Abstract
The asymmetric unit of the title compound, C44H30O2, contains two independent molecules in which the terminal rings of the terphenyl element are inclined at angles of 36.3 (1) and 22.5 (1)° with respect to the central ring and the dihedral angles between the fluorenyl units are 72.3 (1) and 62.8 (1)°. In the crystal, pairs of O—H⋯O hydrogen bonds link the molecules into inversion dimers. The hydroxy H atoms not involved in these hydrogen bonds form O—H⋯π interactions in which the central terphenyl rings act as acceptors. Weak C—H⋯O contacts and π–π [centroid–centroid distance = 4.088 (2) Å] stacking interactions also occur. Taking into account directed non-covalent bonding between the molecules, the crystal is constructed of supramolecular strands extending along the a-axis direction.
Related literature
For the preparation of the starting material for the synthesis of the title compound, see: Staab & Binnig (1967 ▶). For background to organic solid-state inclusion chemistry, see: Atwood et al. (1991 ▶). For the design strategy of host compounds, see: Desiraju (1996 ▶). For diol host inclusion complexes, see: Toda (1996 ▶). For host compound 2,2′-bis(9-hydroxy-9-fluorenyl)biphenyl, see: Weber et al. (1993 ▶); Skobridis, Paraskevopoulos et al. (2011 ▶); Skobridis, Theodorou et al. (2011 ▶). For weak O—H⋯π and C—H⋯O interactions, see: Desiraju & Steiner (1999 ▶). For π–π stacking interactions, see: James (2004 ▶).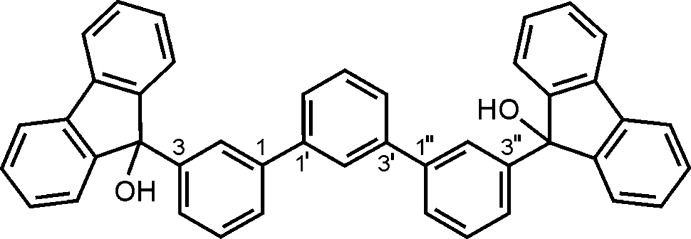
Experimental
Crystal data
C44H30O2
M r = 590.68
Triclinic,

a = 11.2292 (3) Å
b = 12.4823 (3) Å
c = 24.4440 (5) Å
α = 76.070 (1)°
β = 78.080 (1)°
γ = 66.917 (1)°
V = 3034.99 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 150 K
0.32 × 0.18 × 0.06 mm
Data collection
Bruker X8 APEXII CCD diffractometer
55787 measured reflections
13774 independent reflections
9930 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.109
S = 1.03
13774 reflections
845 parameters
4 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.24 e Å−3
Δρmin = −0.22 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813024033/rk2410sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813024033/rk2410Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813024033/rk2410Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the C20–C25 and C20A–C25A rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O2i | 0.86 (1) | 2.07 (1) | 2.894 (2) | 163 (1) |
| O2—H2⋯Cg1ii | 0.84 (1) | 3.42 (1) | 4.163 (2) | 150 (1) |
| O1A—H1A⋯O2A iii | 0.85 (1) | 1.99 (1) | 2.807 (2) | 160 (1) |
| O2A—H2A⋯Cg2iv | 0.85 (2) | 3.46 (1) | 4.169 (2) | 145 (1) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
We thank the NMR Center and Mass Spectrometry Unit of the University of Ioannina for measuring the 1H NMR and HRMS spectra. KS thanks the State Scholarships Foundation (IKY) for supporting this work.
supplementary crystallographic information
1. Comment
In the realm of organic state inclusion chemistry (Atwood et al., 1991), bulkily substituted diols have proven a versatile design strategy for the formation of host compounds (Desiraju, 1996) yielding crystalline host-guest complexes with a variety of guest molecules (Toda, 1996). With regard to that, 2,2'-bis(9-hydroxy-9-fluorenyl)biphenyl is a prototype host structure (Weber et al., 1993; Skobridis, Paraskevopoulos et al., 2010; Skobridis, Theodorou et al., 2011). Following this structural approach, the title compound involving exchange of the central 2,2'-disubstituted biphenyl unit for a 3,3"-disubstituted m-terphenyl moiety was prepared and its crystal structure studied unexpectedly showing a solvent-free crystal species on crystallization from ethanol. The compound was found in the space group P1 with two crystallographically independent molecules in the asymmetric unit (Fig. 1). The twist angles between the aromatic rings of their terpenyl element are 36.3 (1)° (rings A/B), 22.5 (1)° (rings B/C), 33.8 (1)° (rings A'/B') and 17.2° (rings B'/C') while the mean planes of the fluorenyl units are inclined at angles of 72.3 (1)° and 62.8 (1)°, respectively. The crystal structure (Fig. 2 & Fig. 3) is constructed of inversion dimers which are stabilized by classical O–H···O hydrogen bonds. The hydroxy H atoms excluded from strong hydrogen bonding interlink the molecular dimers via O–H···π interactions (Desiraju & Steiner, 1999) with the central rings of the terphenyl units acting as acceptors (O2–H2···C22 = 2.692 (2) Å, 153.3 (1)°; O2A–H2A···C22A = 2.724 (2) Å, 142.5 (1)°). Weak C–H···O contacts (Desiraju & Steiner, 1999) and π–π stacking interactions (James, 2004) (Cg(B)···Cg(B)i, 4.088 (2) Å; symmetry code: (i) 1-x, 2-y, 2-z) complete the pattern of intermolecular interactions.
2. Experimental
The starting compound 3,3"-dibromo-1,1':2',1"-terphenyl was prepared according to a literature procedure (Staab & Binnig, 1967). To a stirred solution of this dibromide (2.13 g, 5.5 mmol) in dry diethyl ether (25 ml), n-BuLi (1.6 N in n-hexane, 7.0 ml, 12 mmol) was added dropwise at 195 K under argon. Stirring of the mixture was continued at 253 K for 15 min. Then, fluorenone (1.98 g, 11.0 mmol) in 25 ml of dry diethyl ether was added and the mixture kept at reflux. After completion of the reaction (24 h), which was monitored by TLC (hexane/ethyl acetate 2:1, Rf 0.55), the mixture was cooled, quenched with saturated NH4Cl solution and extracted with diethyl ether (2 × 30 ml). The combined organic extracts were dried (Na2SO4) and evaporated. The pale yellow oily residue was precipitated by the addition of hexane and purified by flash chromatography on a SiO2 column (hexane/ethyl acetate 2:1) to yield 3.90 g (65 %) of a colourless solid. M.p. = 485-487 K. IR (KBr) 3446, 3037, 1597, 1467, 1448, 1166, 1120, 769, 738. 1H NMR (500 MHz, CDCl3) δ 2.61 (s, br, 2 H, OH), 7.34-7.62 (m, 21 H, ArH), 7.79-7.92 (m, 7 H, ArH). 13C NMR (125 MHz, CDCl3) δ 83.7 (CO), 120.2, 124.3, 124.7, 124.8, 126.3, 128.5, 128.7, 129.0, 129.2, 139.7, 141.2, 141.8, 143.8. 150.4 (Ar). MS (HR-ESI) m/z: found 613.2126 [M+Na], calc. for C44H30O2 + Na: 613.2138. The melting point (uncorrected) was measured on a hot stage microscope. The IR spectrum was recorded on a Perkin Elmer FT-IR 1600 spectrometer. 1H and 13C NMR spectra were measured on a Bruker Avance AV-500 spectrometer using (CH3)4Si as internal standard. The high resolution ESI mass spectrum was obtained using a ThermoFisher Scientific Orbitrap XL spectrometer. Crystals of the title compound suitable for X-ray structural analysis were grown by slow evaporating a solution of the material in ethanol.
3. Refinement
The H atoms for hydroxy groups were obtained from the difference electron density map and refined freely. Other aromatic H atoms were positioned geometrically and allowed to ride on their respective parent atoms, with C–H = 0.95Å and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
Asymmetric unit of the title compound, showing the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.

Packing excerpt of the title compound, showing the O—H···O hydrogen-bonded dimers interlinked via O—H···π interactions. O—H···O and O—H···π contacts are presented as broken lines and broken double lines, respectively. Non-relevant hydrogens are omitted for clarity.
Fig. 3.
A view along the a-axis of the title compound. Hydrogen-bond type contacts are presented as broken lines. Non-relevant hydrogens are omitted for clarity.
Crystal data
| C44H30O2 | Z = 4 |
| Mr = 590.68 | F(000) = 1240 |
| Triclinic, P1 | Dx = 1.293 Mg m−3 |
| Hall symbol: -P 1 | Melting point = 485–487 K |
| a = 11.2292 (3) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 12.4823 (3) Å | Cell parameters from 9885 reflections |
| c = 24.4440 (5) Å | θ = 2.3–28.4° |
| α = 76.070 (1)° | µ = 0.08 mm−1 |
| β = 78.080 (1)° | T = 150 K |
| γ = 66.917 (1)° | Irregular, colourless |
| V = 3034.99 (13) Å3 | 0.32 × 0.18 × 0.06 mm |
Data collection
| Bruker X8 APEXII CCD diffractometer | 9930 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.035 |
| Graphite monochromator | θmax = 27.4°, θmin = 0.9° |
| φ– and ω–scans | h = −14→14 |
| 55787 measured reflections | k = −16→16 |
| 13774 independent reflections | l = −31→31 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.109 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0457P)2 + 0.734P] where P = (Fo2 + 2Fc2)/3 |
| 13774 reflections | (Δ/σ)max < 0.001 |
| 845 parameters | Δρmax = 0.24 e Å−3 |
| 4 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s.planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. The bond lengths of the hydroxy groups (O1–H1, O2–H2, O1A–H1A, O2A–H2A) were restraint to target values of 0.84 (1)°. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.94347 (11) | 0.99066 (10) | 0.89442 (4) | 0.0298 (2) | |
| H1 | 0.9865 (18) | 1.0357 (15) | 0.8911 (8) | 0.058 (6)* | |
| O2 | −0.06412 (10) | 0.85199 (9) | 1.09227 (4) | 0.0270 (2) | |
| H2 | −0.1445 (10) | 0.8725 (18) | 1.0921 (9) | 0.058 (6)* | |
| C1 | 1.12300 (14) | 0.86200 (13) | 0.83697 (6) | 0.0271 (3) | |
| C2 | 1.19755 (16) | 0.77159 (15) | 0.87461 (8) | 0.0379 (4) | |
| H2AA | 1.1594 | 0.7462 | 0.9114 | 0.045* | |
| C3 | 1.33026 (18) | 0.71841 (17) | 0.85737 (10) | 0.0499 (5) | |
| H3 | 1.3832 | 0.6555 | 0.8826 | 0.060* | |
| C4 | 1.38572 (17) | 0.75594 (18) | 0.80409 (10) | 0.0503 (5) | |
| H4 | 1.4764 | 0.7183 | 0.7932 | 0.060* | |
| C5 | 1.31174 (17) | 0.84740 (17) | 0.76627 (8) | 0.0420 (4) | |
| H5 | 1.3508 | 0.8731 | 0.7297 | 0.050* | |
| C6 | 1.17897 (15) | 0.90096 (14) | 0.78287 (7) | 0.0299 (3) | |
| C7 | 1.07732 (15) | 0.99960 (14) | 0.75333 (6) | 0.0292 (3) | |
| C8 | 1.08036 (19) | 1.06728 (16) | 0.69911 (7) | 0.0415 (4) | |
| H8 | 1.1594 | 1.0530 | 0.6739 | 0.050* | |
| C9 | 0.9664 (2) | 1.15561 (17) | 0.68269 (7) | 0.0459 (5) | |
| H9 | 0.9676 | 1.2022 | 0.6457 | 0.055* | |
| C10 | 0.85090 (19) | 1.17759 (15) | 0.71886 (8) | 0.0421 (4) | |
| H10 | 0.7740 | 1.2392 | 0.7066 | 0.051* | |
| C11 | 0.84615 (16) | 1.11050 (14) | 0.77289 (7) | 0.0323 (4) | |
| H11 | 0.7666 | 1.1248 | 0.7977 | 0.039* | |
| C12 | 0.95974 (15) | 1.02239 (13) | 0.78973 (6) | 0.0255 (3) | |
| C13 | 0.97803 (14) | 0.93475 (13) | 0.84594 (6) | 0.0238 (3) | |
| C14 | 0.88925 (13) | 0.86512 (12) | 0.85287 (6) | 0.0218 (3) | |
| C15 | 0.92373 (14) | 0.77027 (13) | 0.82483 (6) | 0.0247 (3) | |
| H15 | 1.0080 | 0.7427 | 0.8040 | 0.030* | |
| C16 | 0.83589 (14) | 0.71609 (13) | 0.82719 (6) | 0.0251 (3) | |
| H16 | 0.8600 | 0.6519 | 0.8077 | 0.030* | |
| C17 | 0.71268 (14) | 0.75488 (12) | 0.85783 (6) | 0.0234 (3) | |
| H17 | 0.6525 | 0.7179 | 0.8588 | 0.028* | |
| C18 | 0.67726 (13) | 0.84809 (12) | 0.88717 (5) | 0.0210 (3) | |
| C19 | 0.76591 (13) | 0.90268 (12) | 0.88367 (6) | 0.0218 (3) | |
| H19 | 0.7416 | 0.9674 | 0.9028 | 0.026* | |
| C20 | 0.54817 (13) | 0.88801 (12) | 0.92254 (6) | 0.0216 (3) | |
| C21 | 0.48126 (14) | 1.00700 (13) | 0.92661 (6) | 0.0243 (3) | |
| H21 | 0.5180 | 1.0644 | 0.9066 | 0.029* | |
| C22 | 0.36119 (14) | 1.04162 (13) | 0.95977 (6) | 0.0258 (3) | |
| H22 | 0.3158 | 1.1229 | 0.9620 | 0.031* | |
| C23 | 0.30668 (14) | 0.95900 (13) | 0.98970 (6) | 0.0251 (3) | |
| H23 | 0.2240 | 0.9842 | 1.0120 | 0.030* | |
| C24 | 0.37232 (13) | 0.83886 (13) | 0.98735 (6) | 0.0223 (3) | |
| C25 | 0.49253 (14) | 0.80609 (13) | 0.95310 (6) | 0.0230 (3) | |
| H25 | 0.5379 | 0.7249 | 0.9506 | 0.028* | |
| C26 | 0.32002 (13) | 0.74718 (13) | 1.02154 (6) | 0.0224 (3) | |
| C27 | 0.40254 (14) | 0.63031 (13) | 1.03636 (6) | 0.0252 (3) | |
| H27 | 0.4936 | 0.6085 | 1.0242 | 0.030* | |
| C28 | 0.35372 (14) | 0.54571 (13) | 1.06855 (6) | 0.0258 (3) | |
| H28 | 0.4110 | 0.4659 | 1.0772 | 0.031* | |
| C29 | 0.22209 (14) | 0.57635 (13) | 1.08829 (6) | 0.0242 (3) | |
| H29 | 0.1893 | 0.5178 | 1.1105 | 0.029* | |
| C30 | 0.13789 (13) | 0.69292 (12) | 1.07553 (6) | 0.0215 (3) | |
| C31 | 0.18631 (14) | 0.77647 (13) | 1.04124 (6) | 0.0224 (3) | |
| H31 | 0.1279 | 0.8551 | 1.0308 | 0.027* | |
| C32 | −0.00592 (13) | 0.72451 (12) | 1.09871 (6) | 0.0225 (3) | |
| C33 | −0.07417 (13) | 0.67342 (13) | 1.06951 (6) | 0.0241 (3) | |
| C34 | −0.08402 (15) | 0.69123 (14) | 1.01235 (6) | 0.0303 (3) | |
| H34 | −0.0481 | 0.7430 | 0.9855 | 0.036* | |
| C35 | −0.14767 (16) | 0.63166 (15) | 0.99506 (7) | 0.0375 (4) | |
| H35 | −0.1549 | 0.6423 | 0.9559 | 0.045* | |
| C36 | −0.20056 (17) | 0.55699 (16) | 1.03447 (8) | 0.0424 (4) | |
| H36 | −0.2440 | 0.5173 | 1.0219 | 0.051* | |
| C37 | −0.19141 (16) | 0.53890 (15) | 1.09183 (8) | 0.0370 (4) | |
| H37 | −0.2283 | 0.4877 | 1.1186 | 0.044* | |
| C38 | −0.12712 (14) | 0.59729 (13) | 1.10932 (6) | 0.0266 (3) | |
| C39 | −0.09714 (14) | 0.59233 (13) | 1.16595 (6) | 0.0259 (3) | |
| C40 | −0.12494 (16) | 0.52828 (14) | 1.21874 (7) | 0.0331 (4) | |
| H40 | −0.1745 | 0.4796 | 1.2228 | 0.040* | |
| C41 | −0.07897 (17) | 0.53695 (15) | 1.26528 (7) | 0.0371 (4) | |
| H41 | −0.0965 | 0.4931 | 1.3015 | 0.044* | |
| C42 | −0.00772 (17) | 0.60871 (15) | 1.25964 (7) | 0.0360 (4) | |
| H42 | 0.0231 | 0.6134 | 1.2920 | 0.043* | |
| C43 | 0.01929 (15) | 0.67407 (14) | 1.20693 (6) | 0.0293 (3) | |
| H43 | 0.0679 | 0.7235 | 1.2031 | 0.035* | |
| C44 | −0.02603 (13) | 0.66525 (12) | 1.16066 (6) | 0.0232 (3) | |
| O1A | 1.41120 (11) | 0.32518 (9) | 0.59705 (5) | 0.0357 (3) | |
| H1A | 1.4660 (16) | 0.3507 (17) | 0.6037 (8) | 0.055 (6)* | |
| O2A | 0.44394 (10) | 0.54003 (10) | 0.39659 (5) | 0.0293 (2) | |
| H2A | 0.3615 (10) | 0.5663 (18) | 0.3979 (9) | 0.068 (7)* | |
| C1A | 1.44187 (15) | 0.15094 (13) | 0.67427 (6) | 0.0257 (3) | |
| C2A | 1.32848 (16) | 0.17143 (14) | 0.71175 (7) | 0.0331 (4) | |
| H2AB | 1.2472 | 0.2245 | 0.6997 | 0.040* | |
| C3A | 1.33615 (18) | 0.11247 (16) | 0.76755 (7) | 0.0417 (4) | |
| H3A | 1.2595 | 0.1261 | 0.7943 | 0.050* | |
| C4A | 1.45487 (19) | 0.03382 (17) | 0.78450 (7) | 0.0443 (4) | |
| H4A | 1.4580 | −0.0072 | 0.8226 | 0.053* | |
| C5A | 1.56883 (17) | 0.01375 (15) | 0.74710 (7) | 0.0369 (4) | |
| H5A | 1.6499 | −0.0396 | 0.7593 | 0.044* | |
| C6A | 1.56194 (15) | 0.07326 (13) | 0.69149 (6) | 0.0269 (3) | |
| C7A | 1.66334 (14) | 0.07059 (12) | 0.64249 (6) | 0.0261 (3) | |
| C8A | 1.79803 (15) | 0.01418 (14) | 0.63803 (7) | 0.0332 (4) | |
| H8A | 1.8391 | −0.0360 | 0.6700 | 0.040* | |
| C9A | 1.87136 (16) | 0.03244 (15) | 0.58611 (8) | 0.0387 (4) | |
| H9A | 1.9636 | −0.0055 | 0.5825 | 0.046* | |
| C10A | 1.81221 (17) | 0.10512 (15) | 0.53940 (8) | 0.0379 (4) | |
| H10A | 1.8642 | 0.1163 | 0.5041 | 0.045* | |
| C11A | 1.67762 (16) | 0.16195 (14) | 0.54350 (7) | 0.0336 (4) | |
| H11A | 1.6372 | 0.2126 | 0.5115 | 0.040* | |
| C12A | 1.60384 (15) | 0.14348 (13) | 0.59493 (6) | 0.0268 (3) | |
| C13A | 1.45695 (14) | 0.19901 (12) | 0.61050 (6) | 0.0263 (3) | |
| C14A | 1.37525 (14) | 0.15922 (13) | 0.58288 (6) | 0.0250 (3) | |
| C15A | 1.41270 (16) | 0.04087 (14) | 0.57924 (7) | 0.0331 (4) | |
| H15A | 1.4936 | −0.0144 | 0.5909 | 0.040* | |
| C16A | 1.33264 (16) | 0.00314 (14) | 0.55870 (7) | 0.0358 (4) | |
| H16A | 1.3590 | −0.0779 | 0.5562 | 0.043* | |
| C17A | 1.21453 (15) | 0.08276 (13) | 0.54183 (6) | 0.0298 (3) | |
| H17A | 1.1602 | 0.0561 | 0.5278 | 0.036* | |
| C18A | 1.17481 (14) | 0.20167 (13) | 0.54526 (6) | 0.0237 (3) | |
| C19A | 1.25665 (14) | 0.23819 (13) | 0.56608 (6) | 0.0244 (3) | |
| H19A | 1.2304 | 0.3191 | 0.5688 | 0.029* | |
| C20A | 1.04741 (14) | 0.28872 (12) | 0.52785 (6) | 0.0229 (3) | |
| C21A | 0.97615 (14) | 0.38363 (13) | 0.55611 (6) | 0.0250 (3) | |
| H21A | 1.0091 | 0.3934 | 0.5867 | 0.030* | |
| C22A | 0.85781 (14) | 0.46352 (13) | 0.53978 (6) | 0.0270 (3) | |
| H22A | 0.8099 | 0.5281 | 0.5592 | 0.032* | |
| C23A | 0.80815 (14) | 0.45033 (13) | 0.49536 (6) | 0.0254 (3) | |
| H23A | 0.7262 | 0.5056 | 0.4849 | 0.030* | |
| C24A | 0.87711 (14) | 0.35678 (12) | 0.46593 (6) | 0.0228 (3) | |
| C25A | 0.99691 (14) | 0.27705 (12) | 0.48319 (6) | 0.0231 (3) | |
| H25A | 1.0454 | 0.2127 | 0.4637 | 0.028* | |
| C26A | 0.82490 (14) | 0.34188 (12) | 0.41805 (6) | 0.0231 (3) | |
| C27A | 0.90496 (14) | 0.27128 (13) | 0.37860 (6) | 0.0271 (3) | |
| H27A | 0.9951 | 0.2310 | 0.3823 | 0.032* | |
| C28A | 0.85477 (15) | 0.25912 (14) | 0.33404 (6) | 0.0305 (3) | |
| H28A | 0.9100 | 0.2081 | 0.3085 | 0.037* | |
| C29A | 0.72513 (15) | 0.32066 (13) | 0.32662 (6) | 0.0286 (3) | |
| H29A | 0.6920 | 0.3138 | 0.2954 | 0.034* | |
| C30A | 0.64318 (14) | 0.39281 (13) | 0.36500 (6) | 0.0239 (3) | |
| C31A | 0.69259 (14) | 0.40052 (13) | 0.41083 (6) | 0.0239 (3) | |
| H31A | 0.6355 | 0.4466 | 0.4380 | 0.029* | |
| C32A | 0.50106 (14) | 0.46201 (13) | 0.35537 (6) | 0.0250 (3) | |
| C33A | 0.42463 (14) | 0.38268 (13) | 0.35927 (7) | 0.0277 (3) | |
| C34A | 0.40304 (16) | 0.29852 (14) | 0.40431 (8) | 0.0376 (4) | |
| H34A | 0.4390 | 0.2827 | 0.4386 | 0.045* | |
| C35A | 0.32739 (18) | 0.23747 (16) | 0.39831 (10) | 0.0502 (5) | |
| H35A | 0.3107 | 0.1799 | 0.4290 | 0.060* | |
| C36A | 0.27647 (19) | 0.25950 (18) | 0.34843 (11) | 0.0563 (6) | |
| H36A | 0.2264 | 0.2159 | 0.3450 | 0.068* | |
| C37A | 0.29705 (17) | 0.34435 (17) | 0.30308 (9) | 0.0482 (5) | |
| H37A | 0.2609 | 0.3597 | 0.2689 | 0.058* | |
| C38A | 0.37171 (15) | 0.40635 (14) | 0.30880 (7) | 0.0317 (4) | |
| C39A | 0.41085 (15) | 0.49906 (14) | 0.26875 (7) | 0.0309 (4) | |
| C40A | 0.38515 (18) | 0.55247 (16) | 0.21365 (7) | 0.0427 (4) | |
| H40A | 0.3326 | 0.5311 | 0.1956 | 0.051* | |
| C41A | 0.4372 (2) | 0.63715 (17) | 0.18554 (7) | 0.0487 (5) | |
| H41A | 0.4207 | 0.6738 | 0.1476 | 0.058* | |
| C42A | 0.51331 (19) | 0.66985 (15) | 0.21146 (7) | 0.0437 (4) | |
| H42A | 0.5478 | 0.7287 | 0.1914 | 0.052* | |
| C43A | 0.53934 (16) | 0.61658 (14) | 0.26691 (7) | 0.0330 (4) | |
| H43A | 0.5918 | 0.6382 | 0.2849 | 0.040* | |
| C44A | 0.48756 (14) | 0.53195 (13) | 0.29503 (6) | 0.0264 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0302 (6) | 0.0376 (6) | 0.0289 (6) | −0.0191 (5) | 0.0048 (4) | −0.0144 (5) |
| O2 | 0.0185 (5) | 0.0238 (6) | 0.0369 (6) | −0.0063 (5) | −0.0019 (4) | −0.0056 (4) |
| C1 | 0.0208 (7) | 0.0281 (8) | 0.0360 (8) | −0.0108 (6) | −0.0019 (6) | −0.0102 (6) |
| C2 | 0.0293 (9) | 0.0366 (9) | 0.0495 (10) | −0.0126 (8) | −0.0115 (8) | −0.0043 (8) |
| C3 | 0.0309 (10) | 0.0397 (11) | 0.0808 (15) | −0.0065 (8) | −0.0203 (10) | −0.0131 (10) |
| C4 | 0.0204 (9) | 0.0529 (12) | 0.0846 (15) | −0.0093 (9) | 0.0009 (9) | −0.0382 (11) |
| C5 | 0.0292 (9) | 0.0527 (11) | 0.0533 (11) | −0.0201 (9) | 0.0110 (8) | −0.0305 (9) |
| C6 | 0.0240 (8) | 0.0346 (9) | 0.0387 (9) | −0.0160 (7) | 0.0050 (6) | −0.0184 (7) |
| C7 | 0.0325 (9) | 0.0355 (9) | 0.0278 (8) | −0.0216 (7) | 0.0033 (6) | −0.0102 (6) |
| C8 | 0.0532 (12) | 0.0542 (11) | 0.0298 (9) | −0.0374 (10) | 0.0058 (8) | −0.0089 (8) |
| C9 | 0.0715 (14) | 0.0488 (11) | 0.0296 (9) | −0.0393 (11) | −0.0130 (9) | 0.0066 (8) |
| C10 | 0.0546 (12) | 0.0328 (9) | 0.0449 (10) | −0.0204 (9) | −0.0213 (9) | 0.0028 (8) |
| C11 | 0.0318 (9) | 0.0283 (8) | 0.0389 (9) | −0.0133 (7) | −0.0058 (7) | −0.0041 (7) |
| C12 | 0.0279 (8) | 0.0254 (8) | 0.0273 (7) | −0.0146 (7) | −0.0010 (6) | −0.0056 (6) |
| C13 | 0.0205 (7) | 0.0258 (8) | 0.0254 (7) | −0.0088 (6) | 0.0002 (6) | −0.0070 (6) |
| C14 | 0.0202 (7) | 0.0225 (7) | 0.0216 (7) | −0.0082 (6) | −0.0021 (5) | −0.0014 (5) |
| C15 | 0.0194 (7) | 0.0283 (8) | 0.0239 (7) | −0.0075 (6) | 0.0018 (6) | −0.0059 (6) |
| C16 | 0.0263 (8) | 0.0244 (8) | 0.0250 (7) | −0.0086 (6) | −0.0011 (6) | −0.0075 (6) |
| C17 | 0.0228 (7) | 0.0256 (8) | 0.0229 (7) | −0.0110 (6) | −0.0029 (6) | −0.0023 (6) |
| C18 | 0.0191 (7) | 0.0223 (7) | 0.0187 (6) | −0.0061 (6) | −0.0028 (5) | 0.0001 (5) |
| C19 | 0.0223 (7) | 0.0207 (7) | 0.0220 (7) | −0.0081 (6) | −0.0021 (5) | −0.0032 (5) |
| C20 | 0.0184 (7) | 0.0259 (8) | 0.0204 (7) | −0.0077 (6) | −0.0023 (5) | −0.0045 (6) |
| C21 | 0.0224 (7) | 0.0268 (8) | 0.0249 (7) | −0.0113 (6) | −0.0027 (6) | −0.0026 (6) |
| C22 | 0.0218 (7) | 0.0235 (8) | 0.0294 (8) | −0.0047 (6) | −0.0040 (6) | −0.0053 (6) |
| C23 | 0.0183 (7) | 0.0304 (8) | 0.0246 (7) | −0.0067 (6) | −0.0004 (6) | −0.0069 (6) |
| C24 | 0.0183 (7) | 0.0275 (8) | 0.0212 (7) | −0.0084 (6) | −0.0026 (5) | −0.0044 (6) |
| C25 | 0.0204 (7) | 0.0241 (7) | 0.0239 (7) | −0.0074 (6) | −0.0017 (6) | −0.0055 (6) |
| C26 | 0.0206 (7) | 0.0270 (8) | 0.0198 (7) | −0.0084 (6) | −0.0016 (5) | −0.0057 (6) |
| C27 | 0.0170 (7) | 0.0306 (8) | 0.0253 (7) | −0.0064 (6) | −0.0005 (6) | −0.0058 (6) |
| C28 | 0.0224 (7) | 0.0239 (8) | 0.0268 (7) | −0.0037 (6) | −0.0034 (6) | −0.0042 (6) |
| C29 | 0.0236 (7) | 0.0253 (8) | 0.0237 (7) | −0.0103 (6) | −0.0018 (6) | −0.0028 (6) |
| C30 | 0.0187 (7) | 0.0267 (8) | 0.0203 (7) | −0.0089 (6) | −0.0019 (5) | −0.0057 (6) |
| C31 | 0.0199 (7) | 0.0229 (7) | 0.0228 (7) | −0.0057 (6) | −0.0022 (5) | −0.0050 (6) |
| C32 | 0.0189 (7) | 0.0212 (7) | 0.0260 (7) | −0.0061 (6) | −0.0021 (6) | −0.0042 (6) |
| C33 | 0.0162 (7) | 0.0239 (8) | 0.0299 (8) | −0.0034 (6) | −0.0032 (6) | −0.0070 (6) |
| C34 | 0.0233 (8) | 0.0306 (8) | 0.0317 (8) | −0.0020 (7) | −0.0064 (6) | −0.0067 (6) |
| C35 | 0.0307 (9) | 0.0405 (10) | 0.0401 (9) | −0.0013 (8) | −0.0155 (7) | −0.0154 (8) |
| C36 | 0.0305 (9) | 0.0443 (10) | 0.0606 (12) | −0.0096 (8) | −0.0159 (8) | −0.0223 (9) |
| C37 | 0.0285 (9) | 0.0357 (9) | 0.0513 (10) | −0.0148 (7) | −0.0034 (7) | −0.0117 (8) |
| C38 | 0.0183 (7) | 0.0271 (8) | 0.0344 (8) | −0.0071 (6) | −0.0011 (6) | −0.0092 (6) |
| C39 | 0.0181 (7) | 0.0242 (8) | 0.0325 (8) | −0.0058 (6) | 0.0026 (6) | −0.0078 (6) |
| C40 | 0.0288 (8) | 0.0286 (8) | 0.0372 (9) | −0.0119 (7) | 0.0080 (7) | −0.0050 (7) |
| C41 | 0.0394 (10) | 0.0331 (9) | 0.0283 (8) | −0.0093 (8) | 0.0058 (7) | −0.0017 (7) |
| C42 | 0.0389 (10) | 0.0383 (9) | 0.0256 (8) | −0.0078 (8) | −0.0032 (7) | −0.0075 (7) |
| C43 | 0.0268 (8) | 0.0318 (8) | 0.0295 (8) | −0.0100 (7) | −0.0019 (6) | −0.0080 (6) |
| C44 | 0.0172 (7) | 0.0235 (7) | 0.0261 (7) | −0.0052 (6) | 0.0005 (6) | −0.0054 (6) |
| O1A | 0.0337 (6) | 0.0204 (6) | 0.0557 (7) | −0.0077 (5) | −0.0239 (6) | 0.0004 (5) |
| O2A | 0.0193 (6) | 0.0333 (6) | 0.0350 (6) | −0.0042 (5) | −0.0057 (5) | −0.0129 (5) |
| C1A | 0.0265 (8) | 0.0222 (7) | 0.0322 (8) | −0.0086 (6) | −0.0100 (6) | −0.0068 (6) |
| C2A | 0.0287 (8) | 0.0328 (9) | 0.0410 (9) | −0.0098 (7) | −0.0058 (7) | −0.0135 (7) |
| C3A | 0.0424 (10) | 0.0525 (11) | 0.0367 (9) | −0.0228 (9) | 0.0034 (8) | −0.0174 (8) |
| C4A | 0.0566 (12) | 0.0546 (12) | 0.0278 (9) | −0.0275 (10) | −0.0092 (8) | −0.0022 (8) |
| C5A | 0.0402 (10) | 0.0379 (10) | 0.0345 (9) | −0.0141 (8) | −0.0168 (7) | 0.0004 (7) |
| C6A | 0.0282 (8) | 0.0253 (8) | 0.0313 (8) | −0.0096 (7) | −0.0124 (6) | −0.0047 (6) |
| C7A | 0.0253 (8) | 0.0209 (7) | 0.0352 (8) | −0.0083 (6) | −0.0105 (6) | −0.0046 (6) |
| C8A | 0.0269 (8) | 0.0263 (8) | 0.0477 (10) | −0.0070 (7) | −0.0141 (7) | −0.0052 (7) |
| C9A | 0.0253 (8) | 0.0328 (9) | 0.0621 (12) | −0.0123 (7) | −0.0021 (8) | −0.0159 (8) |
| C10A | 0.0374 (10) | 0.0377 (10) | 0.0445 (10) | −0.0211 (8) | 0.0060 (8) | −0.0145 (8) |
| C11A | 0.0388 (10) | 0.0325 (9) | 0.0339 (8) | −0.0176 (8) | −0.0076 (7) | −0.0033 (7) |
| C12A | 0.0269 (8) | 0.0246 (8) | 0.0325 (8) | −0.0103 (6) | −0.0094 (6) | −0.0053 (6) |
| C13A | 0.0249 (8) | 0.0204 (7) | 0.0338 (8) | −0.0058 (6) | −0.0121 (6) | −0.0023 (6) |
| C14A | 0.0231 (7) | 0.0259 (8) | 0.0249 (7) | −0.0063 (6) | −0.0086 (6) | −0.0018 (6) |
| C15A | 0.0278 (8) | 0.0262 (8) | 0.0434 (9) | −0.0007 (7) | −0.0185 (7) | −0.0061 (7) |
| C16A | 0.0342 (9) | 0.0250 (8) | 0.0504 (10) | −0.0034 (7) | −0.0186 (8) | −0.0118 (7) |
| C17A | 0.0278 (8) | 0.0308 (9) | 0.0348 (8) | −0.0087 (7) | −0.0120 (7) | −0.0092 (7) |
| C18A | 0.0221 (7) | 0.0273 (8) | 0.0206 (7) | −0.0072 (6) | −0.0058 (6) | −0.0027 (6) |
| C19A | 0.0255 (8) | 0.0231 (7) | 0.0243 (7) | −0.0071 (6) | −0.0075 (6) | −0.0025 (6) |
| C20A | 0.0204 (7) | 0.0246 (8) | 0.0228 (7) | −0.0084 (6) | −0.0051 (6) | 0.0000 (6) |
| C21A | 0.0252 (8) | 0.0283 (8) | 0.0231 (7) | −0.0107 (6) | −0.0053 (6) | −0.0040 (6) |
| C22A | 0.0251 (8) | 0.0254 (8) | 0.0275 (8) | −0.0063 (6) | −0.0012 (6) | −0.0062 (6) |
| C23A | 0.0202 (7) | 0.0250 (8) | 0.0283 (7) | −0.0060 (6) | −0.0057 (6) | −0.0014 (6) |
| C24A | 0.0200 (7) | 0.0237 (7) | 0.0221 (7) | −0.0070 (6) | −0.0033 (5) | −0.0002 (6) |
| C25A | 0.0209 (7) | 0.0230 (7) | 0.0248 (7) | −0.0063 (6) | −0.0037 (6) | −0.0049 (6) |
| C26A | 0.0213 (7) | 0.0223 (7) | 0.0235 (7) | −0.0069 (6) | −0.0055 (6) | 0.0007 (6) |
| C27A | 0.0199 (7) | 0.0291 (8) | 0.0277 (8) | −0.0042 (6) | −0.0059 (6) | −0.0024 (6) |
| C28A | 0.0254 (8) | 0.0342 (9) | 0.0280 (8) | −0.0035 (7) | −0.0036 (6) | −0.0106 (6) |
| C29A | 0.0247 (8) | 0.0334 (9) | 0.0272 (8) | −0.0062 (7) | −0.0081 (6) | −0.0077 (6) |
| C30A | 0.0208 (7) | 0.0246 (8) | 0.0257 (7) | −0.0077 (6) | −0.0058 (6) | −0.0017 (6) |
| C31A | 0.0204 (7) | 0.0235 (7) | 0.0251 (7) | −0.0045 (6) | −0.0046 (6) | −0.0036 (6) |
| C32A | 0.0203 (7) | 0.0250 (8) | 0.0289 (8) | −0.0048 (6) | −0.0059 (6) | −0.0068 (6) |
| C33A | 0.0165 (7) | 0.0232 (8) | 0.0397 (9) | −0.0023 (6) | −0.0015 (6) | −0.0092 (6) |
| C34A | 0.0249 (8) | 0.0270 (9) | 0.0502 (10) | −0.0030 (7) | 0.0047 (7) | −0.0061 (7) |
| C35A | 0.0304 (10) | 0.0282 (9) | 0.0819 (15) | −0.0098 (8) | 0.0161 (10) | −0.0128 (9) |
| C36A | 0.0318 (10) | 0.0431 (11) | 0.1030 (18) | −0.0191 (9) | 0.0077 (11) | −0.0333 (12) |
| C37A | 0.0290 (9) | 0.0500 (12) | 0.0758 (14) | −0.0127 (9) | −0.0101 (9) | −0.0295 (10) |
| C38A | 0.0195 (8) | 0.0298 (8) | 0.0478 (10) | −0.0044 (7) | −0.0079 (7) | −0.0151 (7) |
| C39A | 0.0239 (8) | 0.0311 (8) | 0.0364 (8) | −0.0012 (7) | −0.0123 (7) | −0.0114 (7) |
| C40A | 0.0402 (10) | 0.0451 (11) | 0.0404 (10) | −0.0017 (9) | −0.0201 (8) | −0.0140 (8) |
| C41A | 0.0581 (12) | 0.0435 (11) | 0.0295 (9) | 0.0004 (10) | −0.0147 (8) | −0.0036 (8) |
| C42A | 0.0510 (11) | 0.0330 (10) | 0.0365 (9) | −0.0087 (9) | −0.0020 (8) | −0.0008 (7) |
| C43A | 0.0337 (9) | 0.0296 (9) | 0.0355 (9) | −0.0099 (7) | −0.0052 (7) | −0.0073 (7) |
| C44A | 0.0215 (7) | 0.0246 (8) | 0.0311 (8) | −0.0031 (6) | −0.0082 (6) | −0.0059 (6) |
Geometric parameters (Å, º)
| O1—C13 | 1.4276 (17) | O1A—C13A | 1.4276 (18) |
| O1—H1 | 0.855 (9) | O1A—H1A | 0.852 (9) |
| O2—C32 | 1.4458 (17) | O2A—C32A | 1.4459 (17) |
| O2—H2 | 0.837 (9) | O2A—H2A | 0.849 (9) |
| C1—C2 | 1.378 (2) | C1A—C2A | 1.379 (2) |
| C1—C6 | 1.402 (2) | C1A—C6A | 1.396 (2) |
| C1—C13 | 1.520 (2) | C1A—C13A | 1.529 (2) |
| C2—C3 | 1.392 (2) | C2A—C3A | 1.387 (2) |
| C2—H2AA | 0.9500 | C2A—H2AB | 0.9500 |
| C3—C4 | 1.380 (3) | C3A—C4A | 1.385 (3) |
| C3—H3 | 0.9500 | C3A—H3A | 0.9500 |
| C4—C5 | 1.383 (3) | C4A—C5A | 1.383 (2) |
| C4—H4 | 0.9500 | C4A—H4A | 0.9500 |
| C5—C6 | 1.391 (2) | C5A—C6A | 1.385 (2) |
| C5—H5 | 0.9500 | C5A—H5A | 0.9500 |
| C6—C7 | 1.467 (2) | C6A—C7A | 1.469 (2) |
| C7—C8 | 1.392 (2) | C7A—C8A | 1.389 (2) |
| C7—C12 | 1.399 (2) | C7A—C12A | 1.400 (2) |
| C8—C9 | 1.381 (3) | C8A—C9A | 1.384 (2) |
| C8—H8 | 0.9500 | C8A—H8A | 0.9500 |
| C9—C10 | 1.380 (3) | C9A—C10A | 1.382 (2) |
| C9—H9 | 0.9500 | C9A—H9A | 0.9500 |
| C10—C11 | 1.386 (2) | C10A—C11A | 1.389 (2) |
| C10—H10 | 0.9500 | C10A—H10A | 0.9500 |
| C11—C12 | 1.380 (2) | C11A—C12A | 1.378 (2) |
| C11—H11 | 0.9500 | C11A—H11A | 0.9500 |
| C12—C13 | 1.531 (2) | C12A—C13A | 1.522 (2) |
| C13—C14 | 1.522 (2) | C13A—C14A | 1.523 (2) |
| C14—C19 | 1.3910 (19) | C14A—C19A | 1.384 (2) |
| C14—C15 | 1.394 (2) | C14A—C15A | 1.388 (2) |
| C15—C16 | 1.384 (2) | C15A—C16A | 1.384 (2) |
| C15—H15 | 0.9500 | C15A—H15A | 0.9500 |
| C16—C17 | 1.390 (2) | C16A—C17A | 1.383 (2) |
| C16—H16 | 0.9500 | C16A—H16A | 0.9500 |
| C17—C18 | 1.396 (2) | C17A—C18A | 1.391 (2) |
| C17—H17 | 0.9500 | C17A—H17A | 0.9500 |
| C18—C19 | 1.390 (2) | C18A—C19A | 1.395 (2) |
| C18—C20 | 1.4900 (18) | C18A—C20A | 1.4894 (19) |
| C19—H19 | 0.9500 | C19A—H19A | 0.9500 |
| C20—C25 | 1.391 (2) | C20A—C25A | 1.390 (2) |
| C20—C21 | 1.395 (2) | C20A—C21A | 1.394 (2) |
| C21—C22 | 1.3864 (19) | C21A—C22A | 1.381 (2) |
| C21—H21 | 0.9500 | C21A—H21A | 0.9500 |
| C22—C23 | 1.386 (2) | C22A—C23A | 1.388 (2) |
| C22—H22 | 0.9500 | C22A—H22A | 0.9500 |
| C23—C24 | 1.397 (2) | C23A—C24A | 1.395 (2) |
| C23—H23 | 0.9500 | C23A—H23A | 0.9500 |
| C24—C25 | 1.3984 (19) | C24A—C25A | 1.4002 (19) |
| C24—C26 | 1.489 (2) | C24A—C26A | 1.491 (2) |
| C25—H25 | 0.9500 | C25A—H25A | 0.9500 |
| C26—C27 | 1.394 (2) | C26A—C27A | 1.394 (2) |
| C26—C31 | 1.4056 (19) | C26A—C31A | 1.4027 (19) |
| C27—C28 | 1.382 (2) | C27A—C28A | 1.388 (2) |
| C27—H27 | 0.9500 | C27A—H27A | 0.9500 |
| C28—C29 | 1.384 (2) | C28A—C29A | 1.381 (2) |
| C28—H28 | 0.9500 | C28A—H28A | 0.9500 |
| C29—C30 | 1.390 (2) | C29A—C30A | 1.392 (2) |
| C29—H29 | 0.9500 | C29A—H29A | 0.9500 |
| C30—C31 | 1.3861 (19) | C30A—C31A | 1.387 (2) |
| C30—C32 | 1.5214 (19) | C30A—C32A | 1.5248 (19) |
| C31—H31 | 0.9500 | C31A—H31A | 0.9500 |
| C32—C33 | 1.527 (2) | C32A—C33A | 1.521 (2) |
| C32—C44 | 1.5304 (19) | C32A—C44A | 1.526 (2) |
| C33—C34 | 1.382 (2) | C33A—C34A | 1.381 (2) |
| C33—C38 | 1.401 (2) | C33A—C38A | 1.397 (2) |
| C34—C35 | 1.391 (2) | C34A—C35A | 1.392 (3) |
| C34—H34 | 0.9500 | C34A—H34A | 0.9500 |
| C35—C36 | 1.384 (3) | C35A—C36A | 1.376 (3) |
| C35—H35 | 0.9500 | C35A—H35A | 0.9500 |
| C36—C37 | 1.384 (2) | C36A—C37A | 1.387 (3) |
| C36—H36 | 0.9500 | C36A—H36A | 0.9500 |
| C37—C38 | 1.388 (2) | C37A—C38A | 1.389 (2) |
| C37—H37 | 0.9500 | C37A—H37A | 0.9500 |
| C38—C39 | 1.472 (2) | C38A—C39A | 1.472 (2) |
| C39—C40 | 1.391 (2) | C39A—C40A | 1.385 (2) |
| C39—C44 | 1.398 (2) | C39A—C44A | 1.399 (2) |
| C40—C41 | 1.385 (2) | C40A—C41A | 1.379 (3) |
| C40—H40 | 0.9500 | C40A—H40A | 0.9500 |
| C41—C42 | 1.385 (2) | C41A—C42A | 1.385 (3) |
| C41—H41 | 0.9500 | C41A—H41A | 0.9500 |
| C42—C43 | 1.394 (2) | C42A—C43A | 1.393 (2) |
| C42—H42 | 0.9500 | C42A—H42A | 0.9500 |
| C43—C44 | 1.375 (2) | C43A—C44A | 1.376 (2) |
| C43—H43 | 0.9500 | C43A—H43A | 0.9500 |
| C13—O1—H1 | 110.5 (14) | C13A—O1A—H1A | 110.5 (13) |
| C32—O2—H2 | 108.7 (14) | C32A—O2A—H2A | 109.0 (15) |
| C2—C1—C6 | 121.17 (15) | C2A—C1A—C6A | 121.42 (14) |
| C2—C1—C13 | 128.26 (14) | C2A—C1A—C13A | 127.86 (14) |
| C6—C1—C13 | 110.55 (13) | C6A—C1A—C13A | 110.61 (13) |
| C1—C2—C3 | 118.30 (17) | C1A—C2A—C3A | 118.30 (15) |
| C1—C2—H2AA | 120.8 | C1A—C2A—H2AB | 120.9 |
| C3—C2—H2AA | 120.8 | C3A—C2A—H2AB | 120.9 |
| C4—C3—C2 | 120.78 (18) | C4A—C3A—C2A | 120.38 (16) |
| C4—C3—H3 | 119.6 | C4A—C3A—H3A | 119.8 |
| C2—C3—H3 | 119.6 | C2A—C3A—H3A | 119.8 |
| C3—C4—C5 | 121.25 (17) | C5A—C4A—C3A | 121.42 (16) |
| C3—C4—H4 | 119.4 | C5A—C4A—H4A | 119.3 |
| C5—C4—H4 | 119.4 | C3A—C4A—H4A | 119.3 |
| C4—C5—C6 | 118.55 (17) | C4A—C5A—C6A | 118.44 (16) |
| C4—C5—H5 | 120.7 | C4A—C5A—H5A | 120.8 |
| C6—C5—H5 | 120.7 | C6A—C5A—H5A | 120.8 |
| C5—C6—C1 | 119.95 (16) | C5A—C6A—C1A | 120.03 (15) |
| C5—C6—C7 | 131.22 (15) | C5A—C6A—C7A | 131.35 (14) |
| C1—C6—C7 | 108.82 (13) | C1A—C6A—C7A | 108.61 (13) |
| C8—C7—C12 | 119.53 (16) | C8A—C7A—C12A | 120.10 (14) |
| C8—C7—C6 | 131.96 (15) | C8A—C7A—C6A | 131.28 (14) |
| C12—C7—C6 | 108.52 (13) | C12A—C7A—C6A | 108.57 (13) |
| C9—C8—C7 | 118.76 (16) | C9A—C8A—C7A | 118.76 (15) |
| C9—C8—H8 | 120.6 | C9A—C8A—H8A | 120.6 |
| C7—C8—H8 | 120.6 | C7A—C8A—H8A | 120.6 |
| C10—C9—C8 | 121.35 (16) | C10A—C9A—C8A | 120.90 (16) |
| C10—C9—H9 | 119.3 | C10A—C9A—H9A | 119.6 |
| C8—C9—H9 | 119.3 | C8A—C9A—H9A | 119.6 |
| C9—C10—C11 | 120.57 (17) | C9A—C10A—C11A | 120.72 (16) |
| C9—C10—H10 | 119.7 | C9A—C10A—H10A | 119.6 |
| C11—C10—H10 | 119.7 | C11A—C10A—H10A | 119.6 |
| C12—C11—C10 | 118.45 (16) | C12A—C11A—C10A | 118.74 (15) |
| C12—C11—H11 | 120.8 | C12A—C11A—H11A | 120.6 |
| C10—C11—H11 | 120.8 | C10A—C11A—H11A | 120.6 |
| C11—C12—C7 | 121.34 (14) | C11A—C12A—C7A | 120.77 (15) |
| C11—C12—C13 | 128.05 (13) | C11A—C12A—C13A | 128.44 (14) |
| C7—C12—C13 | 110.56 (13) | C7A—C12A—C13A | 110.69 (13) |
| O1—C13—C1 | 112.25 (12) | O1A—C13A—C12A | 112.08 (12) |
| O1—C13—C14 | 107.04 (11) | O1A—C13A—C14A | 107.06 (11) |
| C1—C13—C14 | 114.50 (12) | C12A—C13A—C14A | 115.11 (12) |
| O1—C13—C12 | 113.36 (12) | O1A—C13A—C1A | 113.48 (12) |
| C1—C13—C12 | 101.52 (11) | C12A—C13A—C1A | 101.39 (11) |
| C14—C13—C12 | 108.22 (12) | C14A—C13A—C1A | 107.77 (12) |
| C19—C14—C15 | 118.63 (13) | C19A—C14A—C15A | 119.01 (14) |
| C19—C14—C13 | 119.65 (12) | C19A—C14A—C13A | 120.41 (13) |
| C15—C14—C13 | 121.47 (12) | C15A—C14A—C13A | 120.29 (13) |
| C16—C15—C14 | 120.32 (13) | C16A—C15A—C14A | 120.22 (14) |
| C16—C15—H15 | 119.8 | C16A—C15A—H15A | 119.9 |
| C14—C15—H15 | 119.8 | C14A—C15A—H15A | 119.9 |
| C15—C16—C17 | 120.51 (14) | C17A—C16A—C15A | 120.35 (15) |
| C15—C16—H16 | 119.7 | C17A—C16A—H16A | 119.8 |
| C17—C16—H16 | 119.7 | C15A—C16A—H16A | 119.8 |
| C16—C17—C18 | 120.05 (14) | C16A—C17A—C18A | 120.43 (14) |
| C16—C17—H17 | 120.0 | C16A—C17A—H17A | 119.8 |
| C18—C17—H17 | 120.0 | C18A—C17A—H17A | 119.8 |
| C19—C18—C17 | 118.71 (13) | C17A—C18A—C19A | 118.47 (13) |
| C19—C18—C20 | 119.99 (13) | C17A—C18A—C20A | 121.33 (13) |
| C17—C18—C20 | 121.29 (13) | C19A—C18A—C20A | 120.20 (13) |
| C18—C19—C14 | 121.76 (13) | C14A—C19A—C18A | 121.51 (14) |
| C18—C19—H19 | 119.1 | C14A—C19A—H19A | 119.2 |
| C14—C19—H19 | 119.1 | C18A—C19A—H19A | 119.2 |
| C25—C20—C21 | 118.43 (12) | C25A—C20A—C21A | 118.57 (13) |
| C25—C20—C18 | 120.20 (12) | C25A—C20A—C18A | 120.79 (13) |
| C21—C20—C18 | 121.36 (12) | C21A—C20A—C18A | 120.64 (13) |
| C22—C21—C20 | 120.10 (13) | C22A—C21A—C20A | 120.16 (14) |
| C22—C21—H21 | 120.0 | C22A—C21A—H21A | 119.9 |
| C20—C21—H21 | 120.0 | C20A—C21A—H21A | 119.9 |
| C23—C22—C21 | 120.77 (14) | C21A—C22A—C23A | 120.65 (14) |
| C23—C22—H22 | 119.6 | C21A—C22A—H22A | 119.7 |
| C21—C22—H22 | 119.6 | C23A—C22A—H22A | 119.7 |
| C22—C23—C24 | 120.54 (13) | C22A—C23A—C24A | 120.75 (13) |
| C22—C23—H23 | 119.7 | C22A—C23A—H23A | 119.6 |
| C24—C23—H23 | 119.7 | C24A—C23A—H23A | 119.6 |
| C23—C24—C25 | 117.72 (13) | C23A—C24A—C25A | 117.56 (13) |
| C23—C24—C26 | 121.86 (12) | C23A—C24A—C26A | 121.23 (12) |
| C25—C24—C26 | 120.38 (13) | C25A—C24A—C26A | 121.21 (13) |
| C20—C25—C24 | 122.42 (13) | C20A—C25A—C24A | 122.31 (13) |
| C20—C25—H25 | 118.8 | C20A—C25A—H25A | 118.8 |
| C24—C25—H25 | 118.8 | C24A—C25A—H25A | 118.8 |
| C27—C26—C31 | 117.89 (13) | C27A—C26A—C31A | 117.68 (13) |
| C27—C26—C24 | 121.07 (12) | C27A—C26A—C24A | 121.73 (13) |
| C31—C26—C24 | 121.02 (12) | C31A—C26A—C24A | 120.58 (13) |
| C28—C27—C26 | 120.87 (13) | C28A—C27A—C26A | 120.92 (13) |
| C28—C27—H27 | 119.6 | C28A—C27A—H27A | 119.5 |
| C26—C27—H27 | 119.6 | C26A—C27A—H27A | 119.5 |
| C27—C28—C29 | 120.51 (13) | C29A—C28A—C27A | 120.46 (14) |
| C27—C28—H28 | 119.7 | C29A—C28A—H28A | 119.8 |
| C29—C28—H28 | 119.7 | C27A—C28A—H28A | 119.8 |
| C28—C29—C30 | 119.92 (13) | C28A—C29A—C30A | 119.84 (14) |
| C28—C29—H29 | 120.0 | C28A—C29A—H29A | 120.1 |
| C30—C29—H29 | 120.0 | C30A—C29A—H29A | 120.1 |
| C31—C30—C29 | 119.37 (13) | C31A—C30A—C29A | 119.39 (13) |
| C31—C30—C32 | 121.83 (12) | C31A—C30A—C32A | 121.58 (13) |
| C29—C30—C32 | 118.79 (12) | C29A—C30A—C32A | 119.03 (13) |
| C30—C31—C26 | 121.34 (13) | C30A—C31A—C26A | 121.59 (13) |
| C30—C31—H31 | 119.3 | C30A—C31A—H31A | 119.2 |
| C26—C31—H31 | 119.3 | C26A—C31A—H31A | 119.2 |
| O2—C32—C30 | 107.55 (11) | O2A—C32A—C33A | 111.49 (12) |
| O2—C32—C33 | 112.49 (11) | O2A—C32A—C30A | 107.26 (11) |
| C30—C32—C33 | 111.75 (11) | C33A—C32A—C30A | 113.03 (12) |
| O2—C32—C44 | 111.42 (11) | O2A—C32A—C44A | 110.87 (11) |
| C30—C32—C44 | 112.09 (11) | C33A—C32A—C44A | 101.93 (12) |
| C33—C32—C44 | 101.58 (11) | C30A—C32A—C44A | 112.30 (12) |
| C34—C33—C38 | 120.92 (14) | C34A—C33A—C38A | 120.90 (16) |
| C34—C33—C32 | 128.55 (14) | C34A—C33A—C32A | 128.75 (15) |
| C38—C33—C32 | 110.50 (12) | C38A—C33A—C32A | 110.35 (13) |
| C33—C34—C35 | 118.52 (15) | C33A—C34A—C35A | 118.49 (18) |
| C33—C34—H34 | 120.7 | C33A—C34A—H34A | 120.8 |
| C35—C34—H34 | 120.7 | C35A—C34A—H34A | 120.8 |
| C36—C35—C34 | 120.49 (16) | C36A—C35A—C34A | 120.77 (18) |
| C36—C35—H35 | 119.8 | C36A—C35A—H35A | 119.6 |
| C34—C35—H35 | 119.8 | C34A—C35A—H35A | 119.6 |
| C35—C36—C37 | 121.35 (16) | C35A—C36A—C37A | 121.13 (18) |
| C35—C36—H36 | 119.3 | C35A—C36A—H36A | 119.4 |
| C37—C36—H36 | 119.3 | C37A—C36A—H36A | 119.4 |
| C36—C37—C38 | 118.46 (16) | C36A—C37A—C38A | 118.52 (19) |
| C36—C37—H37 | 120.8 | C36A—C37A—H37A | 120.7 |
| C38—C37—H37 | 120.8 | C38A—C37A—H37A | 120.7 |
| C37—C38—C33 | 120.26 (15) | C37A—C38A—C33A | 120.18 (17) |
| C37—C38—C39 | 131.01 (14) | C37A—C38A—C39A | 130.96 (16) |
| C33—C38—C39 | 108.69 (13) | C33A—C38A—C39A | 108.86 (14) |
| C40—C39—C44 | 120.15 (15) | C40A—C39A—C44A | 120.02 (16) |
| C40—C39—C38 | 131.16 (15) | C40A—C39A—C38A | 131.49 (16) |
| C44—C39—C38 | 108.67 (12) | C44A—C39A—C38A | 108.48 (13) |
| C41—C40—C39 | 118.68 (15) | C41A—C40A—C39A | 118.79 (17) |
| C41—C40—H40 | 120.7 | C41A—C40A—H40A | 120.6 |
| C39—C40—H40 | 120.7 | C39A—C40A—H40A | 120.6 |
| C40—C41—C42 | 120.83 (15) | C40A—C41A—C42A | 121.30 (16) |
| C40—C41—H41 | 119.6 | C40A—C41A—H41A | 119.3 |
| C42—C41—H41 | 119.6 | C42A—C41A—H41A | 119.3 |
| C41—C42—C43 | 120.74 (16) | C41A—C42A—C43A | 120.18 (17) |
| C41—C42—H42 | 119.6 | C41A—C42A—H42A | 119.9 |
| C43—C42—H42 | 119.6 | C43A—C42A—H42A | 119.9 |
| C44—C43—C42 | 118.45 (15) | C44A—C43A—C42A | 118.63 (16) |
| C44—C43—H43 | 120.8 | C44A—C43A—H43A | 120.7 |
| C42—C43—H43 | 120.8 | C42A—C43A—H43A | 120.7 |
| C43—C44—C39 | 121.13 (14) | C43A—C44A—C39A | 121.08 (14) |
| C43—C44—C32 | 128.29 (13) | C43A—C44A—C32A | 128.56 (14) |
| C39—C44—C32 | 110.54 (13) | C39A—C44A—C32A | 110.36 (13) |
| C6—C1—C2—C3 | −0.8 (2) | C6A—C1A—C2A—C3A | −0.1 (2) |
| C13—C1—C2—C3 | −178.89 (16) | C13A—C1A—C2A—C3A | 175.92 (15) |
| C1—C2—C3—C4 | 0.4 (3) | C1A—C2A—C3A—C4A | −1.0 (3) |
| C2—C3—C4—C5 | 0.2 (3) | C2A—C3A—C4A—C5A | 1.5 (3) |
| C3—C4—C5—C6 | −0.4 (3) | C3A—C4A—C5A—C6A | −0.8 (3) |
| C4—C5—C6—C1 | 0.1 (2) | C4A—C5A—C6A—C1A | −0.3 (2) |
| C4—C5—C6—C7 | 178.64 (16) | C4A—C5A—C6A—C7A | −179.59 (16) |
| C2—C1—C6—C5 | 0.6 (2) | C2A—C1A—C6A—C5A | 0.7 (2) |
| C13—C1—C6—C5 | 178.96 (14) | C13A—C1A—C6A—C5A | −175.89 (14) |
| C2—C1—C6—C7 | −178.32 (15) | C2A—C1A—C6A—C7A | −179.80 (14) |
| C13—C1—C6—C7 | 0.08 (17) | C13A—C1A—C6A—C7A | 3.56 (17) |
| C5—C6—C7—C8 | 2.8 (3) | C5A—C6A—C7A—C8A | −5.3 (3) |
| C1—C6—C7—C8 | −178.51 (17) | C1A—C6A—C7A—C8A | 175.30 (16) |
| C5—C6—C7—C12 | −177.63 (16) | C5A—C6A—C7A—C12A | 177.18 (16) |
| C1—C6—C7—C12 | 1.08 (17) | C1A—C6A—C7A—C12A | −2.18 (17) |
| C12—C7—C8—C9 | 0.0 (2) | C12A—C7A—C8A—C9A | 0.6 (2) |
| C6—C7—C8—C9 | 179.56 (17) | C6A—C7A—C8A—C9A | −176.65 (15) |
| C7—C8—C9—C10 | 0.1 (3) | C7A—C8A—C9A—C10A | −0.1 (2) |
| C8—C9—C10—C11 | −0.4 (3) | C8A—C9A—C10A—C11A | 0.2 (3) |
| C9—C10—C11—C12 | 0.7 (3) | C9A—C10A—C11A—C12A | −0.7 (2) |
| C10—C11—C12—C7 | −0.6 (2) | C10A—C11A—C12A—C7A | 1.2 (2) |
| C10—C11—C12—C13 | −177.73 (15) | C10A—C11A—C12A—C13A | 177.29 (15) |
| C8—C7—C12—C11 | 0.3 (2) | C8A—C7A—C12A—C11A | −1.1 (2) |
| C6—C7—C12—C11 | −179.35 (14) | C6A—C7A—C12A—C11A | 176.70 (14) |
| C8—C7—C12—C13 | 177.85 (14) | C8A—C7A—C12A—C13A | −177.87 (13) |
| C6—C7—C12—C13 | −1.80 (17) | C6A—C7A—C12A—C13A | −0.06 (17) |
| C2—C1—C13—O1 | 55.8 (2) | C11A—C12A—C13A—O1A | −53.1 (2) |
| C6—C1—C13—O1 | −122.45 (13) | C7A—C12A—C13A—O1A | 123.37 (14) |
| C2—C1—C13—C14 | −66.5 (2) | C11A—C12A—C13A—C14A | 69.6 (2) |
| C6—C1—C13—C14 | 115.25 (14) | C7A—C12A—C13A—C14A | −113.97 (14) |
| C2—C1—C13—C12 | 177.18 (16) | C11A—C12A—C13A—C1A | −174.42 (15) |
| C6—C1—C13—C12 | −1.08 (16) | C7A—C12A—C13A—C1A | 2.02 (16) |
| C11—C12—C13—O1 | −60.3 (2) | C2A—C1A—C13A—O1A | 59.9 (2) |
| C7—C12—C13—O1 | 122.35 (13) | C6A—C1A—C13A—O1A | −123.76 (14) |
| C11—C12—C13—C1 | 179.10 (15) | C2A—C1A—C13A—C12A | −179.77 (15) |
| C7—C12—C13—C1 | 1.75 (16) | C6A—C1A—C13A—C12A | −3.40 (16) |
| C11—C12—C13—C14 | 58.26 (19) | C2A—C1A—C13A—C14A | −58.50 (19) |
| C7—C12—C13—C14 | −119.08 (13) | C6A—C1A—C13A—C14A | 117.87 (13) |
| O1—C13—C14—C19 | 28.10 (17) | O1A—C13A—C14A—C19A | −18.55 (18) |
| C1—C13—C14—C19 | 153.19 (13) | C12A—C13A—C14A—C19A | −143.86 (14) |
| C12—C13—C14—C19 | −94.41 (15) | C1A—C13A—C14A—C19A | 103.86 (15) |
| O1—C13—C14—C15 | −157.72 (13) | O1A—C13A—C14A—C15A | 167.68 (14) |
| C1—C13—C14—C15 | −32.64 (19) | C12A—C13A—C14A—C15A | 42.36 (19) |
| C12—C13—C14—C15 | 79.77 (16) | C1A—C13A—C14A—C15A | −69.92 (17) |
| C19—C14—C15—C16 | 0.8 (2) | C19A—C14A—C15A—C16A | 0.5 (2) |
| C13—C14—C15—C16 | −173.41 (13) | C13A—C14A—C15A—C16A | 174.32 (15) |
| C14—C15—C16—C17 | −0.5 (2) | C14A—C15A—C16A—C17A | −0.2 (3) |
| C15—C16—C17—C18 | −0.9 (2) | C15A—C16A—C17A—C18A | 0.0 (3) |
| C16—C17—C18—C19 | 1.8 (2) | C16A—C17A—C18A—C19A | −0.1 (2) |
| C16—C17—C18—C20 | −177.17 (12) | C16A—C17A—C18A—C20A | −179.40 (14) |
| C17—C18—C19—C14 | −1.5 (2) | C15A—C14A—C19A—C18A | −0.5 (2) |
| C20—C18—C19—C14 | 177.53 (12) | C13A—C14A—C19A—C18A | −174.35 (13) |
| C15—C14—C19—C18 | 0.1 (2) | C17A—C18A—C19A—C14A | 0.3 (2) |
| C13—C14—C19—C18 | 174.49 (12) | C20A—C18A—C19A—C14A | 179.63 (13) |
| C19—C18—C20—C25 | −142.93 (14) | C17A—C18A—C20A—C25A | −34.0 (2) |
| C17—C18—C20—C25 | 36.04 (19) | C19A—C18A—C20A—C25A | 146.67 (14) |
| C19—C18—C20—C21 | 36.10 (19) | C17A—C18A—C20A—C21A | 145.90 (14) |
| C17—C18—C20—C21 | −144.94 (14) | C19A—C18A—C20A—C21A | −33.4 (2) |
| C25—C20—C21—C22 | −1.1 (2) | C25A—C20A—C21A—C22A | 0.2 (2) |
| C18—C20—C21—C22 | 179.90 (13) | C18A—C20A—C21A—C22A | −179.69 (13) |
| C20—C21—C22—C23 | 0.7 (2) | C20A—C21A—C22A—C23A | 0.2 (2) |
| C21—C22—C23—C24 | 0.5 (2) | C21A—C22A—C23A—C24A | −0.6 (2) |
| C22—C23—C24—C25 | −1.2 (2) | C22A—C23A—C24A—C25A | 0.5 (2) |
| C22—C23—C24—C26 | 176.48 (13) | C22A—C23A—C24A—C26A | −179.85 (13) |
| C21—C20—C25—C24 | 0.3 (2) | C21A—C20A—C25A—C24A | −0.3 (2) |
| C18—C20—C25—C24 | 179.33 (13) | C18A—C20A—C25A—C24A | 179.59 (13) |
| C23—C24—C25—C20 | 0.9 (2) | C23A—C24A—C25A—C20A | 0.0 (2) |
| C26—C24—C25—C20 | −176.89 (13) | C26A—C24A—C25A—C20A | −179.71 (13) |
| C23—C24—C26—C27 | −155.25 (14) | C23A—C24A—C26A—C27A | 161.96 (14) |
| C25—C24—C26—C27 | 22.4 (2) | C25A—C24A—C26A—C27A | −18.4 (2) |
| C23—C24—C26—C31 | 23.0 (2) | C23A—C24A—C26A—C31A | −17.1 (2) |
| C25—C24—C26—C31 | −159.38 (13) | C25A—C24A—C26A—C31A | 162.58 (13) |
| C31—C26—C27—C28 | 1.1 (2) | C31A—C26A—C27A—C28A | −0.4 (2) |
| C24—C26—C27—C28 | 179.40 (13) | C24A—C26A—C27A—C28A | −179.53 (14) |
| C26—C27—C28—C29 | −2.1 (2) | C26A—C27A—C28A—C29A | 2.6 (2) |
| C27—C28—C29—C30 | 0.3 (2) | C27A—C28A—C29A—C30A | −1.9 (2) |
| C28—C29—C30—C31 | 2.4 (2) | C28A—C29A—C30A—C31A | −1.0 (2) |
| C28—C29—C30—C32 | −178.89 (13) | C28A—C29A—C30A—C32A | 178.74 (14) |
| C29—C30—C31—C26 | −3.4 (2) | C29A—C30A—C31A—C26A | 3.2 (2) |
| C32—C30—C31—C26 | 177.94 (13) | C32A—C30A—C31A—C26A | −176.55 (13) |
| C27—C26—C31—C30 | 1.6 (2) | C27A—C26A—C31A—C30A | −2.4 (2) |
| C24—C26—C31—C30 | −176.63 (13) | C24A—C26A—C31A—C30A | 176.67 (13) |
| C31—C30—C32—O2 | −15.88 (18) | C31A—C30A—C32A—O2A | 6.98 (18) |
| C29—C30—C32—O2 | 165.48 (12) | C29A—C30A—C32A—O2A | −172.73 (13) |
| C31—C30—C32—C33 | 108.04 (15) | C31A—C30A—C32A—C33A | −116.31 (15) |
| C29—C30—C32—C33 | −70.61 (16) | C29A—C30A—C32A—C33A | 63.98 (17) |
| C31—C30—C32—C44 | −138.68 (14) | C31A—C30A—C32A—C44A | 129.03 (14) |
| C29—C30—C32—C44 | 42.67 (18) | C29A—C30A—C32A—C44A | −50.67 (18) |
| O2—C32—C33—C34 | 63.47 (18) | O2A—C32A—C33A—C34A | −62.11 (19) |
| C30—C32—C33—C34 | −57.62 (19) | C30A—C32A—C33A—C34A | 58.8 (2) |
| C44—C32—C33—C34 | −177.30 (14) | C44A—C32A—C33A—C34A | 179.56 (14) |
| O2—C32—C33—C38 | −118.49 (13) | O2A—C32A—C33A—C38A | 116.84 (13) |
| C30—C32—C33—C38 | 120.42 (13) | C30A—C32A—C33A—C38A | −122.23 (13) |
| C44—C32—C33—C38 | 0.74 (15) | C44A—C32A—C33A—C38A | −1.49 (15) |
| C38—C33—C34—C35 | −0.1 (2) | C38A—C33A—C34A—C35A | 0.1 (2) |
| C32—C33—C34—C35 | 177.76 (14) | C32A—C33A—C34A—C35A | 178.95 (14) |
| C33—C34—C35—C36 | 0.5 (2) | C33A—C34A—C35A—C36A | 0.7 (2) |
| C34—C35—C36—C37 | −0.3 (3) | C34A—C35A—C36A—C37A | −1.1 (3) |
| C35—C36—C37—C38 | −0.2 (2) | C35A—C36A—C37A—C38A | 0.6 (3) |
| C36—C37—C38—C33 | 0.6 (2) | C36A—C37A—C38A—C33A | 0.2 (2) |
| C36—C37—C38—C39 | −177.10 (15) | C36A—C37A—C38A—C39A | 179.37 (16) |
| C34—C33—C38—C37 | −0.4 (2) | C34A—C33A—C38A—C37A | −0.5 (2) |
| C32—C33—C38—C37 | −178.64 (13) | C32A—C33A—C38A—C37A | −179.59 (14) |
| C34—C33—C38—C39 | 177.73 (13) | C34A—C33A—C38A—C39A | −179.89 (13) |
| C32—C33—C38—C39 | −0.48 (16) | C32A—C33A—C38A—C39A | 1.06 (17) |
| C37—C38—C39—C40 | −0.4 (3) | C37A—C38A—C39A—C40A | 0.2 (3) |
| C33—C38—C39—C40 | −178.32 (15) | C33A—C38A—C39A—C40A | 179.42 (16) |
| C37—C38—C39—C44 | 177.87 (16) | C37A—C38A—C39A—C44A | −179.38 (16) |
| C33—C38—C39—C44 | −0.02 (16) | C33A—C38A—C39A—C44A | −0.12 (17) |
| C44—C39—C40—C41 | −1.1 (2) | C44A—C39A—C40A—C41A | 0.5 (2) |
| C38—C39—C40—C41 | 177.07 (15) | C38A—C39A—C40A—C41A | −179.00 (16) |
| C39—C40—C41—C42 | 0.6 (2) | C39A—C40A—C41A—C42A | −0.5 (3) |
| C40—C41—C42—C43 | 0.1 (2) | C40A—C41A—C42A—C43A | 0.4 (3) |
| C41—C42—C43—C44 | −0.3 (2) | C41A—C42A—C43A—C44A | −0.3 (2) |
| C42—C43—C44—C39 | −0.2 (2) | C42A—C43A—C44A—C39A | 0.3 (2) |
| C42—C43—C44—C32 | −178.00 (14) | C42A—C43A—C44A—C32A | −179.63 (15) |
| C40—C39—C44—C43 | 0.9 (2) | C40A—C39A—C44A—C43A | −0.4 (2) |
| C38—C39—C44—C43 | −177.61 (13) | C38A—C39A—C44A—C43A | 179.16 (14) |
| C40—C39—C44—C32 | 179.03 (13) | C40A—C39A—C44A—C32A | 179.53 (14) |
| C38—C39—C44—C32 | 0.51 (16) | C38A—C39A—C44A—C32A | −0.87 (17) |
| O2—C32—C44—C43 | −62.81 (19) | O2A—C32A—C44A—C43A | 62.61 (19) |
| C30—C32—C44—C43 | 57.77 (19) | C33A—C32A—C44A—C43A | −178.62 (15) |
| C33—C32—C44—C43 | 177.20 (14) | C30A—C32A—C44A—C43A | −57.4 (2) |
| O2—C32—C44—C39 | 119.23 (13) | O2A—C32A—C44A—C39A | −117.36 (14) |
| C30—C32—C44—C39 | −120.18 (13) | C33A—C32A—C44A—C39A | 1.41 (15) |
| C33—C32—C44—C39 | −0.75 (15) | C30A—C32A—C44A—C39A | 122.66 (14) |
Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C20–C25 and C20A–C25A rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O2i | 0.86 (1) | 2.07 (1) | 2.894 (2) | 163 (1) |
| O2—H2···Cg1ii | 0.84 (1) | 3.42 (1) | 4.163 (2) | 150 (1) |
| O1A—H1A···O2Aiii | 0.85 (1) | 1.99 (1) | 2.807 (2) | 160 (1) |
| O2A—H2A···Cg2iv | 0.85 (2) | 3.46 (1) | 4.169 (2) | 145 (1) |
Symmetry codes: (i) −x+1, −y+2, −z+2; (ii) −x, −y+2, −z+2; (iii) −x+2, −y+1, −z+1; (iv) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2410).
References
- Atwood, J. L., Davies, J. E. D. & MacNicol, D. D. (1991). Editors. Inclusion Compounds, Vol. 4. Oxford Universty Press.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Desiraju, G. R. (1996). Comprehensive Supramolecular Chemistry, edited by D. D. MacNicol, F. Toda & R. Bishop, pp. 1–22. Oxford: Elsevier.
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond in Structural Chemistry and Biology, ch. 2. Oxford University Press.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- James, S. L. (2004). Encyclopedia of Supramolecular Chemistry, edited by J. L. Atwood & J. W. Steed, pp. 1093–1099. Boca Raton: CRC Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Skobridis, K., Paraskevopoulos, G., Theodorou, V., Seichter, W. & Weber, E. (2011). Cryst. Growth Des. 11, 5275–5288. [DOI] [PMC free article] [PubMed]
- Skobridis, K., Theodorou, V., Seichter, W. & Weber, E. (2011). Cryst. Growth Des. 10, 862–869.
- Staab, H. A. & Binnig, A. (1967). Chem. Ber. 11, 293–305.
- Toda, F. (1996). Comprehensive Supramolecular Chemistry edited by D. D. MacNicol, F. Toda & R. Bishop, pp. 465–516. Oxford: Elsevier.
- Weber, E., Skobridis, K., Wierig, A., Stathi, S., Nassimbeni, L. R. & Niven, M. L. (1993). Angew. Chem. Int. Ed. 32, 606–608.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813024033/rk2410sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813024033/rk2410Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813024033/rk2410Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




