. 10 Lyon: International Agency for Research on Cancer; 2010. Available from: http://www.iarc.fr/en/publications/eresources/cancerbases/index.php [accessed 5 June 2013].
- 5.Matsuno RK, Costantino JP, Ziegler RG, Anderson GL, Li H, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103:951–61. doi: 10.1093/jnci/djr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–84. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead M. The concepts and principles of equity and health. Copenhagen: World Health Organization, Regional Office for Europe; 1990 (EUR/ICP/RPD 414). Available from: salud.ciee.flacso.org.ar/flacso/optativas/equity_and_health.pdf [accessed 5 June 2013]. [Google Scholar]
- 8.Knaul FM, Bhadelia A, Gralow J, Arreola-Ornelas H, Langer A, Frenk J. Meeting the emerging challenge of breast and cervical cancer in low- and middle-income countries. Int J Gynaecol Obstet. 2012;119(Suppl 1):S85–8. doi: 10.1016/j.ijgo.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Namasivayam A, Osuorah DC, Syed R, Antai D. The role of gender inequities in women’s access to reproductive health care: a population-level study of Namibia, Kenya, Nepal, and India. Int J Womens Health. 2012;4:351–64. doi: 10.2147/IJWH.S32569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen G, Östlin P. Unequal, unfair, ineffective and inefficient, gender inequity in health: why it exists and how we can change it Stockholm: Karolinska Institute; 2007. Available from: http://www.who.int/social_determinants/resources/csdh_media/wgekn_final_report_07.pdf [accessed 5 June 2013].
- 11.Tsu VD, Levin CE. Making the case for cervical cancer prevention: what about equity? Reprod Health Matters. 2008;16:104–12. doi: 10.1016/S0968-8080(08)32411-2. [DOI] [PubMed] [Google Scholar]
- 12.Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the World Health Survey. PLoS ONE. 2012;7:e48834. doi: 10.1371/journal.pone.0048834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaul FM, Frenk J, Shulman L. Closing the cancer divide: a blueprint to expand access in low and middle income countries Boston: Harvard Global Equity Initiative; 2011. Available from: http://www.gavialliance.org/library/publications/searchtext/hgei/ [accessed 5 June 2013].
- 14.Lu B, Kumar A, Castellsagué X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89:821–830B. doi: 10.2471/BLT.11.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binagwaho A, Wagner CM, Gatera M, Karema C, Nutt CT, Ngabo F. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90:623–8. doi: 10.2471/BLT.11.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladner J, Besson MH, Hampshire R, Tapert L, Chirenje M, Saba J. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC Public Health. 2012;12:370. doi: 10.1186/1471-2458-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GAVI Alliance [Internet]. Human papillomavirus vaccine support. Geneva: GAVI; 2012. Available from: http://www.gavialliance.org/support/nvs/human-papillomavirus-vaccine-support/ [accessed 5 June 2013].
- 19.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–51. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 20.Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130:e249–56. doi: 10.1542/peds.2011-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi S, Denny L, Irwin KL, Jeronimo J, Lopalco PL, Monsonego J, et al. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128:2765–74. doi: 10.1002/ijc.25915. [DOI] [PubMed] [Google Scholar]
- 23.Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:221–32. doi: 10.1016/j.bpobgyn.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Cervical Cancer Action [Internet]. Progress in cervical cancer prevention: the CCA report card. Atlanta: CCA; 2012. http://www.cervicalcanceraction.org/pubs/pubs.php [accessed 5 June 2013].
- 25.Sankaranarayanan R, Rajkumar R, Esmy PO, Fayette JM, Shanthakumary S, Frappart L, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–43. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 28.Jeronimo J. 165 new screening algorithms for population-based programs. Int J Gynecol Obstet 2012;119:S202. [Google Scholar]
- 29.McTiernan A, Porter P, Potter JD. Breast cancer prevention in countries with diverse resources. Cancer. 2008;113(Suppl):2325–30. doi: 10.1002/cncr.23829. [DOI] [PubMed] [Google Scholar]
- 30.Beral V, Alexander M, Duffy S, Ellis IO, Given-Wilson R, Holmberg L, et al. The number of women who would need to be screened regularly by mammography to prevent one death from breast cancer. J Med Screen. 2011;18:210–2. doi: 10.1258/jms.2011.011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittra I. Breast cancer screening in developing countries. Prev Med. 2011;53:121–2. doi: 10.1016/j.ypmed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer. 2010;126:976–84. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 33.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Prabhakar J, Augustine P, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–80. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 34.US Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26, W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 35.Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, et al. National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060–96. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 36.Tsu VD, Winkler JL, Anderson BO, Sarria G, Jeronimo J. Coordinated training on early detection and diagnosis of breast cancer across different levels of health workers: an example from Peru. In: Shetty MK, editor. Breast and gynecological cancers: an integrated approach for screening and early diagnosis in developing countries New York: Springer; 2013. Available from: http://link.springer.com/content/pdf/10.1007%2F978-1-4614-1876-4_14.pdf# [accessed 5 June 2013]. [Google Scholar]
- 37.Knaul FM, Nigenda G, Lozano R, Arreola-Ornelas H, Langer A, Frenk J. Breast cancer in Mexico: a pressing priority. Reprod Health Matters. 2008;16:113–23. doi: 10.1016/S0968-8080(08)32414-8. [DOI] [PubMed] [Google Scholar]
- 38.Caleffi M, Ribeiro RA, Bedin AJ, Jr, Viegas-Butzke JM, Baldisserotto FD, Skonieski GP, et al. Adherence to a breast cancer screening program and its predictors in underserved women in southern Brazil. Cancer Epidemiol Biomarkers Prev. 2010;19:2673–9. doi: 10.1158/1055-9965.EPI-10-0338. [DOI] [PubMed] [Google Scholar]
- 39.Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(Suppl):2221–43. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 40.Eniu A, Carlson RW, El Saghir NS, Bines J, Bese NS, Vorobiof D, et al. Breast Health Global Initiative Treatment Panel Guideline implementation for breast healthcare in low- and middle-income countries: treatment resource allocation. Cancer. 2008;113(Suppl):2269–81. doi: 10.1002/cncr.23843. [DOI] [PubMed] [Google Scholar]
- 41.Programme of Action for Cancer Therapy. Progress report: autumn/winter 2011 Vienna: International Atomic Energy Agency; 2011. Available from: cancer.iaea.org/documents/PACTProgressReport2011_2.pdf [accessed 5 June 2013]. [Google Scholar]
- 42.Stalsberg H, Awuah B, Ibarra JA, Nsiah-Asare A. Re-establishing a surgical pathology service in Kumasi, Ghana: case report and discussion of barriers and key elements of a successful collaboration between low- and high-resource countries. Cancer. 2008;113(Suppl):2338–46. doi: 10.1002/cncr.23830. [DOI] [PubMed] [Google Scholar]
- 43.Sohani AR, Sohani MA. Static digital telepathology: a model for diagnostic and educational support to pathologists in the developing world. Anal Cell Pathol (Amst) 2012;35:25–30. doi: 10.3233/ACP-2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masood S, Vass L, Ibarra JA, Jr, Ljung B-M, Stalsberg H, Eniu A, et al. Breast Health Global Initiative Pathology Focus Group Breast pathology guideline implementation in low- and middle-income countries. Cancer. 2008;113(Suppl):2297–304. doi: 10.1002/cncr.23833. [DOI] [PubMed] [Google Scholar]
- 45.Comprehensive cervical cancer prevention and control: a healthier future for girls and women Geneva: World Health Organization; 2013. [Google Scholar]
- 46.Political declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. In: General Assembly of the United Nations [Internet]. Sixty-sixth session of the General Assembly of the United Nations, New York, 19–20 September 2012. New York: United Nations; 2012 (A/RES/66/2). [Google Scholar]
- 47.A draft comprehensive global monitoring framework, including indicators, and a set of voluntary global targets for the prevention and control of noncommunicable diseases. Geneva: World Health Organization; 2012 (A/NCD/INF./1). Available from: http://apps.who.int/gb/ncds/pdf/A_NCD_INF1-en.pdf [accessed 5 June 2013].