Abstract
Liposomes are composed of lipid bilayer membranes that encapsulate an aqueous volume. A major challenge in the development of liposomes for drug delivery is the control of size and size distribution. In conventional methods, lipids are spontaneously assembled into heterogeneous bilayers in a bulk phase. Additional processing by extrusion or sonication is required to obtain liposomes with small size and a narrow size distribution. Microfluidics is an emerging technology for liposome synthesis, because it enables precise control of the lipid hydration process. Here, we describe a number of microfluidic methods that have been reported to produce micro/nanosized liposomes with narrower size distribution in a reproducible manner, focusing on the use of continuous-flow microfluidics. The advantages of liposome formation using the microfluidic approach over traditional bulk-mixing approaches are discussed.
1. Introduction
Liposomes are vesicular structures consisting of one or more lipid bilayer membranes that encapsulate an aqueous volume. Hydrophilic drugs can be loaded into the interior aqueous core of liposomes, whereas lipophilic and amphiphilic drugs can be incorporated into the lipid bilayers (Bangham et al., 1965; Jesorka and Orwar, 2008; Meure et al., 2008). Due to these properties, liposomes are widely used as drug and gene delivery vehicles (Torchilin, 2005). As drug delivery vehicles, liposomes can provide metabolic protection, prolong circulation time, reduce toxicity, control drug release, and enhance cell/tissue specificity of delivery. Several liposomal drugs, for example Doxil (PEGylated liposomal doxorubicin), have reached clinical use (Abraham et al., 2005). In addition to conventional drugs, liposomes hold great promises as delivery vehicles for oligonucleotide-based therapeutics, including siRNA (Kawakami et al., 2008; Yu et al., 2009). Properties such as uniform particle size and good colloidal stability are essential for liposomes to be developed as in vivo drug carriers. Size characteristics of liposomes have a critical effect on the capacity of drug loading, in vivo biodistribution and clearance rate, etc. For instance, liposomes with a diameter of 50–200 nm have been shown to exhibit a slower clearance rate than larger ones (Li and Huang, 2008; Liu et al., 1992). Therefore, it is essential for production methods of liposomes to reproducibly generate particle size distributions within a certain size range.
A number of methods have been developed to produce nano/microsized liposomes, such as thin-film hydration (Amselem et al., 1990; Bhalerao and Raje Harshal, 2003), ethanol injection (Batzri and Korn, 1973; Pons et al., 1993), and detergent dialysis methods (Cardoza et al., 1984; Zumbuehl and Weder, 1981). However, the conventional bulk production of liposomes mainly relies on self-assembly of lipids in a bulk phase, which is heterogeneous and uncontrolled. The resultant liposomes are polydispersed in size and are multilamellar. Further post-processing by extrusion, freeze–thaw, sonication, and/or high-pressure homogenization is often required (Johnson et al., 1971; Purmann et al., 1993).
Microfluidics is a versatile technology to manipulate liquid flows in channels with dimensions of tens to hundreds of micrometers (Stone et al., 2004; Whitesides, 2006). It can provide rapid and tunable mixing, a homogenous reaction environments and a high-throughput experimental platform. Therefore, it is an attractive technology for a variety of applications in chemical synthesis and biological analysis (Jahn et al., 2008; Whitesides, 2006). The exquisite control of flow and mixing conditions in microfluidics has been applied for altering particle size and improving homogeneity of particle size distributions as well (Jahn et al., 2008). Jahn et al. (2004, 2007) developed a microfluidic hydrodynamic focusing (MHF) method for controlled liposome formation. Our group has successfully extended MHF technology for producing polymer-DNA (polyplex) (Koh et al., 2009a) and lipid-polymer-DNA (lipopolyplex) nanoparticles (Koh et al., 2009b) using three-inlet or five-inlet MHF devices. Additionally, we reported a simple and low-cost alternative method with the key feature of microfluidics for production of liposomes.
Here the use of microfluidics for production of liposomes is reviewed, emphasizing the production of nanosized liposomes based on continuous-flow microfluidics. The advantages of liposome formation using microfluidics compared to traditional bulk mixing (BM) are also discussed.
2. Conventional Technologies for Production of Liposomes
There is a wide variety of traditional methods used to prepare liposomes, including thin-film hydration, detergent dialysis, reverse-phase evaporation, and ethanol injection (Jesorka and Orwar, 2008; Meure et al., 2008; Szoka and Papahadjopoulos, 1978; Düzgüneş, 2003). In these methods, lipids are generally dissolved in a transfer medium, followed by the removal of the medium. The liposomal particles are spontaneously self-assembled in the bulk phase by the hydration of a thin-film, or during the removal of the transfer medium, such as an organic solvent or a detergent solution. The self-assembly of lipid vesicles typically occurs under an environment with characteristic dimension of millimeters or centimeters, which results in local concentration fluctuations of lipids and payloads. Thus, the prepared vesicles are initially multilamellar and heterogeneous. To alter particle size and minimize the polydispersity of the size distributions, one or two postprocessing procedures, such as freeze–thaw, sonication, extrusion, and high-pressure homogenization are required (Castile and Taylor, 1999; Meure et al., 2008; Plum et al., 1988). For example, the extrusion method can be used to prepare liposomes with controlled sizes determined by the pore size of a track-etched polycarbonate membrane. However, this method is limited by the requirement of highly specialized equipment, and is time-consuming.
In summary, limitations of conventional preparation methods include complexity and length of procedures, low drug encapsulation efficiency and polydisperse size distributions. Recently, additional new procedures have been reported for producing liposomes to address the issues in conventional production technologies. The new procedures include freeze-drying of a monophase solution (Li and Deng, 2004), MHF method (Jahn et al., 2004), and supercritical fluid method (Frederiksen et al., 1997; Otake et al., 2001).
3. Microfluidic Technologies for Synthesis of Nanoparticles
Microfluidics is a technology that enables precise control and manipulation of fluids and fluid interfaces at the micrometer scale. In microfluidic chips, the fluid streams can merge and form well-defined interface by laminar flow, as opposed to the typically chaotic flows in BM. According to the difference in manipulation modes of flow, microfluidics is categorized into two classes: continuous-flow microfluidics (Jahn et al., 2008) and digital (droplet-based) microfluidics (Chatterjee et al., 2006). In continuous-flow microfluidics, liquid flow is continuously manipulated through microfabricated channels, whereas discrete and controllable droplets are manipulated in droplet-based microfluidics. Continuous-flow microfluidic is generally more suitable for producing nanoparticles (Jahn et al., 2008).
Over the past decade, microfluidics technologies have been developed as an important tool in chemical synthesis and biological analysis (Whitesides, 2006). In parallel, there has been an increasing interest in the use of microfluidics as a novel platform for the preparation of nano- and microparticles. The rapid and tunable mixing provided by microfluidic makes it suitable for the controlled synthesis of nanoparticles, including CdSe quantum dots (QDs) (Schabas et al., 2008), titanium dioxide nanorods (Cottam et al., 2007), and polymeric nanoparticles (Karnik et al., 2008). In addition, MHF devices have been used for the self-assembly of nanoparticles, including liposomes (Jahn et al., 2004, 2007). For example, microfluidics was used to synthesize complexes between calf thymus DNA and cationic lipids (Otten et al., 2005) and dendrimers (Dootz et al., 2006). Langer and coworkers (Karnik et al., 2008) used MHF microfluidics to control nanoprecipitation of poly(lactic-co-glycolic acid)-b-poly(ethylene glycol) (PLGA-PEG) diblock copolymers as a model polymeric material for drug delivery. Polymeric nanoparticles with smaller size and narrower size distribution were obtained by varying the flow rates, polymer composition, and polymer concentration. Nanoparticles yielded by MHF synthesis had a Z-average diameter of about 24 nm at PLGA-PEG concentration of 50 mg/ml, which was smaller than the Z-average diameter of 31 nm obtained by conventional slow-BM. They also observed that with a decrease of mixing time by increasing the flow ratio (0.03–0.1), the size of nanoparticles generated by the MHF method decreased from 26 to 20 nm at 20 mg/ml PLGA-PEG, which was smaller than the 30–35 nm size prepared by BM method. Moreover, the polymeric nanoparticles prepared by MHF showed much narrower distribution, and improved drug loading and release properties (Karnik et al., 2008).
4. Microfluidic Technologies for Production of Liposomes
Confinement and well-defined mixing in microfluidics makes it attractive for production of liposomes ranging from tens of nanometers to tens of micrometers in diameter. The self-assembly in microfluidics can be controlled by varying liquid flow rates, ratios of cross-flows and the composition and concentration of lipids, resulting in tunable sizes, and narrower size distributions. Recently, several studies on liposome production in microfluidics have been reported (Jahn et al., 2008).
4.1. Giant liposomes produced by droplet-based microfluidics
Giant liposomes or giant unilamellar vesicles (GUVs) (over 10 μm in diameter) have a size comparable to that of cells and can encapsulate biomaterials such as DNA and proteins. Giant liposomes, therefore, are used as a model to mimic cell membranes for studing the interaction of the cell membrane with other molecules (Chatterjee et al., 2006; Kuribayashi et al., 2006). Several methods have been developed for production of this type of liposomes. Basically, these involve the swelling of dried lipid, with or without enhancement by an electric field.
Recently, droplet-based microfluidic systems have been used to produce giant liposomes. Tan et al. (2006) presented a novel method to control the formation of liposomal vesicles by a shear-focusing-based droplet microfluidic systems. Using this microfluidic system, they were able to encapsulate large biological materials ranging from cancer cells and yeast cells to nanosized proteins. The resultant vesicles showed long-term stability (>26 days) and high encapsulation efficiency. Stachowiak et al. (2008) also developed a microfluidics method for simultaneously creating and loading giant liposomes based on a pulsed microfluidic jet. In the microfluidic jetting, a planar lipid bilayer is deformed into a vesicle that is filled with the solution from the jet. Compared to other conventional giant liposome production methods, this method can rapidly and controllably generate multiple monodisperse and unilamellar vesicles. They demonstrated that 500 nm particles can be encapsulated into GUVs in a highly reproducible manner and functional pore proteins can also be incorporated into the vesicle membrane simultaneously.
4.2. Nanosized liposome formation by MHF
Jahn et al. (2004) first reported on the controlled synthesis of submicrometer-sized liposomes through MHF. As depicted in Fig. 7.1A, isopropyl alcohol (IPA) containing the dissolved lipids flows through the center inlet channel, and an aqueous solution flows through the two side inlet channels. The stream of lipids in IPA is hydrodynamically focused by two aqueous streams at the cross junction of the microfluidic chip. The flow rates of the IPA and buffer streams are adjusted to control the width of the interface, that is the degree of hydrodynamic focusing. The liposome formation is based on a diffusion-driven process in which the dissolved lipids self-assemble into liposomes as IPA quickly diffuses and dilutes into two aqueous streams at the interfacial region. Thus, the size and size distribution of liposomes can be controlled through adjusting lipids concentrations and flow conditions. By altering the flow rate of IPA (from 2.4 to 59.8 mm/s), the size of resultant liposome can be readily controlled over the range of 100–300 nm (Jahn et al., 2004). Basically, the mechanism of liposome formation in the MHF method is very similar to that in the ethanol injection method. In contrast to ethanol injection, the flow ratio between the lipid-solved stream and buffer streams becomes an important control condition in the MHF method.
Figure 7.1.
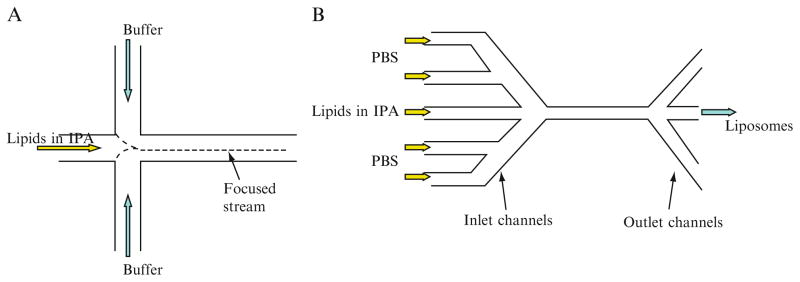
Schematic diagram of microfluidic channels for liposome formation: (A) three-inlet design; (B) five-inlet design.
Recently, Jahn et al. (2007) made significant modifications in their microfluidic system to greatly improve the control over size and size distribution. As shown in Fig. 7.1B, their new microfluidics device has five-inlet channels and three-outlet channels, which are fabricated in a silicon wafer. The resulting microfluidics channels have a rectangular cross section with a depth of 100 μm and a width of 46 or 64 μm. The lipid IPA solution is injected into the center channel of the microfluidics network, while phosphate-buffered saline (PBS) is injected into two side channels intersecting with the center channel. Owing to this three-outlet design, relatively high liposome concentration can be produced at the center point in the channel once the focused IPA stream is diluted to the critical concentration for formation of the more stable liposomes along the interfacial region.
Liposome formation under different shear forces was investigated by changing the buffer volumetric flow rate (VFR) of PBS from 15 to 90 μl/min and the VFR of IPA from 1 to 6 μl/min. The flow rate ratio (FRR), defined as VFR of PBS to VFR of IPA, ranged from 10 to 60. They observed the precise control of size over a mean diameter from 50 to 150 nm by manipulating the flow conditions, especially in the FRR. As the FRR increases sixfold (from 10 to 60), the liposome size decreases in diameter from approximately 120–50 nm and the standard deviation decreases from 0.4 to 0.2. Liposome size and size distribution showed little change when VFR of IPA was varied from 30 to 180 μl/min at a constant FRR of 30. This suggests that liposome formation in MHF is more dependent on the width of the focused alcohol stream and its diffusive mixing with the aqueous stream than on the shear forces at the interfaces. The narrow liposome size and homogeneous size distribution can be facilely manipulated by reducing the width of the alcohol stream or the length scale of liquid mixing to a few micrometers.
4.3. Formation of liposomes containing oligonucleotides by MHF
Oligonucleotides (ONs), including antisense oligodeoxynucleotides (AS-ODN) and small interfering RNA (siRNA), have been emerging as therapeutic agents for treatment of numerous diseases (Dykxhoorn and Lieberman, 2006; Kurreck, 2003). Their applications in the clinic have been severely limited by the lack of safe and efficient delivery systems. To efficiently deliver ONs, liposomes are widely recognized as one of the most promising vehicles. However, it is still a challenge to produce homogeneous ON liposomes with narrow size distribution. Our group developed an MHF technology to produce DNA/polyethylenimine polyplexes for gene delivery (Koh et al., 2009a). Recently, we have been extending the MHF technology to production of ON liposomes (Koh et al., 2009b).
The five-inlet microfluidic device consists of three-inlet ports and one-outlet port (Fig. 7.2). The inlet ports are each connected to sterile syringes containing lipids ethanol solution, protamine or ON aqueous solutions. As shown in Fig. 7.2, a fluid stream is split into two side streams at inlet port 1 or 2, while a fluid stream directly entered the center microchannel through inlet port 3. The resulting ON liposomes solution is collected at the outlet port. The MHF device is fabricated in a poly(methyl methacrylate) (PMMA) plate using a modified microfabrication protocol described elsewhere (Koh et al., 2003). The channel patterns in MHF device is first designed in AutoCAD (Autodesk, San Rafael, CA) and then is fabricated by a high-precision computer numerically controlled (CNC) machine (Aerotech, Inc.) according to the transferred g-code program from AutoCAD. The closed channels are formed by thermally laminating a 45-μm thick PMMA film onto the fabricated microfluidics chip. After lamination, fluidic connectors are bonded onto the inlet/outlet openings in PMMA plate by applying a UV curing adhesive under the exposure of UV irradiation (Novacure 2100, EFXO Corp., Quebec, Canada) for 10 s. The degree of hydrodynamic focusing is controlled by adjusting the FRR of the side streams (lipids and protamine solutions) to the middle stream (ON solution). Two programmable syringe pumps (Pump 33, Harvard Apparatus, Holliston, MA) are used to manipulate the fluid flow rates independently.
Figure 7.2.
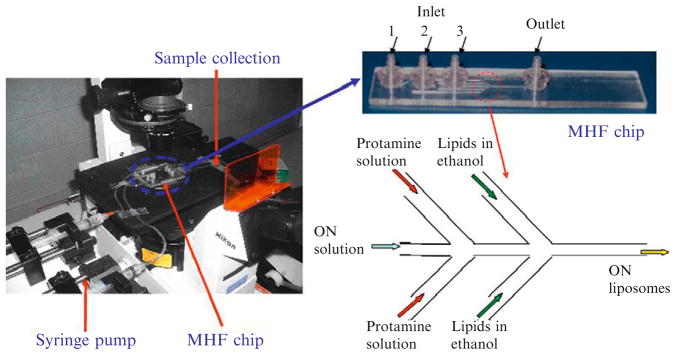
Photographs of five-inlet MHF methods to prepare liposomes containing oligonucleotides.
G3139, an AS-ODN against human Bcl-2, is incorporated in liposomes to evaluate the difference between G3139 liposomes produced by the MHF method and that prepared by the BM method. For all G3139 containing liposomes, the final weight ratio of G3139:protamine:lipids (DC-Chol/egg PC/PEG-DSPE at a molar ratio of 30/68/2) is 1:0.3:12.5 and the ethanol concentration of 40% is maintained during nanoparticle synthesis. The flow rates for G3139, protamine, and lipids streams are 20, 20, and 450 μl/min, respectively. Our results demonstrate that MHF-produced G3139 liposomes have similar nanostructures but smaller size (114.8 ± 12.7 nm) and tighter size distribution comparing to BM-produced G3139 liposomes (152.7 ± 22.1 nm). Importantly, G3139 liposomes produced by the MHF method facilitated better delivery into K562 cells and also downregulated an apoptotic protein, Bcl-2 more efficiently as compared to the BM method.
To implement MHF in a laboratory setting, syringe pumps and a microfluidic chip are required. A number of commercial suppliers are now available that can provide suitable chips. For example, Translume Inc. (Ann Arbor, MI) offers all-glass microfluidic chips with T- and Y-channel designs with luer connectors with 30, 100, and 300 μm channel widths. These chips can be connected easily to a pair of syringe pumps for MHF production of liposomes.
4.4. A facile microfluidic method for production of liposomes
Although the advantages of microfluidics for liposome production have been demonstrated by Jahn et al. (2004, 2007) and in our group, this technology requires microfabrication expertise and facilities, which may be unavailable and unaffordable in many biomedical research laboratories. To facilely use microfluidics in the laboratory, we further developed a simple and low-cost method with the key features of microfluidics, but without the need for microfabrication (Pradhan et al., 2008).
A microfluidics injection device driven by a syringe-pump is used to produce liposomes under various conditions. As demonstrated in Fig. 7.3A, the syringe carrying water is injected to an elbow connecter by plastic tubing. The other syringe containing the lipids dissolved in ethanol is connected by a needle. The internal diameters of inlet/outlet connectors are 2 mm and the internal diameter of the tubing is 1.5 mm. Needles of a series of gauges (internal diameters of 1.194, 0.838, 0.495, 0.241, and 0.191 mm) are selected for the control of shear force of lipid flow. In a typical setup, 1 ml lipid ethanol solution and 10 ml deionized water are loaded into a 1 and 10 ml syringe, respectively. Syringes are mounted on an infusion syringe pump (model 975, Harvard Apparatus, Holliston, MA, USA) and moved at the same speed (Fig. 7.3B).
Figure 7.3.
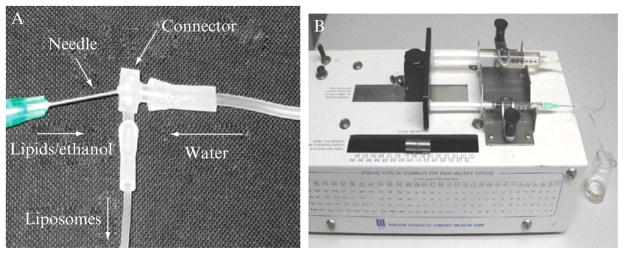
Photographs of a facile microfluidic device for production of liposomes.
Microfluidics is characterized by laminar flow, which means that the Reynolds number (Re) should be lower than the value of 2000. In a typical device, the calculated Re value is 13.5, which is much lower than 2000 and indicates strictly laminar flow. The chaotic mixing in this device is significantly different from the laminar mixing in classic microfluidics devices. As the ethanol in the lipid stream diffuses and dilutes into the water stream, the lipids tend to assemble into liposomes. Stable liposomes are eventually formed when the mixture reaches equilibrium.
This microfluidic injection method is similar to the conventional ethanol injection method, but it enables continuous manipulation of particle size by providing well-defined mixing and a constant water-to-ethanol flow ratio (10:1). More importantly, this method does not require the costly micro-fabrication equipment and advanced manipulation skills. The key features of a microfluidics system are kept in this nonmicrofabricated method. The average size of the liposomes produced by using this method can be conveniently and repeatedly tuned by varying the type of lipid, lipid concentration, flow rate, and temperature. Thus, the fluidity of the specific lipid composition can modulate the size of the produced liposomes. Mixtures containing cholesterol, which is known to rigidify the liquid crystalline lipid bilayers, produce larger liposomes. Lipids in the gel (solid) phase, which exhibit considerably lower fluidity than the liquid crystalline phase, generate liposomes of significantly larger size (cf. 90 nm for the liquid crystalline egg phosphatidylcholine versus 250 nm for the solid hydrogenated soy phosphatidylcholine at the same concentration and flow rate) (Pradhan et al., 2008).
In contrast to other liposome manufacturing techniques, this method is simple, quick, and affordable, and it can be readily adopted in any biomedical or pharmaceutical laboratory.
5. Concluding Remarks
Microfluidics is a relatively novel technology for the production of micro- and nanosized liposomes. The characteristics of laminar flow and tunable mixing in microfluidics systems have distinctive advantages in liposome formation over traditional methods, such as thin-film hydration and reverse-phase evaporation. Reproducible control of particle size and size distribution can be implemented in continuous microfluidics flow systems. Using hydrodynamic focusing in microfluidic channels, nano-sized liposomes with smaller size and narrower size distribution are easily formed by varying flow parameters. They include flow rate, flow ratio, concentration of lipids solution, and temperature as well as characteristic length in microfluidics channels. However, the problem of scaling up liposome production needs to be addressed during the implementation of microfluidics technology for practical application. Additionally, the ultrafine structure of MFH-produced liposomes needs to be further investigated. Microfluidics provides a new platform for the development and optimization of liposomes in the emerging field of nanomedicine. It can control liposome self-assembly and potentially lead to applications in instant liposome synthesis as part of point-of-care personalized therapeutics.
Acknowledgments
This work was supported by grants EEC 0425626 from the National Science Foundation and R21 CA131832 and R01 CA135243 from the National Institutes of Health. The authors thank Dr. B. Tenchov and Dr. R. Koynova for their helpful suggestions.
References
- Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- Amselem S, Gabizon A, Barenholz Y. Optimization and upscaling of doxorubicin-containing liposomes for clinical use. J Pharm Sci. 1990;79:1045–1052. doi: 10.1002/jps.2600791202. [DOI] [PubMed] [Google Scholar]
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973;298:1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Bhalerao SS, Raje Harshal A. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev Ind Pharm. 2003;29:451–467. doi: 10.1081/ddc-120018380. [DOI] [PubMed] [Google Scholar]
- Cardoza JD, Kleinfeld AM, Stallcup KC, Mescher MF. Hairpin configuration of H-2kk in liposomes formed by detergent dialysis. Biochemistry. 1984;23:4401–4409. doi: 10.1021/bi00314a025. [DOI] [PubMed] [Google Scholar]
- Castile JD, Taylor KMG. Factors affecting the size distribution of liposomes produced by freeze-thaw extrusion. Int J Pharm. 1999;188:87–95. doi: 10.1016/s0378-5173(99)00207-0. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Hetayothin B, Wheeler AR, King DJ, Garrell RL. Droplet-based microfluidics with nonaqueous solvents and solutions. Lab Chip. 2006;6:199–206. doi: 10.1039/b515566e. [DOI] [PubMed] [Google Scholar]
- Cottam BF, Krishnadasan S, deMello AJ, deMello JC, Shaffer MSP. Accelerated synthesis of titanium oxide nanostructures using microfluidic chips. Lab Chip. 2007;7:167–169. doi: 10.1039/b616068a. [DOI] [PubMed] [Google Scholar]
- Dootz R, Otten A, Koster S, Struth B, Pfohl T. Evolution of DNA compaction in microchannels. J Phys Condens Matter. 2006;18:S639–S652. [Google Scholar]
- Düzgüneş N. Preparation and quantitation of small unilamellar liposomes and large unilamellar reverse-phase evaporation liposomes. Methods Enzymol. 2003;367:23–27. doi: 10.1016/S0076-6879(03)67003-5. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Lieberman J. Knocking down disease with siRNAs. Cell. 2006;126:231–235. doi: 10.1016/j.cell.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen L, Anton K, van Hoogevest P, Keller HR, Leuenberger H. Preparation of liposomes encapsulating water-soluble compounds using supercritical carbon dioxide. J Pharm Sci. 1997;86:921–928. doi: 10.1021/js960403q. [DOI] [PubMed] [Google Scholar]
- Jahn A, Vreeland WN, Gaitan M, Locascio LE. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J Am Chem Soc. 2004;126:2674–2675. doi: 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23:6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- Jahn A, Reiner JE, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Preparation of nanoparticles by continuous-flow microfluidics. J Nanopart Res. 2008;10:925–934. [Google Scholar]
- Jesorka A, Orwar O. Liposomes: Technologies and analytical applications. Annu Rev Anal Chem. 2008;1:801–832. doi: 10.1146/annurev.anchem.1.031207.112747. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Bangham AD, Hill MW, Korn ED. Single bilayer liposomes. Biochim Biophys Acta. 1971;233:820–826. doi: 10.1016/0005-2736(71)90184-2. [DOI] [PubMed] [Google Scholar]
- Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Higuchi Y, Hashida M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci. 2008;97:726–745. doi: 10.1002/jps.21024. [DOI] [PubMed] [Google Scholar]
- Koh CG, Tan W, Zhao MQ, Ricco AJ, Fan ZH. Integrating polymerase chain reaction, valving, and electrophoresis in a plastic device for bacterial detection. Anal Chem. 2003;75:4591–4598. doi: 10.1021/ac0343836. [DOI] [PubMed] [Google Scholar]
- Koh CG, Kang XH, Xie YB, Fei ZZ, Guan JJ, Yu B, Zhang XL, Lee LJ. Delivery of polyethylenimine (PEI)/DNA complexes assembled in a microfluidics device. Mol Pharm. 2009a doi: 10.1021/mp900016q. (in press) [DOI] [PubMed] [Google Scholar]
- Koh CG, Zhang XL, Liu SJ, Golan S, Yu B, Yang XJ, Guan JJ, Yan J, Talmon Y, Muthasamy R, Chan KK, Byrd J. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J Control Release. 2009b doi: 10.1016/j.jconrel.2009.08.019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribayashi K, Tresset G, Coquet P, Fujita H, Takeuchi S. Electroformation of giant liposomes in microfluidic channels. Meas Sci Technol. 2006;17:3121–3126. [Google Scholar]
- Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- Li C, Deng Y. A novel method for the preparation of liposomes: Freeze drying of monophase solutions. J Pharm Sci. 2004;93:1403–1414. doi: 10.1002/jps.20055. [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- Liu D, Mori A, Huang L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta. 1992;1104:95–101. doi: 10.1016/0005-2736(92)90136-a. [DOI] [PubMed] [Google Scholar]
- Meure LA, Foster NR, Dehghani F. Conventional and dense gas techniques for the production of liposomes: A review. AAPS Pharm Sci Technol. 2008;9:798–809. doi: 10.1208/s12249-008-9097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Imura T, Sakai H, Abe M. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir. 2001;17:3898–3901. [Google Scholar]
- Otten A, Koster S, Struth B, Snigirev A, Pfohl T. Microfluidics of soft matter investigated by small-angle X-ray scattering. J Synchrotron Radiat. 2005;12:745–750. doi: 10.1107/S0909049505013580. [DOI] [PubMed] [Google Scholar]
- Plum G, Korber C, Rau G. Freeze/thaw response of single bilayer liposomes. Cryobiology. 1988;25:520. [Google Scholar]
- Pons M, Foradada M, Estelrich J. Liposomes obtained by the ethanol injection method. Int J Pharm. 1993;95:51–56. [Google Scholar]
- Pradhan P, Guan J, Lu D, Wang PG, Lee LJ, Lee RJ. A facile microfluidic method for production of liposomes. Anticancer Res. 2008;28:943–947. [PubMed] [Google Scholar]
- Purmann T, Mentrup E, Kreuter J. Preparation of suv-liposomes by high-pressure homogenization. Eur J Pharm Biopharm. 1993;39:45–52. [Google Scholar]
- Schabas G, Yusuf H, Moffitt MG, Sinton D. Controlled self-assembly of quantum dots and block copolymers in a microfluidic device. Langmuir. 2008;24:637–643. doi: 10.1021/la703297q. [DOI] [PubMed] [Google Scholar]
- Stachowiak JC, Richmond DL, Li TH, Liu AP, Parekh SH, Fletcher DA. Unilamellar vesicle formation and encapsulation by microfluidic jetting. Proc Natl Acad Sci USA. 2008;105:4697–4702. doi: 10.1073/pnas.0710875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech. 2004;36:381–411. [Google Scholar]
- Szoka F, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YC, Hettiarachchi K, Siu M, Pan YR, Lee AP. Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. J Am Chem Soc. 2006;128:5656–5658. doi: 10.1021/ja056641h. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbuehl O, Weder HG. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid-detergent mixed micelles. Biochim Biophys Acta. 1981;640:252–262. doi: 10.1016/0005-2736(81)90550-2. [DOI] [PubMed] [Google Scholar]


