Abstract
Traumatic brain injury (TBI) causes multiple long-term defects including a loss of working memory that is frequently incapacitating. Administrations of mesenchymal stem/stromal cells (MSCs) previously produced beneficial effects in models of TBI as well as other disease models. In several models, the beneficial effects were explained by the MSCs being activated to express TSG-6, a multifunctional protein that modulates inflammation. In a mouse model of TBI, we found the initial mild phase of the inflammatory response persisted for at least 24 hour and was followed by secondary severe response that peaked at 3 days. Intravenous human MSCs or TSG-6 during initial mild phase decreased neutrophil extravasation, expression of matrix metalloproteinase 9 by endothelial cells and neutrophils, and the subsequent blood brain barrier leakage in secondary phase. Administration of TSG-6 also decreased the lesion size at 2 weeks. Importantly, the acute administration of TSG-6 within 24 hour of TBI was followed 6 to 10 weeks later by improvements in memory, depressive-like behavior and the number of newly born-neurons. The data suggested that acute administration of TSG-6 may be an effective therapy for decreasing some of the long-term consequences of TBI.
Keywords: TSG-6, mesenchymal stem cells, traumatic brain injury, blood brain barrier, neutrophils, matrix metalloproteinase
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability, particularly in young adults who fall victim to motor vehicle accidents, falls, sporting injuries, and physical assaults (Coronado et al., 2011). Aside from the enormous personal burden, TBI generates substantial economic costs, estimated to be more than $55 billion dollars per year in the United States (Maas et al., 2008). Despite advances in clinical care, a vast majority of the survivors of severe TBI are not be able to live independently with a loss of working memory the most troubling symptom cited by patients (Myburgh et al., 2008). TBI invokes a complex inflammatory response by the innate immune system mediated primarily through microglia and astrocytes but also involving cross talk with invading neutrophils, macrophages and T cells (Ransohoff and Brown, 2012). The inflammatory response is both harmful and helpful in that it can cause excessive destruction of tissues, and it clears necrotic and apoptotic cells. A number of anti-inflammatory agents have been tested in TBI but all have been disappointing. Glucorticoids were used clinically to decrease brain edema but failed in a large clinical trial because of increased mortality (Edwards et al., 2005; Roberts et al., 2004). Also, glucocorticoids were shown to aggravate retrograded memory deficits in a TBI model (Chen et al., 2009). Non-steroidal anti-inflammatory drugs produced mixed results in models for TBI with some reports indicating improvements (Kovesdi et al., 2012; Thau Zuchman et al., 2012) but others indicating deleterious effects such as worsened cognitive outcomes (Browne et al., 2006). Strategies to reduce inflammation by targeting toll-like receptor (TLR) ligands, TLR receptors or pro-inflammatory cytokines have also proven ineffective (Rivest, 2011).
Mesenchymal stem/stromal cells (MSCs) potentially offer a novel therapy for multiple central nervous system pathologies such as stroke, Parkinson's disease, experimental autoimmune encephalomyelitis, amyotrophic lateral sclerosis, and TBI (Chopp et al., 2008; Mahmood et al., 2004; Menge et al., 2012; Ohtaki et al., 2008; Parr et al., 2007; Pati et al., 2011; Prockop, 2007; Uccelli and Prockop, 2010; Zietlow et al., 2008). However, their mode of action has not been clearly defined. We initially observed that administration of human MSCs limited injury to the brains of mice after transient global ischemia by modulating neuroinflammation (Ohtaki et al., 2008). We also showed that MSCs suppressed endotoxin-induced glial activation in organotypic hippocampal slice cultures (Foraker et al., 2012). More recently, we reported that some of the therapeutic effects of MSCs can be explained by activation of the cells to express the inflammation modulatory protein TSG-6 in animal models for myocardial infarction (Lee et al., 2009), peritonitis (Choi et al., 2011) and chemical injury of the cornea (Oh et al., 2010). TSG-6 protein is a multifunctional protein that consists of a hyaluronan-binding Link domain and CUB module arranged in a contiguous fashion (Milner et al., 2006). The protein is normally up-regulated in many pathological contexts (Getting et al., 2002; Mahoney et al., 2005; Milner and Day, 2003; Szanto et al., 2004; Wisniewski et al., 1996). TSG-6 was shown to be a potent inhibitor of neutrophil migration in an in vivo model of acute inflammation (Wisniewski et al., 1996). Also transgenic mice with null alleles for TSG-6 demonstrated enhanced neutrophil extravasation when challenged with proteoglycan-induced arthritis (Szanto et al., 2004). One of the many actions of TSG-6 is to interrupt the inflammatory cascade of proteases by binding to inter-α-inhibitor and enhancing its inhibitory activity (Mahoney et al., 2005). Another effect is to bind to and thereby inactivate pro-inflammatory fragments of hyaluronan (Wisniewski and Vilcek, 1997). In addition, our research group found that the protein modulated TLR2/TNF-α signaling in resident macrophages during the initial mild phase of inflammation (Phase I inflammation) (Choi et al., 2011; Oh et al., 2010). As a result, the protein decreased the secondary cytokine storm that is triggered by resident macrophages and that ushers in the massive inflammatory response to tissue injury (Phase II inflammation) (Choi et al., 2011; Oh et al., 2010). In a mouse model for chemical injuries of the cornea (Oh et al., 2010), administration of TSG-6 during the Phase I of inflammation, but not during Phase II, effectively suppressed the Phase II response and prevented pathological changes. Chemical injuries of the cornea resemble TBI in that steroids are used with caution and other anti-inflammatory agents are contra-indicated because they activate proteases that cause melting of the corneal stroma (Flach, 2000; Lin et al., 2000). Accordingly, we tested the hypothesis that acute administration of TSG-6 during the Phase I inflammatory response may be beneficial in TBI.
Materials and Methods
Controlled cortical impact injury (CCI)
Male C57BL/6j mice were purchased from Jackson Laboratories and were 2-3 months old at the time of CCI. All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Texas A&M Health Science Center College of Medicine. A controlled cortical impact device (eCCI Model 6.3; Custom Design and Fabrication at Virginia Commonwealth University Medical Center, Richmond, VA) was used to administer a unilateral brain injury as described (Mihara et al., 2011). Mice were anesthetized with 4% sevoflurane and O2 and the head was mounted in a stereotactic frame. The head was held in a horizontal plane, a midline incision was used for exposure, and a 4 mm craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at the midpoint between bregma and lambda, 2 mm lateral to the midline, overlying the temporo-parietal cortex. Animals received a single impact with the instrument set to deliver a deformation of 0.8 mm depth with a velocity of 4.5 m/seconds and a dwell time of 250 ms using a 3 mm diameter impactor tip. After the injury, a disk made from dental cement was adhered to the skull using Vetbond tissue adhesive (3M, St. Paul, MN). The scalp was fastened with sutures. The animal was transferred to a heated recovery cage to be monitored for full recovery from the anesthesia. Sham injured animals were similarly anesthetized and craniectomy performed without cortical injury.
Preparation and culture of human MSCs (hMSCs)
hMSCs from normal healthy donors were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (http://medicine.tamhsc.edu/irm/msc-distribution.html). The cells were prepared as previously described (Colter et al., 2000; Sekiya et al., 2002; Wolfe et al., 2008) with protocols approved by an Institutional Review Board of Texas A&M Health Science Center College of Medicine. Frozen vials of passage-1 hMSCs (about 1 × 106) were thawed, plated on 150 cm2 dishes in 20 mL complete MSC medium: α-MEM (GIBCO/BRL, Grand Island, NY, USA); 16.6% fetal bovine serum (lot selected for rapid growth; Atlanta Biologicals, Norcross, GA); 100 units/mL penicillin (GIBCO/BRL); 100 μg/mL streptomycin (GIBCO/BRL); and 2 mM L-glutamine (GIBCO/BRL), and incubated at 37°C with 5% humidified CO2. After 24 h, the medium was removed and adherent, viable cells were washed with phosphate-buffered saline (PBS), and harvested with 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA) (GIBCO/BRL) at 37°C for 5 minutes. For expansion, cells were plated at 100 cells/cm2 in complete MSC medium and incubated with a medium change every 3–4 days. The cells (passage-2) were then incubated until they reached 70% confluence (approximately 7 days and about 7 population doublings) at which time they were harvested with trypsin/EDTA. The cells were expanded second time under the same conditions to prepare passage 3 hMSCs that were used for experiments.
I.V. Infusion of hMSCs and TSG-6
The mice were placed in a tail vein injection restrainer with warming water bath (40°C), which restrained the animal and gently warmed the tail while allowing access to the tail vein. The hMSCs (106 cells/mouse) or TSG-6 protein (50 μg/mouse, purchased from R&D systems, Minneapolis, MN) in a volume of 200 μl PBS were injected using a 27G needle at 6 hours after CCI. Some mice were treated with 50 μg/mouse TSG-6 protein again 24 hour after CCI. PBS (200 μl) was injected into control mice.
Fluorescence immunohistochemistry
Twenty-four hour after CCI, mice were anesthetized and perfused with PBS and 4% paraformaldehyde (PFA). The brains were removed, stored in fresh 4% PFA overnight, protected in 20% sucrose, frozen in O.C.T. media (Sakura Finetek, Torrance, CA), sectioned (12 μm), and mounted onto slides. The sections were blocked with 5% normal horse serum (NHS, Vector Laboratories, Burlingame, CA) and 0.3% triton-X (Sigma-Aldrich) in PBS (blocking buffer), and incubated with several combinations of primary antibodies (Table 1) in blocking buffer at 4°C. The next day, the sections were washed three times with PBS and incubated with secondary antibodies (Table 1) for 90 minutes at room temperature. After washing, the sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 minutes. Fluorescent images were acquired using a spinning disc fluorescent microscope (Olympus, Center Valley, PA) with Slidebook 3I software (Intelligent Imaging Innovations, Denver, CO).
Table 1. Antibodies used for immunostaining.
| Antibody | Antigen | Host | Clone | Company | Dilution | Target |
|---|---|---|---|---|---|---|
| NeuN | purified cell nuclei from mouse brain |
mouse | A60 | Millipore | 1000 | neurons |
| Ly6G/Ly6C | mouse Ly-6G and Ly-6C |
rat | RB6-8C5 | BD Biosciences |
100 | neutrophils |
| MMP9 | mouse MMP9 | goat | polyclonal (AF909) |
R&D systems | 500 | MMP9 |
| vWF | human von Willebrand Factor |
rabbit | Polyclonal (AB7356) |
Millipore | 50 | brain blood vessels endothelial cells |
| DCX | Human Doublecortin |
goat | C18 (sc-8066) | Santa Cruz | 250 | New born neurons |
| mouse IgG | mouse IgG | goat | Invitrogen | 500 | Alexa 488 labeled 2nd antibody | |
| rat IgG | rat IgG | goat | Invitrogen | 500 | Alexa 488 labeled 2nd antibody | |
| goat IgG | goat IgG | donkey | Invitrogen | 500 | Alexa 592 labeled 2nd antibody | |
| rabbit IgG | rabbit IgG | goat | Invitrogen | 500 | Alexa 488 labeled 2nd antibody | |
| Goat IgG | Goat IgG | horse | Vector Labs | 200 | Biotin labeled 2nd antibody |
Enzyme-Linked Immuno Sorbent Assay (ELISA) for myeloperoxidase (MPO)
For protein extraction, the injured brain hemisphere was homogenized with disperser (T10; IKA Wilmington, NC) in lysis buffer containing 200 mM NaCl, 5 mM EDTA, 10 mM Tris-HCl (pH 7.4), 10% glycerin, 1 mM phenylmethanesulfonylfluoride (PMSF) and protease inhibitor cocktail (Thermo Scientific). The samples were sonicated on ice and centrifuged twice (15,000 × g at 4 °C for 20 minutes). The supernatant was assayed for protein with the BradFord reagent (Ameresco, Solon, OH), and for myeloperoxidase by ELISA (MPO ELISA kit; HyCult Biotech, Plymouth Meeting, PA).
Evan's blue blood brain barrier (BBB) permeability analysis
Evans Blue was used to assess the BBB permeability as this dye has a very high affinity for serum albumin (Rawson, 1942). Seventy-two hours after CCI injury, 5% Evans Blue (Sigma-Aldrich, St. Louis, MO) in saline was injected via tail vein (4 mL/kg). The dye was allowed to circulate for 2 hour. Animals were anesthetized with lethal dose of ketamine/xylazine mixture and then transcardially perfused with saline, followed by 4% PFA. The brains were harvested and cut into 2 mm sections. After they were photographed, the sections were divided into contralateral and ipsilateral hemispheres. The sections were incubated in 400 μL formamide (Sigma-Aldrich) at 55°C for 24 h and samples were centrifuged at 20,000 g for 20 minutes. The supernatant was collected, and the OD at 620 nm was measured using a micro plate reader (BMG LABTECK; Fluostar Omega, Ortenberg, Germany) to determine the amount of Evans Blue in each sample. All values were normalized to hemisphere weight.
Histological examination
Mice were anesthetized and transcardially perfused with saline and 4% PFA and their brains processed and cut into 25 μm sections described above. Sections were stained with Gill's hematoxylin and eosin (Shandon rapid-chrome frozen section staining kit, Thermo scientific, Waltham, MA) and coverslipped. For volumetric assessment of lesion, images of seven brain sections, taken every 0.5 mm from 0.5 mm to 3.5 mm posterior to bregma, were captured using a stereomicroscope (SMZ800, Nikon, Melville, NY) and digitalized with an image analysis system (Image J, NIMH, Bethesda, MD). The area of the lesion in each section was calculated by subtracting the size of ipsilateral cortex from the control contralateral cortex. The lesion volume was computed by integrating the lesion area of each section measured at each coronal level and the distance between two sections (0.5 mm). The volume of the lesion in the hippocampus was measured by same method as described above from five sections taken every 0.5 mm from 1.0 mm to 3.0 mm posterior to bregma. In addition, sections from 1.5 mm posterior to bregma were immunostained with anti-NeuN antibody (Table 1) for overnight at 4°C, washed in PBS and incubated with a secondary antibody (Table 1) for 90 minutes at room temperature. Sections were counterstained with DAPI (Sigma-Aldrich).
Zymograms
Mice were killed at 24 h post-CCI. The brains were rapidly removed, and damaged brain tissue within the traumatized hemisphere was homogenized in lysis buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). Soluble and insoluble extracts were separated by centrifugation (20,000 g, 30 minutes at 4°C). After the protein concentration was measured by BradFord reagent (Ameresco), samples containing 20 μg total protein were analyzed by gel zymography using precast gelatin gels (10% Zymogram Gelatin Gels; Invitrogen/Novex). With constant gentle agitation, gels were renatured in Novex Zymogram Renauring buffer (Invitrogen/Novex) for 30 minutes at room temperature, developed in Novex Zymogram Developing Buffer (Invitrogen/Novex) overnight at 37 °C, stained with Colloidal Blue (Invitrogen/Novex), and extensively washed with distilled water (>20 hours) to yield uniform background signal. Digital images of stained wet gels were captured using a scanner. The images were analyzed with the densitometry using Image J software.
Behavior Tests
Behavior test was performed as described previously (Parihar et al., 2011). Experimental time-line was shown in Fig. 6. Elevated plus maze test and open field test were performed to assess anxiety-like behavior. Morris water maze test, Y maze spontaneous alternation test and novel object recognition test were performed to evaluate memory function. Depression-like behavior was analyzed by forced swim test and novelty suppressed feeding test. Details on each behavior test are described in below.
Fig. 6.
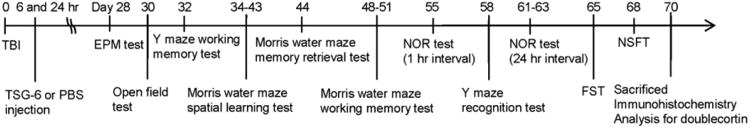
Schematic schedule of behavioral tests performed for sham or TBI mice treated with vehicle or TSG-6.
Morris water maze test
Mice underwent learning and memory testing during the daylight period. The water maze tank (a circular plastic pool measuring 120 cm in diameter and 60 cm in height) was filled with 30°C water containing milk to a 30 cm height and extra-maze cues were placed on the walls of the room. Before training periods, mice were allowed to swim for 45-seconds in the pool without platform in order to become familiar with swimming. Swim speed and total travel distance were calculated in this time. Mice were first trained to find the circular platform (10 cm in diameter) submerged in water within one of the 4 quadrants using spatial cues. The movement of mouse in the water maze tank was continuously video-tracked and recorded using the computerized ANY-Maze video-tracking system. The training comprised 9 sessions over 9 days with 4 acquisition trials per session. Each trial lasted 90-seconds and the inter-trial interval was 60-seconds. During different trials, the mouse was placed in the water facing the wall of the pool in a pseudo-random manner so that each trial commenced from a different start location. Once the mouse reached the platform, it was allowed to stay there for 15-seconds. When a mouse failed to find the platform within the ceiling period of 90-seconds, it was guided into the platform where it stayed for 15-seconds. The location of platform remained constant across all days and trials for an individual animal. After each trial, the mouse was wiped thoroughly with dry towels, air-dried and placed in the home cage. During the 9 days acquisition period, the latency to reach the platform was measured as an indicator of learning ability. The latency to find the platform was recorded for every trial. From these, the mean latency to reach the platform in every session was calculated. One-day after the above 9-day learning paradigm, mice were subjected to 45-seconds retention (probe) test. For this, the platform was removed and mice were released from the quadrant opposite to the original position of the platform. Number of entries into the platform area and dwell time in the platform quadrant was measured. Typically, mice that are capable of easily retrieving the learned memory head straight to the platform area after release, spend most of the trial (45-seconds) searching within the quadrant (or area) where the platform was originally placed and exhibit many platform area crossings. Thus, mice exhibiting increased numbers of platform area entries and greater dwell time in the platform area are considered to have superior memory than mice exhibiting fewer platform area entries greater latency to reach the platform area, reduced dwell time in the platform area.
The procedures described above are for the assessment of trial-independent learning (that is, the goal does not move from trial to trial during a given phase of testing). To assess working memory, a different method was performed. In this procedure, the platform was relocated every day and the animal was given four trials per day. On each day, the first trial represented a sample trial. During the sample trial, the animal must learn the new location of the platform by trial-and-error. Trial 2-4 began after a 15-seconds inter-trial interval. The latency to find the platform was recorded for every trial. From these, the mean latency to reach the platform in each trial for 4 days was calculated. If the animal recalls the sample trial, it swims a shorter path to the goal on the following trial. As the platform was moved daily, no learning of platform position from the previous day could be transferred to the next day's problem; hence, recall on each day during Trial 3-4 was dependent on that day's sample trial and measured only working memory.
Y-maze test
Y maze spontaneous alternation is a behavioral test for measuring the willingness of rodents to explore new environments. The first session measures working memory in mice by scoring the number of alternations which the mouse does in Y-maze when the animal visits all three arms without going into same arm twice in a row. The experimental apparatus consisted of Y-shaped maze with three gray opaque plastic arms at a 120° angle from each other. ANY-maze video tracking system was used to record and analyze the animal's movement within the maze. After introduction to the center of the maze, the animal was allowed to freely explore the three arms for 5 minutes. Over the course of multiple arm entries, the subject should show a tendency to enter a less recently visited arm. The number of arm entries and the number of triads was recorded in order to calculate the percentage of alternation.
The second session includes two trials. During trial 1, one of the arms of the maze was blocked, allowing for a 5 minutes exploration of only two arms of the maze. After a 30 minutes delay, trial 2 was started. During trial 2, all three arms were available for another 5 minutes exploration. Trial 2 takes advantage of the innate tendency of mice to explore novel unexplored areas (e.g., the previously blocked arm). The time spent in novel unexplored areas of each animal was measured. Mice with intact recognition memory prefer to explore a novel arm over the familiar arms, whereas mice with impaired spatial memory enter all arms randomly. Thus, trial 2 represents a classic test for spatial recognition memory.
Novelty suppressed feeding test (NSFT)
All mice were subjected to fasting for twenty-four hours before the commencement of the test but water was provided ad libitum. During the test, food pellets (regular chow) was placed on a circular piece of white filter paper positioned in the center of an open field (45 × 45 cm) that was filled with approximately 2 cm of animal bedding. Each mouse was removed from its home cage and placed in a corner of the open field. The test lasted for 10 minutes. The latency to the first bite of the food pellet was recorded (defined as the mouse sitting on its haunches and biting the pellet with the use of its forepaws). It is well known that latency to the first bite is much shorter in normal mice than depressed mice. The overall latency to the first bite determines the extent of depressive-like behavior in individual mice.
Forced swim test (FST)
Each mouse was first placed in a glass beaker (having an inner diameter of 10 cm and depth of 15 cm) filled with tap water (∼25°C) to a depth of 10 cm. The depth of water used ensured that the animal could not touch the bottom of the container with their hind paws. The FST was conducted in a single session comprising 6 minutes and data were collected every minute for swimming, climbing (or struggling) and immobility (or floating) during the procedure. Swimming in the FST is defined as the horizontal movement of the animal in the swim chamber and climbing refers to the vertically directed movement with forepaws mostly above the water along the wall of the swim chamber. On the other hand, immobility or floating is defined as the minimum movement necessary to keep the head above the water level. Mice were removed from the water at the end of 6 minutes and gently dried and placed back in their home cages. From the collected data, the total time spent in immobility for the trial duration was calculated for every mouse and utilized as an index of depressive-like behavior.
Doublecortin (DCX) immunohistochemistry
Serial sections (every fifteenth) through the entire hippocampus were selected in each animal belonging to different groups and processed for DCX immunostaining using a goat polyclonal antibody to DCX (Table 1) using the avidin-biotin complex (ABC) method, as detailed in a previous study (Rao et al., 2005).
Quantification of the numbers of newly born neurons (DCX positive neurons) in the hippocampus
In order to determine the status of hippocampal neurogenesis, stereological quantifications of DCX positive cells in the SGZ–GCL were performed using serial, total seven sections (every fifteenth) immunostained for DCX. Numbers of DCX positive immature neurons in the SGZ-GCL were measured via the optical fractionator counting method using a Stereo Investigator system (Microbrightfield Bioscience, Williston, VT) as detailed in previous study (Rao and Shetty, 2004).
Statistical analysis
All data are represented as mean ± SEM. One-way ANOVA with a Holm multiple comparison test was carried out with JSTAT software to determine statistically significant differences for all data. One-way repeated measures ANOVA with Newman-Keuls multiple comparison test was also carried out with Prizm software for water maze spatial learning test to investigate statistically significant progressive decreases in latency to reach the hidden platform. p < 0.05 was considered to be significant.
Results
Time course for neutrophil infiltration produced by TBI
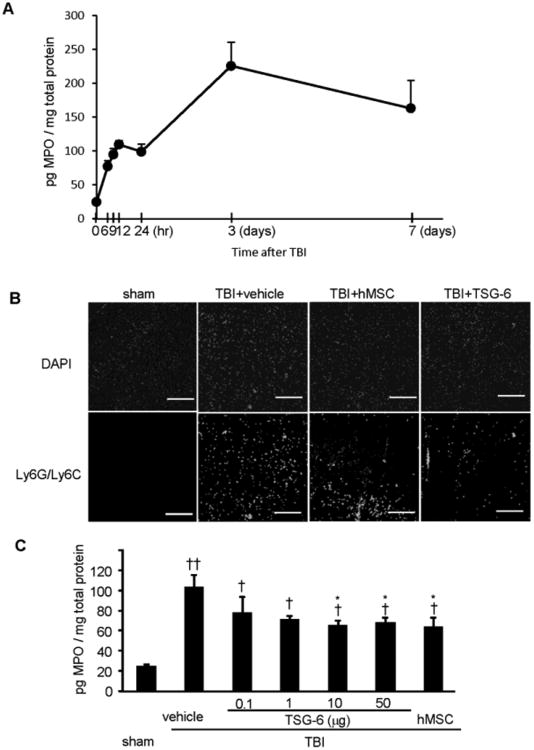
We produced controlled cortical impact injury in immunocompetent male mice (C57BL/6j) and followed neutrophil infiltration by ELISAs for myeloperoxidase (MPO in Fig. 1A). As expected, there was an initial small Phase I response and then a larger Phase II response as is seen following acute injury to other tissues (Choi et al., 2011; Oh et al., 2010). However, the time course was more protracted than seen in peripheral organs such as the cornea or the peritoneum. The peaks of Phase I in zymosan-induced peritonitis model and cornea chemical injury model were 2 and 4 hours, respectively. Phase II peaked in peritonitis model and cornea injury model between 8 and 24 hours (Choi et al., 2011; Oh et al., 2010). Here, Phase I of TBI model persisted for at least 24 hour. Phase II also appeared to be more extended in time in that it peaked about 72 hour. The results suggested therefore that the therapeutic window for treating TBI with TSG-6 might be longer than the window of about 4 hour observed with chemical injuries of the cornea (Fig. 7 in reference 24).
Fig. 1.
TSG-6 and MSCs reduced neutrophil infiltration after TBI. hMSCs (106 cells/mouse) or TSG-6 protein (50 μg/mouse) was administrated 6 hour after TBI. (A) Time course of neutrophil infiltration as reflected by assays of myeloperoxidase (MPO). n= 6 mice/each point. (B) Representative images of Ly6G/Ly6C-stained neutrophil infiltration in the superficial pericontusional area of cortex from sham operated or TBI mouse treated with vehicle, hMSC or TSG-6 at 24 hours after CCI. Sections were counter stained with DAPI. Scale bars = 200 μm. (C) The MPO assayed by ELISA in injured brains of mice treated with vehicle, MSC or indicated dose of TSG-6 at 24 hours after CCI. n = 5 mice/group. All data are represented as mean ± SEM. †p < 0.05, ††P < 0.01 versus the sham group, *p <0.05 versus the vehicle group.
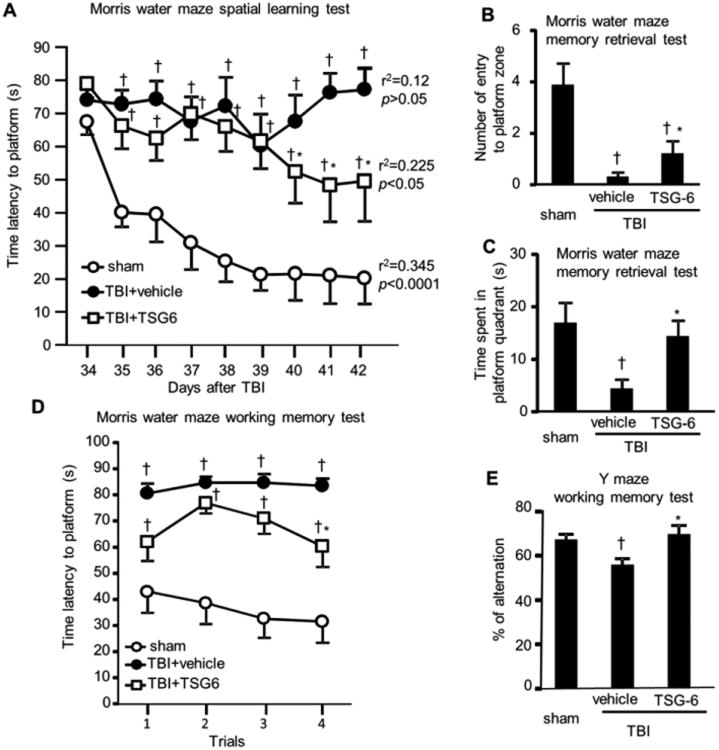
Fig. 7.
Protective effect of TSG-6 protein on cognitive function. TSG-6 protein (50 μg/mouse) was administered twice at 6 and 24 hour after TBI. Effect of TSG-6 on learning was assessed in Morris water maze (A). Probe (memory retention) test was performed at 24 h after the last learning session (B, C). The defects in working memory were assessed by Morris water maze test (D) and Y-maze spontaneous alternation test (E). Behavioral tests were performed as described in Fig. 6. N = 9 or 10 mice/group. All data are represented as mean ± SEM. †P < 0.05 versus the sham group, *p < 0.05 versus the vehicle group.
Effects of TSG-6 and MSCs on neutrophil infiltration and matrix metalloproteinase 9 (MMP9) 24 hour after TBI
We then tested whether intravenous administration of hMSCs or TSG-6 at 6 hour after TBI reduced neutrophil infiltration. Both were effective as assayed by immunohisotochemical staining for a neutrophil marker (Ly6G/Ly6C) in cortical sections from injured mice at 24 hour following an injury (Fig. 1B). Similar results were obtained by assays for MPO, a semi-quantitative assay of neutrophil infiltration (Fig. 1 C). Statistically significant decreases of MPO expression levels were observed from administration of 10 and 50 μg/mouse of TSG-6 (Fig. 1C).
Previous studies demonstrated that leukocyte-derived MMP9 mediated BBB breakdown after focal cerebral ischemia (Gidday et al., 2005) and MMP9 contributed to the pathophysiology of TBI (Wang et al., 2000). At 24 hour after TBI, immunohistochemistry of brain sections demonstrated a high level of MMP9 in the entire damaged cortex compared to sham injured cortex (Fig. 2A). In contrast, there was a marked reduction in the level of MMP9 expression in the brains of injured mice treated with hMSCs or TSG-6 (Fig. 2A). Gel zymography confirmed the observations (Fig. 2B-D, P < 0.05).
Fig. 2.
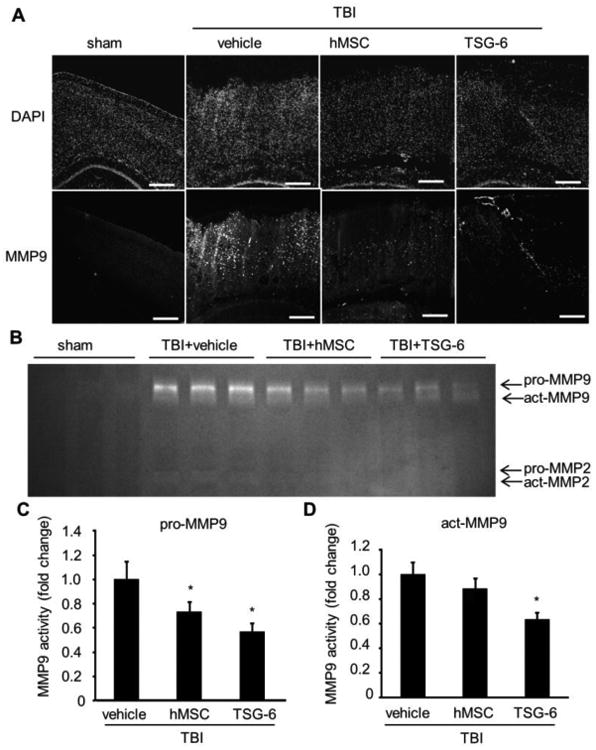
TSG-6 attenuated TBI-induced expression of matrix metalloproteinase-9 (MMP9) after TBI. hMSCs (106 cells/mouse) or TSG-6 protein (50 μg/mouse) was administrated IV 6 hour after TBI. (A) Representative images of MMP9 immunostained brain from sham operated or injured mouse treated with vehicle, hMSC or TSG-6 at 24 hours after CCI. Counter staining was performed with DAPI. Images were taken from contusion-pericontusion area. Scale bars = 500 μm. (B) Representative zymogram of MMP9 and MMP2 activity in ipsilateral cortex from sham operated or injured mouse treated with vehicle, hMSC or TSG-6 at 24 hours after CCI. (C, D) Graphical representation of the average values obtained by densitometric analysis of zymograms for pro-MMP9 (C) or activated-MMP9. (D). n = 5 mice/group. All data are represented as mean ± SEM. * p < 0.05 versus the vehicle group.
The injured cortex was also assayed to identify the cells expressing MMP9. MMP9 co-localized with Ly6G/Ly6C immunopositive neutrophils in brain sections after TBI (Fig. 3A). Of interest was that cells co-expressing MMP9 and Ly6G/Ly6C were also observed in brains of mice treated with either hMSCs or TSG-6 but the number was dramatically decreased (Fig. 3A). Strong MMP9 immunoreactivity also co-localized with blood vessel endothelial cells labeled with von Willibrand Factor (vWF) (Figure 3B). Treatment with either hMSCs or TSG-6 decreased expression of MMP9 in the endothelial cells (Fig. 3B).
Fig. 3.
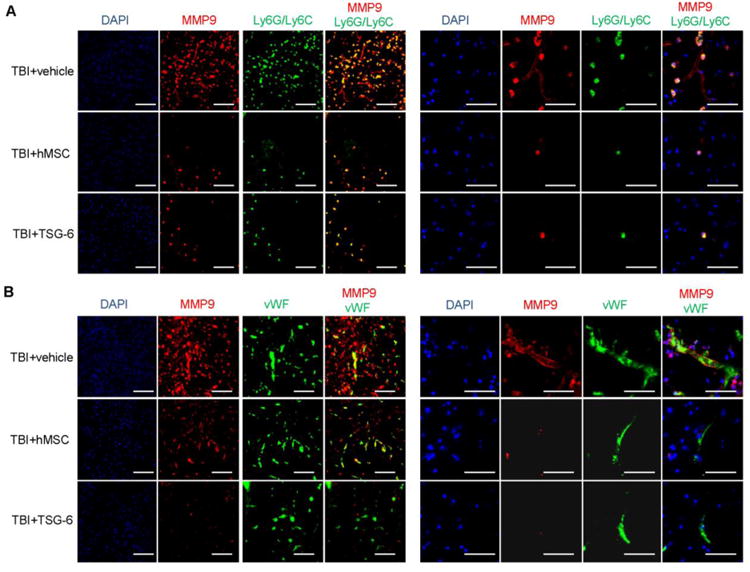
Neutrophils that infiltrated the brain and blood vessel endothelial cells expressed MMP9 at 24 hour after TBI. hMSCs (106 cells/mouse) or TSG-6 protein (50 μg/mouse) were administrated 6 hour after TBI. (A) Representative sections showing double-immunofluorescence labeling of cortical sections from the injured mouse treated with vehicle, hMSC or TSG-6 at 24 hour after TBI. The images were taken from superficial pericontusional area of cortex. Co-labeling of MMP9 (red) with marker for neutrophils (Ly6G/Ly6C, green). Left: ×20 magnification (scale bars=100 μm), Right: ×60 magnification (scale bars = 50 μm). (B) Images of representative sections showing double-immunofluorescence detection of cortex sections from the injured mice that received vehicle, hMSCs or TSG-6 at 24 hour after TBI. The images were taken from superficial pericontusional area of cortex. Co-labeling of MMP9 (red) with von Willibrand Factor in blood vessel endothelial cells (vWF, green). Left: ×20 magnification (scale bars=100 μm), Right: ×60 magnification (scale bars = 50 μm).
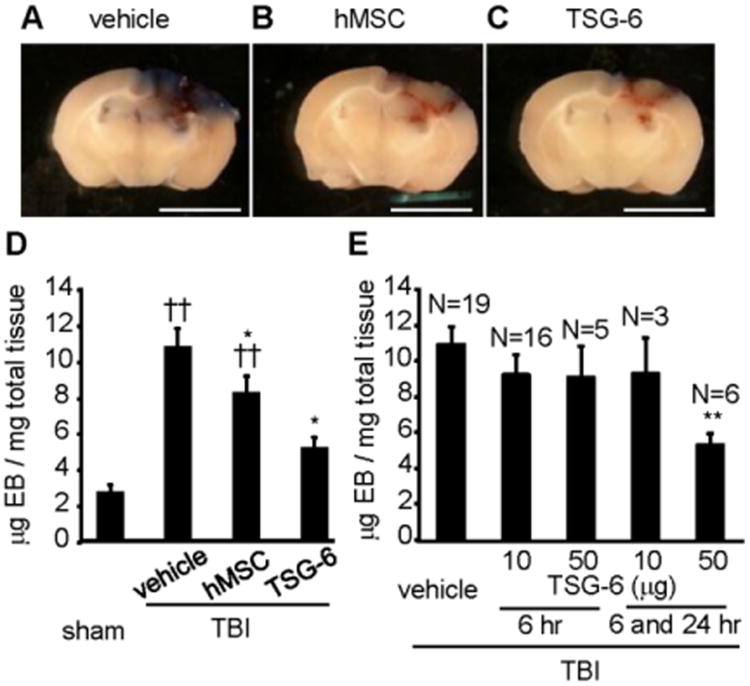
Intravenous administration of TSG-6 protein decreased BBB permeability 3 days after TBI
As expected from the decrease in neutrophil infiltration and MMP9 activity (Gidday et al., 2005; Wang et al., 2000), intravenous administration of either hMSC or TSG-6 significantly decreased BBB leakage on day 3 compared with control TBI mice as assayed by leakage of albumin-bound Evans blue into the parenchyma of the brain (Fig. 4A-C). A single administration of 106 hMSCs at 6 hour after TBI was effective. However, two administrations of 50 μg TSG-6, one at 6 and another at 24 hour, were required to obtain a significant effect (Fig. 4E).
Fig. 4.
IV-injected hMSCs or TSG-6 protein decreased blood brain barrier (BBB) permeability in mice 3 days after TBI. hMSCs (106 cells/mouse) were administrated 6 hour after TBI. TSG-6 protein at a dose of 50 μg/mouse was administered twice at 6 and 24 hour after TBI (A-D). In assay (E), TSG-6 was administrated at 10 or 50 μg/mouse dose once or twice. (A-C) Representative brain slices of the site of cortical contusion injury after administration of vehicle (A), hMSCs (B) or TSG-6 (C) and recovered 3 days post TBI. Blue represents Evans Blue dye extravasation at the site of injury. Scale bars = 5 mm. (D) Quantitative data of Evans blue level in the ipsilateral cerebral hemisphere tissue of mice from sham operated group (n=8) and injured group treated vehicle (n=21), hMSCs (n=14) or TSG-6 (n=6) at 3 days after CCI. hMSCs (106 cells/mouse) were administrated 6 hour after TBI. TSG-6 protein at a dose of 50 μg/mouse was administered twice at 6 and 24 hour after TBI. (E) Dose-dependency and time window of TSG-6 treatment in BBB breakdown following TBI. Numbers of samples are indicated above the columns. Evans Blue dye was extracted from brain at 3 days after CCI. All data are represented as mean ± SEM. ††p < 0.01 versus the sham group, *p < 0.05, **p < 0.01 versus the vehicle group.
Reduced lesion size at 2 weeks after TBI mice
After 2 weeks, TBI in untreated mice produced a lesion (including cavity) that was extensive, spreading from the cortex through the hippocampus and connecting to the lateral ventricles (Fig. 5A). Treatment with hMSCs or TSG-6 tended to reduce the size of the lesion but the TSG-6 appeared to be more effective (Fig. 5B and C). Quantitative analyses showed that total lesion volume in the whole hemisphere was reduced by 40% following TSG-6 administration (Fig. 5D, p < 0.05). In the hippocampus, injured control mice lost 2.40 ± 0.44 cm3 of tissue, compared to 1.45 ± 0.33 cm3 for injured mice that received TSG-6 (Fig. 5E, p < 0.05).
Fig. 5.
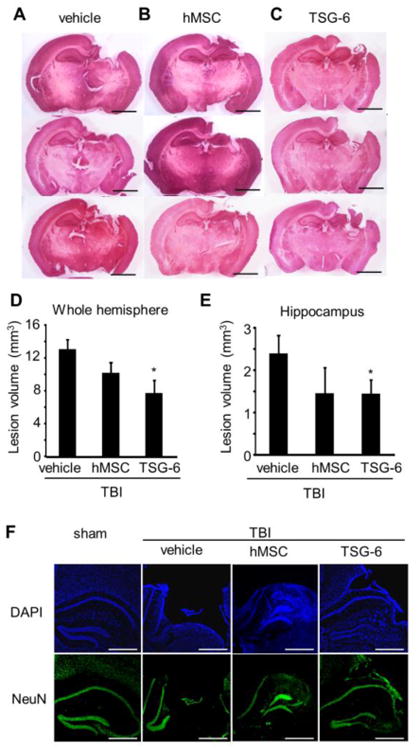
Intravenous injection of TSG-6 protein protected against TBI induced tissue loss in vivo after TBI. hMSCs (106 cells/mouse) were administrated 6 hour after TBI. TSG-6 protein at dose of 50 μg/mouse was administrated twice at 6 and 24 hour after TBI. (A-C) Representative H&E stained sections from injured mice treated with vehicle (A), MSC (B) or TSG-6 (C) at 14 days after TBI. Scale bars = 2 mm. (D, E) Lesion volume of whole hemisphere (D) and hippocampus (E) treated with vehicle (n=8), hMSCs (n=5), or TSG-6 (n=5) at 14 days after TBI. (F) Representative images of NeuN and DAPI stained hippocampus of mice 14 days after TBI. Scale bars = 500 μm. All data are represented as mean ± SEM. *p < 0.05 versus the vehicle group.
To investigate further the hippocampal injury, we stained neurons with NeuN, a marker for mature neurons. The CA1 and CA3 pyramidal cell layers of control TBI mice were entirely destroyed (Fig. 5F). hMSC or TSG-6 treatment significantly attenuated this damage.
Improved cognitive function at 6 to 7 weeks after TBI
We also tested whether treatment with TSG-6 during the Phase I of inflammation had a long-term effect on cognitive function. Because we obtained more promising results with TSG-6, we continued experiments only with TSG-6 treatment. As expected, assays in the Morris water maze (see experimental schedule in Fig. 6) demonstrated that TBI in the mice caused severe defects in spatial learning (Fig. 7A). Average latency values to reach the submerged platform over nine training sessions decreased progressively in sham surgery animal group (p < 0.0001, r2=0.345; learning session 1 ‘S1’ versus S2-S9, p<0.01-0.001) but did not change in TBI-vehicle treated group (p>0.05, r2=0.12). However, mice treated with TSG-6 during the first 24 hour after TBI demonstrated better learning ability. Latency values in these mice showed statistically significant decreases over learning sessions (p<0.05, r2=0.225; S1 versus S8 or S9, p<0.05). These results showed that TBI-vehicle treated group could not learn the place of platform at all, but TBI-TSG-6 mice began to remember the location of the platform after repeated learning trials between days 34 and 40. Though TBI-TSG-6 mice took longer to learn than the sham group, significant improvement in learning was observed. Also, TBI-TSG-6 mice successfully retrieved memory in the probe test (Fig. 7B and C). The working memory test in the Morris water maze (Fig. 7D) showed similar results to spatial leaning test. TBI-vehicle treated mice could not memorize the place of platform in 4 trials, but TBI-TSG-6 mice were able to locate the hidden platform with shorter latency values in the fourth trial. In addition, they demonstrated improvement in the Y-maze working memory test (Fig. 7E). Furthermore, motor functions such as swim speed and total distance traveled in water maze test and number of entries into each arm in Y-maze test were unchanged (Supplementary Fig. 1A-C). We also assessed ability for recognition memory using Y-maze test. TBI mice showed reduced recognition memory function but TSG-6 treatment did not alleviate this deficit (Supplementary Fig. 1D). In novel object recognition (NOR) test, TBI mice did not show deficits for both short- and long- term recognition memory (Supplementary Fig. 1E and F).
Decreased depressive-like behavior at 9 weeks after TBI
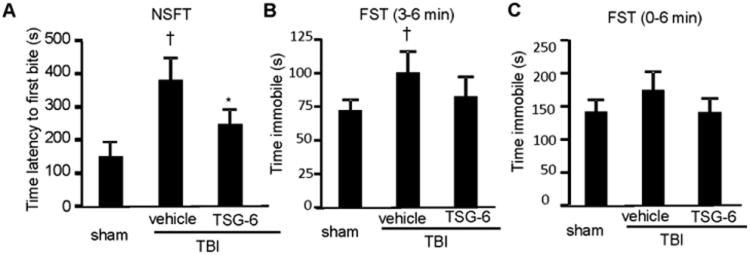
In addition, we tested the mice for depressive-like behavior 9 weeks after TBI. The treatment with TSG-6 within the first 24 of TBI improved results in the novelty suppressed feeding test (NSFT; Fig. 8A). Furthermore, in the last 3 minutes of forced swim test (FST), control TBI mice exhibited increased immobility (or floating) behavior (Fig. 8B). TSG-6 treatment after TBI reduced this depressive-like behavior to levels seen in sham control mice (Fig. 8B). Similar trend was seen when floating behavior was assessed for the entire duration of FST (Fig. 8C). Unexpectedly, there was only a small increase in depressive-like behavior in the mouse model of TBI employed in this study, therefore, only a little improvement was seen with the TSG-6 treatment. We also assessed anxiety-like behavior by elevated plus maze and open field tests but no anxiety-like behavior was observed in mice after TBI (Supplementary Fig. 1G-L).
Fig. 8.
TSG-6 protein decreases depressive-like behavior. TSG-6 protein (50 μg/mouse) was administered twice at 6 and 24 hour after TBI. Depressive-like behavior was assessed by novelty suppressed feeding test (NSFT) (A) and forced swim test (FST) (B, C). The total time spent in immobility for the entire trial duration (C) or last 3 minutes (B) was calculated in FST. Behavioral tests were performed as described in Fig. 6. N = 9 or 10 mice/group. All data are represented as mean ± SEM. †P < 0.05 versus the sham group, *P < 0.05 versus the vehicle group.
Increased neurogenesis in the hippocampus at 10 weeks after TBI
Some of the cognitive deficits associated with inflammation may be related to decreased neurogenesis in the hippocampus (Kohman and Rhodes, 2013). Therefore, we performed immunostaining for doublecortin (DCX), a marker of newly born immature neurons, of brains obtained 10 weeks after TBI. In hippocampus ipsilateral to TBI, DCX positive newly born neurons in the subgranular zone (SGZ)-granule cell layer (GCL) were decreased by 85% after TBI (Fig. 9A and C). TSG-6 treatment increased the newly born neurons by 1.8-fold (p < 0.05). This protective effect of TSG-6 was apparent in the posterior region of hippocampus (2.0 fold increase compared to vehicle treated group (p < 0.05. Fig. 9D). In the anterior region of hippocampus, the structure of hippocampus was better preserved in the TSG-6 treated group (Fig. 9B), but the number of DCX positive neurons was comparable to control TBI mice (Fig. 9E). These data suggest that TSG-6 modulated inflammation in peripheral damaged area. Interestingly, though CA1 and CA3 pyramidal cell layers were lost in TBI-vehicle administrated mice but preserved in TBI-TSG-6 administrated mice (Fig. 5A, C and F, Fig. 9 A and B), volumes of DG-SGZ-GCL were comparable between two groups (Fig. 9F). Administration of TSG-6 improved the density of DCX-positive newly born neurons however (Fig. 9G). These data suggest that TSG-6 not only protected CA1 and CA3 cell layers, but also maintained neural stem/progenitor cell activity in DG-SGZ-GCL via modulating inflammation. DCX positive newly born neurons in contralateral side of hippocampus were also increased in TBI mice receiving TSG-6 (Fig. 10A, B and C). This was particularly conspicuous in the anterior region of hippocampus (Fig. 10E). These data showed that TSG-6 treatment after TBI facilitated improved neurogenesis. Hence, it is likely that TSG-6 improved cognitive and mood function at least in part by up-regulation of hippocampal neurogenesis.
Fig. 9.
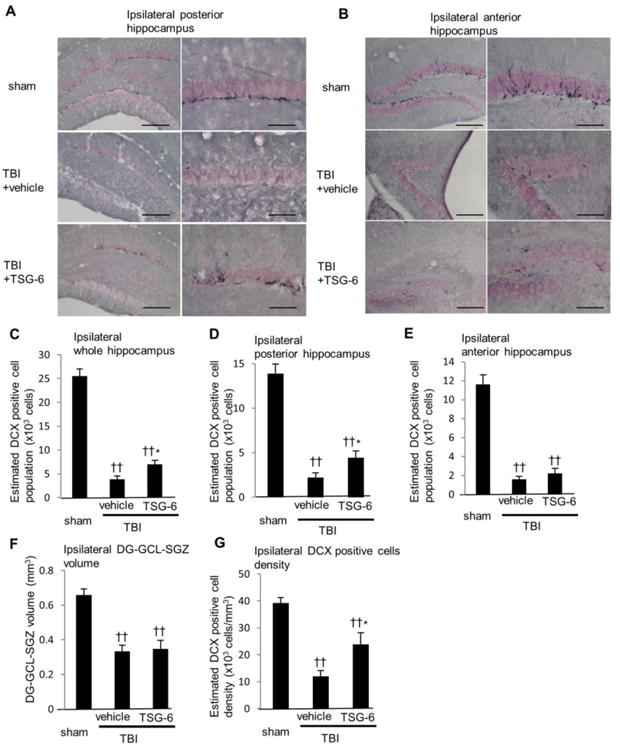
Administration of TSG-6 protein maintained neurogenesis in the hippocampus at 10 weeks after TBI. TSG-6 protein (50 μg/mouse) was administered twice at 6 and 24 hour after TBI. (A, B) Images of representative sections showing distribution of newly born neurons expressing doublecortin (DCX) in the subgranular zone-granule cell layer (SGZ–GCL) of ipsilateral posterior (A) and anterior (B) hippocampus at 10 weeks after TBI from sham and injured mice that received vehicle or TSG-6. Left: ×10 magnification (scale bars=200 μm), Right: ×20 magnification (scale bars = 100 μm). (C-E) Numbers of DCX positive newly born neurons. The total numbers of DCX positive neurons were estimated from unbiased stereological cell counts of the subgranular zone-granule cell layer (SGZ-GCL). Seven sections were collected from the whole hippocampus, one at each 450 μm. The number of DCX positive neurons was calculated from all seven sections (C), posterior three sections (D) or anterior four sections (E). The volume of dentate gyrus (DG) SGZ-GCL was calculated by Stereo Investigator system from seven sections (F). The density of DCX positive cells was calculated as dividing the number of DCX-positive cells by the volume of DG-SGZ-GCL (G). n = 9 or 10 mice/group. All data are represented as mean ± SEM. ††p<0.01 versus the sham group, *p < 0.05 versus the vehicle group.
Fig. 10.
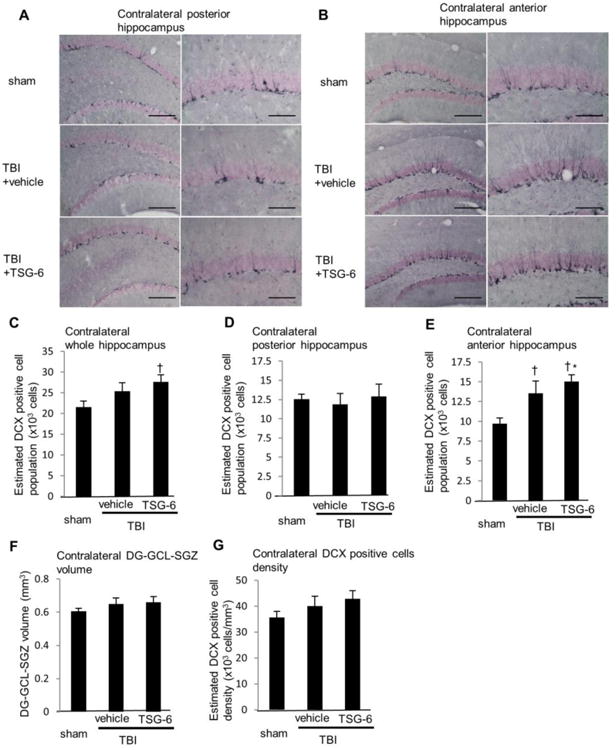
Administration of TSG-6 protein increased neurogenesis in the hippocampus at 10 weeks after TBI. TSG-6 protein (50 μg/mouse) was administered twice at 6 and 24 hour after TBI. (A, B) Images of representative sections showing distribution of newly born neurons expressing doublecortin (DCX) in the subgranular zone-granule cell layer (SGZ–GCL) of contralateral posterior (A) and anterior (B) hippocampus at 10 weeks after TBI from sham and injured mice that received vehicle or TSG-6. Left: ×10 magnification (scale bars=200 μm), Right: ×20 magnification (scale bars = 100 μm). (C-E) Numbers of DCX positive newly born neurons. The total numbers of DCX positive neurons were estimated from unbiased stereological cell counts of the subgranular zone-granule cell layer (SGZ-GCL). Seven sections were collected from the whole hippocampus, one at each 450 μm. The number of DCX positive neurons was calculated from all seven sections (C), posterior three sections (D) or anterior four sections (E). The volume of dentate gyrus (DG) SGZ-GCL was calculated by Stereo Investigator system from seven sections (F). The density of DCX positive cells was calculated as dividing the number of DCX-positive cells by the volume of DG-SGZ-GCL (G). n = 9 or 10 mice/group. All data are represented as mean ± SEM. †p<0.05 versus the sham group, *p < 0.05 versus the vehicle group.
Discussion
There have been multiple attempts to use anti-inflammatory agents in animal models and clinical trials of TBI. Essentially all have failed (Ransohoff and Brown, 2012; Rivest, 2011). The approach here to test TSG-6 in TBI is novel in two respects. One is that the therapy was administered acutely and only during the first 24 hour following TBI. Therefore the therapy was targeted to a time when inflammation is more likely to be harmful than helpful. The second novelty in the approach is that the protein employed does not fit the usual definition of an anti-inflammatory agent: in response to acute tissue injury in both the cornea (Oh et al., 2010) and the peritoneum (Choi et al., 2011), it acted during the initial Phase I of the inflammatory response by binding directly to or through hyaluronan to CD44 in a manner that modulated TLR2/NF-k B signaling in resident macrophages, the sentinel cells in most tissues that receive the initial signals of damage associated molecular patterns (DAMPs) (Medzhitov, 2010). In both cornea and peritonitis models, TSG-6 did not completely inhibit inflammation. In the corneal injury model in which the time window was explored (Oh et al., 2010), it had little if any effect when administered after 6 hour and at the onset of the large Phase II of the inflammatory response (Oh et al., 2010). Therefore TSG-6 is probably better classified as an inflammation modulatory protein than an anti-inflammatory agent. The results demonstrated that the initial mild Phase I of the inflammatory response following TBI is more protracted than in other tissues and persisted for at least 24 hour. In effect, a longer time is required in the brain for normal sequence of events in inflammation, i. e. the time required for sensor cells (macrophages, dendritic cells, and mast cells in peripheral tissues) to respond to DAMPs from injured cells and to release mediators (cytokines, chemokines, bioactive amines, ecasonoids and proteolytic products such as bradykinin) that usher in the massive edema and invasion of neutrophils, macrophages and lymphocytes that characterize inflammation (Medzhitov, 2010). The longer time for Phase I suggested that the time window for therapy with TSG-6 might be as long as 24 hour. Intravenous administration of either human MSCs or TSG-6 about 6 hour after TBI were about equally effective in decreasing the inflammatory response in terms of neutrophil infiltration and the level of MMP9 activity in endothelial cells and invading neutrophils at 24 hour. However, two administrations of TSG-6, one at 6 and one at 24 hour, were more effective than a single administration of either hMSCs or TSG-6 in BBB maintenance at day 3 and preserving neural tissue 2 weeks after the TBI. Most importantly, the two administrations of TSG-6 during the first 24-hour after TBI improved memory with repeated learning trials in the water maze, depressive-like behavior, and neurogenesis in the hippocampus after 6 weeks. The results in the water maze test were particularly impressive since TSG-6 treated mice not only learned to remember the location of the platform but also successfully retrieved the learned memory in the probe test whereas the vehicle treated TBI mice did not.
The results reported here are consistent with previous observation on TBI. An influx of peripheral neutrophils occurs following TBI, with a time course that correlates with BBB disruption (Ghajar, 2000). Timely resolution of leukocyte extravasation is essential to reduce damage to healthy tissue. A previous study showed that neutrophil depletion reduced BBB breakdown, axon injury, and inflammation after intracerebral hemorrhage (Moxon-Emre and Schlichter, 2011). Our results showed that TSG-6 treatment after TBI significantly decreased neutrophil infiltration as well as BBB permeability. These observations demonstrate that early TSG-6 administration after TBI can modulate inflammation. TSG-6 was reported to modulate the adhesion of neutrophils to the endothelium (Cao et al., 2004). In addition, our research group found that TSG-6 decreased zymosan/TLR2/NFk-B signaling in resident macrophages and thereby modulated the initial phase of inflammatory responses (Choi et al., 2011). Similar results were obtained in a rodent model of chemical injury to the cornea (Oh et al., 2010). One of the critical observations was that TSG-6 did not act as a classical anti-inflammatory agent since it was only effective if administered during the initial and mild phase I of the inflammatory reaction (Oh et al., 2010). Also of interest was that TSG-6 exerted similar neutrophil inhibitory effects in different models of inflammation and regardless of whether it is administered intravenously or directly into a site of inflammation (Getting et al., 2002; Lee et al., 2009; Roddy et al., 2011; Wisniewski et al., 1996). It thus seems likely that TSG-6 acts via the circulation to influence a fundamental process of neutrophil recruitment and extravasation.
Common consequences of TBI include personality changes, cognitive problems and a reduced quality of life calling for long-term rehabilitation and treatment (Masel and DeWitt, 2010). The mechanisms underlying TBI-induced cognitive and behavioral impairments are unclear. Neuroinflammation was recently reported to decrease neurogenesis and impair aspects of cognitive function (Russo et al., 2011). On the other hand, activation of more chronic inflammatory pathways was reported to be important for regenerative responses (Schmidt et al., 2005). The inflammatory process thus poses a double-edged sword with both negative and positive consequences to the post-injury process (Rivest, 2011). Acute administration of TSG-6 rescued both tissue damage and neurogenesis. Interestingly, TSG-6 also up-regulated neurogenesis in hippocampus contralateral to injury as long as 10 weeks after TBI.
The mechanism whereby TSG-6 modulated inflammation in the TBI model may or may not be the same as its mechanism of action in peripheral tissues. Macrophages are not present in the central nervous system; their function is largely sub-served by microglia and in part by astrocytes. Microglia express TLRs and TLRs in the brain and the genes were up-regulated by TBI (Hua et al., 2011). Therefore microglia, or specific subset of microglia, may respond to TSG-6 in a manner similar to resident macrophages in other tissues. Alternatively, TSG-6 may be acting primarily on the monocytes/macrophages that invade the brain as the blood brain barrier is disrupted by TBI. Also, some of the many other actions of TSG-6 may be involved. The protein was discovered as cDNA clone number 6 isolated after cultures of fibroblasts with stimulated with TNF-α (Wisniewski and Vilcek, 2004). It was subsequently shown to be expressed by a variety of cells in response to stimulation by pro-inflammatory cytokines (Fülöp et al., 1997; Milner et al., 2006; Milner and Day, 2003; Szanto et al., 2004; Wisniewski and Vilcek, 2004). TSG-6 can stabilize the extracellular matrix and thereby limit the invasion of inflammatory cells by binding to hyaluronan, heparin, heparan sulfate, thrombospondins-1 and -2, and fibronectin (Baranova et al., 2011; Blundell et al., 2005; Kuznetsova et al., 2005; Kuznetsova et al., 2008; Mahoney et al., 2005). In addition, it can inhibit the cascade of proteases released by inflammation by its complex catalytic interaction with inter-α-inhibitor (Rugg et al., 2005; Scavenius et al., 2011; Zhang et al., 2012), or by forming ternary complexes with mast cell trypases and heparin (Nagyeri et al., 2011). In apparently independent interactions, TSG-6 also reduces the migration of neutrophils through endothelial cells (Cao et al., 2004), and inhibits FGF-2 induced angiogenesis through an interaction with pentraxin (Leali et al., 2012). It is not clear which of these effects may be involved in suppressing inflammation after TBI. TSG-6 remains an attractive therapeutic agent, in part because no toxicities were reported in the many experiments performed in rodents with recombinant TSG-6 (Milner et al., 2006; Wisniewski and Vilcek, 2004).
Conclusions
Here we provided evidence that acute treatment with TSG-6 is highly effective in not only decreasing the initial injury to the brain but also in decreasing the long-term memory and behavioral disabilities observed in a mouse model for TBI. The results therefore suggest that acute administration of TSG-6 is potentially an attractive therapy for patients with TBI.
Supplementary Material
Highlights.
Administration of TSG-6 within 24 hours of TBI improves long-term memory.
TSG-6 reduced neutrophil infiltration after TBI.
Administration of TSG-6 protein decreased blood brain barrier (BBB) permeability.
Administration of TSG-6 protein maintained neurogenesis in the hippocampus.
Intravenous injection of TSG-6 protein protected against TBI induced tissue loss.
Acknowledgments
This study is supported in part by NIH grant P40OD011050.
Abbreviations
- BBB
blood brain barrier
- CCI
controlled cortical impact injury
- DCX
doublecortin
- DG
dentate gyrus
- FST
forced swim test
- GCL
granule cell layer
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- MSC
mesenchymal stem cells
- NOR
novel object recognition
- NSFT
novelty suppressed feeding test
- SGZ
subgranular zone
- TBI
traumatic brain injury
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baranova N, et al. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J Biol Chem. 2011;286:25675–25686. doi: 10.1074/jbc.M111.247395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell C, et al. Towards a structure for a TSG-6.hyaluronan complex by modeling and NMR spectroscopy: insights into other members of the link module superfamily. J Biol Chem. 2005;280:18189–18201. doi: 10.1074/jbc.M414343200. [DOI] [PubMed] [Google Scholar]
- Browne K, et al. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Exp Neurol. 2006;201:301–307. doi: 10.1016/j.expneurol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cao TV, et al. Inhibitory effects of TSG-6 Link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation. 2004;11:615–24. doi: 10.1080/10739680490503438. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. Glucocorticoids aggravate retrograde memory deficiency associated with traumatic brain injury in rats. J Neurotrauma. 2009;26:253–260. doi: 10.1089/neu.2007.0504. [DOI] [PubMed] [Google Scholar]
- Choi H, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–8. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, et al. Plasticity and remodeling of brain. Journal of the Neurological Sciences. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Colter DC, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, et al. Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- Edwards P, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- Flach A. Topically applied nonsteroidal anti-inflammatory drugs and corneal problems: an interim review and comment. Ophthalmology. 2000;107:1224–1226. doi: 10.1016/s0161-6420(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Foraker JE, et al. Cross-talk between human mesenchymal stem/progenitor cells (MSCs) and rat hippocampal slices in LPS-stimulated cocultures: the MSCs are activated to secrete prostaglandin E2. Journal of Neurochemistry. 2012;119:1052–63. doi: 10.1111/j.1471-4159.2011.07511.x. [DOI] [PubMed] [Google Scholar]
- Fülöp C, et al. Coding sequence, exon-intron structure and chromosomal localization of murine TNF-stimulated gene 6 that is specifically expressed by expanding cumulus cell-oocyte complexes. Gene. 1997;202:95–102. doi: 10.1016/s0378-1119(97)00459-9. [DOI] [PubMed] [Google Scholar]
- Getting SJ, et al. The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-alpha -inhibitor-independent manner. The Journal of Biological Chemistry. 2002;277:51068–76. doi: 10.1074/jbc.M205121200. [DOI] [PubMed] [Google Scholar]
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–9. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- Gidday JM, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. American Journal of Physiology. 2005;289:H558–68. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Hua F, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. Journal of Neuroinflammation. 2011;8:42–42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman R, Rhodes J. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27:22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi E, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Frontiers in Neurology. 2012;3:111–111. doi: 10.3389/fneur.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova S, et al. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha-trypsin inhibitor. J Biol Chem. 2005;280:30899–30908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova S, et al. TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biology. 2008;27:201–210. doi: 10.1016/j.matbio.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leali D, et al. Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction: a biological rheostat for fibroblast growth factor 2-mediated angiogenesis. Arterioscler Thromb Vasc Biol. 2012;32:696–703. doi: 10.1161/ATVBAHA.111.243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, et al. Corneal melting associated with use of topical nonsteroidal anti-inflammatory drugs after ocular surgery. Arch Ophthalmol. 2000;118:1129–1132. [PubMed] [Google Scholar]
- Maas AIR, et al. Moderate and severe traumatic brain injury in adults. Lancet Neurology. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Mahmood A, et al. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. Journal of Neurotrauma. 2004;21:33–9. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, et al. Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: implications for the inhibition of plasmin in extracellular matrix microenvironments. The Journal of Biological Chemistry. 2005;280:27044–55. doi: 10.1074/jbc.M502068200. [DOI] [PubMed] [Google Scholar]
- Masel B, DeWitt D. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Menge T, et al. Mesenchymal Stem Cells Regulate Blood-Brain Barrier Integrity Through TIMP3 Release After Traumatic Brain Injury. Science Translational Medicine. 2012;4:161ra150–161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara Y, et al. Expression and localization of the orexin-1 receptor (OX1R) after traumatic brain injury in mice. J Mol Neurosci. 2011;43:162–8. doi: 10.1007/s12031-010-9438-6. [DOI] [PubMed] [Google Scholar]
- Milner CM, et al. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. Journal of Cell Science. 2003;116:1863–73. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. Journal of Neuropathology and Experimental Neurology. 2011;70:218–35. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- Myburgh J, et al. Epidemiology and 12-month outcomes from traumatic brain injury in australia and new zealand. J Trauma. 2008;64:854–862. doi: 10.1097/TA.0b013e3180340e77. [DOI] [PubMed] [Google Scholar]
- Nagyeri G, et al. TSG-6 protein, a negative regulator of inflammatory arthritis, forms a ternary complex with murine mast cell tryptases and heparin. J Biol Chem. 2011;286:23559–23569. doi: 10.1074/jbc.M111.222026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16875–80. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, et al. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Molecular Psychiatry. 2011;16:171–83. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, et al. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplantation. 2007;40:609–19. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Pati S, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells and Development. 2011;20:89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clinical Pharmacology and Therapeutics. 2007;82:241–3. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Ransohoff R, Brown M. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. The European Journal of Neuroscience. 2005;21:464–76. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. The European Journal of Neuroscience. 2004;19:234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rawson RH. The binding of T-1824 and structurally related diazo dyes by the plasma proteins. American Journal of Physiology. 1942;138:708–717. 708. [Google Scholar]
- Rivest S. The promise of anti-inflammatory therapies for CNS injuries and diseases. Expert Review of Neurotherapeutics. 2011;11:783–786. doi: 10.1586/ern.11.64. [DOI] [PubMed] [Google Scholar]
- Roberts I, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- Roddy GW, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells (Dayton, Ohio) 2011;29:1572–9. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- Rugg M, et al. Characterization of complexes formed between TSG-6 and inter-alpha-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J Biol Chem. 2005;280:25674–25686. doi: 10.1074/jbc.M501332200. [DOI] [PubMed] [Google Scholar]
- Russo I, et al. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavenius C, et al. Human inter-α-inhibitor is a substrate for factor XIIIa and tissue transglutaminase. Biochim Biophys Acta. 2011;1814:1624–1630. doi: 10.1016/j.bbapap.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Schmidt O, et al. Closed head injury--an inflammatory disease? Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Sekiya I, et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells (Dayton, Ohio) 2002;20:530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Szanto S, et al. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis and Rheumatism. 2004;50:3012–22. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- Thau Zuchman O, et al. The anti-inflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma. 2012;29:375–384. doi: 10.1089/neu.2010.1673. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Current Opinion in Immunology. 2010;22:768–74. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–42. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Reviews. 1997;8:143–156. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Wisniewski HG, et al. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156:1609–15. [PubMed] [Google Scholar]
- Wisniewski H, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Reviews. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Wolfe M, et al. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs) Methods in Molecular Biology (Clifton N J) 2008;449:3–25. doi: 10.1007/978-1-60327-169-1_1. [DOI] [PubMed] [Google Scholar]
- Zhang S, et al. Constitutive expression of inter-α-inhibitor (IαI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J Biol Chem. 2012;287:12433–12444. doi: 10.1074/jbc.M112.342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietlow R, et al. Human stem cells for CNS repair. Cell and Tissue Research. 2008;331:301–22. doi: 10.1007/s00441-007-0488-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.