Abstract
Background
Cancer patients seek information about lifestyle and dietary changes which may optimize outcomes. Using data from two large National Cancer Institute-sponsored clinical trials, we report on the dietary and lifestyle practices of patients receiving therapy for stage III colon or metastatic colorectal cancer.
Methods
Self-administered questionnaires were completed by patients undergoing chemotherapy for stage III colon cancer (n = 1095) and metastatic colorectal cancer (n = 875). Descriptive statistical analyses were performed to evaluate anthropometrics, diet and lifestyle in each cohort.
Results
Median body mass index was comparable for stage III and metastatic patients (27.3 vs. 26.5 kg/m2). Stage III patients reported a modestly higher median level of physical activity than metastatic patients (4.6 vs. 3.4 metabolic equivalent task-hours/week). Ten percent of stage III and 9% of metastatic patients reported ongoing cigarette use. Avoidance of alcohol was reported by 47% of stage III and 43% of metastatic patients. Dietary patterns for both groups were comparable with more than 80% of stage III and metastatic patients failed to meet the recommended daily intake of vegetables, fruits, and milk products. Usage of at least 2 multivitamin per week was reported by 49% of stage III and 40% of metastatic patients. Two percent of stage III and 5% of metastatic patients reported vitamin D supplement use.
Conclusions
We observed notable similarities in dietary and lifestyle behaviors between stage III colon and metastatic colorectal cancer patients actively receiving chemotherapy. Future research should aim to elucidate the effect of these behaviors on patient outcomes.
INTRODUCTION
Colorectal cancer is the fourth most common malignancy and the second leading cause of cancer-related death in the United States, with 143,460 new cases and 51,690 deaths anticipated in 2011.[1] The influence of diet and other lifestyle factors on the risk of developing colon or rectum cancer has been established in epidemiologic and scientific research.[2,3] However, current understanding of the influence of these factors on outcomes of patients with established cancer is limited. Studies have previously shown that colorectal cancer patients are highly motivated to seek information beyond standard treatment options and are interested in making healthy lifestyle changes.[4,5,6] As a result, research evaluating the impact of dietary and lifestyle factors on treatment efficacy and overall survival has generated increasing interest.
Several studies have characterized dietary and lifestyle behaviors among colorectal cancer patients.[4,5,7,8,9] Two studies assessed the prevalence and predictors of healthy lifestyle changes in diet, physical activity, and supplement use in patients after a diagnosis of localized or regional colorectal, breast, or prostate cancer.[4,5] Three studies have directly addressed the prevalence and frequency of vitamin and supplement use after diagnosis,[7,8] as well as longitudinal changes in use from pre- to post-diagnosis,[4] among colorectal cancer patients with localized disease. Additionally, a recent Canadian study reported on diet and physical activity behaviors among a small convenience sample of patients with localized and advanced colorectal cancer receiving chemotherapy.[9] Although these studies begin to demonstrate the prevalence of specific diet and lifestyle behaviors among colorectal cancer patients, no study has comprehensively analyzed and reported on the prevalence and frequency of a wide-range of dietary and lifestyle habits among a large sample of colorectal cancer patients with consideration of potential differences based on stage of disease.
Using two large National Cancer Institute (NCI)-sponsored clinical trials, we collected extensive data on dietary patterns, physical activity, smoking status, and the use of aspirin, vitamins, and other supplements. We describe the dietary patterns and lifestyle habits of patients with stage III colon cancer and metastatic colorectal cancer who were actively receiving chemotherapy as participants in phase III clinical trials. The purpose of this comparison was to apprise clinicians of the behavioral patterns of their patients with advanced colon cancer and to inform future clinical research focused on the influence of dietary and lifestyle factors and colorectal cancer outcomes.
METHODS
Study Population
Subjects were patients diagnosed with either stage III colon cancer or metastatic colorectal cancer who were actively receiving chemotherapy as part of a Cancer and Leukemia Group B (CALGB) protocol. The first cohort consisted of participants with stage III colon cancer enrolled in the phase III adjuvant therapy trial comparing weekly fluorouracil (FU) and leucovorin to weekly irinotecan, FU, and leucovorin after curative-intent surgery (CALGB 89803).[10] The second cohort was comprised of patients with previously untreated, metastatic adenocarcinoma of the colon or rectum, enrolled in the CALGB phase III trial of irinotecan, 5-FU, and leucovorin or oxaliplatin, 5-FU, and leucovorin combined with either bevacizumab, cetuximab, or a combination of bevacizumab and cetuximab (CALGB 80405). Questionnaire completion was a requirement of the protocol for CALGB 89803 patients, while patients enrolled in CALGB 80405 had the option to complete a separate consent to participate in the questionnaire element of the study.
Total enrollment for CALGB 89803, between April 1999 and May 2001, was 1,264 participants, of which 98% of eligible patients (n = 1095) completed the questionnaire. Ineligible patients included those enrolled prior to the diet and lifestyle amendment to the protocol (n = 87) and patients who experienced cancer recurrence, died, or were removed from the protocol prior to administration of the questionnaire (n = 59). Enrollment for CALGB 80405 during the period between activation on September 15, 2005 to August 6, 2009, included 1,451 participants, of which 74% (n = 1067) consented to participate in the diet and lifestyle study. Among those who consented, 82% (n = 875) completed and returned the questionnaire, 25 patients were in the process of completing the questionnaire at the time of this analysis and 167 patients failed to complete the questionnaire due to withdrawal of initial consent to participate (n = 34), removal from protocol prior to administration of the questionnaire (n = 27), death (n = 8), and other unknown reasons (n = 98). All patients signed an informed consent document approved by each site’s institutional review board.
Dietary and Lifestyle Assessment
The self-administered Willett food frequency questionnaire assessing diet and lifestyle habits was given to patients in the CALGB 89803 cohort midway through their adjuvant therapy (approximately 3 months into treatment, which would be roughly 5 months after diagnosis) and in the CALGB 80405 cohort within one month after initiating chemotherapy.[11] The questionnaire administered to each cohort contained the same basic elements with incorporation of minor modifications in the later version used for the CALGB 80405 cohort. The questionnaire collected comprehensive dietary information on consumption of 149 food and beverage items, as well as data concerning lifestyle behaviors including physical activity, cigarette smoking, aspirin use, and vitamin and supplement use. The questionnaire has been extensively tested and validated among large populations, including patients receiving chemotherapy.[11,12]
Body Mass Index (BMI)
BMI analyses utilized patients’ self-reported current height and weight indicated on the questionnaire. BMI was calculated by dividing the patient’s squared height (meters) into their weight (kilograms).
Physical Activity Assessment
Questions utilized to assess physical activity have been described and extensively validated in previous research.[13,14,15,16] To measure physical activity, participants were asked “During the past 2 months, what was your average time per week spent at each of the following recreational activities?” Patients reported duration of participation (ranging from 0 to 11 or more hours per week) in nine leisure-time activities as well as number of flights of stairs climbed daily and their normal walking pace. Data regarding sedentary activity was not collected. Physical activities on the questionnaire were individually assigned a metabolic equivalent task (MET) score, consistent with previously validated calculations.[13,17] One MET is equivalent to the energy expenditure of sitting quietly for 1 hour. MET scores for walking were assigned based on the walking pace reported, while a leisurely to moderate intensity score was selected for other activities. Scores for MET-hours per week for each physical activity were calculated from the reported hours per week engaged in that activity multiplied by the assigned MET score. A total MET-hours per week was derived by summing MET scores from each individual activity.
Alcohol Use and Cigarette Smoking Assessment
Average consumption (ranging from never to 6 or more servings per day) of regular beer, light beer, red wine, white wine, and liquor, over the previous three months, was self-reported. Alcohol consumption was calculated by converting each frequency of use response into an equivalent drinks per week value. Total alcohol consumption per week was calculated as the sum of all individual alcoholic beverage drinks per week values.
Participants’ cigarette use, past and present, was also assessed on the questionnaire with responses to the question, “Have you smoked 20 packs of cigarettes or more in your lifetime?” The percentage of participants reporting their smoking status as never, current, or past was evaluated. For those who were current smokers, the average number of cigarettes per day was reported. For those who were past smokers, the timeframe of smoking cessation was reported.
Dietary Intake Assessment: Food Groups
Patients reported average daily intake during the immediate past three months for 149 food and beverage items. For each item, a commonly used unit or portion size was indicated (e.g., one egg, slice of bread, or 8 oz. glass). For assessment, food and beverage items were broken down into 12 predefined food groups, based on common nutritional profiles. For each item there were up to 10 possible response choices, which ranged from never to 6 or more servings per day. Servings per week for items within each food group were individually calculated and subsequently summed to obtain the total median servings per week for each group.
The percentage of individuals meeting the current recommended daily intake, according to the United States Department of Agriculture (USDA), of vegetables (5 servings per day), fruits (4 servings per day), and milk products (3 cups per day) was also calculated.[18]
Dietary Change Assessment
The questionnaire assessed change in dietary consumption patterns for 8 food and beverage items, including vegetables, fruits, fish, red meat, whole grains, whole milk, sugar, and alcohol, by asking participants whether their consumption of each item decreased, increased, or remained the same over the past year. The questionnaire did not specifically identify those dietary changes that may have been related to symptoms preceding the cancer diagnosis or following colorectal surgery. The percentage of patients, from each cohort, reporting each type of change in consumption was calculated.
Vitamin and Supplement Use Assessment
Patients were asked to report their history of vitamin, mineral, and supplement use. The questionnaire inquired about regular use of multi-vitamins (at least 2 tablets per week) and the use of specific individual vitamins, minerals, fiber and non-vitamin dietary supplements. For analysis, the percentage of patients indicating current vitamin and mineral use of the above items was assessed, as well as the current mean total numbers of vitamins, supplements, and non-vitamin dietary supplements taken daily by patients.
Aspirin Use Assessment
Aspirin usage questions varied slightly between the questionnaires for CALGB 89803 and 80405. For CALGB 89803, patients were asked to report their current frequency of aspirin use (0 days/month, 1–3 days/month, 1–2 days/week, 3–4 days/week, 5–6 days/week, and daily use) and their current aspirin intake per week (0 tablets/week, 0.5–2 tablets/week, 3–5 tablets/week, 6–14 tablets/week, and ≥15 tablets/week). For CALGB 80405, patients were asked whether they currently take “baby” or low dose aspirin (<325 mg/tablet) and whether they take aspirin or aspirin-containing products (325 mg/tablet or more). If a patient indicated current use, they subsequently elaborated on use by reporting how many days per week (1, 2–3, 4–5, or 6+ days), and how many tablets per week (1–2, 3–5, 6–14, or 15+ tablets). Due to differences in the type of questions asked, for data analysis only the percentage of patients from each cohort reporting daily aspirin use and use of 3 or more aspirin tablets per week was assessed.
Statistical Analyses
Data were analyzed using SAS Version 9.1 (SAS Institute Inc., Cary, NC). Statistical analyses included descriptive statistics only. Data from each patient cohort were analyzed separately, with data from all treatment arms within the study combined. Mean, median, and frequency data are presented graphically and in table format.
RESULTS
Patient Characteristics and Lifestyle Habits
Data were collected and analyzed from 1,095 stage III colon cancer patients and 875 metastatic colorectal cancer patients. Median BMI for stage III patients was 27.3 kg/m2 while median BMI for metastatic patients was 26.5 kg/m2. The proportion of patients from each cohort who were underweight, normal, overweight, and obese, according to WHO classifications[19], were similar (Table 1).
Table 1.
Distribution of body mass index, physical activity, smoking and alcohol consumption for stage III colon cancer and metastatic colorectal cancer patients receiving chemotherapy
| Stage III CALGB 89803 (N = 1095) |
Metastatic CALGB 80405 (N = 875) |
||
|---|---|---|---|
|
Body mass index* |
Median (kg/m2) (95% Range) | 27.3 (19.3–40.0) | 26.5 (17.6–40.8) |
| < 18.5 kg/m2 | 2 % | 5 % | |
| 18.5 – 24.9 kg/m2 | 32 % | 31 % | |
| 25 – 29.9 kg/m2 | 36 % | 36 % | |
| ≥ 30 kg/m2 | 30 % | 27 % | |
|
Physical activity |
Median (MET-hours/week) (95% Range) | 4.6 (0.0–77.3) | 3.4 (0.0–68.2) |
| < 3 MET-hours / week | 40 % | 47 % | |
| 3 – 8.9 MET-hours/week | 27 % | 23 % | |
| 9 – 17.9 MET-hours/week | 12% | 13% | |
| 18 – 26.9 MET-hours/week | 7% | 5% | |
| ≥ 27 MET-hours/week | 14% | 12% | |
|
Smoking history |
Never smoker | 46 % | 48 % |
| Current smoker | 10 % | 9 % | |
| Past smoker | 44 % | 43 % | |
|
Alcohol consumption |
Median (drinks/week) (95% Range) | 0.2 (0.0–21.5) | 0.5 (0.0–19.0) |
| 0 | 47 % | 43 % | |
| > 0 to 1 | 20 % | 25 % | |
| > 1 to 7 | 21 % | 23 % | |
| > 7 to 14 | 5 % | 5 % | |
| > 14 to 21 | 4 % | 2 % | |
| > 21 | 3 % | 2 % |
Kg = kilograms; m2 = meters squared; MET = metabolic equivalent task units
Categories based on WHO classification [19]
Stage III patients reported more overall leisure-time physical activity than metastatic patients, with median metabolic equivalent task (MET)-hours per week of 4.6 and 3.4, respectively. The distribution of total MET-hours per week is presented in Table 1. An appreciable percentage of patients, 40% of stage III and 47% of metastatic patients would be classified as inactive, expending fewer than 3 MET-hours per week, which in the equivalent of less than 1 hour per week of recreational walking at an average pace.[13,20] Nonetheless, 21% of stage III patients and 17% of metastatic patients reported expenditure of at least 18 MET-hours per week, which is equivalent to walking at an average pace for at least one hour, six days per week.
Cigarette smoking status for both patient cohorts is reported in Table 1. Nearly half of all patients reported no history of cigarette smoking. The percentage of patients who reported themselves as current smokers was relatively small, and similar among the two cohorts, with current smokers representing 10% of stage III and 9% of metastatic patients. Alcohol consumption was relatively similar between the two cohorts (Table 1). Nearly half of all participants, 47% of stage III and 43% of metastatic patients, reported no alcohol intake during the 3 months prior to questionnaire completion. Two-thirds of both patient cohorts reported consumption of less than one drink per week, while moderate alcohol consumption (>1 to 7 drinks/week) was reported by 21% of stage III and 23% of metastatic patients.
Dietary Consumption Patterns
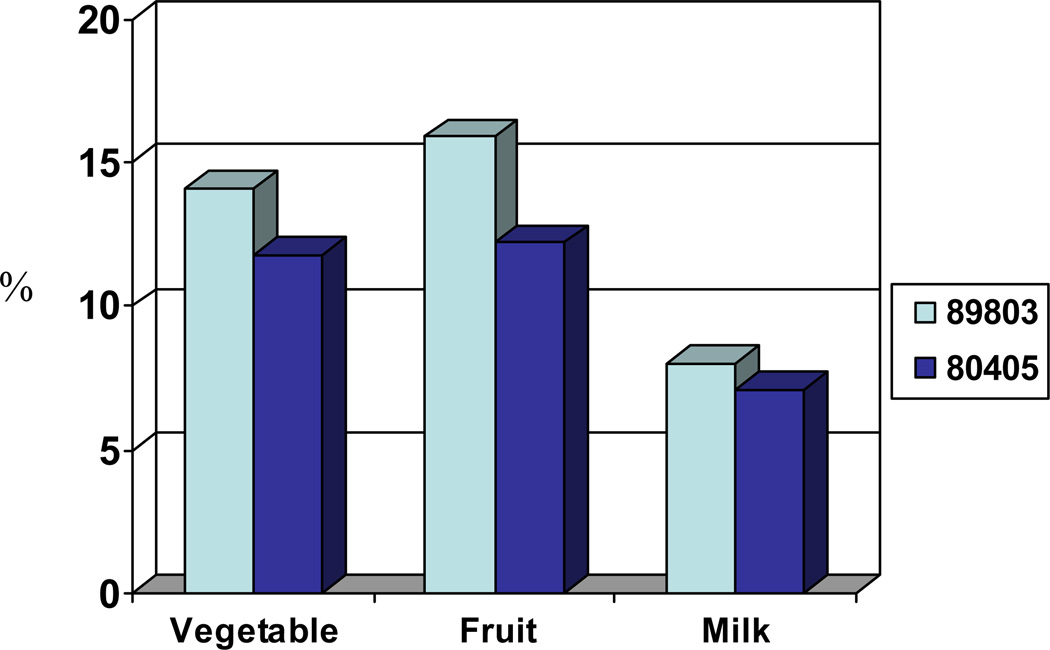
Median servings per week of 12 predefined food groups are reported in Table 2. Overall, the data reflect that stage III and metastatic patients have relatively comparable dietary consumption patterns. More than 80% of stage III and metastatic patients failed to meet the USDA recommended daily intake of vegetables, fruits, and milk products (Figure 1).
Table 2.
Major Food Groups: Median Serving/Week Reported For Each Group
| Median Servings per Week (95% Range) |
||
|---|---|---|
| Food Group | Stage III (N = 1095) |
Metastatic (N = 875) |
| Vegetables | 19.1 (5.2–55.0) | 16.7 (3.8–56.0) |
| Fruits | 15.1 (2.0–46.3) | 13.0 (2.0–43.2) |
| Fish | 1.4 (0.0–6.0) | 1.4 (0.0–6.0) |
| Red Meat | 3.2 (0.0–12.0) | 2.9 (0.0–13.0) |
| Poultry | 2.2 (0.0–9.0) | 2.2 (0.0–11.3) |
| Processed Meats | 2.0 (0.0–10.0) | 2.0 (0.0–12.5) |
| Milk | 9.9 (1.0–34.0) | 9.5 (0.9–32.6) |
| Whole Grains | 8.8 (0.2–38.7) | 4.2 (0.0–19.7) |
| Refined Grains | 20.1 (3.9–65.8) | 13.7 (3.6–46.5) |
| Nuts | 1.4 (0.0–17.7) | 2.0 (0.0–18.1) |
| Sweets | 8.3 (0.6–41.5) | 7.1 (0.8–39.7) |
| Sodas | 3.2 (0.0–27.5) | 2.4 (0.0–28.0) |
The whole grains group included brown rice, bulgar, oatmeal, popcorn, whole grain breads (i.e., whole wheat, oatmeal, rye, etc.), and whole grain cereals. As per USDA guidelines, wheat germ, oat bran, and other bran were not included in this group.
The milk group included skim milk, low-fat milk, whole milk, yogurt, hard natural cheeses, soft cheeses, processed cheeses, frozen yogurt, and ice cream. As per USDA guidelines, cream cheese, cream, butter, and margarine were not included in this group.
Figure 1.
Percentage of patients meeting recommended daily intake for each identified food group.[18]
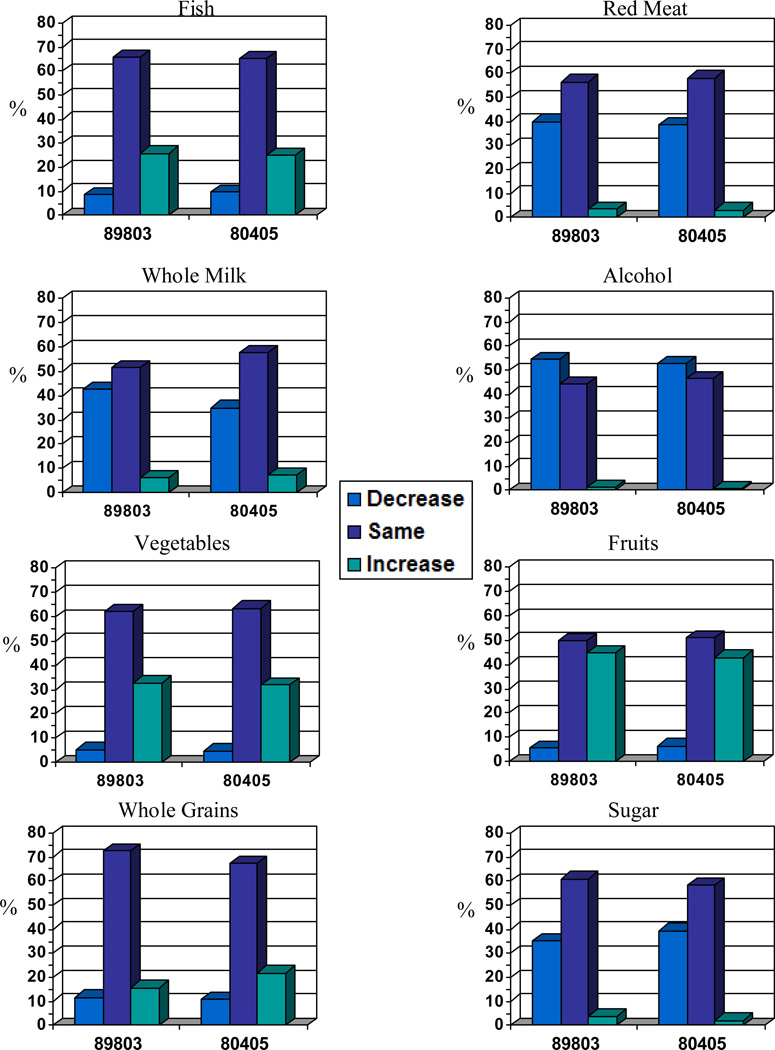
Both questionnaires asked patients to report on changes in consumption of eight food and beverage items since diagnosis and the results are presented graphically for each cohort in Figure 2. As reflected in the graphs, changes in dietary patterns were remarkably consistent between stage III and metastatic patients, with increased consumption of fruits, vegetables, fish, and whole grains, and decreased consumption of alcohol, red meat, sugar, and whole milk reported.
Figure 2.
Dietary change data indicating change in use (i.e., increase, decrease, remain the same) of identified food and beverage items over the past year.
Vitamin, Supplement, & Aspirin Use
The percentage of patients indicating current use of commonly marketed vitamins and supplements is displayed in Table 3. Intake of at least 2 multivitamin tablets weekly was reported by 49% of stage III and 40% of metastatic patients. The mean total number of vitamins currently taken was 1.5 (standard deviation (SD) = 1.9) for stage III patients and 1.1 (SD = 1.6) for metastatic patients. With respect to specific vitamin supplements, 2% of stage III and 5% of metastatic patients reported vitamin D supplement use. Calcium and vitamin C were reported as commonly taken vitamins among both stage III and metastatic patients, with Vitamin E use also prevalent among stage III patients only. Non-vitamin dietary supplement use was less common than vitamin use and less prevalent among metastatic than stage III patients, although not a statistically significant difference, with mean total numbers of 0.4 (SD = 1.0) and 1.1 (SD = 0.7), respectively.
Table 3.
Prevalence of Vitamin, Mineral & Supplement Use
| Cohort |
||
|---|---|---|
| Variable | Stage III (N = 1095) |
Metastatic (N = 875) |
| Mean number of vitamins daily (SD) | 1.5 (± 1.9) | 1.1 (± 1.6) |
| Percentage (95% CI) |
||
| Multivitamins | 49 % (46–52%) | 40 % (37–43%) |
| Vitamin A | 3 % (2–4%) | 2 % (1–4%) |
| Beta-Carotene | 2 % (2–3%) | 1 % (1–2%) |
| Vitamin B6 | 6 % (5–8%) | 4 % (3–6%) |
| Vitamin C | 17 % (15–19%) | 13 % (10–15%) |
| Vitamin E | 21 % (18–23%) | 5 % (4–7%) |
| Folic Acid | 6 % (4–7%) | 4 % (3–5%) |
| Calcium | 22 % (19–24%) | 18 % (16–21%) |
| Selenium | 4 % (3–5%) | 2 % (1–3%) |
| Zinc | 4 % (3–5%) | 3 % (2–5%) |
| Vitamin D | 2 % (2–3%) | 5 % (4–7%) |
| Vitamin B12 | 5 % (4–6%) | 5 % (4–7%) |
| B-Complex Vitamins | 5 % (4–6%) | 3 % (2–4%) |
| Use of ≥ 4 vitamins and/or NVDS (Excluding Multivitamins) |
11 % (9–13%) | 8 % (6–10%) |
| Mean number of NVDS daily (SD) | 1.1 (± 0.7) | 0.4 (± 1.0) |
| Mean number of any supplements daily (SD) |
1.3 (± 2.3) | 1.1 (± 2.0) |
NVDS = Non-vitamin dietary supplements (e.g., Echinacea, fish oil, flaxseed oil, Ginkgo biloba, ginseng, green tea, milk thistle, saw palmetto, etc.)
Aspirin use questions varied slightly between the questionnaires for each cohort, with baby aspirin use only assessed for metastatic patients. The percentage of stage III patients reporting daily aspirin use (dose not specified) was 14%, while the percentage of metastatic patients reporting daily baby aspirin use and daily standard aspirin use were 13% and 3%, respectively. Analysis of average tablet intake per week indicated that 14% of stage III patients reported taking 3 or more aspirin tablets per week, 11% of metastatic patients reported use of 3 or more baby tablets (<325 mg/tablet) per week, and only 4% of metastatic patients indicated taking 3 or more standard tablets (325 mg/tablet or more) per week.
DISCUSSION
Utilizing a well-validated diet and lifestyle questionnaire, we were able to comprehensively measure the prevalence and frequency of various lifestyle factors including dietary intake, BMI, physical activity, cigarette smoking, alcohol consumption, vitamin use, and medication use for patients with different stages of colorectal cancer receiving chemotherapy. We observed similarities in dietary patterns and lifestyle behaviors between two large cohorts of stage III colon and metastatic colorectal cancer patients.
Several previous studies have investigated the prevalence of healthy diet and lifestyle behaviors among adult cancer patients, as well as changes in health-related behaviors from pre-to post-diagnosis.[4,5,21,22] However, these studies often included a heterogeneous patient population with varying cancer types, stages of disease, and non-standardized treatment and follow-up care. To our knowledge, this is the first study to extensively describethe prevalences of a wide-range of health-related dietary patterns and lifestyle behaviors among large cohorts of only colorectal cancer patients with stage III and metastatic disease who were receiving chemotherapy.
Our findings appear relatively consistent with previous literature on dietary patterns and lifestyle habits among mixed stages of colorectal cancer patients. The BMI distributions among our patient cohorts were consistent with previous research assessing BMI in colon cancer patients,[23,24,25] and were largely similar to the frequencies of overweight and obesity observed in the 2005–2006 National Health and Nutrition Examination Survey (NHANES), which reported 33% of U.S. adults 20 years and older are overweight and 34% are obese.[26] The substantial percentage of obese patients in our study cohorts does heighten the concern raised by recent studies associating obesity with colon cancer recurrence and mortality in stage II and III patients.[23,24,25,27,28,29] While cachexia has been described previously as a predictor of poor prognosis amongst patients with advanced gastrointestinal cancers, data investigating an association between BMI and cancer-related mortality in patients with advanced colon cancer are not yet available. Observational studies have demonstrated that physical activity confers improvements in physical function, quality of life, cancer-related fatigue and possibly reduces cancer recurrences.[13,30,31,32] Though the amount of physical activity required to achieve a potential colorectal cancer-related benefit is not known, the American Cancer Society (ACS) Guidelines for Nutrition and Physical Activity for Cancer Survivors recommend that cancer survivors avoid inactivity and resume normal activity as soon as possible following diagnosis or treatment. For aerobic activity, it is recommended that cancer survivors follow the 2008 U.S Department of Health and Human Services (US DHHS) Physical Activity Guidelines for Americans, with specific exercise program adaptations based on disease and treatment-related adverse effects.[32] The US DHHS guidelines suggest that for substantial health benefits adults and older adults should engage in at least 150 minutes a week of moderate-intensity or 75 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination, along with muscle-strengthening activities of moderate or high intensity that involve all major muscle groups 2 or more days a week.[33] Data from these two cohorts suggest that most colorectal cancer patients with stage III or metastatic disease fall far short of those levels.
Appreciable changes towards self-reported healthier dietary patterns were observed in both cohorts. These modifications are consistent with previous research suggesting that 40% of colon cancer patients make one or more healthy dietary change(s) following diagnosis[5] and a significant amount of patients increase vegetable and fruit/juice consumption following diagnosis.[4] Nonetheless, more than 80% of stage III and metastatic patients did not meet the US standard recommended daily intake of vegetables, fruits or milk products. Patients were surveyed while on chemotherapy and may have required diet alterations towards a low residue diet if they were experiencing chemotherapy-related diarrhea, which may account for some of the lower than recommended intake.
Vitamin and mineral supplement use was common among our patients with roughly half (45%) reporting multivitamin use, consistent with levels (38–43%) reported in previous research.[4,8] Previous studies suggest that cancer patients are greatly interested in whether use of vitamins can reduce adverse effects of treatment or improve survival.[4,5] Despite the widespread usage of vitamins among cancer patients, data on efficacy or interaction with traditional therapies are lacking. In CALGB 89803, we recently reported that multivitamin usage was not associated with cancer recurrences or survival.[35] In addition, preliminary observational studies in colorectal cancer patients have reported an improved survival among subjects with higher plasma levels of 25-OH-vitamin D or predicted vitamin D levels.[36,37,38] However, in the current study, only 2% of stage III patients and 5% of metastatic patients reported use of specific vitamin D supplements. Ongoing randomized, placebo-controlled trials are assessing the role of vitamin D supplementation in colorectal cancer patients.
The study included several potential limitations. First, patients who are enrolled in a randomized controlled trial may differ from the cancer population at large, as participants must meet eligibility criteria, be selected as appropriate candidates, and be motivated to participate. Additionally, although our response rate to the questionnaires was high, we cannot be certain that those who completed the questionnaire have the same lifestyle behaviors as those who declined to participate. However, we believe our findings are generalizable to stage III and metastatic colorectal cancer patients as the study included a large sample size of patients from both community and academic medical centers across North America, and reasonable variation in dietary patterns and lifestyle habits among participants was observed.
We must also consider the possibility that use of a self-report measure may introduce reporting bias, as patients may indicate more socially desirable dietary patterns and health-related behaviors. We have previously reported on a validation study of the food frequency questionnaire in cancer patients receiving chemotherapy which demonstrated similar validity when compared to a population without cancer.[12]
In summary, results from this study advance our current understanding of health-related behaviors among stage III and metastatic colorectal cancer patients. By comprehensively exploring and comparing dietary consumption patterns and lifestyle habits of these two cohorts, we were able to identify areas of disparity as well as areas of concern that should be considered and addressed by clinicians and patients. Moreover, with recent research implicating the influence of Western dietary patterns,[39] physical activity,[13,27,30,31] obesity,[23,24,25,27,28,29] and aspirin use[40] on recurrence and survival in early stage colon cancer patients, motivated stage III and metastatic colorectal cancer patients represent a group that could benefit from counseling and targeted interventions towards healthy habits to improve patient outcomes. However, the influence of many lifestyle factors on patient outcomes is still largely unknown, and ongoing research in this area is needed to ultimately build an understanding of how diet and lifestyle factors impact prognosis and survival.
CLINICAL PRACTICE POINTS.
Several previous studies have investigated the prevalence of healthy diet and lifestyle behaviors among adult cancer patients, as well as changes in health-related behaviors from pre- to post-diagnosis. In this study, we comprehensively analyze and report on the prevalence and frequency of a wide-range of dietary and lifestyle habits among a large sample of patients with stage III and metastatic colorectal cancer. While appreciable changes towards self-reported healthier dietary patterns were observed, our findings evoke ample opportunity for clinicians to provide informed guidance for this patient population that is highly motivated to make lifestyle changes that complement standard oncologic therapies. These findings indicate that a substantial portion of patients undergoing therapy for advanced colorectal cancer are obese., 40% of stage III and 47% of metastatic patients were classified as inactive with fewer than 3 MET-hours per week. More than 80% of stage III and metastatic patients did not meet the US standard recommended daily intake of vegetables, fruits or milk products. Approximately 10% continued to smoke following diagnosis. Finally, vitamin and mineral supplement use is common, with roughly half (45%) reporting multivitamin use and 11% of patients with stage III disease and 8% of patients with metastatic disease reporting use of ≥ 4 vitamins and/or non-vitamin dietary supplements; however, this widespread usage of vitamins and supplements among cancer patients is largely unsupported by data on efficacy or therapeutic interactions. Future research is needed to improve our understanding of how diet and lifestyle factors impact prognosis and survival in patients with advanced colorectal cancer.
Acknowledgement of research support
The research for CALGB 89803 and 80405 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, M.D., Chair) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601) as well as support from Pharmacia & Upjohn Company, now Pfizer Oncology, sanofi-Aventis and Bristol Myers Squibb. These analyses were also supported by R01CA118553 (PI, Fuchs). R01CA14922 (PI, Meyerhardt), and P50 CA127003 (PI: Fuchs). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 3.Martinez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 4.Satia JA, Campbell MK, Galanko JA, James A, Carr C, et al. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13:1022–1031. [PubMed] [Google Scholar]
- 5.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, et al. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 6.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, et al. Types of alternative medicine used by patients with breast, colon, or prostate cancer: predictors, motives, and costs. J Altern Complement Med. 2002;8:477–485. doi: 10.1089/107555302760253676. [DOI] [PubMed] [Google Scholar]
- 7.Greenlee H, White E, Patterson RE, Kristal AR. Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. J Altern Complement Med. 2004;10:660–666. doi: 10.1089/acm.2004.10.660. [DOI] [PubMed] [Google Scholar]
- 8.Sandler RS, Halabi S, Kaplan EB, Baron JA, Paskett E, et al. Use of vitamins, minerals, and nutritional supplements by participants in a chemoprevention trial. Cancer. 2001;91:1040–1045. [PubMed] [Google Scholar]
- 9.Stephenson LE, Bebb DG, Reimer RA, Culos-Reed SN. Physical activity and diet behaviour in colorectal cancer patients receiving chemotherapy: associations with quality of life. BMC Gastroenterol. 2009;9:60. doi: 10.1186/1471-230X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, et al. Journal of Clinical Oncology. Vol. 22. ASCO Annual Meeting Proceedings (Post-Meeting Edition); 2004. Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cancer (intergroup trial CALGB C89803) p. 3500. 2004. [Google Scholar]
- 11.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Heseltine D, Campos H, Holmes MD, Willett WC, et al. Assessment of a dietary questionnaire in cancer patients receiving cytotoxic chemotherapy. J Clin Oncol. 2005;23:8453–8460. doi: 10.1200/JCO.2005.02.5460. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 14.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 15.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans. 6th ed. Washington DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 19.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 20.Colditz GA, Feskanich D, Chen WY, Hunter DJ, Willett WC. Physical activity and risk of breast cancer in premenopausal women. Br J Cancer. 2003;89:847–851. doi: 10.1038/sj.bjc.6601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27:246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 23.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, 3rd, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 24.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 25.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. National Center for Health Statistics. Hyattsville, MD: US Department of Health and Human Services; 2008. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–80 through 2005–2006. [Google Scholar]
- 27.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinicrope FA, Foster NR, Yoon HH, Smyrk TC, Kim GP, et al. Association of Obesity With DNA Mismatch Repair Status and Clinical Outcome in Patients With Stage II or III Colon Carcinoma Participating in NCCTG and NSABP Adjuvant Chemotherapy Trials. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.39.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt J, Giovannucci E, Ogino S, Kirkner G, Chan A, et al. Physical Activity and Male Colorectal Cancer Survival. Arch Int Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 33.Physical Activity Guidelines for Americans. US Department of Health and Human Services. 2008 [Google Scholar]
- 34.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 35.Ng K, Meyerhardt JA, Chan JA, Niedzwiecki D, Hollis DR, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 28:4354–4363. doi: 10.1200/JCO.2010.28.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 37.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101:916–923. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Jama. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 40.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. Jama. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]