Abstract
Angiogenesis is mediated by signaling through receptor tyrosine kinases (RTKs), Src family kinases and adhesion receptors such as integrins, yet the mechanism how these signaling pathways regulate one another remains incompletely understood. The RTK modulator, Sprouty4 (Spry4) inhibits endothelial cell functions and angiogenesis, but the mechanisms remain to be fully elucidated. In this study, we demonstrate that Spry4 regulates angiogenesis in part by regulating endothelial cell migration. Overexpression of Spry4 in human endothelial cells inhibited migration and adhesion on vitronectin (VTN), whereas knockdown of Spry4 enhanced these behaviors. These activities were shown to be c-Src-dependent and Ras-independent. Spry4 disrupted the crosstalk between vascular endothelial growth factor-2 and integrin αVβ3, the receptor for VTN. Spry4 overexpression resulted in decreased integrin β3 protein levels in a post-transcriptional manner in part by modulating its tyrosine phosphorylation by c-Src. Conversely, knockdown of Spry4 resulted in increased integrin β3 protein levels and tyrosine phosphorylation. Moreover, in vivo analysis revealed that Spry4 regulated integrin β3 levels in murine embryos and yolk sacs. Our findings identify an unanticipated role for Spry4 in regulating c-Src activity and integrin β3 protein levels, which contributes to the regulation of migration and adhesion of endothelial cells. Thus, targeting Spry4 may be exploited as a target in anti-angiogenesis therapies.
Keywords: Sprouty, c-Src, integrins, angiogenesis, endothelial cell migration
Introduction
Angiogenesis is critical to normal physiological processes such as wound healing and post-ischemic tissue regeneration as well as pathological conditions such as cancer and rheumatoid arthritis [1,2]. Angiogenesis is initiated by angiogenic growth factors such as vascular endothelial cell growth factor (VEGF), binding to their cognate receptors in coordination with integrin receptors binding to extracellular matrix (ECM) components [3–5]. Modulation of angiogenesis is considered to be a promising target for pharmacological interventions in patients with cancer [6,7], macular degeneration and complications of diabetes [8,9]. Integrin αVβ3 is expressed at low levels on quiescent endothelial cells in vivo, but is significantly elevated during angiogenesis [10–12]. Vascular development is defective in the absence of integrin αVβ3, as evidenced by impaired maturation of coronary capillaries in integrin β3-null mice [13]. On the other hand, DiYF-knockin mice expressing a signaling defective integrin β3 exhibit an impaired angiogenic response, a phenotype similar to that produced by integrin αVβ3-blocking antibodies or peptides [14]. In vitro studies show that engagement of integrin αVβ3 by its ligand vitronectin (VTN) increases activation of VEGF receptor-2 (VEGFR-2) signaling, which enhances angiogenic responses [15]. The cooperative interaction between integrin αVβ3 and VEGFR-2 is mediated in part by c-Src, where VEGFR-2 activation results in c-Src-mediated tyrosine phosphorylation of integrin β3 and enhanced affinity to VTN. Moreover, deficiency in VTN, the ligand of integrin αVβ3, is associated with increased wound fibrinolysis and decreased microvascular angiogenesis [16].
While much has been learned about the activation of integrins and receptor tyrosine kinases (RTKs) in endothelial cells, much less is known about the mechanisms that attenuate these signals. The Sprouty (Spry) family of proteins was originally identified as feedback inhibitors of fibroblast growth factor receptor signaling [17–19] and subsequent studies showed that other RTK signaling pathways such as VEGF are also inhibited by Spry [20–22]. Four mammalian Spry genes have been identified, and all of them are expressed in endothelial cells. Spry1, Spry2 and Spry4 have all been shown to potently inhibit VEGF signaling in endothelial cells [23–25], although the mechanisms are not well understood. It was reported that Spry4 physically interacts with Raf1 and blocks its activation by VEGF through phospholipase Cγ and protein kinase C pathways [26], although other pathways were not investigated. Gene targeting studies in mice show that simultaneous deletion of Spry2 and Spry4 is embryonic lethal due to severe vascular defects, whereas deletion of either Spry alone has no effect on vascular development [27]. Adenovirus-mediated overexpression of Spry4 inhibits sprouting and branching of small vessels resulting in abnormal embryonic development, and also inhibits endothelial cell proliferation by arresting cell cycle progression without inducing apoptosis [24]. Spry4-null mice show mild limb defects and are born without obvious vascular defects, but show increased vascular density in several tissues and accelerated neovascularization after hindlimb ischemia [28,29]. However, the signaling pathways involved in enhanced vascularization of Spry4-null mice have not been fully elucidated.
In the present study, we report that Spry4 inhibits endothelial cell migration and adhesion in part by inhibiting c-Src activation and by decreasing integrin β3 protein levels. These data indicate that Spry4 is a negative regulator of angiogenesis, in part, by regulating c-Src activation and integrin β3 stability. Because integrins have been targets for anti-angiogenic therapies, our data suggest that targeting Spry4 may also be exploited to treat cardiovascular disorders and solid tumors.
Materials and methods
Mice
The Maine Medical Center Research Institute (MMCRI) Transgenic Mouse Core generated the conditional Spry4 transgenic (CAGGFP-Spry4) mice. The CAGCAT-Z vector containing a chicken β-actin gene promoter-loxP-chloramphenicol acetyltransferase (CAT) gene-loxP-LacZ was modified by replacing the CAT gene with the open reading frame of green fluorescent protein [30]. The LacZ cassette was then replaced with a Myc/His tagged open reading frame of mouse Spry4. Ophir Klein (UCSF) generously provided conditional Spry4f/f mice. Conditional overexpression or targeted-deletion of Spry4 in endothelial cells was achieved by mating female CAGGFP-Spry4 or Spry4f/f mice to male VE-cad-Cre or Tie2-Cre mice (Jackson Laboratory) [31]. The resulting bitransgenic and knockout mice were genotyped by polymerase chain reaction as previously described [32]. The Institutional Animal Care and Use Committee at MMCRI approved all experiments involving mice.
Primary murine endothelial cell isolation
Lungs of adult mice were aseptically dissected and washed with phosphate buffered saline (PBS, Thermo Scientific). After cut into small pieces with scissors, lung tissues were digested with collagenase (0.2 %, Sigma) on a rocker at 37 °C overnight. Tissue pieces were further separated into single cell suspensions by passing through 23 gauge needles and the cells were cultured in Dulbecco’s modified eagle medium (DMEM, Thermo Scientific) containing fetal bovine serum (FBS, 10 %, Atlanta Biologicals) at 37 °C for 6 days. Cells were then trypsinized and endothelial cells were isolated with magnetic beads (BD) conjugated with anti-PECAM antibodies (BD) and grown in DMEM containing FBS (20 %) on VTN (0.5 g/ml, BD).
Cells culture and viruses
Human umbilical vein endothelial cells (HUVECs, Lonza) and human aortic endothelial cells (HAECs, Lonza) were maintained according to the supplier’s instructions and used between passage 4 and 9. 293T cells were culture in DMEM supplemented with FBS (10 %). Spry4 adenovirus (AdSpry4) and integrin β3 adenovirus (Adβ3) were generated in our laboratory using the pAdLox system, and acZ adenovirus (AdLacZ) and Cre adenovirus (AdCre) were prepared as previously described [33]. Spry4 shRNA lentiviruses (LentishSpry4) and non-targeting lentivirus (LentiNT) were purchased from Open Biosystems. Constitutively active c-Src adenovirus (AdCASrc) and dominant negative c-Src adenovirus (AdDNSrc) were generously provided by Dr. Alejandro Adam and Dr. Kevin Pumiglia (Albany Medical Center). Adenoviruses were used at 1 × 103 vp/cell, and lentiviruses were used at 1 × 104 vp/cell.
Retinal angiogenesis assay
Eyes from P5 mice were excised, washed with PBS and fixed in paraformaldehyde (PFA, 4 %, Sigma) at room temperature (RT) for 5 min. Retinas were then dissected and further fixed with PFA (4 %) at RT for 2–5 h. Samples were then permeabilized and blocked in bovine serum albumin (BSA, 1 %, Roche) supplemented with Triton X-100 (0.2 %, Sigma) at 4 °C overnight. Retinas were washed twice with PBS, and incubated in Isolectin B4 Alexa Fluor® dye conjugates (5 μg/ml, Invitrogen) at 4 °C overnight. Samples were then washed five times with PBS, flat mounted and analyzed by DFC 340 FX inverted fluorescent microscope (Leica) according to manufacturer’s instructions. Images were further analyzed and processed using ImageJ software (NIH) according to the user’s guide.
Boyden chamber assay
Modified Boyden chamber migration assays were performed as previously described [34]. Briefly, serum starved endothelial cells were seeded as 1 × 105 cells/well in 100 μl of serum-free endothelial basal medium (EBM-2, Lonza) into 5.0 μm pore size polycarbonate transwells (Falcon) pre-coated with VTN (0.5 μg/ml), and the transwells were inserted into a 24-well plates (Falcon) containing 600 μl of EBM-2. Cells were incubated at 37 °C for 1 h, and then VEGF-A (R&D) was added into the lower chambers at a final concentration of 20 ng/ml. Six hours later, cells on the upper surface of transwells were removed and cells that had crossed the pores were stained with DAPI (2 μg/ml, SIGMA). Endothelial cell migration was quantified by measuring the numbers of migrating cells at 5 distinct positions using DFC 340 FX inverted fluorescent microscope according to manufacturer’s instructions. All assays were performed in triplicates.
Wound healing assay
Cells were grown to confluence on 6-well plates (Falcon) pre-coated with VTN (0.5 μg/ml). After treated with mitomycin C (10 μg/ml, Sigma) for 20 min at 37 °C, monolayers were washed with EBM-2, scratched with a pipet tip and cultured in endothelial complete medium (EGM-2, Lonza) at 37 °C. Photographs were taken with Axiovert 40C microscope (Zeiss) at the indicated times. Endothelial cell migration was quantitated by measuring the width of the cell-free zone (distance between the edges of the injured monolayer) at 5 distinct positions, and all assays were performed in triplicates.
Adhesion assays
Black 96-well plates (Nunc) were coated with VTN (0.5 μg/ml) at 37 °C for 1 h. Equal number of cells were seeded onto each well and incubated in EBM-2 at 37 °C for 30 min. The adherent cells were stained with CyQUANT NF dye (Invitrogen) at 37 °C for 1 h. The fluorescent signal from labeled cells was captured and analyzed using a Modulus microplate reader (Turner Biosystems) according to the manufacturer’s instructions.
Immunoprecipitation and Immunoblot analysis
Immunoprecipitation and immunoblot analysis was performed and quantified as described [31]. Briefly, for immunoprecipitation, equal amounts of cell lysate were incubated with the indicated antibodies overnight at 4 °C with rotation. Protein A/G-agarose beads (20 μl, Santa Cruz) were then added to the lysates to capture the immune complexes at 4 °C with rotation for 1 h. For immunoblotting, equal volume of proteins were loaded into SDS-polyacrylamide gels, separated by electrophoresis, and transferred onto nitrocellulose membranes (Bio-Rad). Antibodies against c-Myc (9E10), β-tubulin and diphosphorylated ERK1/2 were purchased from Sigma. Antibodies against phospho-Src (Tyr416), phospho-AKT (Ser473), AKT and VEGFR-2 (55B11) were purchased from Cell Signaling. Antibodies against c-Src, ERK1 (C-16), Spry4 (H-100), phospho-integrin β3 (Tyr747), integrin β3 (H-96), PECAM-1 (H-300), HDAC2 (H-54) and integrin αV (H-75) were purchased from Santa Cruz. The immunoblotting images were analyzed and quantified by ImageJ software. The data are presented as relative expression of the experimental groups compared to the control after normalization to the loading controls. All experiments were performed at least three times.
RT-PCR and real-time quantitative PCR analysis
Total RNA was extracted using an RNeasy mini kit (Qiagen) following the manufacturer’s instructions. First strand cDNA was synthesized using the NEB first stand synthesis system (New England Biolabs). RT-PCR analysis was performed using PCR MasterMix (5Prime) and real-time quantitative PCR analysis was performed using SYBR green and iCycle system (Bio-Rad). The comparative Ct method was used to calculate the relative abundance of mRNA compared with that of GAPDH expression.
Flow cytometry
Both embryos and yolk sacs were isolated at E9.5. After incubating in tryspin-EDTA (0.25%, Thermo) at 37 °C for 30 min, tissues were further separated into single cell suspensions through 23 gauge needles and cells were collected by centrifugation at 1200 rpm for 4 min. After blocking with rat IgG (1%, BD) in BSA (0.1%) at 4 °C for 5 min, anti-mouse/rat integrin β3 PE and anti-mouse PECAM APC conjugated antibodies (eBioscience) were added and incubated at 4 °C for 30 min. Cells were then analyzed with a FACS Calibur (BD). The data were analyzed by FlowJo software.
Statistical analysis
All values were expressed as mean ± S.D. Statistical significance was determined with 2-tailed Student’s t test. Statistical significance was defined as P < 0.05 and indicated with asterisk.
Results
Spry4 overexpression in endothelial cells inhibits in vivo angiogenesis
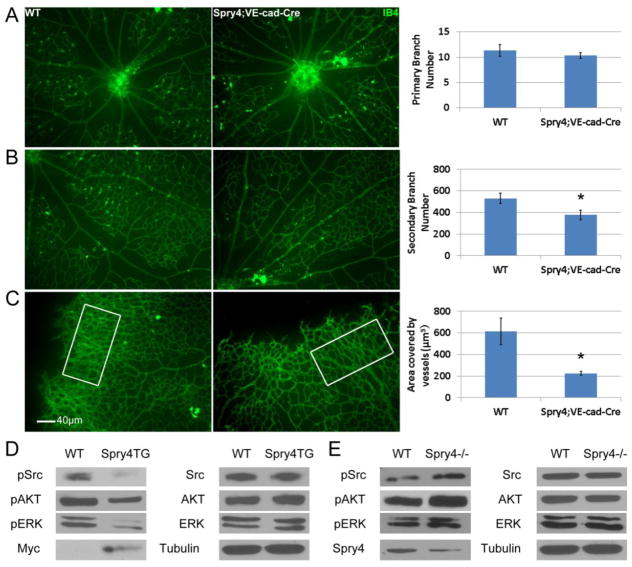
Previous studies showed that injection of a Spry4 encoding adenovirus into the mouse embryonic vasculature inhibited vascular development [24]. Global gene targeting of Spry4 in the mouse results increased vascular density in some tissues, and resistance to hindlimb ischemia due to enhanced neovascularization [29]. Because neither of these previous studies targeted the endothelial cells specifically, we developed a conditional Spry4 transgenic mouse (Suppl. Fig. 1). Conditional CAGGFP-Spry4 female mice were crossed with VE-cad-Cre male transgenic mice and retinas from P5 CAGGFP-Spry4;VE-cad-Cre mice compared to CAGGFP-Spry4 Cre-negative mice. Overexpression of Spry4 in VE-cadherin expressing endothelial cells had little effect on primary branching (Fig. 1A), however, secondary branching and overall vascular density were reduced in retinas from CAGGFP-Spry4;VE-cad-Cre mice (Fig. 1B&C). These data indicate that endothelial cell-specific overexpression of Spry4 impairs angiogenesis in vivo. Conversely, previous studies showed deletion of Spry4 increased angiogenesis [29]. Taken together, these data show that Spry4 is a regulator of angiogenesis in vivo.
Fig. 1. Specific overexpression of Spry4 in endothelial cells inhibits retina angiogenesis.
Representative photographs show IB4 staining of different parts of retinas from P5 wild-type (WT) and Spry4;VE-cad-Cre transgenic mice. Spry4 has no effect on primary branching from the center of retina (A), but significantly represses secondary branching (B) and vessel density (C). (n = 6; * p < 0.05). Murine Primary endothelial cells were isolated from the lungs of Spry4;VE-cad-Cre (Spry4TG), Spry4f/f:VE-cad-Cre (Spry4−/−) mice and their Cre-negative littermates. Deletion or overexpression of Spry4 was achieved by transducing cells in vitro with AdCre. Cells were use 24–48 h after Cre-mediated recombination. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples. Spry4 overexpression inhibits phosphorylation of c-Src, AKT and ERK in murine primary endothelial cells (D), whereas Spry4 knockout enhances their phosphorylation (E).
Inhibition of endothelial cell migration by Spry4 is Ras-independent and c-Src-dependent
It was previously reported that Spry4 inhibited Ras-independent angiogenic signaling [28], however other signals were not fully elucidated. Therefore, to investigate what signaling pathways may be suppressed by Spry4, we used murine primary endothelial cells isolated from the lungs of CAGGFP-Spry4;VE-cad-Cre, Spry4f/f;VE-cad-Cre mice or their Cre-negative littermates. These cells adhered and grew best on VTN coated dishes (data not shown) compared to other ECM proteins. Therefore all in vitro experiments were conducted using VTN, except where indicated. Immunoblot analysis shows that overexpression of Spry4 inhibits phosphorylation of c-Src, AKT and ERK (Fig. 1D), whereas knockout of Spry4 enhances phosphorylation of these signaling proteins (Fig. 1E). Exogenously expressed Spry4 proteins were marked with a Myc tag on its C-terminus.
Endothelial cell migration is an essential component of angiogenesis, and previous studies showed that Spry4 inhibited Ras/ERK signaling in endothelial cells [24]. Therefore, we sought to determine the effect of Spry4 on this pathway in the regulation of endothelial cell migration using wound healing assays. Overexpression of Spry4 in HUVECs by adenovirus transduction significantly inhibited both cell migration and ERK activation. Transduction of HUVECs with AdCARas resulted in increased ERK activation but had little effect on migration. Moreover, co-transduction of HUVECs with AdSpry4 and AdCARas resulted in inhibition of cell migration while leaving ERK activation intact (Suppl. Fig. 2A–C). Because the cells were pre-treated with mitomycin C to inhibit proliferation, the differences we observed were due to cell migration and not proliferation. This suggested that Spry4 inhibited HUVEC migration through a Ras/ERK-independent mechanism. To determine which pathways might be affected by Spry4 to inhibit endothelial cell migration, we treated HUVECs with several chemical inhibitors to analyze signaling pathways impacted by Spry4. The Src family kinase (SFK) inhibitor PP2 significantly inhibited wound healing compared to DMSO controls, whereas the mitogen-activated protein kinase MEK inhibitor U0126 and the phosphoinositide 3-kinase inhibitor Ly294002 had little effect on cell migration (Suppl. Fig. 2D–F).
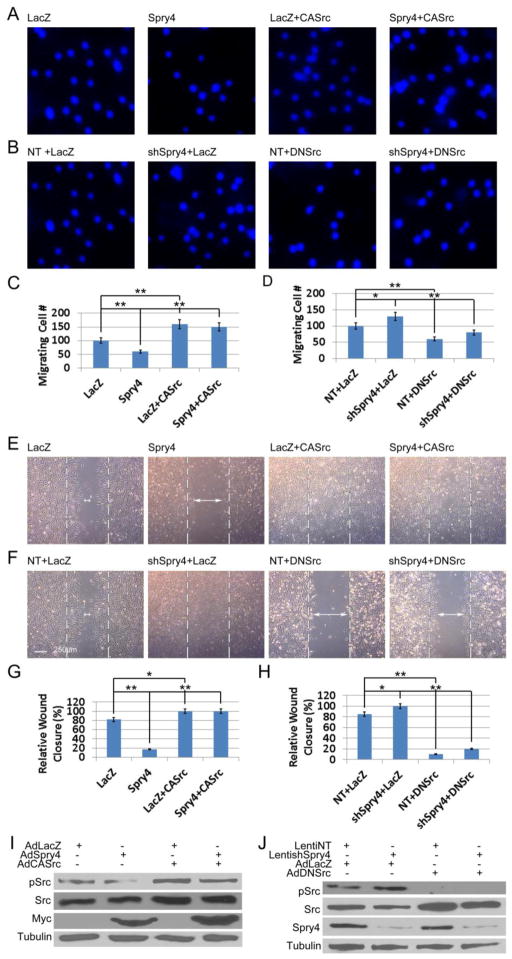
Because Spry4 inhibited c-Src activation in endothelial cells, and because the SFK inhibitor PP2 inhibited cell migration in a manner similar to Spry4, we sought to determine the mechanistic interaction between Spry4 and c-Src in regulating endothelial cell migration. Results of Boyden chamber migration assays showed that transduction of HAECs with AdSpry4 resulted in a marked decrease in cell migration, whereas AdCASrc increased cell migration relative to the LacZ control (Fig. 2A&C). Interestingly, co-transduction of AdSpry4 and AdCASrc showed that CASrc was able to overcome the inhibitory effects of Spry4 expression on HAEC migration (Fig. 2A&C). On the other hand, AdDNSrc inhibited HAEC migration. Knockdown of Spry4 resulted in an increase in cell migration relative to NT control treated cells, and DNSrc reversed this effect (Fig. 2B&D). In addition, wound-healing assays using HUVECs gave similar results (Fig. 2E–H). Immunoblot analysis of HUVEC lysates confirmed Spry4 expression levels and c-Src phosphorylation (Fig. 2I&J). Both gain- and loss-of-function experiments indicated that Spry4 regulates endothelial cell migration via modulating c-Src activation.
Fig. 2. Spry4 regulates human endothelial cell migration on VTN via modulating c-Src activation.
(A) HAECs were transduced with AdLacZ or AdSpry4 with or without AdCASrc, and subjected to Boyden chamber assays 24 h later. Representative photographs show DAPI staining of migrating cells 6 h after addition of VEGF (20 ng/ml) to the bottom well. (B) HAECs were transduced with LentiNT or LentishSpry4, both of which have puromycin drug resistance marker for selecting, and selected with puromycin (0.5 μg/ml) for 5 days. After transduction with AdLacZ or AdDNSrc, the cells were subjected to Boyden chamber assays as in A. (C, D) Quantification of migrating HAECs; n = 6; data are means ± S.D. * p < 0.05; ** p < 0.01. (E) Confluent HUVECs were grown on VTN and transduced with AdLacZ or AdSpry4 with or without AdCASrc. Twenty-four h later cells were treated with mitomycin C (10 μg/ml) for 20 min before subjected to scratch wound-healing assay. (F) Confluent HUVECs grown on VTN were transduced with LentiNT or LentishSpry4, and selected with puromycin (0.5 μg/ml) for 5 days. Cells were then transduced with AdLacZ or AdDNSrc and subjected to wound healing assays as in E. Representative photographs are shown. Dashed lines indicate where the edge of the wound at time 0, and arrowheads indicate the gap between cells 24 h later. (G, H) Quantification of relative wound closure; n = 6; data are means ± S.D. * p < 0.05; ** p < 0.01. (I, J) Cell lysates of the transduced HUVECs subjected to scratch assays were analyzed by immunoblotting. Representative immunoblots are shown. The exogenous Spry4 proteins were marked with a Myc tag on the C-terminus. Equal amount of cell lysate were loaded for all samples.
Spry4 interacts with c-Src in endothelial cells
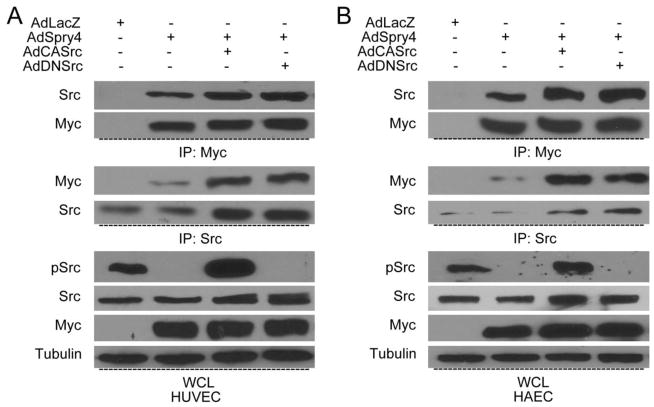
Previous studies showed that Spry4 binds to Raf1 and TESK1 to inhibit their kinase activity [26,35]. Thus, we hypothesize that Spry4 may also regulates the activation of c-Src through protein-protein interactions. To address this, we tried to detect the interaction between these two proteins in endothelial cells by co-immunoprecipitation. Because endogenous Spry4 protein levels are low in normal human primary endothelial cells, we overexpressed exogenous Spry4 using adenoviral expression. As expected, Spry4 co-immunoprecipitated with endogenous c-Src in both HUVECs and HAECs (Fig. 3A&B). When either constitutively active or dominantly negative forms of c-Src were co-transduced with Spry4, there was little impact on the interaction between Spry4 and c-Src (Fig. 3A&B), suggesting that the activation of c-Src is not required for the binding of Spry4 and c-Src. Moreover, immunoprecipitation and immunoblot analysis showed that Spry4 also interacted with c-Src as well as its dominant negative and constitutively active forms in 293T cells (Suppl. Fig. 3). These results suggest that the regulation of c-Src activation may be due in part to its interaction with Spry4.
Fig. 3. Exogenously overexpressed Spry4 interacts with c-Src.
HUVECs (A) and HEACs (B) were transduced with different adenovirus combinations as indicated, and lysed 24 h later. Cell lysates were immunoprecipitated with anti-Myc or anti-c-Src antibodies. Immune complexes and whole cell lysate were resolved by SDS-PAGE and analyzed for c-Src and Myc using specific antibodies. Representative immunoblots are shown. Equal amount of immune complexes and cell lysate were loaded for all samples.
Spry4 regulates integrin 3 protein levels and its interaction with VEGFR-2 via modulating its phosphorylation by c-Src
Integrin αVβ3 is a receptor for VTN on endothelial cells, and plays important roles during vasculogenesis and angiogenesis [36]. Furthermore, previous studies showed that VEGF mediates strong reciprocal activation between VEGFR-2 and integrin β3 in HUVECs grown on VTN [37]. Because this crosstalk is in part dependent on c-Src, but not other Src family members, and VEGFR-2 activation results in c-Src-mediated tyrosine phosphorylation of integrin β3 on Y747 and Y759 and enhanced VEGFR-2 signaling, we sought to determine whether Spry4 had an effect on tyrosine phosphorylation of c-Src and integrin β3 in response to VEGF-A stimulation of HUVECs. Overexpression of Spry4 decreased tyrosine phosphorylation of c-Src and integrin β3 in response to VEGF stimulation, which was rescued by DNCsk (Suppl. Fig. 4A). Knockdown of Spry4 increased tyrosine phosphorylation of c-Src and integrin β3, which persisted longer than controls (Suppl. Fig. 4B). Furthermore, phosphorylation of c-Src and integrin β3 induced by knockdown of Spry4 was repressed by the SFK inhibitor PP2 (Suppl. Fig. 4B). Interestingly, overexpression of Spry4 reduced integrin β3 protein levels, which may account in part for the decrease in integrin β3 tyrosine phosphorylation. Knockdown of Spry4 enhanced integrin β3 protein expression. Moreover, DNCsk increased integrin β3 protein levels, whereas PP2 decreased integrin β3 protein expression. Taken together, these results suggest that Spry4 regulates integrin β3 phosphorylation and protein levels in part through regulation of c-Src activity.
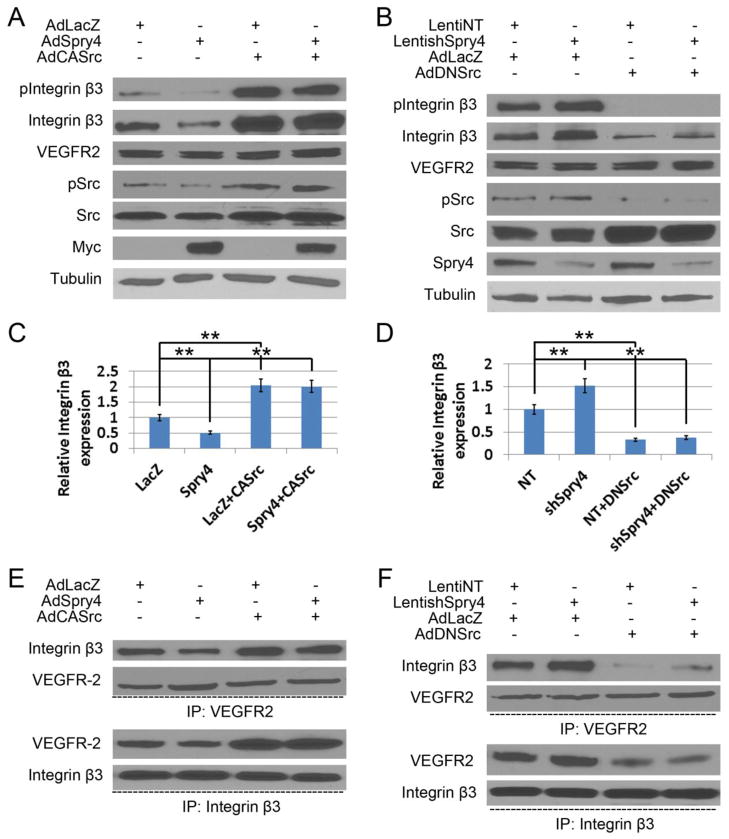
To confirm that inhibition of c-Src activation by Spry4 was responsible for the decrease in integrin β3 protein levels, and to investigate how Spry4 modulates the crosstalk between VEGFR-2 and integrin β3, we transduced HUVECs with AdSpry4, AdCASrc or both. Forced expression of Spry4 reduced integrin β3 phosphorylation and expression as expected, and co-expression of CASrc with Spry4 rescued the effects of Spry4 (Fig. 4A&C). In addition, knockdown of Spry4 increased integrin β3 phosphorylation and protein levels and DNSrc reversed these effects (Fig. 4B&D). These results suggest that DNSrc can suppress increased integrin β3 phosphorylation and expression as a result of Spry4 deletion. By immunoprecipitation of equal amount of either VEGFR-2 or integrin β3 with limiting volume of antibodies, we show that Spry4 overexpression impaired the interaction of VEGFR-2 and integrin β3, and that expression CASrc can rescue this interaction (Fig. 4E). Conversely, Spry4 knockdown enhanced the interaction of VEGFR-2 and integrin β3, and DNSrc repressed the interaction (Fig. 4F). Taken together, Spry4 regulates both integrin β3 protein levels and binding to VEGFR-2 via modulating its phosphorylation by c-Src.
Fig. 4. Spry4 modulates integrin β3 expression and its interaction with VEGFR-2 via c-Src.
(A) HUVECs grown on VTN were transduced with AdLacZ or AdSpry4 with or without AdCASrc, and lysed 24 h later and subjected to immunoblotting with the indicated antibodies. (B) HUVECs grown on VTN were transduced with LentiNT or LentishSpry4, and selected with puromycin (0.5 μg/ml) for 5 days. After transduction with AdLacZ or AdDNSrc, the cells were lysed 24 h later and subjected to immunoblotting with the indicated antibodies. (C, D) Quantification of relative integrin β3 expression normalized to tubulin and relative to LacZ or NT controls; n = 6; data are means ± S.D. ** p < 0.01. (E, F) Cell lysates were immunoprecipitated with anti-VEGFR-2 or anti-integrin β3 antibodies. Immune complexes were then resolved by SDS-PAGE and immunoblotted with VEGFR-2, integrin β3 and Src specific antibodies. Representative immunoblots are shown. Equal amount of immune complexes and cell lysates were loaded for all samples.
Spry4 modulates Integrin β3 protein stability via regulating its phosphorylation by c-Src
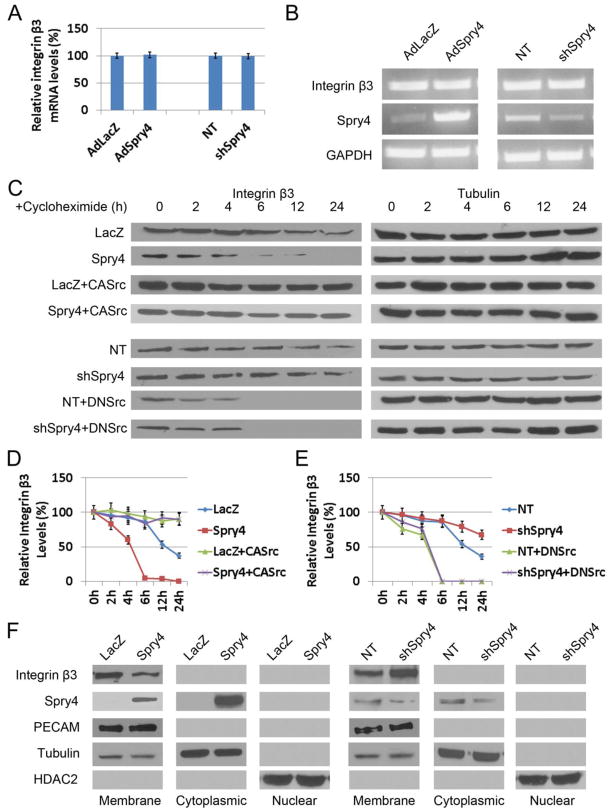
Because Spry4 overexpression decreased, and Spry4 knockdown increased integrin β3 protein levels, we investigated the mechanisms by which this might occur. Both real-time RT-qPCR (Fig. 5A) and RT-PCR (Fig. 5B) analysis of HUVECs showed that overexpression or knockdown of Spry4 had little effect on integrin β3 mRNA levels. This suggests that Spry4 regulates integrin β3 expression by a post-transcriptional or post-translational mechanism. To determine the effect of Spry4 on integrin β3 protein stability, we treated HUVECs with the protein synthesis inhibitor cycloheximide. Immunoblot analysis showed Spry4 overexpression significantly shortened the half-life of integrin β3 proteins, whereas CASrc reversed this effect (Fig. 5C&D). Moreover, knockdown of Spry4 with shRNAs extended the half-life of integrin β3 proteins, and DNSrc reversed this effect (Fig. 5C&E). These results suggest that Spry4 regulates integrin β3 protein levels via modulating its stability and degradation. We also investigated the subcellular localization of integrin β3 in HUVECs where Spry4 was overexpressed or knocked down. Immunoblot analysis showed that Spry4 overexpression reduced the amount of integrin β3 in the membrane fraction while having no effect on PECAM levels (Fig. 5F). Spry4 was present in both the membrane and cytoplasmic fractions. Knockdown of Spry4 resulted in an increase in integrin β3 in the membrane fraction compared to non-targeting control (Fig. 5F). Taken together, these data show that Spry4 regulates integrin β3 protein levels by increasing integrin β3 turnover, and that Spry4 reduces membrane bound as well as total levels of integrin β3 which in part may contribute to reduced cell migration in Spry4 expressing endothelial cells.
Fig. 5. Spry4 regulates the protein stability of integrin β3 via c-Src.
(A) Real-time qPCR analyses reveals no effect of Spry4 on integrin β3 mRNA levels; n = 3; data are means ± S.D. (B) RT-PCR analysis show that mRNA levels of integrin β3 in HUVECs that either overexpressing or depleted of Spry4 by shRNAs are unaffected. (C) HUVECs were transduced as indicated, and cell lysates were prepared at different time points as indicated after cycloheximide (10 μg/ml) treatment. Equal amounts of cell lysate were loaded and analyzed by immunoblotting. Representative immunoblots are shown. (D, E) Quantification of relative integrin β3 protein levels normalized to tubulin and relative to the levels at 0 hr of each group; n = 3; data are means ± S.D. (F) Subcellular factions of HUVECs either overexpressing or depleted of Spry4 by shRNAs were prepared and analyzed by immunoblotting. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples.
Spry4 regulates endothelial cell migration and adhesion via modulating Integrin β3 expression
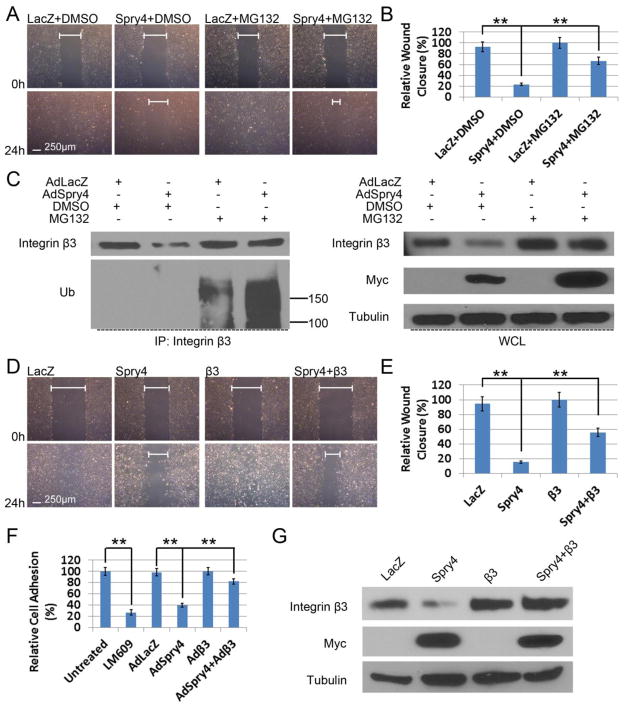
Our data indicate that Spry4 regulates integrin β3 protein levels by a post-transcriptional mechanism. Therefore, we treated HUVECs overexpressing Spry4 with the proteasome inhibitor MG132. Treatment of HUVECs overexpressing Spry4 with MG132 resulted in a partial rescue of the inhibitory effect of Spry4 on endothelial cells migration (Fig. 6A&B). The rescue of HUVEC migration by MG132 correlated with a restoration of integrin β3 protein levels (Fig. 6C). Furthermore, when proteasome degradation was blocked by MG132, Spry4 overexpression increased the ubiquitination levels of integrin β3 proteins (Fig. 6C). Similar effects were also observed in HAECs (Suppl. Fig. 5A&B). Moreover, Spry4 overexpression significantly shortened the half-life of integrin β3 proteins in HAECs, whereas MG132 reversed this effect (Suppl. Fig. 5C). These data further support a role for Spry4 in the regulation of endothelial cell migration and adhesion, in part through regulating integrin β3 protein ubiquitination and degradation.
Fig. 6. Spry4 regulates human endothelial cell migration and adhesion on VTN via modulating integrin β3 protein levels.
(A) MG132 rescues migration of HUVECs overexpressing Spry4. Confluent HUVECs grown on VTN were transduced with AdLacZ or AdSpry4 and subjected to wound healing assays with DMSO or MG132 (5 μM) 24 h later. Confluent monolayers were pre-treated with mitomycin C (10 μg/ml) for 20 min, scratched, and photographed at the indicated times. (B) Quantification of relative wound closure; n = 6; data are means ± S.D. ** p < 0.01. (C) HUVECs were transduced with the indicated adenoviruses and treated with MG132 as described in A, and analyzed by immunoprecipitation and immunoblotting. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples. (D) Confluent HUVECs grown on VTN were transduced with adenoviruses as indicated and subjected to wound healing assays 24 h later as described in A. (E) Quantification of relative wound closure; n = 6; data are means ± S.D. ** p < 0.01. (F) Overexpression of exogenous integrin β3 rescues inhibition of cell adhesion by Spry4. HUVECs were transduced with adenoviruses as indicated and subjected to adhesion assays on VTN (0.5 μg/ml) 24 h later. Cell adhesion was quantified relative to untreated control; n = 3; data are means ± S.D. ** p < 0.01. The integrin αVβ3-blocking antibody LM609 was used as a positive control. (G) Cell lysates of the transduced HUVECs subjected to scratch assays were analyzed by immunoblotting to confirm exogenous integrin β3 expression. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples.
To confirm integrin β3 is a target in the regulation of endothelial cell migration and adhesion by Spry4, we performed rescue experiments by overexpressing exogenous integrin β3. Forced expression of integrin β3 in HUVECs by adenoviral transduction partially rescued Spry4-mediated inhibition of cell migration on VTN (Fig. 6D&E). In addition, Spry4 overexpression in HUVECs mimicked the function of the integrin αVβ3 blocking antibody, LM609, on cell adhesion to VTN, further establishing a role for Spry4 in regulating integrin αVβ3-mediated attachment to VTN (Fig. 6F). Moreover, exogenous expression of integrin β3 restored adhesion of HUVECs overexpressing Spry4 (Fig. 6F&G). Taken together, these data suggest that Spry4 regulates integrin β3 protein levels in endothelial cells via a proteasome-dependent mechanism, which in part regulates endothelial cell migration and adhesion.
Spry4 regulates integrin β3 expression in murine endothelial cells both ex vivo and in vivo
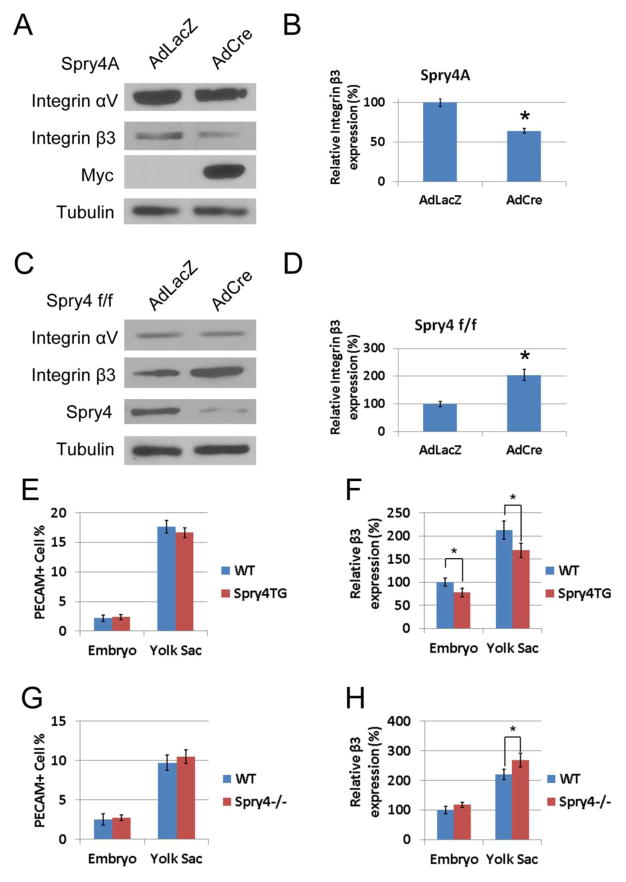
Primary endothelial cells were isolated from conditional transgenic CAGGFP-Spry4 and Spry4f/f gene targeted mice. Overexpression or deletion of Spry4 in murine primary endothelial cells was achieved by Cre-mediated recombination using AdCre. Immunoblotting of endothelial cell lysates from CAGGFP-Spry4 mice that had been induced to overexpress Spry4 showed a significant decrease in integrin β3 protein levels relative to AdLacZ transduced control cells (Fig. 7A&B). Conversely, AdCre-mediated deletion of Spry4 in murine primary endothelial cells from Spry4f/f mice showed a significant increase in integrin β3 protein levels (Fig. 7C&D). Similar to the results obtained from HUVECs (data not shown), increased or decreased expression of Spry4 in murine primary endothelial cells showed little effect on the expression of integrin αV (Fig. 7A&C). We also investigated whether Spry4 regulates integrin β3 protein levels in vivo. Conditional CAGGFP-Spry4 and Spry4f/f female mice were crossed with Tie2-Cre male mice. Embryos and yolk sacs were collected at E9.5 and analyzed by flow cytometry. While conditional overexpression or knockout of Spry4 in Tie2-positive cells had little effect on endothelial cell number as indicated by the number of PECAM-positive cells (Fig. 7E&G), integrin β3 staining intensity was decreased in PECAM-positive cells from CAGGFP-Spry4;Tie2-Cre transgenic embryos and yolk sacs relative to Cre-negative controls (Fig. 7F). Conversely, integrin β3 staining intensity was increased in PECAM-positive cells from Spry4f/f;Tie2-Cre yolk sacs relative to Cre-negative controls, whereas in embryos the increase did not reach statistical significance probably due to a lower overall percentage of endothelial cells in embryos (Fig. 7H). These results indicated that Spry4 plays an important role in regulating integrin β3 protein levels both ex vivo and in vivo.
Fig. 7. Spry4 regulates integrin β3 protein levels both ex vivo and in vivo.
Murine primary endothelial cells were isolated from the lungs of CAGGFP-Spry4 and Spry4f/f mice. Cells were then transduced with AdLacZ or AdCre to either overexpress (A, B) or delete (C, D) Spry4. The expression of integrin subunits αV and β3 were analyzed by immunoblotting. The images were quantified by ImageJ and normalized to tubulin levels; n = 3; data are means ± S.D. * p < 0.05. Conditional CAGGFP-Spry4 transgenic (E, F) or Spry4f/f (G, H) knockout female mice were mated with male Tie2-Cre transgenic mice. Both embryos and yolk sacs at E9.5 were dissociated into single cell suspensions and analyzed by flow cytometry. The level of integrin β3 in PECAM-positive cells was quantified; n = 5; data are geometric means ± S.D. * p < 0.05. Spry4 transgene significantly inhibits integrin β3 protein levels in both embryos and yolk sacs, whereas Spry4 deletion increases integrin β3 protein levels.
Discussion
Although the role of Spry4 in regulating angiogenesis has been investigated previously, the mechanisms by which this occur remains to be fully elucidated. In this study, we used human primary endothelial cells and conditional transgenic and gene-targeted mice to investigate the regulation of angiogenesis by Spry4. Angiogenesis is mediated through the complex interaction between endothelial cells and signals in the microenvironment such as VEGF and the ECM [2,3,38]. It was first reported that Spry4 negatively regulated RTK-mediated Ras/MAPK activation in endothelial cells and that activation of this pathway is required for angiogenesis [24,39]. It was subsequently shown that Spry4 deficiency in mice enhanced angiogenesis by potentiating Ras-independent angiogenic signals [27,28]. Despite these studies, the mechanisms by which Spry4 regulates endothelial cell migration and adhesion remains to be established. Here, we report for the first time that Spry4 inhibits c-Src signaling and regulates integrin β3 protein levels to regulate endothelial cell migration and adhesion, two critical steps in the angiogenic cascade. Our results are consistent with previous reports that Spry4 regulates Ras-independent signals, and we identify c-Src as one of the potential Ras-independent signals regulated by Spry4.
We observed a decrease in total integrin β3 protein but not mRNA levels in endothelial cells overexpressing Spry4. This suggests that Spry4 regulates integrin β3 by a post-transcriptional mechanism. Indeed, treatment of endothelial cells overexpressing Spry4 with the proteasome inhibitor MG132 restored integrin β3 protein to control levels, suggesting that Spry4 mediates proteasomal degradation of integrin β3. We also found that knockdown of Spry4 increased integrin β3 protein levels without affect mRNA levels, supporting a role for Spry4 in regulating integrin β3 stability and turnover.
Little is known about the degradative pathways that regulate integrin protein levels. Following endocytosis integrins are targeted to the early endosomes where they are sorted into different compartments such as late endosomes and lysosomes where they are degraded, or sorted to other compartments for recycling [40]. Integrin β5 is degraded upon recruitment of c-Cbl after activation of FGFR2 in osteoblasts [41]. Furthermore, it was recently reported that degradation of integrin β1 is dependent upon ubiquitination, and integrin β1 is a required component of fibroblast migration [42]. While Spry1 and Spry2 have been shown to bind c-Cbl through a conserved N-terminal sequence and are degraded by a proteasome-dependent mechanism [43–45], Spry4 does not bind, nor is it ubiquitinated by c-Cbl [46], although it contains a similar N-terminal sequence including a canonical conserved tyrosine residue. In addition, we have previously shown that Spry1 and Spry2 are ubiquitinated by Siah2, and degraded in a proteasome dependent manner, whereas Spry4 is not [47]. Thus, Spry4 may have a unique role among Spry family members with regard to its own stability and the regulation of the stability of other proteins. Although both our gain- and loss-of-function studies show that Spry4 plays a role in regulating integrin β3 stability and turnover, additional study will be required to define the precise mechanism by which this occurs.
It has been reported that integrin αVβ3 forms complexes with VEGFR-2, which results in enhanced downstream signaling mediated by VEGF-A, and that adhesion to VTN together with stimulation with VEGF-A results in c-Src-mediated tyrosine phosphorylation of integrin β3 [15]. Studies also showed that c-Src is the major kinase responsible for the tyrosine phosphorylation of integrin β3, which is required for VEGF-A induced activation of integrin β3 for high affinity binding to VTN, cell adhesion, and maximal formation of the VEGFR-2: integrin αVβ3 signaling complex [37]. Our results show that overexpression of Spry4 results in both decreased integrin β3 tyrosine phosphorylation and protein levels, and knockdown of Spry4 has opposite effects. Furthermore, we show that Spry4 modulates the interaction between VEGFR-2 and integrin αVβ3; Spry4 overexpression decreases the interaction and knockdown of Spry4 increases it. These findings also confirm that c-Src-induced tyrosine phosphorylation of integrin β3 is required for the interaction between these two important transmembrane proteins on endothelial cell surface.
It was reported that Spry4 regulates Raf1 and TESK1 kinase activities by directly associating with these proteins [26,35]. We show in the present study that Spry4 interacts with c-Src as shown by immunoprecipitation, suggesting that this association may play a role in the inhibition of c-Src activity. VEGF stimulation of endothelial cells results in recruitment of c-Src to VEGFR-2 where c-Src becomes activated. Because there is no obvious difference between the interactions of Spry4 with either active or inactive form of c-Src, we speculate that Spry4 through its association with c-Src sequesters it in a manner that prevents its activation by VEGFR-2. This would subsequently prevent tyrosine phosphorylation of integrin β3, and consequently reduce its association with VEGFR-2 and the affinity of integrin αVβ3 for VTN, resulting in decreased cell adhesion and migration. Immunoblotting of subcellular fractions showed that Spry4 was present in both cytoplasmic and membrane compartments, indicating the possibility of its interaction with c-Src at the membrane. Moreover, immunoprecipitation also revealed that Spry4 also interacted with c-Src in 293T cells, which express very little VEGFR-2 or integrin αVβ3. These data suggest that the association between Spry4 and c-Src may be constitutive and not limited to VEGF signaling. We cannot rule out the association of other signaling proteins with the Spry4:c-Src complex that may contribute to reduced c-Src activation and inhibition of adhesion and migration. Additional study will be required to further define the nature of the inhibition of c-Src by Spry4.
Gene targeting of Spry4 in the mouse does not produce any overt embryonic vascular phenotypes [32]. However, adult Spry4−/− mice have increased vascular density in some tissues, are resistant to hindlimb ischemia-induced tissue damage, and show enhanced neovascularization of transplanted tumors[27]. The results of our experiments using mouse embryos and primary murine endothelial cells indicate that Spry4 has a role in regulating integrin β3 levels in vivo. It has been shown that SFKs are required for VEGF-induced angiogenesis in vitro and in vivo [48,49], and that vascular development and angiogenic responses are defective in integrin β3-null mice and DiYF-knockin mice, which express an integrin β3 mutant where Tyr747 and 759 have been mutated to Phe and are defective in signaling [13,14]. Therefore, we hypothesize that the enhanced angiogenic responses observed in Spry4−/− mice are due in part to increased c-Src activation based upon our observations that Spry4 knockdown in HUVEC results in increased migration and adhesion, two important components of angiogenesis. Furthermore, because integrin αVβ3, a major mediator of angiogenesis, is increased in Spry4f/f;Tie2-Cre PECAM-positive cells this may also contribute to enhanced angiogenesis on a Spry4 null background.
Overall, the results of this study show that Spry4 regulates migration and adhesion of endothelial cells on VTN in part through inhibition of c-Src signaling, and increasing the turnover of integrin β3 by a proteasome-dependent mechanism. These data provide new mechanistic information on the role of Spry4 in modulating c-Src signaling and integrin β3 protein levels to regulate adhesion and migration, two critical steps in the angiogenic cascade. Our data also suggest a potential mechanism for increased vascular density and enhanced angiogenesis in Spry4−/− mice. These novel observations demonstrate new roles for Spry4 as a negative regulator of angiogenic signaling, and will inform new targets for therapy in vascular disease and cancer.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HL065301 and P30RR030927/P30GM103392 (transgenic mouse, confocal microscopy, DNA sequencing, and viral vector cores) to R. Friesel, PI, and P20RR181789/P20GM103465 (FACS, and cell phenotyping cores) to D.M. Wojchowski, PI. Yan Gong was the recipient of a predoctoral fellowship from the Founders Affiliate of the American Heart Association. IP was supported by NIH grant R01 HL35627 and a grant from the Maine Cancer Foundation. PB was supported by NIH grant R01 CA91645, CPHV was support by NIH grant R01 HL083151. All investigators gratefully acknowledge institutional support from the Maine Medical Center. We also acknowledge the skillful technical assistance of Lindsey Gower in support of this work.
Footnotes
Conflict of interest The authors have declared that no conflict of interest exists.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 3.Brooks PC. Role of integrins in angiogenesis. Eur J Cancer. 1996;32A:2423–2429. doi: 10.1016/s0959-8049(96)00381-4. [DOI] [PubMed] [Google Scholar]
- 4.Ravelli C, Mitola S, Corsini M, Presta M. Involvement of alphavbeta3 integrin in gremlin-induced angiogenesis. Angiogenesis. 2013;16:235–243. doi: 10.1007/s10456-012-9309-6. [DOI] [PubMed] [Google Scholar]
- 5.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 7.Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263–276. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- 8.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 9.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 10.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 11.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 12.Serini G, Napione L, Bussolino F. Integrins team up with tyrosine kinase receptors and plexins to control angiogenesis. Curr Opin Hematol. 2008;15:235–242. doi: 10.1097/MOH.0b013e3282fa745b. [DOI] [PubMed] [Google Scholar]
- 13.Weis SM, Lindquist JN, Barnes LA, Lutu-Fuga KM, Cui J, et al. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang YC, Tsou R, Gibran NS, Isik FF. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery. 2000;127:696–704. doi: 10.1067/msy.2000.105858. [DOI] [PubMed] [Google Scholar]
- 17.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 18.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Webster JB, Kovalenko D, Nadeau RJ, Zubanova O, et al. Sprouty genes are expressed in osteoblasts and inhibit fibroblast growth factor-mediated osteoblast responses. Calcif Tissue Int. 2006;78:233–240. doi: 10.1007/s00223-005-0231-4. [DOI] [PubMed] [Google Scholar]
- 20.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 21.Edwin F, Anderson K, Ying C, Patel TB. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol. 2009;76:679–691. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202. doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- 23.Impagnatiello MA, Weitzer S, Gannon G, Compagni A, Cotten M, et al. Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol. 2001;152:1087–1098. doi: 10.1083/jcb.152.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SH, Schloss DJ, Jarvis L, Krasnow MA, Swain JL. Inhibition of angiogenesis by a mouse sprouty protein. J Biol Chem. 2001;276:4128–4133. doi: 10.1074/jbc.M006922200. [DOI] [PubMed] [Google Scholar]
- 25.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, et al. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Cell Cycle. 2003;2:281–282. [PubMed] [Google Scholar]
- 27.Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, et al. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902. doi: 10.1016/j.bbrc.2006.11.107. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi K, Ishizaki T, Ayada T, Sugiyama Y, Wakabayashi Y, et al. Sprouty4 deficiency potentiates Ras-independent angiogenic signals and tumor growth. Cancer Sci. 2009;100:1648–1654. doi: 10.1111/j.1349-7006.2009.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi K, Sasaki K, Watari K, Yasukawa H, Imaizumi T, et al. Suppression of Sproutys has a therapeutic effect for a mouse model of ischemia by enhancing angiogenesis. PLoS One. 2009;4:e5467. doi: 10.1371/journal.pone.0005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Harkins LK, Zubanova O, Harrington A, Kovalenko D, et al. Overexpression of Spry1 in chondrocytes causes attenuated FGFR ubiquitination and sustained ERK activation resulting in chondrodysplasia. Dev Biol. 2008;321:64–76. doi: 10.1016/j.ydbio.2008.05.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Gong Y, Friesel R. Spry1 is expressed in hemangioblasts and negatively regulates primitive hematopoiesis and endothelial cell function. PLoS One. 2011;6:e18374. doi: 10.1371/journal.pone.0018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, et al. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalenko D, Yang X, Chen PY, Nadeau RJ, Zubanova O, et al. A role for extracellular and transmembrane domains of Sef in Sef-mediated inhibition of FGF signaling. Cell Signal. 2006;18:1958–1966. doi: 10.1016/j.cellsig.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 35.Tsumura Y, Toshima J, Leeksma OC, Ohashi K, Mizuno K. Sprouty-4 negatively regulates cell spreading by inhibiting the kinase activity of testicular protein kinase. Biochem J. 2005;387:627–637. doi: 10.1042/BJ20041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 37.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 39.Tefft D, Lee M, Smith S, Crowe DL, Bellusci S, et al. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am J Physiol Lung Cell Mol Physiol. 2002;283:L700–706. doi: 10.1152/ajplung.00372.2001. [DOI] [PubMed] [Google Scholar]
- 40.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 41.Kaabeche K, Guenou H, Bouvard D, Didelot N, Listrat A, et al. Cbl-mediated ubiquitination of alpha5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J Cell Sci. 2005;118:1223–1232. doi: 10.1242/jcs.01679. [DOI] [PubMed] [Google Scholar]
- 42.Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, et al. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19:148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Egan JE, Hall AB, Yatsula BA, Bar-Sagi D. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc Natl Acad Sci U S A. 2002;99:6041–6046. doi: 10.1073/pnas.052090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fong CW, Leong HF, Wong ES, Lim J, Yusoff P, et al. Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function. J Biol Chem. 2003;278:33456–33464. doi: 10.1074/jbc.M301317200. [DOI] [PubMed] [Google Scholar]
- 45.Hall AB, Jura N, DaSilva J, Jang YJ, Gong D, et al. hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr Biol. 2003;13:308–314. doi: 10.1016/s0960-9822(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 46.Wong ES, Fong CW, Lim J, Yusoff P, Low BC, et al. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadeau RJ, Toher JL, Yang X, Kovalenko D, Friesel R. Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J Cell Biochem. 2007;100:151–160. doi: 10.1002/jcb.21040. [DOI] [PubMed] [Google Scholar]
- 48.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 49.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.