Abstract
Voltage sensors trigger the closed–open transitions in the pore of voltage-gated ion channels. To probe the transmission of voltage sensor signalling to the channel pore of CaV1.2, we investigated how elimination of positive charges in the S4 segments (charged residues were replaced by neutral glutamine) modulates gating perturbations induced by mutations in pore-lining S6 segments. Neutralisation of all positively charged residues in IIS4 produced a functional channel (IIS4N), while replacement of the charged residues in IS4, IIIS4 and IVS4 segments resulted in nonfunctional channels. The IIS4N channel displayed activation kinetics similar to wild type. Mutations in a highly conserved structure motif on S6 segments (“GAGA ring”: G432W in IS6, A780T in IIS6, G1193T in IIIS6 and A1503G in IVS6) induce strong left-shifted activation curves and decelerated channel deactivation kinetics. When IIS4N was combined with these mutations, the activation curves were shifted back towards wild type and current kinetics were accelerated. In contrast, 12 other mutations adjacent to the GAGA ring in IS6–IVS6, which also affect activation gating, were not rescued by IIS4N. Thus, the rescue of gating distortions in segments IS6–IVS6 by IIS4N is highly position-specific. Thermodynamic cycle analysis supports the hypothesis that IIS4 is energetically coupled with the distantly located GAGA residues. We speculate that conformational changes caused by neutralisation of IIS4 are not restricted to domain II (IIS6) but are transmitted to gating structures in domains I, III and IV via the GAGA ring.
Keywords: Calcium channel, Voltage sensor, Pore, Mutational analysis, Channel gating
Introduction
The entry of Ca2+ through voltage-gated Ca2+ channels has direct effects on muscle contraction, release of hormones and neurotransmitters, hearing, vision, gene expression and other important physiological functions [8]. L-type Ca2+ channel dysfunction can result from structural aberrations within their pore-forming α1-subunits causing hypokalemic periodic paralysis and malignant hyperthermia sensitivity (CaV1.1), Timothy syndrome (CaV1.2), sinoatrial node function and hearing (CaV1.3) and incomplete congenital stationary night blindness (CaV1.4) [46].
The pore-forming α1-subunits of voltage-gated Ca2+ channels are composed of four homologous domains formed by six transmembrane segments (S1–S6) that are linked together on a single polypeptide [6]. The voltage-sensing machinery is formed by multiple charged amino acids located in segment S4 and adjacent structures of each domain. Segments S6 of each domain form the conducting pore of the channel.
Hodgkin and Huxley [19] hypothesised that activation of voltage-gated ion channels is initiated by movement of charged particles across the membrane. This charge movement (called “gating current”) was later directly measured for sodium channels [1]. Crystal structures of ion channels revealed that four domains in NaV or in KV comprise six transmembrane segments (S1–S6) that are assembled in such a way that S1–S4 form the voltage-sensing module and the remaining S5 and S6 segments form the ion-conducting pore [26, 29]. In principle, the voltage-sensing and ion-conducting modules can function independently of one another [26]: voltage sensors can exist without ion pores [27, 32, 36] and two-transmembrane-segment channels (two TM) function without voltage sensors [7].
Gating-sensitive positions in the pore of CaV have been identified through extensive site-directed mutagenesis studies [20, 24, 34, 35, 50, 57]. Changes in stability of the open and/or closed states induced by pore mutations [29, 55, 56] or e.g. Zn2+ bridges constraining the S4 movement [31] are manifested in shifts of the activation curve and accompanying deceleration of deactivation kinetics. Interestingly, such gating distortions are characteristic of a CACNA1F mutation identified in an X-linked retinal disorder [16] and the Timothy syndrome [10, 42, 43].
Biophysical models describe voltage sensor movements and pore opening in KV as separate transitions [4, 21, 25, 39, 51]. Based on these assumptions, the kinetic properties of mutants with gating disturbances in pore-forming S6 segments of NaV (e.g. NaChBac [55, 56]) and CaV [2] have successfully been quantified.
The opening of the Shaker KV channel involves multiple activation steps as the S4 segments move from the resting state towards the activated state, which is followed by a concerted opening transition of the S6 gate. This concept of Ledwell and Aldrich [25] is supported by biophysical studies in Shaker KV [5, 9, 14, 22, 25, 28, 37–41, 45, 47, 52, 53].
Surprisingly, in a concatenated Shaker KV, a single voltage sensor is sufficient to open and close the channel [13]. Furthermore, a recent molecular dynamics simulation of a potassium channel revealed that the downward movement of a single S4 segment can initiate channel closure [23]. Thus, if channel closure is a concerted movement of all four S6 gates then one S4 segment either directly or allosterically controls gating structures in all four domains.
Previously, a number of strong activation determinants were identified in pore-forming segments S6 of CaV1.2 [10, 20, 24]. In order to get insights into the link between the voltage-sensing S4 segments and pore residues of CaV1.2, we combined these pore mutations in all four S6 segments with a voltage-sensing S4 segment where the charged residues were replaced by neutral glutamines. Our results suggest that conformational changes in a single S4 segment (IIS4) are transmitted to specific residues (GAGA ring [10]) in all four pore-forming S6 segments.
Materials and methods
Mutagenesis
Substitutions in S4 and S6 segments of the CaV1.2 α1-subunit (GenBank™ X15539) were introduced using the QuikChange® Lightning Site-Directed Mutagenesis Kit (Stratagene) with mutagenic primers according to the manufacturer’s instructions. All constructs were checked by restriction site mapping and sequencing.
Cell culture and transient transfection
Human embryonic kidney tsA-201 cells were grown at 5 % CO2 and 37 °C to 80 % confluence in Dulbecco’s modified Eagle’s/F-12 medium supplemented with 10 % (v/v) foetal calf serum and 100 units/ml penicillin/streptomycin. Cells were split with trypsin/EDTA and plated on 35 mm Petri dishes (Falcon) at 30–50 % confluence ~16 h before transfection. Subsequently, tsA-201 cells were co-transfected with cDNAs encoding wild type or mutant CaV1.2 α1-subunits with auxiliary β2a [30] as well as α2-δ1 [11] subunits and GFP to identify transfected cells.
The transfection of tsA-201 cells was performed using the FUGENE6 Transfection Reagent (Roche) following standard protocols. tsA-201 cells were used until passage number 15. No variation in channel gating related to different cell passage numbers was observed.
Ionic current recordings and data acquisition
Barium currents (IBa) through voltage-gated Ca2+ channels were recorded at 22–25 °C by patch-clamping [15] using an Axopatch 200A patch clamp amplifier (Axon Instruments, Foster City) 36–48 h after transfection. The extracellular bath solution (in millimolars: BaCl2 5, MgCl2 1, HEPES 10 and choline-Cl 140) was titrated to pH7.4 with methanesulphonic acid. Patch pipettes with resistances of 1 to 4 MΩ were made from borosilicate glass (Clark Electro-medical Instruments, UK) and filled with pipette solution (in millimolars: CsCl 145, MgCl2 3, HEPES 10 and EGTA 10), titrated to pH7.25 with CsOH. All data were digitised using a DIGIDATA 1200 interface (Axon Instruments, Foster City), smoothed by means of a four-pole Bessel filter and saved to disc. One hundred-millisecond current traces were sampled at 10 kHz and filtered at 5 kHz; tail currents were sampled at 50 kHz and filtered at 10 kHz. Leak currents were subtracted digitally using average values of scaled leakage currents elicited by a 10-mV hyperpolarizing pulse or electronically by means of an Axopatch 200 amplifier (Axon Instruments, Foster City). Series resistance and offset voltage were routinely compensated for. The pClamp software package (Version 7.0 Axon Instruments, Inc.) was used for data acquisition and preliminary analysis. Microcal Origin 7.0 was used for analysis and curve fitting.
The voltage dependence of activation was determined from I to V curves and fitted to
The time courses of current activation and deactivation were fitted to a mono-exponential function:
; where I(t) is current at time t, A is the amplitude coefficient, τ is the time constant, and C the steady-state current. Data are given as mean±S.E. Time constants were plotted versus voltage (e.g. Fig. 1c). The left branch of the bell-shaped curve of the time constants corresponds to channel deactivation and the right branch to the activation (see [19]). At voltages where channel activation and deactivation overlap (peak of the bell-shaped dependence), the data are given as averaged values of both time constants.
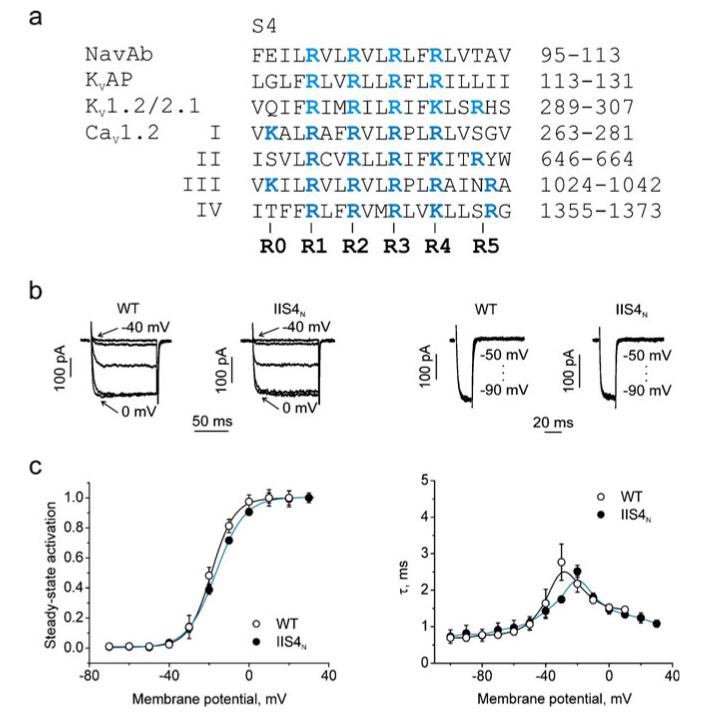
Fig. 1.
Charge neutralisation in segment IIS4 of CaV1.2 has minor effects on activation gating: a alignment of S4 segments of NaVAb (protein databank identifier, 3RVY), KVAP (protein data bank identifier, 1ORS), KV1.2/2.1 (protein data bank identifier, 2R9R) and CaV1.2 (accession number, P15381). Charged residues are shown in blue. b Left panel shows representative families of IBa through wild type channel and through the channel with the “charge-neutralised” IIS4 segment (IIS4N). Barium currents were evoked during depolarization starting from −40 with 10 mV increments from a holding potential of −100 mV. Right panel shows representative tail currents. Currents were activated during a 20-ms conditioning depolarization to 0 mV. Deactivation was recorded during subsequent repolarisations with 10 mV increments starting from −100 mV. c Left panel, averaged activation curves of wild type (n=9) and IIS4N (n=11) channels. Right panel, voltage-dependent time constants of channel activation/deactivation
Structural changes (point mutations) in Ca2+ channels may influence activation and inactivation properties (e.g. [10, 20, 24, 49]). To minimise effects of inactivation on the estimation of the rate of channel activation and deactivation, the α1 subunit of CaV1.2 was co-expressed with the auxiliary β2a subunit known to substantially slow the inactivation kinetics [17]. Furthermore, in order to remove Ca2+-dependent inactivation, the experiments were performed with Ba2+ as charge carrier.
Modelling
The homology model of CaV1.2 is based on the NaVAb crystal structure [29] (protein databank identifier: 3RVY). Modeller 9v7 [12] was used to generate the model. Modelling criteria have been published previously [44].
Results
Charge neutralisation in IIS4 induces only small gating disturbances in CaV1.2
The location of the arginine and lysine residues (which we refer to as R1–R5) in the S4 segments is shown in Fig. 1a, b. We will use the designation S4N for a mutant with a “charge-neutralised” S4 segment in which all the positions R1–R5 are replaced by glutamines. Currents were measurable only through construct IIS4N (Table 1), while mutants IS4N, IIIS4N and IVS4N did not result in expression of channels that conducted barium currents. This finding can either be explained by insufficient trafficking of the constructs to the membrane or by an impaired function of the channels. Applying a more negative holding potential (−140 mV) had no effect suggesting that these constructs are not in an inactivated state.
Table 1.
Midpoints and slope factors (k) of the activation curves and free energy changes during channel opening (ΔG, ΔΔG=ΔGmut/IIS4n–ΔGIIS4n–ΔGmut)
| Mutant | V0.5, mV | k, mV | ΔG, kcal/mol | ΔΔG, kcal/mol |
|---|---|---|---|---|
| wt | −18.8±0.9 (n=9) | 5.7±0.9 | ||
| R1Q/R2Q/R3Q/R4Q/R5Q (IIS4N)a | −17.5±0.8 (11) | 6.7±0.7 | 0.39±0.36 | |
| GAGA mutations | ||||
| G432Wb | −33.7±0.6 (8) | 4.7±0.4 | −2.25±0.48 | |
| G432N | −36.7±0.6 (6) | 6.1±0.5 | −1.58±0.43 | |
| A780Tb | −44.0±0.6 (5) | 5.1±0.6 | −3.10±0.67 | |
| G1193Tc | −49.8±1.0 (7) | 5.1±0.6 | −3.76±0.74 | |
| A1503Gb | −41.4±0.8 (5) | 5.8±0.5 | −2.23±0.48 | |
| G432W/IIS4N | −19.3±0.7 (8) | 5.8±0.7 | −0.01±0.40 | 1.84±0.72* |
| G432N/IIS4N | −22.1±0.7 (6) | 7.7±0.5 | 0.24±0.33 | 1.43±0.65 * |
| A780T/IIS4N | −32.0±0.7 (10) | 5.1±0.7 | −1.73±0.64 | 0.96±0.99 |
| G1193T/IIS4N | −20.8±0.7 (5) | 7.5±0.6 | 0.32±0.35 | 3.68±0.90* |
| A1503G/IIS4N | −19.8±0.7 (6) | 7.5±0.7 | 0.38±0.35 | 2.22±0.70* |
| GAGA–1 mutations | ||||
| L431P | 8.1±0.7 (5) | 7.4±1.0 | 2.56±0.33 | |
| L431V | −8.4±1.3 (4) | 6.7±0.8 | 1.19±0.34 | |
| L779P | −31.2±0.8 (6) | 6.1±0.6 | −1.02±0.45 | |
| V1192P | −13.8±0.7 (5) | 6.6±1.0 | 0.70±0.37 | |
| V1502P | −3.6±0.7 (6) | 5.4±0.6 | 1.52±0.32 | |
| L431P/IIS4N | No measurable currents observed | |||
| L431V/IIS4N | −10.1±0.9 (4) | 7.7±0.6 | 1.15±0.33 | −0.43±0.60 |
| L779P/IIS4N | −32.1±1.5 (5) | 5.4±1.6 | −1.50±1.06 | −0.87±1.21 |
| V1192P/IIS4N | −13.0±1.0 (5) | 5.5±1.1 | 0.56±0.42 | −0.53±0.67 |
| V1502P/IIS4N | −5.4±1.0 (7) | 6.6±1.0 | 1.44±0.33 | −0.48±0.59 |
| GAGA+1 mutations | ||||
| V433C | −8.2±0.8 (5) | 5.0±0.6 | 0.96±0.35 | |
| V433L | −30.1±0.6 (5) | 6.1±0.5 | −0.95±0.39 | |
| I781T | −47.1±0.9 (5) | 7.4±0.7 | −1.75±0.48 | |
| F1194C | −6.8±0.9 (5) | 4.4±0.6 | 1.02±0.36 | |
| V1504T | −11.6±0.7 (7) | 4.9±0.6 | 0.55±0.36 | |
| V433C/IIS4N | −12.4±0.7 (7) | 5.7±0.6 | 0.65±0.35 | −0.70±0.62 |
| V433L/IIS4N | −31.1±1.0 (4) | 5.7 ±0.6 | −1.25±0.47 | −0.70±0.71 |
| I781T/IIS4N | −54.8±0.6 (9) | 6.7±0.4 | −2.79±0.42 | −1.44±0.74* |
| F1194C/IIS4N | −5.7±0.9 (5) | 5.6±0.6 | 1.33±0.33 | −0.09±0.61 |
| V1504T/IIS4N | −14.0±0.9 (5) | 5.4±0.6 | 0.41±0.38 | −0.54±0.64 |
| GAGA+2 mutations | ||||
| L434T | −27.6±0.8 (6) | 6.1±0.7 | −0.71±0.44 | |
| L434V | −30.2±0.7 (6) | 6.3±0.6 | −0.87±0.41 | |
| A782P | −43.0±0.9 (6) | 6.7±0.8 | −1.81±0.55 | |
| V1195Tc | −28.9±0.7 (7) | 5.1±0.7 | −1.37±0.55 | |
| I1505P | −8.7±0.8 (5) | 5.5±0.6 | 0.99±0.34 | |
| L434T/IIS4N | −27.4±0.8 (6) | 6.4±0.7 | −0.57±0.42 | −0.25±0.71 |
| L434V/IIS4N | −30.6±0.8 (6) | 6.8±0.6 | −0.69±0.39 | −0.22±0.68 |
| A782P/IIS4N | −50.2±0.8 (5) | 7.4±0.6 | −2.00±0.45 | −0.58±0.80 |
| V1195T/IIS4N | −25.3±1.0 (6) | 4.5±0.8 | −1.28±0.66 | −0.30±0.94 |
| I1505P/IIS4N | −7.5±0.9 (5) | 5.1±0.8 | 1.06±0.36 | −0.32±0.61 |
Channel gating of IIS4N was quite similar to the wild type (wt); no significant shift in the activation curve was observed (ΔV=1.2±1.2 mV, Fig. 1). At first glance, these results suggest that one or more of the other segments (IS4, IIIS4 and IVS4) play a role in voltage-dependent gating, while IIS4 has only a minor impact. However, combining IIS4N with mutations in S6 segments revealed that it had a significant impact on channel activation.
Charge neutralisation in segment IIS4 rescues gating disturbances in the GAGA ring in segments IS6–IVS6
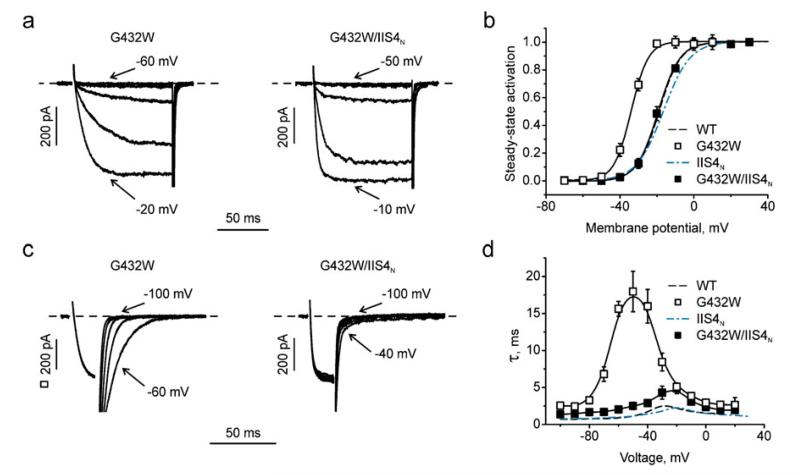
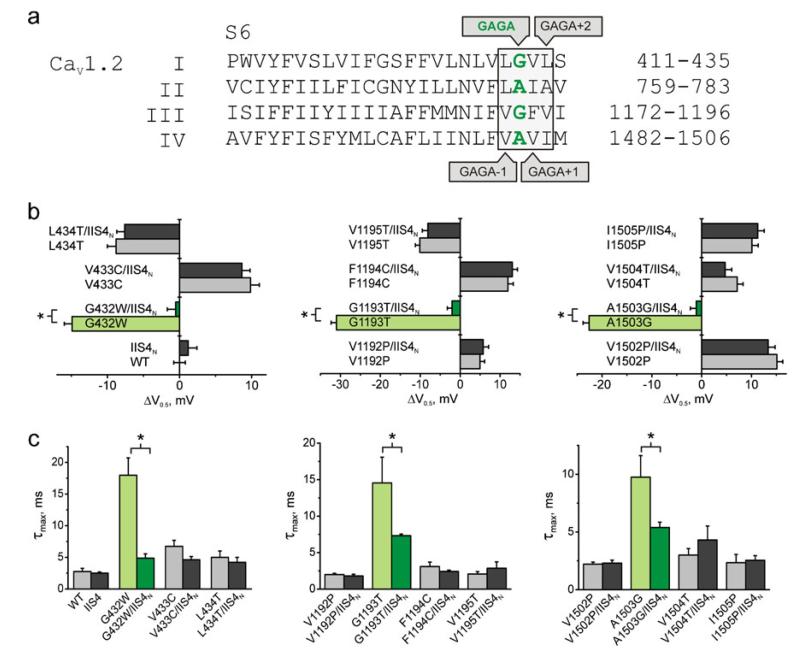
First, we combined IIS4N with perturbing pore mutations in a highly conserved structure motif (“GAGA ring” [10]) of small residues in homologous positions of all four domains (Gly-432 (IS6), Ala-780 (IIS6), Gly-1193 (IIIS6) and Ala-1503 (IVS6)). All of these mutations have been shown to shift the activation curve to the left and to decelerate deactivation (see also [2, 20, 24, 35, 48]). For example, mutant G432W activates and deactivates (Fig. 2a, c, d) with the slowest activation/deactivation time constant of about 18 ms observed at −50 mV (Fig. 2d). However, gating disturbances induced by G432W in IS6 were “rescued” by combination with IIS4N. In G432W/IIS4N, the shift of the activation curve compared to wild type (ΔV=V0.5,mut–V0.5,wt) was eliminated (ΔV=−0.5±1.1 mV (G432W/IIS4N) vs. −14.9±1.0 mV (G432W), Fig. 2) and current kinetics were correspondingly faster with a maximal activation/deactivation time constant of about 5 ms at −20 mV (Fig. 2a, c, d). A similar result was obtained with G432N, shifting the activation curve to the left. Combining G432N with IIS4N reversed this shift (ΔV=−3.3±1.1 mV (G432N/IIS4N) vs. by 17.9±1.1 mV (G432N)) and also accelerated deactivation (see Supplemental Fig. 1). Comparable rescue effects on gating disturbances were observed for constructs G1193T/IIS4N and A1503G/IIS4N. Substantial shifts in channel activation (ΔV=−31.0±1.3 mV in G1193T and −22.6±1.2 mV in A1503G) were reduced to −2.0±1.1 mV and −1.0±1.1 mV in G1193T/IIS4N and A1503G/IIS4N, respectively (coloured bars in Fig. 3b, Table 1). Current kinetics were also considerably faster; the maximal (slowest) time constants of current activation and deactivation for these constructs are given as coloured columns in Fig. 3c.
Fig. 2.
Charge neutralisation in IIS4 rescues a gating disturbances induced by G432W in segment IS6. a, c Representative activation (a) and tail currents (c) traces through G432W and G432W/IIS4N mutant channels. b, d Averaged activation curves (b) and voltage dependence of the activation/deactivation time constants (d) of WT, G432W, IIS4N and G432W/IIS4N
Fig. 3.
Charge neutralisation in domain II (IIS4) rescues gating disturbances in GAGA positions of domains I, III and IV. a Alignment of S6 segments of CaV1.2 (accession number, P15381). The “GAGA” ring of small conserved residues in IS6–IVS6 is highlighted in green. Mutated residues are boxed. b, c Shift of activation curve (b) and peak activation/deactivation time constants (c) of the studied pore mutations in segments IS6 (left), IIIS6 (middle) and IVS6 (right) either alone or in combination with IIS4N. Statistically significantly different (t test) values are marked with asterisks. Error bars indicate SEM
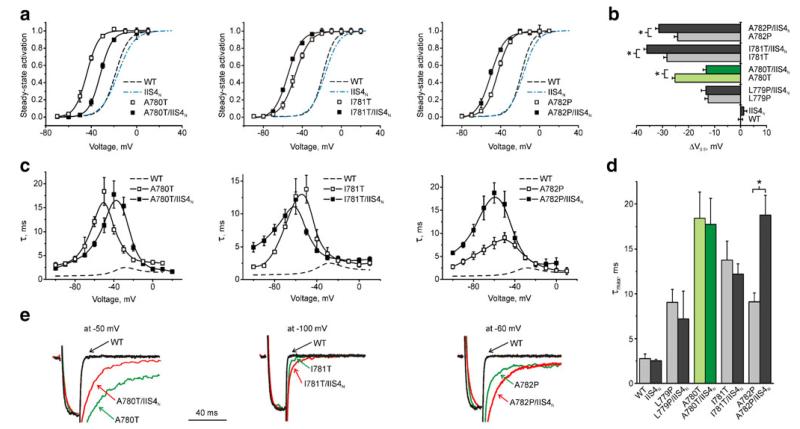
Interestingly, combining IIS4N with the gating disturbing A780T in IIS6 (a pore mutation in its “own” domain II) shifted the activation curve only partially towards wild type (ΔV= −25.2±1.1 mV (A780T) vs. ΔV=−13.2±1.1 mV in A780T/IIS4N), without inducing significant changes in current kinetics (see shifts of the voltage dependence in Fig. 4c, d).
Fig. 4.
Interaction of IIS4 with IIS6. a Averaged activation curves of WT (n=9), IIS4N (n=11) and mutations A780T (left), I781T (middle) and A782P (right) in domain II (n=5–10). b Shifts of the activation curves induced by IIS6 mutation (light bars) and in combination with IIS4N (dark bars). Statistically significant differences (unpaired t test) are marked with asterisks. c Voltage dependence of the activation/deactivation time constants for indicated constructs. d Maximal activation/deactivation time constants of IIS6 mutations alone (light bars) and in combination with IIS4N (dark bars). e Selected tail currents through WT, A780T and A780T/IIS4N mutant channels at the indicated voltages
Charge neutralisation in IIS4 does not affect gating disturbances in positions neighbouring to GAGA
Mutations of residues upstream and downstream of the GAGA positions in segments IS6, IIIS6 and IVS6 (G432, G1193 and A1503, see alignment in Fig. 3a) resulted in a wide spectrum of gating phenotypes. Gating disturbances induced either leftward or rightward shifts of the activation curve (ranging from −28.3±1.2 mV (I781T) to +26.9±1.2 mV (L431P)). None of these mutants was, however, sensitive to combination with IIS4N. This is evident from Fig. 3 illustrating the steady-state activation (Fig. 3b) and the maximal time constants (Fig. 3c). Neither the shifts of the activation curves nor the maximal time constants of the activation/deactivation were reversed towards wild type kinetics.
Charge neutralisation in segment IIS4 modulates kinetics of IIS6 pore mutants
That IIS4N only partially rescued gating disturbances induced by A780T is not unexpected. If a hyperpolarisation-driven downward movement of IIS4 initiates pore closure by moving IIS6 towards its closed position, then replacement of the charged arginines would inevitably decelerate the gate movement (channel closure) in domain II.
In line with such a scenario, constructs I781T/IIS4N and A782P/IIS4N displayed additional shifts of the activation curves compared to I781T and A782P. In I781T, ΔV amounted −28.3±1.2 mV while charge neutralisation (I781T/IIS4N) further increased ΔV to −36.0±1.1 mV (Fig. 4a, c middle column). Likewise, the shift of the activation curve in A782P/IIS4N (ΔV=−31.4±1.2 mV) was also not rescued but increased compared to A782P ( −24.2± 1.2 mV, Fig. 4a, c right column).
Interestingly, charge neutralisation in IIS4 decelerated deactivation of both constructs at negative voltages (from −60 to −100 mV), with little effect on kinetics of channel activation (from −40 to +30 mV). In L779P/IIS4N, the leftward shift of the activation curve was less affected by charge neutralisation compared to the L779P (ΔV=−13.3±1.7 mV (L779P/IIS4N) vs. −12.4±1.2 mV (L779P), Table 1) suggesting that this residue does not interact with IIS4.
Mutant cycle analysis reveals energetic coupling between IIS4 and residues of the GAGA ring
The position-specific effects of S4 neutralisation in domain II on gating perturbations in distant S6 segments were investigated by means of mutant cycle analysis. This approach enables the estimation of interaction energies between the mutated pore residues in segments IS6–IVS6 and the voltage sensor in domain II (IIS4) on the basis of the free energy changes [18, 33, 51, 54]. The interaction energies were estimated by calculating the free energies as described in Kudrnac et al. [24] according to , and ΔΔG = ΔGmut/IIS4 – ΔGmut – ΔGIIS4, with V0.5 representing the voltage of steady-state (equilibrium) half-activation and k the slope factor (Table 1).
If the residues in segments IS6–IVS6 do not interact with the IIS4N segment, then the change in free energy (ΔGmut/IIS4) of the combined construct should be equal to the sum of the changes in free energy (ΔGmut and ΔGIIS4). If the residues are energetically coupled, then the change in free energy for the combination mutant would be significantly different from the sum of the two separate mutations.
Our data show that mutations in positions G432 (IS6), G1193 (IIIS6) and A1503 (VIS6) are energetically coupled with segment IIS4. This is evident from the non-additive shifts of the activation curves of the combined constructs (S6mut/IIS4N), which strongly suggest interdependence of the gating perturbations in IIS4 and the S6 residues (e.g. ΔΔG=3.68±0.90 kcal/mol for G1193T/IIS4N, see also Table 1). Analysis of the other combined constructs shows that mutations in IIS4 do not interact with a series of other mutations in IS6, IIIS6 or IVS6 (GAGA−1, GAGA+1 or GAGA+2), with ΔΔG ≈0 kcal/mol. We therefore conclude that the conformational changes in the voltage sensor IIS4 and the S6 residues outside the GAGA ring are energetically independent. While ΔΔG of construct A780T/IIS4N was not significantly different from 0 (ΔΔG=0.96±0.99 kcal/mol), the next residue downstream I781T was found to be energetically coupled to IIS4N (ΔΔG of I781T/IIS4N= −1.44±0.74 kcal/mol, Table 1).
Discussion
If we translate the Hodgkin–Huxley model for potassium currents into current terminology, we can say that all four gating units (S4 segments) must be in an activated position before the channel can open [19]. This hypothesis was supported by Horn et al. [21] showing that immobilisation of only one S4 segment in its down position prevents Shaker activation. Interestingly, potassium channel closure (deactivation) seems to require only one S4 in its resting position [13, see also 23]. Here we demonstrate that a single voltage sensor (IIS4) in an asymmetric CaV1.2 interacts with a ring of conserved small residues (GAGA ring) in all four S6 segments.
Figure 1 illustrates that charge neutralisation of IIS4 (replacing residues R1–R5 by glutamines, IIS4N) has no detectable effects on channel kinetics. In order to resolve potential IIS4N effects on current kinetics, we combined these mutations with S6 gating perturbations inducing pronounced shifts of the activation curve. In part, we utilised previously studied CaV mutations (see [3, 10, 20, 24, 35, 48]) inducing ΔV>10 mV regardless of the physicochemical properties of the substituent (see marks and subscripts to Table 1). The resulting S6mut/IIS4N constructs emerged as tools to analyse voltage sensor/S6 interactions.
IIS4 interactions with segments IS6–IVS6
As shown in Figs. 2, 3 and 4, combining IIS4N with mutations in the gating-sensitive regions of pore-forming S6 segments produced three kinetic phenotypes. First, four of the S6 mutants (G432W (IS6), G1193T (IIIS6), A1503G (VIS6) and partially A780T (IIS6)) were rescued by IIS4N (coloured bars on Figs. 3 and 4, Supplemental Fig. 1). This is evident from the unexpected backwards shifts of the activation curves towards wild type (Fig. 3b) and accompanying acceleration of channel kinetics (Fig. 3c). Thermodynamic cycle analysis supports the hypothesis that IIS4 interacts with GAGA residues (e.g. ΔΔG(G432W/IIS4N)=1.84±0.72 kcal/mol, ΔΔG(G432N/IIS4N)=1.43±0.65 kcal/mol, ΔΔG(G1193T/IIS4N)=3.68±0.90 kcal/mol and ΔΔG (A1503G/IIS4N)=2.22±0.70 kcal/mol, see Table 1). Interestingly, the rightward shift of the activation curve in A780T/IIS4N was less pronounced (Fig. 4a, left column) and ΔΔG not significantly different from zero (Table 1). We speculate that these peculiarities are caused by domain-specific interactions between IIS4 and IIS6 (see detailed discussion below).
Second, pore mutants in segments IS6, IIIS6 and IVS6 upstream and downstream of the GAGA ring (see Fig. 3a) were not significantly affected by charge neutralisation in IIS4 (Fig. 3b, c; Supplemental Fig. 1). The effect of IIS4N in these constructs was comparable to the one observed for wild type (IIS4N, Fig. 1). Additional experiments were carried out to analyse a potential “mutation dependence” of the position-specific rescue effect. As illustrated in Fig. 1 (in Supplemental Material), only substitution G432N in segment IS6 was rescued (G432N/IIS4N, green bars, Supplemental Fig. 1) while alternative mutations of neighbouring residues (L431V, V433L and L434V) were not sensitive to IIS4N (compare with similar findings in Fig. 3b, c).
Third, combining IIS4N with I781T and I782P augmented the gating disturbances as evident from the additional shift of the activation curve to the left (Fig. 4a) and deceleration of deactivation kinetics (Fig. 4c). If we envisage the voltage sensor predominantly “pushing” the gating structures of CaV1.2 into the closed conformation [2], then charge neutralisation of IIS4 would decelerate channel closure at negative voltages (i.e. slow deactivation). Such “expected gating changes” were observed only for constructs I781T/IIS4N and A782P/IIS4N (Fig. 4).
Dual role of A780?
Interestingly, the activation curve of A780T/IIS4N was shifted towards the wild type position, but only half way (Fig. 4a, left column). This distinguishes A780T from the other GAGA mutants. We speculate that IIS4N would potentially shift the activation curve of A780T/IIS4N to the right (Fig. 3b). A780 is, however, also part of the IIS6 gating structure where combinations with IIS4N induce leftward shifts (compare with other IIS6 mutants I781T and A782P, Fig. 4). Such opposing effects could explain an incomplete backward shift of the activation curve of A780T/IIS4N (Fig. 4a).
Conclusions and outlook
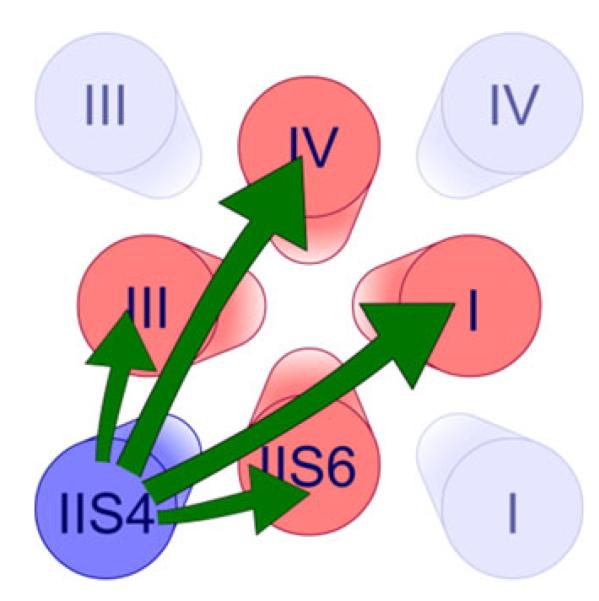
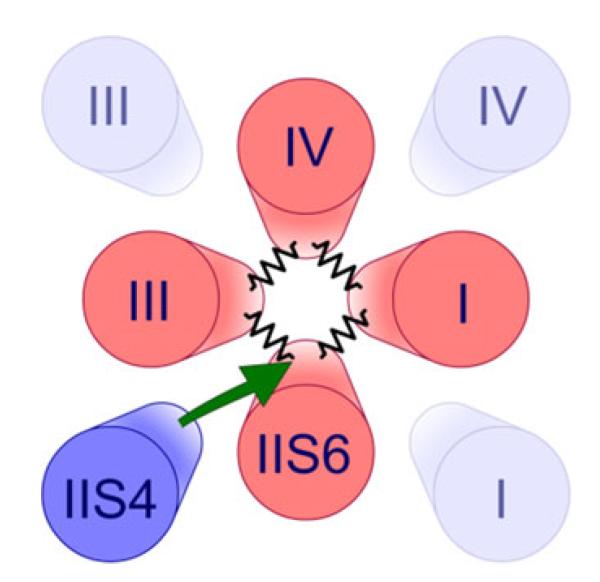
The principal finding of our study is that segment IIS4 interacts with a ring of conserved small residues in segments IS6–IVS6 (GAGA ring [10]). In general, the interaction of a single voltage sensor with all four S6 (and associated gate structures) suggests either interactions of IIS4 with each of them (Scheme 1) or, alternatively, interactions with the gate structures in domain II that are cooperatively linked to other domains (Scheme 2).
Scheme 1.
Hypothetical long range interactions of segment IIS4 with all four S6 segments
Scheme 2.
Cooperative gating model: A single voltage sensor (IIS4) interacts with an S6 segment which facilitates the stabilisation of closed state formation by all four S6 segments
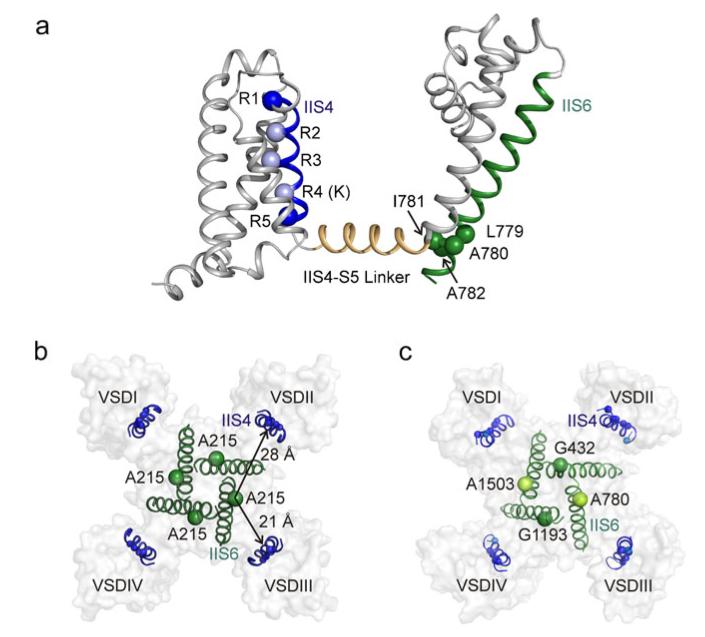
The distances between the GAGA residues (corresponding to AAAA in the crystal structure of a homologous bacterial sodium channel NaVAb [29]) and the voltage sensor segments are illustrated in Fig. 5b. Such long range interactions between segment IIS4 and S6 residues in domains I, III and IV cannot be excluded but would have to occur via different distances (IIS4–IS6, 21 Å; IIS4–IIIS6, 40 Å and IIS4–IVS6, 36 Å) and different pathways in a highly specific manner.
Fig. 5.
CaV1.2 homology model based on NaVAb crystal structure [29] illustrates the location of the GAGA ring with respect to S4. a Blue spheres represent the arginine (R650, R653, R656 and R662) and lysine (K659) positions in segment IIS4. A previously identified gating-sensitive motif (LAIA [20]) in IIS6 is shown as green spheres. The linker helix is coloured orange. Figures generated with Pymol (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC). In analogy to KV and NaV crystal structures, we speculate that in the closed conformation, LAIA residues [20] in the bundle crossing region of S6 interact with the voltage sensor domain via the S4–S5 linker (e.g. I781 is in direct contact with the linker which would enable to force the IIS6 segment towards its closed position; see details in Discussion section on IIS4–IIS6 interaction). b, c Bottom view of the pore domain and S4 segments of NaVAb crystal structure and CaV1.2 homology model coloured as in panel A. The GAGA ring [10] (corresponding to AAAA ring in NaVAb) is shown as green spheres. The distances between the AAAA ring in the NaVAb crystal structure (corresponding to GAGA in CaV1.2) with respect to the voltage-sensing domains are indicated
We favour a “cooperative gating model” illustrated in Scheme 2. Cooperative interactions between individual amino acids of pore-forming S6 segments of CaV1.2 were previously identified [24]. Moreover, in the closed state, the small residues of the GAGA ring are apparently in tight contact with neighbouring bulky amino acids which may stabilise the closed channel state [10]. Channel closure is initiated by downward movement of an S4 segment and subsequent translocation of the corresponding S6 towards the resting position via the S4–S5 linkers (Fig. 5a). In its closing position, a single S6 segment is stabilised by cooperative interactions (e.g. hydrophobic) with gate structures of neighbouring domains (see [3]). In other words, a single S6 segment in its closing position increases the probability of closed state formation by all four S6 segments. The effect that only conformational changes in the GAGA positions are reversed by charge neutralisation in IIS4 warrants further research.
We speculate that in CaV1.2 (in analogy to KV) any single S4 segment may initiate channel closure under hyperpolarizing voltage [13]. To enable channel opening, however, all four S4 segments must be activated [21]. Phillips and Swartz [31] have shown that KV opens while S4 segments are still moving (i.e. they do not reach their fully activated position). We therefore further speculate that opening of CaV1.2 occurs when all four S4 segment left the resting state.
In wild type CaV1.2, S6 segments form a tight closed conformation, with the GAGA ring playing a key role. Hence, substitution of any of the GAGA residues by a bulky tryptophan (G432W), polar threonine (A780T) or a helix-bending proline (A780P) disturbs the fine-tuned closure mechanism, as is evident from the slow deactivation. Such a slowing of S6 gating would affect the corresponding S4 transitions to the resting state. Correspondingly, translocation of all S4 segments in their resting position interferes with the pore closure of the mutants. In such a scenario, removal of a “disturbing IIS4 interaction” with the cooperative GAGA ring might relieve spatial constraints thereby providing more flexibility for pore-closing interactions [3]. Cooperative interactions are not restricted to interactions within the GAGA ring. Contributions of residues in segments IS6 and IIS6 to activation gating in CaV1.2 have previously been demonstrated for Ser-435 (IS6) and Ile-781 (IIS6) [24], suggesting that paired interactions between additional S6 gating structures may stabilise the closed state.
Replacing charged residues in segments IS4–IVS4 with neutral glutamines resulted in only one functional construct (IIS4N, Table 1) which warrants further research. Interestingly, Gagnon and Bezanilla [13] observed substantial reduction of the current amplitudes if the charged residues of S4 segments in a symetric potassium channel concatamer were subsequently substituted by glutamine or asparagine, suggesting that neutralisation of charged residues in S4 segments my affect expression density, membrane targeting or the functionality of these constructs.
Supplementary Material
Acknowledgments
The research was funded by the Austrian Science Fund (FWF): grants P22600-B12 and W1232.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00424-012-1144-5) contains supplementary material, which is available to authorized users.
References
- 1.Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 2.Beyl S, Kügler P, Kudrnac M, Hohaus A, Hering S, Timin E. Different pathways for activation and deactivation in CaV1.2: a minimal gating model. J Gen Physiol. 2009;134:231–241. doi: 10.1085/jgp.200910272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyl S, Depil K, Hohaus A, Stary-Weinzinger A, Timin E, Shabbir W, Kudrnac M, Hering S. Physicochemical properties of pore residues predict activation gating of CaV1.2: a correlation mutation analysis. Pflugers Arch. 2011;461:53–63. doi: 10.1007/s00424-010-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 5.Bezanilla F, Perozo E, Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994;66:1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- 9.Del Camino D, Kanevsky M, Yellen G. Status of the intracellular gate in the activated-not-open state of Shaker K+ channels. J Gen Physiol. 2005;126:419–428. doi: 10.1085/jgp.200509385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depil K, Beyl S, Stary-Weinzinger A, Hohaus A, Timin E, Hering S. Timothy mutation disrupts the link between activation and inactivation in Ca(V)1.2 protein. J Biol Chem. 2011;286:31557–31564. doi: 10.1074/jbc.M111.255273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 12.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M-Y, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics Chapter. 2006;5 doi: 10.1002/0471250953.bi0506s15. Unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnon DG, Bezanilla F. A single charged voltage sensor is capable of gating the Shaker K+ channel. J Gen Physiol. 2009;133:467–483. doi: 10.1085/jgp.200810082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackos DH, Chang T-H, Swartz KJ. Scanning the intracellular S6 activation gate in the shaker K+ channel. J Gen Physiol. 2002;119:521–532. doi: 10.1085/jgp.20028569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Hemara-Wahanui A, Berjukow S, Hope CI, Dearden PK, Wu S-B, Wilson-Wheeler J, Sharp DM, Lundon-Treweek P, Clover GM, Hoda J-C, Striessnig J, Marksteiner R, Hering S, Maw MA. A CACNA1F mutation identified in an X-linked retinal disorder shifts the voltage dependence of CaV1.4 channel activation. Proc Natl Acad Sci U S A. 2005;102:7553–7558. doi: 10.1073/pnas.0501907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hering S. Beta-subunits: fine tuning of Ca(2+) channel block. Trends Pharmacol Sci. 2002;23:509–513. doi: 10.1016/s0165-6147(02)02104-1. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo P, MacKinnon R. Revealing the architecture of a K + channel pore through mutant cycles with a peptide inhibitor. Science. 1995;268:307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- 19.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol Lond. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohaus A, Beyl S, Kudrnac M, Berjukow S, Timin EN, Marksteiner R, Maw MA, Hering S. Structural determinants of L-type channel activation in segment IIS6 revealed by a retinal disorder. J Biol Chem. 2005;280:38471–38477. doi: 10.1074/jbc.M507013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn R, Ding S, Gruber HJ. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshi T, Zagotta WN, Aldrich RW. Shaker potassium channel gating. I: transitions near the open state. J Gen Physiol. 1994;103:249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen MØ, Jogini V, Borhani DW, Leffler AE, Dror RO, Shaw DE. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 24.Kudrnac M, Beyl S, Hohaus A, Stary A, Peterbauer T, Timin E, Hering S. Coupled and independent contributions of residues in IS6 and IIS6 to activation gating of CaV1.2. J Biol Chem. 2009;284:12276–12284. doi: 10.1074/jbc.M808402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledwell JL, Aldrich RW. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J Gen Physiol. 1999;113:389–414. doi: 10.1085/jgp.113.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 27.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 28.Pathak M, Kurtz L, Tombola F, Isacoff E. The cooperative voltage sensor motion that gates a potassium channel. J Gen Physiol. 2005;125:57–69. doi: 10.1085/jgp.200409197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 31.Phillips LR, Swartz KJ. Position and motions of the S4 helix during opening of the Shaker potassium channel. J Gen Physiol. 2010;136:629–644. doi: 10.1085/jgp.201010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganathan R, Lewis JH, MacKinnon R. Spatial localization of the K+ channel selectivity filter by mutant cycle-based structure analysis. Neuron. 1996;16:131–139. doi: 10.1016/s0896-6273(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 34.Raybaud A, Dodier Y, Bissonnette P, Simoes M, Bichet DG, Sauvé R, Parent L. The role of the GX9GX3G motif in the gating of high voltage-activated Ca2+ channels. J Biol Chem. 2006;281:39424–39436. doi: 10.1074/jbc.M607405200. [DOI] [PubMed] [Google Scholar]
- 35.Raybaud A, Baspinar E-E, Dionne F, Dodier Y, Sauvé R, Parent L. The role of distal S6 hydrophobic residues in the voltage-dependent gating of CaV2.3 channels. J Biol Chem. 2007;282:27944–27952. doi: 10.1074/jbc.M703895200. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 37.Schoppa NE, Sigworth FJ. Activation of Shaker potassium channels I. Characterization of voltage-dependent transitions. J Gen Physiol. 1998;111:271–294. doi: 10.1085/jgp.111.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoppa NE, Sigworth FJ. Activation of Shaker potassium channels II. Kinetics of the V2 mutant channel. J Gen Physiol. 1998;111:295–311. doi: 10.1085/jgp.111.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoppa NE, Sigworth FJ. Activation of Shaker potassium channels III. An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J Gen Physiol. 1998;111:399–420. doi: 10.1085/jgp.111.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J Gen Physiol. 1998;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca (V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. (Keating MT) discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stary A, Shafrir Y, Hering S, Wolschann P, Guy HR. Structural model of the CaV 1.2 pore. Channels Austin. 2008;2:210–215. doi: 10.4161/chan.2.3.6158. [DOI] [PubMed] [Google Scholar]
- 45.Stefani E, Toro L, Perozo E, Bezanilla F. Gating of Shaker K+ channels: I. Ionic and gating currents. Biophys J. 1994;66:996–1010. doi: 10.1016/S0006-3495(94)80881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Striessnig J, Bolz HJ, Koschak A. Channelopathies in CaV1.1, CaV1.3, and CaV1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sukhareva M, Hackos DH, Swartz KJ. Constitutive activation of the Shaker KV channel. J Gen Physiol. 2003;122:541–556. doi: 10.1085/jgp.200308905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tadross MR, Ben Johny M, Yue DT. Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Ca (v)1.3 channels. J Gen Physiol. 2010;135:197–215. doi: 10.1085/jgp.200910308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talavera K, Nilius B. Evidence for common structural determinants of activation and inactivation in T-type Ca2+ channels. Pflugers Arch. 2006;453:189–201. doi: 10.1007/s00424-006-0129-7. [DOI] [PubMed] [Google Scholar]
- 50.Wall-Lacelle S, Hossain MI, Sauvé R, Blunck R, Parent L. Double mutant cycle analysis identified a critical leucine residue in the IIS4S5 linker for the activation of the Ca(V)2.3 calcium channel. J Biol Chem. 2011;286:27197–27205. doi: 10.1074/jbc.M111.237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yifrach O, MacKinnon R. Energetics of pore opening in a voltage-gated K(+) channel. Cell. 2002;111:231–239. doi: 10.1016/s0092-8674(02)01013-9. [DOI] [PubMed] [Google Scholar]
- 52.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zagotta WN, Hoshi T, Dittman J, Aldrich RW. Shaker potassium channel gating. II: transitions in the activation pathway. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zandany N, Ovadia M, Orr I, Yifrach O. Direct analysis of cooperativity in multisubunit allosteric proteins. Proc Natl Acad Sci U S A. 2008;105:11697–11702. doi: 10.1073/pnas.0804104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Scheuer T, Catterall WA. Reversed voltage-dependent gating of a bacterial sodium channel with proline substitutions in the S6 transmembrane segment. Proc Natl Acad Sci U S A. 2004;101:17873–17878. doi: 10.1073/pnas.0408270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Yarov-Yarovoy V, Scheuer T, Catterall WA. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhen X-G, Xie C, Fitzmaurice A, Schoonover CE, Orenstein ET, Yang J. Functional architecture of the inner pore of a voltage-gated Ca2+ channel. J Gen Physiol. 2005;126:193–204. doi: 10.1085/jgp.200509292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.