Abstract
The asymmetric unit of the title salt, C8H10N5 +·C7H7O3S−, consists of two amino[(1H-benzimidazol-2-yl)amino]methaniminium cations and two 4-methylbenzenesulfonate anions. The cations are each stabilized by intramolecular N—H⋯N hydrogen bonds between the free amino groups and the imine N atoms of the benzimidazole units, forming S(6) ring motifs. In the crystal, cations and anions are linked by N—H⋯O and C—H⋯O hydrogen bonds, forming a three-dimensional supramolecular framework. Two strong π–π stacking interactions [centroid–centroid distances = 3.4112 (14) and 3.4104 (14) Å] also occur between the centroids of the imidazole rings of like cations.
Related literature
For the synthesis of guanidine-containing compounds, see: Wu et al. (2002 ▶); Hopkins et al. (2002 ▶); Kilburn et al. (2002 ▶); Manimala & Anslyn (2002 ▶). For pharmaceutical and chemical applications of guanidines, see: Han et al. (2008 ▶); Hannon & Anslyn (1993 ▶); Ekelund et al. (2001 ▶); Kovacevic & Maksic (2001 ▶); Costa et al. (1998 ▶). For graph-set motifs, see: Bernstein et al. (1995 ▶) and for standard bond lengths, see: Allen et al. (1987 ▶).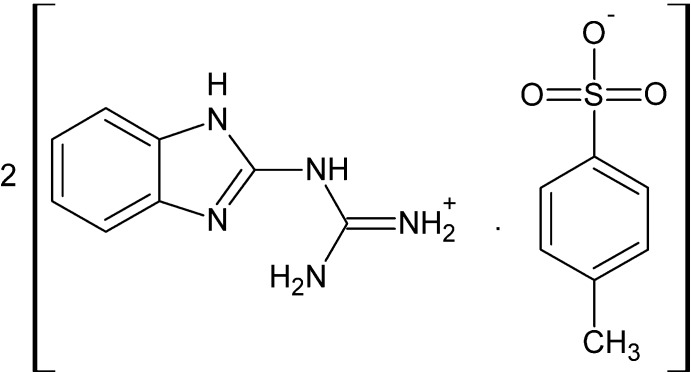
Experimental
Crystal data
C8H10N5 +·C7H7O3S−
M r = 347.41
Monoclinic,

a = 12.3821 (4) Å
b = 17.8077 (7) Å
c = 14.5112 (5) Å
β = 90.013 (2)°
V = 3199.7 (2) Å3
Z = 8
Mo Kα radiation
μ = 0.23 mm−1
T = 100 K
0.35 × 0.10 × 0.04 mm
Data collection
Bruker APEXII CCD diffractometer
20618 measured reflections
5679 independent reflections
4108 reflections with I > 2σ(I)
R int = 0.044
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.109
S = 1.05
5679 reflections
469 parameters
15 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.33 e Å−3
Δρmin = −0.38 e Å−3
Data collection: APEX2 (Bruker, 2013 ▶); cell refinement: SAINT (Bruker, 2013 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶); software used to prepare material for publication: WinGX (Farrugia, 2012 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813024975/sj5349sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813024975/sj5349Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813024975/sj5349Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—HN1⋯O1i | 0.86 (2) | 2.11 (2) | 2.948 (3) | 166 (2) |
| N3—HN3⋯O6ii | 0.86 (2) | 1.95 (2) | 2.805 (3) | 173 (2) |
| N6—HN6⋯O6iii | 0.88 (2) | 2.09 (2) | 2.944 (3) | 164 (2) |
| N8—HN8⋯O1iv | 0.87 (2) | 1.93 (2) | 2.799 (2) | 177 (2) |
| N4—H4A⋯N2 | 0.87 (2) | 1.97 (2) | 2.686 (3) | 139 (2) |
| N4—H4B⋯O3 | 0.87 (2) | 2.06 (2) | 2.909 (3) | 166 (2) |
| N5—H5A⋯O5ii | 0.89 (2) | 1.98 (2) | 2.871 (3) | 176 (3) |
| N5—H5B⋯O2 | 0.88 (2) | 1.99 (2) | 2.863 (3) | 169 (3) |
| N9—H9A⋯N7 | 0.86 (2) | 1.98 (2) | 2.683 (3) | 138 (2) |
| N9—H9B⋯O5v | 0.85 (2) | 2.06 (2) | 2.906 (3) | 174 (2) |
| N10—H10A⋯O4v | 0.88 (2) | 2.00 (2) | 2.863 (3) | 167 (3) |
| N10—H10B⋯O3iv | 0.89 (2) | 1.99 (2) | 2.872 (3) | 179 (3) |
| C7—H7C⋯O4vi | 0.98 | 2.53 | 3.283 (3) | 133 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  .
.
Acknowledgments
Manchester Metropolitan University, Erciyes University and Sohag University are gratefully acknowledged for supporting this study. The authors also thank José Romero Garzón, Centro de Instrumentación Científica, Universidad de Granada, for the data collection.
supplementary crystallographic information
1. Comment
Guanidines are structurally novel molecules reported to exhibit remarkable biological and pharmacological activities, which are affected by the guanidine functionality (Han et al., 2008; Hannon & Anslyn, 1993). Guanidino-containing drugs such as metaiodobenzylguanidine, MIBG, and methylglyoxalbisguanylhydrazone, MGBG, were shown several decades ago to have antitumor properties and have been subjected to intense preclinical and clinical evaluation (Ekelund et al., 2001). Guanidines are also known as useful basic catalysts (Kovacevic & Maksic, 2001; Costa et al., 1998). The synthesis of guanidine derivatives has also attracted continued research interests in recent years, resulting in many new efficient synthetic methods and guanidinylation reagents for different classes of guanidine compounds (Wu et al., 2002; Hopkins et al., 2002; Kilburn et al., 2002; Manimala & Anslyn, 2002). Against this background, we report herein the the synthesis and crystal structure of the title compound.
As seen as in Fig. 1, the asymmetric unit contains two amino(1H-benzimidazol-2-ylamino)methaniminium) cations and two 4-methylbenzenesulfonate anions. The bond lengths in the title compound are within the normal range (Allen et al., 1987).
In the cations, intramolecular N4—H4A···N2 and N9—H9A···N7 hydrogen bonds generate six-membered S(6) rings in each cation (Bernstein et al., 1995). In the crystal, a three-dimensional supramolecular framework is formed via intermolecular N—H···O and C—H···O hydrogen bonds between the cations and anions (Table 1, Fig. 2). Furthermore, two strong π-π stacking interactions [Cg1···Cg1 (-x, 1 - y, -z) = 3.4112 (14) Å and Cg4···Cg4 (1 - x, 1 - y, 2 - z) = 3.4104 (14) Å] also occur between the imidazole rings of like cations (Cg1 and Cg4 are the centroids of the N1/C8/C13/N2/C14 and N6/C23/C28/N7/C29 ring respectively).
2. Experimental
A mixture of 175 mg (1 mmol) 1-(1H-benzimidazol-2-yl)guanidine and 191 mg (1 mmol) of 4-methylbenzenesulfonyl chloride was heated under reflux in 50 ml ethanol together with few drops of triethylamine for 6 h. The solid product started to be deposited during heating and filtered off after completion. The crude solid was washed with ethanol and recrystallized to afford colourless plates suitable for X-ray difraction (M.p. 539–541 K).
3. Refinement
The C-bound H atoms were placed at geometrically idealized positions with C—H = 0.95 and 0.98 Å for aromatic and methyl H-atoms, respectively. The C-bound H-atoms were refined using a riding model with Uiso(H) = 1.2Ueq(Caromatic) and 1.5Ueq(Cmethyl). The N-bound H atoms were located in a difference Fourier map and their positions were refined with distance restraints [N—H = 0.88 (2) Å] and with Uiso(H) = 1.2Ueq(N). The presence of pseudosymmetry in the structure suggests the orthorhombic space group Pbcn, but attempts to refine the structure in this space group resulted in an unsatisfactory model.
Figures
Fig. 1.
The asymmetric unit of the title compound with the atom numbering scheme. Displacement ellipsoids for non-H atoms are drawn at the 50% probability level.
Fig. 2.
View of the packing and hydrogen bonding (dashed lines) along the a axis of the title compound.
Crystal data
| C8H10N5+·C7H7O3S− | F(000) = 1456 |
| Mr = 347.41 | Dx = 1.442 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9624 reflections |
| a = 12.3821 (4) Å | θ = 2.5–25.1° |
| b = 17.8077 (7) Å | µ = 0.23 mm−1 |
| c = 14.5112 (5) Å | T = 100 K |
| β = 90.013 (2)° | Plate, colourless |
| V = 3199.7 (2) Å3 | 0.35 × 0.10 × 0.04 mm |
| Z = 8 |
Data collection
| Bruker APEXII CCD diffractometer | 4108 reflections with I > 2σ(I) |
| Radiation source: sealed tube | Rint = 0.044 |
| Graphite monochromator | θmax = 25.1°, θmin = 2.5° |
| φ and ω scans | h = −14→14 |
| 20618 measured reflections | k = −17→21 |
| 5679 independent reflections | l = −17→17 |
Refinement
| Refinement on F2 | 15 restraints |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.044 | W = 1/[Σ2(FO2) + (0.0495P)2 + 1.3622P] where P = (FO2 + 2FC2)/3 |
| wR(F2) = 0.109 | (Δ/σ)max < 0.001 |
| S = 1.05 | Δρmax = 0.33 e Å−3 |
| 5679 reflections | Δρmin = −0.38 e Å−3 |
| 469 parameters |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | −0.06286 (16) | 0.53699 (11) | 0.14245 (14) | 0.0175 (6) | |
| N2 | 0.09357 (15) | 0.48502 (11) | 0.09774 (13) | 0.0169 (6) | |
| N3 | −0.03173 (16) | 0.40955 (11) | 0.18217 (14) | 0.0180 (6) | |
| N4 | 0.12756 (17) | 0.34452 (13) | 0.15854 (15) | 0.0213 (7) | |
| N5 | −0.01669 (17) | 0.28656 (13) | 0.22877 (15) | 0.0228 (7) | |
| C8 | −0.00584 (19) | 0.59345 (14) | 0.09792 (15) | 0.0175 (8) | |
| C9 | −0.0290 (2) | 0.66825 (15) | 0.08044 (17) | 0.0225 (8) | |
| C10 | 0.0487 (2) | 0.70888 (15) | 0.03340 (18) | 0.0267 (9) | |
| C11 | 0.1448 (2) | 0.67604 (14) | 0.00378 (18) | 0.0239 (8) | |
| C12 | 0.1676 (2) | 0.60121 (14) | 0.02124 (16) | 0.0210 (8) | |
| C13 | 0.09108 (19) | 0.56002 (14) | 0.06984 (16) | 0.0169 (7) | |
| C14 | 0.00195 (18) | 0.47560 (13) | 0.14059 (16) | 0.0158 (7) | |
| C15 | 0.02761 (19) | 0.34569 (14) | 0.18891 (16) | 0.0181 (8) | |
| N6 | 0.56311 (16) | 0.46290 (12) | 1.14251 (14) | 0.0176 (7) | |
| N7 | 0.40627 (15) | 0.51515 (11) | 1.09786 (13) | 0.0165 (6) | |
| N8 | 0.53226 (16) | 0.59052 (11) | 1.18216 (14) | 0.0174 (6) | |
| N9 | 0.37236 (17) | 0.65557 (12) | 1.15834 (15) | 0.0206 (7) | |
| N10 | 0.51667 (17) | 0.71349 (13) | 1.22845 (15) | 0.0239 (7) | |
| C23 | 0.50569 (19) | 0.40666 (14) | 1.09794 (16) | 0.0174 (7) | |
| C24 | 0.5292 (2) | 0.33155 (14) | 1.08030 (18) | 0.0230 (8) | |
| C25 | 0.4513 (2) | 0.29117 (15) | 1.03352 (18) | 0.0256 (8) | |
| C26 | 0.3551 (2) | 0.32380 (14) | 1.00391 (18) | 0.0240 (8) | |
| C27 | 0.33247 (19) | 0.39879 (14) | 1.02117 (16) | 0.0208 (8) | |
| C28 | 0.40902 (19) | 0.43994 (14) | 1.06964 (16) | 0.0170 (7) | |
| C29 | 0.49835 (18) | 0.52464 (14) | 1.14061 (15) | 0.0162 (8) | |
| C30 | 0.47239 (19) | 0.65446 (14) | 1.18866 (16) | 0.0168 (8) | |
| S1 | 0.21220 (5) | 0.14511 (3) | 0.19245 (4) | 0.0171 (2) | |
| O1 | 0.25453 (12) | 0.08701 (10) | 0.25384 (11) | 0.0209 (5) | |
| O2 | 0.09531 (12) | 0.14896 (9) | 0.19288 (11) | 0.0208 (5) | |
| O3 | 0.26233 (12) | 0.21792 (9) | 0.21165 (11) | 0.0199 (5) | |
| C1 | 0.25080 (18) | 0.12068 (13) | 0.07911 (17) | 0.0182 (8) | |
| C2 | 0.1867 (2) | 0.14390 (16) | 0.00683 (19) | 0.0307 (9) | |
| C3 | 0.2188 (2) | 0.13075 (18) | −0.08296 (19) | 0.0363 (10) | |
| C4 | 0.3145 (2) | 0.09439 (17) | −0.10240 (19) | 0.0312 (9) | |
| C5 | 0.3776 (2) | 0.07199 (19) | −0.0290 (2) | 0.0385 (10) | |
| C6 | 0.3467 (2) | 0.08403 (17) | 0.06154 (19) | 0.0321 (9) | |
| C7 | 0.3494 (2) | 0.0806 (2) | −0.2003 (2) | 0.0464 (13) | |
| S2 | 0.71207 (5) | 0.14502 (3) | 0.80768 (4) | 0.0170 (2) | |
| O4 | 0.59550 (13) | 0.14897 (10) | 0.80726 (11) | 0.0211 (5) | |
| O5 | 0.76233 (12) | 0.21802 (9) | 0.78844 (11) | 0.0210 (5) | |
| O6 | 0.75459 (13) | 0.08684 (10) | 0.74612 (11) | 0.0218 (5) | |
| C16 | 0.8464 (2) | 0.08440 (17) | 0.93848 (19) | 0.0330 (9) | |
| C17 | 0.8775 (2) | 0.07195 (19) | 1.0293 (2) | 0.0397 (10) | |
| C18 | 0.8143 (2) | 0.09440 (17) | 1.10254 (18) | 0.0295 (9) | |
| C19 | 0.7187 (2) | 0.13093 (18) | 1.08268 (19) | 0.0353 (10) | |
| C20 | 0.6868 (2) | 0.14404 (16) | 0.99331 (19) | 0.0301 (9) | |
| C21 | 0.75051 (19) | 0.12081 (14) | 0.92102 (16) | 0.0178 (8) | |
| C22 | 0.8491 (2) | 0.0808 (2) | 1.2004 (2) | 0.0486 (13) | |
| HN1 | −0.1196 (16) | 0.5434 (14) | 0.1755 (16) | 0.0210* | |
| HN3 | −0.0980 (14) | 0.4078 (15) | 0.1986 (17) | 0.0220* | |
| H4A | 0.148 (2) | 0.3840 (12) | 0.1274 (17) | 0.0260* | |
| H4B | 0.1687 (18) | 0.3051 (12) | 0.1641 (18) | 0.0260* | |
| H5A | −0.0860 (14) | 0.2874 (16) | 0.2457 (18) | 0.0270* | |
| H5B | 0.021 (2) | 0.2448 (12) | 0.2250 (19) | 0.0270* | |
| H9 | −0.09500 | 0.69050 | 0.09980 | 0.0270* | |
| H10 | 0.03620 | 0.76050 | 0.02100 | 0.0320* | |
| H11 | 0.19580 | 0.70560 | −0.02910 | 0.0290* | |
| H12 | 0.23300 | 0.57890 | 0.00080 | 0.0250* | |
| HN6 | 0.6213 (15) | 0.4571 (15) | 1.1766 (16) | 0.0210* | |
| HN8 | 0.5993 (14) | 0.5904 (15) | 1.2000 (16) | 0.0210* | |
| H9A | 0.353 (2) | 0.6174 (12) | 1.1260 (16) | 0.0250* | |
| H9B | 0.3349 (19) | 0.6944 (12) | 1.1706 (18) | 0.0250* | |
| H10A | 0.478 (2) | 0.7546 (12) | 1.2263 (19) | 0.0290* | |
| H10B | 0.5849 (14) | 0.7141 (16) | 1.2466 (18) | 0.0290* | |
| H24 | 0.59530 | 0.30930 | 1.09940 | 0.0280* | |
| H25 | 0.46380 | 0.23950 | 1.02110 | 0.0310* | |
| H26 | 0.30410 | 0.29410 | 0.97130 | 0.0290* | |
| H27 | 0.26710 | 0.42120 | 1.00060 | 0.0250* | |
| H2 | 0.12040 | 0.16900 | 0.01870 | 0.0370* | |
| H3 | 0.17410 | 0.14710 | −0.13220 | 0.0440* | |
| H5 | 0.44430 | 0.04750 | −0.04100 | 0.0460* | |
| H6 | 0.39100 | 0.06730 | 0.11090 | 0.0390* | |
| H7A | 0.33560 | 0.12570 | −0.23730 | 0.0690* | |
| H7B | 0.30860 | 0.03830 | −0.22580 | 0.0690* | |
| H7C | 0.42680 | 0.06900 | −0.20160 | 0.0690* | |
| H16 | 0.89080 | 0.06800 | 0.88910 | 0.0400* | |
| H17 | 0.94410 | 0.04730 | 1.04120 | 0.0480* | |
| H19 | 0.67400 | 0.14740 | 1.13190 | 0.0420* | |
| H20 | 0.62060 | 0.16920 | 0.98150 | 0.0360* | |
| H22A | 0.80090 | 0.04390 | 1.22900 | 0.0730* | |
| H22B | 0.84580 | 0.12800 | 1.23500 | 0.0730* | |
| H22C | 0.92330 | 0.06170 | 1.20100 | 0.0730* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0175 (11) | 0.0148 (11) | 0.0202 (11) | 0.0030 (9) | 0.0011 (8) | −0.0053 (9) |
| N2 | 0.0175 (11) | 0.0146 (11) | 0.0187 (10) | 0.0009 (9) | −0.0013 (8) | −0.0022 (9) |
| N3 | 0.0129 (10) | 0.0180 (12) | 0.0232 (11) | 0.0025 (9) | 0.0019 (8) | 0.0001 (9) |
| N4 | 0.0207 (12) | 0.0157 (13) | 0.0275 (12) | 0.0043 (9) | 0.0027 (9) | 0.0042 (10) |
| N5 | 0.0198 (11) | 0.0176 (13) | 0.0311 (12) | 0.0049 (10) | 0.0052 (9) | 0.0061 (10) |
| C8 | 0.0206 (13) | 0.0171 (14) | 0.0149 (12) | 0.0004 (10) | −0.0044 (9) | −0.0027 (10) |
| C9 | 0.0251 (14) | 0.0175 (14) | 0.0249 (13) | 0.0049 (11) | −0.0062 (11) | −0.0048 (11) |
| C10 | 0.0340 (16) | 0.0160 (15) | 0.0300 (14) | 0.0006 (12) | −0.0085 (12) | 0.0008 (12) |
| C11 | 0.0266 (14) | 0.0202 (14) | 0.0250 (13) | −0.0053 (11) | −0.0048 (11) | 0.0034 (12) |
| C12 | 0.0209 (13) | 0.0218 (15) | 0.0204 (13) | −0.0014 (11) | −0.0038 (10) | −0.0014 (11) |
| C13 | 0.0204 (13) | 0.0124 (13) | 0.0178 (12) | 0.0007 (10) | −0.0059 (10) | −0.0037 (10) |
| C14 | 0.0170 (12) | 0.0141 (13) | 0.0162 (12) | 0.0004 (10) | −0.0025 (9) | −0.0028 (10) |
| C15 | 0.0180 (13) | 0.0197 (15) | 0.0167 (12) | 0.0020 (11) | −0.0008 (10) | −0.0011 (10) |
| N6 | 0.0163 (11) | 0.0157 (12) | 0.0208 (11) | 0.0034 (9) | −0.0005 (8) | 0.0031 (9) |
| N7 | 0.0190 (11) | 0.0135 (11) | 0.0171 (10) | 0.0001 (9) | 0.0012 (8) | 0.0004 (9) |
| N8 | 0.0142 (10) | 0.0154 (12) | 0.0227 (11) | 0.0021 (9) | −0.0021 (8) | 0.0005 (9) |
| N9 | 0.0200 (12) | 0.0127 (12) | 0.0290 (12) | 0.0035 (9) | −0.0014 (9) | −0.0056 (10) |
| N10 | 0.0198 (12) | 0.0177 (13) | 0.0342 (13) | 0.0039 (10) | −0.0042 (10) | −0.0051 (10) |
| C23 | 0.0201 (13) | 0.0146 (13) | 0.0174 (12) | −0.0003 (10) | 0.0049 (10) | 0.0027 (10) |
| C24 | 0.0250 (14) | 0.0169 (14) | 0.0272 (14) | 0.0042 (11) | 0.0062 (11) | 0.0025 (11) |
| C25 | 0.0318 (15) | 0.0137 (14) | 0.0312 (15) | −0.0006 (12) | 0.0112 (12) | −0.0005 (12) |
| C26 | 0.0268 (14) | 0.0190 (14) | 0.0262 (14) | −0.0059 (12) | 0.0044 (11) | −0.0033 (12) |
| C27 | 0.0185 (13) | 0.0205 (15) | 0.0234 (13) | −0.0014 (11) | 0.0050 (10) | 0.0014 (11) |
| C28 | 0.0203 (13) | 0.0150 (13) | 0.0156 (12) | 0.0009 (10) | 0.0052 (10) | 0.0030 (10) |
| C29 | 0.0178 (13) | 0.0161 (14) | 0.0148 (12) | 0.0009 (10) | 0.0021 (9) | 0.0030 (10) |
| C30 | 0.0184 (13) | 0.0166 (14) | 0.0153 (12) | −0.0007 (10) | 0.0007 (10) | 0.0001 (10) |
| S1 | 0.0165 (3) | 0.0142 (3) | 0.0205 (3) | 0.0006 (2) | 0.0007 (2) | 0.0022 (3) |
| O1 | 0.0190 (9) | 0.0171 (10) | 0.0267 (9) | 0.0007 (7) | −0.0013 (7) | 0.0067 (8) |
| O2 | 0.0166 (9) | 0.0212 (10) | 0.0245 (9) | 0.0000 (7) | 0.0024 (7) | 0.0013 (8) |
| O3 | 0.0222 (9) | 0.0130 (9) | 0.0245 (9) | −0.0008 (7) | −0.0026 (7) | 0.0001 (7) |
| C1 | 0.0182 (13) | 0.0117 (13) | 0.0246 (13) | −0.0023 (10) | 0.0032 (10) | 0.0000 (11) |
| C2 | 0.0279 (15) | 0.0370 (17) | 0.0272 (14) | 0.0119 (13) | 0.0009 (12) | −0.0022 (13) |
| C3 | 0.0344 (17) | 0.049 (2) | 0.0254 (15) | 0.0067 (14) | −0.0032 (12) | −0.0034 (14) |
| C4 | 0.0267 (15) | 0.0379 (18) | 0.0291 (15) | −0.0119 (13) | 0.0026 (12) | −0.0130 (13) |
| C5 | 0.0242 (15) | 0.051 (2) | 0.0404 (18) | 0.0084 (14) | 0.0052 (13) | −0.0144 (15) |
| C6 | 0.0251 (15) | 0.0411 (19) | 0.0302 (15) | 0.0109 (13) | −0.0043 (12) | −0.0029 (14) |
| C7 | 0.0329 (17) | 0.073 (3) | 0.0332 (17) | −0.0122 (16) | 0.0085 (13) | −0.0214 (17) |
| S2 | 0.0163 (3) | 0.0140 (3) | 0.0206 (3) | 0.0006 (2) | −0.0008 (2) | −0.0022 (3) |
| O4 | 0.0170 (9) | 0.0209 (10) | 0.0253 (9) | 0.0016 (7) | −0.0014 (7) | −0.0022 (8) |
| O5 | 0.0213 (9) | 0.0137 (10) | 0.0279 (9) | −0.0003 (7) | 0.0010 (7) | −0.0009 (8) |
| O6 | 0.0196 (9) | 0.0186 (10) | 0.0271 (9) | 0.0019 (7) | 0.0010 (7) | −0.0054 (8) |
| C16 | 0.0260 (15) | 0.0418 (19) | 0.0311 (15) | 0.0109 (13) | 0.0044 (12) | 0.0083 (14) |
| C17 | 0.0252 (16) | 0.053 (2) | 0.0410 (18) | 0.0095 (14) | −0.0048 (13) | 0.0168 (16) |
| C18 | 0.0275 (15) | 0.0349 (18) | 0.0262 (14) | −0.0088 (13) | −0.0032 (11) | 0.0110 (13) |
| C19 | 0.0334 (16) | 0.048 (2) | 0.0245 (15) | 0.0070 (14) | 0.0019 (12) | 0.0014 (14) |
| C20 | 0.0271 (15) | 0.0367 (17) | 0.0266 (14) | 0.0116 (13) | −0.0002 (11) | 0.0016 (13) |
| C21 | 0.0194 (13) | 0.0114 (13) | 0.0226 (13) | −0.0029 (10) | −0.0044 (10) | 0.0016 (10) |
| C22 | 0.0365 (18) | 0.075 (3) | 0.0342 (17) | −0.0139 (17) | −0.0102 (14) | 0.0238 (18) |
Geometric parameters (Å, º)
| S1—O2 | 1.4490 (16) | C9—H9 | 0.9500 |
| S1—O3 | 1.4642 (17) | C10—H10 | 0.9500 |
| S1—O1 | 1.4624 (18) | C11—H11 | 0.9500 |
| S1—C1 | 1.767 (3) | C12—H12 | 0.9500 |
| S2—C21 | 1.766 (2) | C23—C24 | 1.393 (4) |
| S2—O6 | 1.4659 (18) | C23—C28 | 1.397 (3) |
| S2—O5 | 1.4681 (17) | C24—C25 | 1.381 (4) |
| S2—O4 | 1.4451 (17) | C25—C26 | 1.393 (4) |
| N1—C14 | 1.356 (3) | C26—C27 | 1.387 (4) |
| N1—C8 | 1.388 (3) | C27—C28 | 1.389 (3) |
| N2—C14 | 1.305 (3) | C24—H24 | 0.9500 |
| N2—C13 | 1.396 (3) | C25—H25 | 0.9500 |
| N3—C15 | 1.357 (3) | C26—H26 | 0.9500 |
| N3—C14 | 1.386 (3) | C27—H27 | 0.9500 |
| N4—C15 | 1.314 (3) | C1—C6 | 1.379 (3) |
| N5—C15 | 1.321 (3) | C1—C2 | 1.379 (4) |
| N1—HN1 | 0.86 (2) | C2—C3 | 1.382 (4) |
| N3—HN3 | 0.855 (18) | C3—C4 | 1.380 (4) |
| N4—H4A | 0.87 (2) | C4—C7 | 1.505 (4) |
| N4—H4B | 0.87 (2) | C4—C5 | 1.380 (4) |
| N5—H5A | 0.893 (18) | C5—C6 | 1.385 (4) |
| N5—H5B | 0.88 (2) | C2—H2 | 0.9500 |
| N6—C29 | 1.361 (3) | C3—H3 | 0.9500 |
| N6—C23 | 1.388 (3) | C5—H5 | 0.9500 |
| N7—C28 | 1.401 (3) | C6—H6 | 0.9500 |
| N7—C29 | 1.309 (3) | C7—H7B | 0.9800 |
| N8—C29 | 1.384 (3) | C7—H7C | 0.9800 |
| N8—C30 | 1.362 (3) | C7—H7A | 0.9800 |
| N9—C30 | 1.315 (3) | C16—C17 | 1.391 (4) |
| N10—C30 | 1.319 (3) | C16—C21 | 1.376 (4) |
| N6—HN6 | 0.88 (2) | C17—C18 | 1.379 (4) |
| N8—HN8 | 0.870 (18) | C18—C19 | 1.381 (4) |
| N9—H9B | 0.85 (2) | C18—C22 | 1.504 (4) |
| N9—H9A | 0.86 (2) | C19—C20 | 1.376 (4) |
| N10—H10B | 0.885 (19) | C20—C21 | 1.376 (4) |
| N10—H10A | 0.88 (2) | C16—H16 | 0.9500 |
| C8—C9 | 1.386 (4) | C17—H17 | 0.9500 |
| C8—C13 | 1.400 (3) | C19—H19 | 0.9500 |
| C9—C10 | 1.384 (4) | C20—H20 | 0.9500 |
| C10—C11 | 1.394 (4) | C22—H22A | 0.9800 |
| C11—C12 | 1.386 (4) | C22—H22B | 0.9800 |
| C12—C13 | 1.391 (3) | C22—H22C | 0.9800 |
| O2—S1—C1 | 106.62 (10) | C25—C26—C27 | 121.2 (2) |
| O3—S1—C1 | 106.28 (10) | C26—C27—C28 | 117.5 (2) |
| O1—S1—O3 | 111.02 (10) | N7—C28—C23 | 109.9 (2) |
| O1—S1—C1 | 107.21 (10) | N7—C28—C27 | 129.5 (2) |
| O1—S1—O2 | 112.88 (9) | C23—C28—C27 | 120.6 (2) |
| O2—S1—O3 | 112.38 (9) | N7—C29—N8 | 125.5 (2) |
| O6—S2—C21 | 107.36 (11) | N6—C29—N7 | 114.7 (2) |
| O4—S2—O5 | 112.31 (10) | N6—C29—N8 | 119.8 (2) |
| O5—S2—C21 | 106.21 (11) | N8—C30—N10 | 118.1 (2) |
| O5—S2—O6 | 110.96 (10) | N8—C30—N9 | 120.2 (2) |
| O4—S2—O6 | 113.00 (10) | N9—C30—N10 | 121.8 (2) |
| O4—S2—C21 | 106.55 (11) | C25—C24—H24 | 122.00 |
| C8—N1—C14 | 105.87 (19) | C23—C24—H24 | 122.00 |
| C13—N2—C14 | 104.01 (19) | C24—C25—H25 | 119.00 |
| C14—N3—C15 | 125.4 (2) | C26—C25—H25 | 119.00 |
| C8—N1—HN1 | 125.5 (17) | C25—C26—H26 | 119.00 |
| C14—N1—HN1 | 127.1 (16) | C27—C26—H26 | 119.00 |
| C14—N3—HN3 | 116.2 (18) | C28—C27—H27 | 121.00 |
| C15—N3—HN3 | 118.0 (18) | C26—C27—H27 | 121.00 |
| C15—N4—H4A | 115.7 (16) | S1—C1—C6 | 121.45 (19) |
| C15—N4—H4B | 122.2 (15) | S1—C1—C2 | 118.58 (18) |
| H4A—N4—H4B | 122 (2) | C2—C1—C6 | 119.8 (2) |
| C15—N5—H5A | 120.4 (18) | C1—C2—C3 | 120.1 (2) |
| H5A—N5—H5B | 123 (2) | C2—C3—C4 | 121.3 (3) |
| C15—N5—H5B | 115.2 (16) | C5—C4—C7 | 121.3 (2) |
| C23—N6—C29 | 105.77 (19) | C3—C4—C5 | 117.7 (3) |
| C28—N7—C29 | 103.93 (19) | C3—C4—C7 | 121.1 (2) |
| C29—N8—C30 | 125.0 (2) | C4—C5—C6 | 122.1 (2) |
| C29—N6—HN6 | 126.1 (17) | C1—C6—C5 | 119.1 (2) |
| C23—N6—HN6 | 126.6 (18) | C1—C2—H2 | 120.00 |
| C29—N8—HN8 | 114.7 (17) | C3—C2—H2 | 120.00 |
| C30—N8—HN8 | 120.1 (18) | C4—C3—H3 | 119.00 |
| C30—N9—H9B | 117.1 (16) | C2—C3—H3 | 119.00 |
| C30—N9—H9A | 115.7 (16) | C4—C5—H5 | 119.00 |
| H9A—N9—H9B | 127 (2) | C6—C5—H5 | 119.00 |
| C30—N10—H10B | 122.5 (18) | C1—C6—H6 | 120.00 |
| H10A—N10—H10B | 122 (2) | C5—C6—H6 | 120.00 |
| C30—N10—H10A | 115.1 (16) | H7B—C7—H7C | 110.00 |
| C9—C8—C13 | 122.2 (2) | C4—C7—H7A | 109.00 |
| N1—C8—C13 | 105.3 (2) | C4—C7—H7B | 110.00 |
| N1—C8—C9 | 132.5 (2) | C4—C7—H7C | 109.00 |
| C8—C9—C10 | 116.7 (2) | H7A—C7—H7B | 109.00 |
| C9—C10—C11 | 121.8 (2) | H7A—C7—H7C | 109.00 |
| C10—C11—C12 | 121.4 (2) | C17—C16—C21 | 119.2 (2) |
| C11—C12—C13 | 117.5 (2) | C16—C17—C18 | 121.8 (2) |
| N2—C13—C8 | 110.0 (2) | C17—C18—C19 | 117.5 (2) |
| C8—C13—C12 | 120.5 (2) | C17—C18—C22 | 121.2 (2) |
| N2—C13—C12 | 129.6 (2) | C19—C18—C22 | 121.2 (2) |
| N1—C14—N3 | 119.8 (2) | C18—C19—C20 | 121.5 (2) |
| N1—C14—N2 | 114.9 (2) | C19—C20—C21 | 120.2 (2) |
| N2—C14—N3 | 125.3 (2) | S2—C21—C16 | 121.26 (19) |
| N4—C15—N5 | 121.7 (2) | S2—C21—C20 | 118.83 (19) |
| N3—C15—N5 | 118.3 (2) | C16—C21—C20 | 119.7 (2) |
| N3—C15—N4 | 119.9 (2) | C17—C16—H16 | 120.00 |
| C10—C9—H9 | 122.00 | C21—C16—H16 | 120.00 |
| C8—C9—H9 | 122.00 | C16—C17—H17 | 119.00 |
| C11—C10—H10 | 119.00 | C18—C17—H17 | 119.00 |
| C9—C10—H10 | 119.00 | C18—C19—H19 | 119.00 |
| C10—C11—H11 | 119.00 | C20—C19—H19 | 119.00 |
| C12—C11—H11 | 119.00 | C19—C20—H20 | 120.00 |
| C13—C12—H12 | 121.00 | C21—C20—H20 | 120.00 |
| C11—C12—H12 | 121.00 | C18—C22—H22A | 109.00 |
| N6—C23—C24 | 132.2 (2) | C18—C22—H22B | 109.00 |
| N6—C23—C28 | 105.6 (2) | C18—C22—H22C | 109.00 |
| C24—C23—C28 | 122.2 (2) | H22A—C22—H22B | 109.00 |
| C23—C24—C25 | 116.4 (2) | H22A—C22—H22C | 110.00 |
| C24—C25—C26 | 122.1 (2) | H22B—C22—H22C | 109.00 |
| O1—S1—C1—C2 | 152.0 (2) | C13—C8—C9—C10 | 0.1 (4) |
| O2—S1—C1—C2 | 30.8 (2) | N1—C8—C9—C10 | 179.8 (2) |
| O3—S1—C1—C2 | −89.3 (2) | N1—C8—C13—C12 | 179.1 (2) |
| O1—S1—C1—C6 | −32.8 (2) | C8—C9—C10—C11 | 1.0 (4) |
| O2—S1—C1—C6 | −153.9 (2) | C9—C10—C11—C12 | −0.9 (4) |
| O3—S1—C1—C6 | 86.0 (2) | C10—C11—C12—C13 | −0.2 (4) |
| O5—S2—C21—C20 | 89.1 (2) | C11—C12—C13—C8 | 1.2 (3) |
| O6—S2—C21—C20 | −152.1 (2) | C11—C12—C13—N2 | −179.0 (2) |
| O6—S2—C21—C16 | 32.8 (2) | C28—C23—C24—C25 | −0.4 (4) |
| O4—S2—C21—C16 | 154.2 (2) | N6—C23—C24—C25 | 179.7 (2) |
| O5—S2—C21—C16 | −85.9 (2) | C24—C23—C28—N7 | 179.0 (2) |
| O4—S2—C21—C20 | −30.8 (2) | N6—C23—C28—N7 | −1.0 (3) |
| C14—N1—C8—C9 | −178.3 (3) | N6—C23—C28—C27 | 179.2 (2) |
| C14—N1—C8—C13 | 1.4 (2) | C24—C23—C28—C27 | −0.8 (4) |
| C8—N1—C14—N3 | 178.5 (2) | C23—C24—C25—C26 | 1.1 (4) |
| C8—N1—C14—N2 | −1.8 (3) | C24—C25—C26—C27 | −0.8 (4) |
| C13—N2—C14—N3 | −179.0 (2) | C25—C26—C27—C28 | −0.4 (4) |
| C14—N2—C13—C8 | −0.3 (3) | C26—C27—C28—C23 | 1.2 (3) |
| C13—N2—C14—N1 | 1.3 (3) | C26—C27—C28—N7 | −178.6 (2) |
| C14—N2—C13—C12 | 180.0 (2) | S1—C1—C2—C3 | 175.1 (2) |
| C14—N3—C15—N5 | −178.4 (2) | C2—C1—C6—C5 | 0.8 (4) |
| C15—N3—C14—N2 | 3.6 (4) | C6—C1—C2—C3 | −0.3 (4) |
| C14—N3—C15—N4 | 3.5 (4) | S1—C1—C6—C5 | −174.4 (2) |
| C15—N3—C14—N1 | −176.6 (2) | C1—C2—C3—C4 | 0.1 (4) |
| C23—N6—C29—N8 | 178.4 (2) | C2—C3—C4—C7 | −179.7 (3) |
| C23—N6—C29—N7 | −1.5 (3) | C2—C3—C4—C5 | −0.4 (4) |
| C29—N6—C23—C24 | −178.6 (3) | C3—C4—C5—C6 | 1.0 (5) |
| C29—N6—C23—C28 | 1.5 (2) | C7—C4—C5—C6 | −179.8 (3) |
| C28—N7—C29—N8 | −179.1 (2) | C4—C5—C6—C1 | −1.1 (5) |
| C29—N7—C28—C27 | 180.0 (3) | C17—C16—C21—C20 | −0.3 (4) |
| C29—N7—C28—C23 | 0.2 (3) | C17—C16—C21—S2 | 174.8 (2) |
| C28—N7—C29—N6 | 0.8 (3) | C21—C16—C17—C18 | 0.7 (5) |
| C30—N8—C29—N6 | −176.5 (2) | C16—C17—C18—C19 | −0.8 (5) |
| C29—N8—C30—N9 | 3.6 (4) | C16—C17—C18—C22 | −179.8 (3) |
| C30—N8—C29—N7 | 3.4 (4) | C17—C18—C19—C20 | 0.5 (4) |
| C29—N8—C30—N10 | −178.4 (2) | C22—C18—C19—C20 | 179.5 (3) |
| N1—C8—C13—N2 | −0.7 (3) | C18—C19—C20—C21 | −0.1 (4) |
| C9—C8—C13—N2 | 179.0 (2) | C19—C20—C21—C16 | 0.0 (4) |
| C9—C8—C13—C12 | −1.2 (4) | C19—C20—C21—S2 | −175.2 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—HN1···O1i | 0.86 (2) | 2.11 (2) | 2.948 (3) | 166 (2) |
| N3—HN3···O6ii | 0.86 (2) | 1.95 (2) | 2.805 (3) | 173 (2) |
| N6—HN6···O6iii | 0.88 (2) | 2.09 (2) | 2.944 (3) | 164 (2) |
| N8—HN8···O1iv | 0.87 (2) | 1.93 (2) | 2.799 (2) | 177 (2) |
| N4—H4A···N2 | 0.87 (2) | 1.97 (2) | 2.686 (3) | 139 (2) |
| N4—H4B···O3 | 0.87 (2) | 2.06 (2) | 2.909 (3) | 166 (2) |
| N5—H5A···O5ii | 0.89 (2) | 1.98 (2) | 2.871 (3) | 176 (3) |
| N5—H5B···O2 | 0.88 (2) | 1.99 (2) | 2.863 (3) | 169 (3) |
| N9—H9A···N7 | 0.86 (2) | 1.98 (2) | 2.683 (3) | 138 (2) |
| N9—H9B···O5v | 0.85 (2) | 2.06 (2) | 2.906 (3) | 174 (2) |
| N10—H10A···O4v | 0.88 (2) | 2.00 (2) | 2.863 (3) | 167 (3) |
| N10—H10B···O3iv | 0.89 (2) | 1.99 (2) | 2.872 (3) | 179 (3) |
| C2—H2···O2 | 0.95 | 2.57 | 2.929 (3) | 103 |
| C7—H7C···O4vi | 0.98 | 2.53 | 3.283 (3) | 133 |
| C20—H20···O4 | 0.95 | 2.57 | 2.928 (3) | 102 |
Symmetry codes: (i) −x, y+1/2, −z+1/2; (ii) x−1, −y+1/2, z−1/2; (iii) x, −y+1/2, z+1/2; (iv) −x+1, y+1/2, −z+3/2; (v) −x+1, −y+1, −z+2; (vi) x, y, z−1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5349).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2013). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Costa, M., Chiusoli, G. P., Taffurelli, D. & Dalmonego, G. (1998). J. Chem. Soc. Perkin Trans. 1, pp. 1541–1546.
- Ekelund, S., Nygren, P. & Larsson, R. (2001). Biochem. Pharmacol. 61, 1183–1193. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Han, J.-J., Xu, Y.-F., Su, Y.-P., She, X.-P. & Pan, X.-F. (2008). Catal. Commun. 9, 2077–2079.
- Hannon, C. L. & Anslyn, E. V. (1993). Bioorg. Chem. Front. 3, 193–255.
- Hopkins, T. P., Dener, J. M. & Boldi, A. M. (2002). J. Comb. Chem 4, 167–174. [DOI] [PubMed]
- Kilburn, J. P., Lau, J. & Jones, R. C. F. (2002). Tetrahedron, 58, 1739–1743.
- Kovacevic, B. & Maksic, Z. B. (2001). Org. Lett. 3, 1523–1526. [DOI] [PubMed]
- Manimala, J. C. & Anslyn, E. V. (2002). Tetrahedron Lett. 43, 565–567.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wu, Y.-Q., Hamilton, S. K., Wilkinson, D. E. & Hamilton, G. S. (2002). J. Org. Chem. 67, 7553–7556. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813024975/sj5349sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813024975/sj5349Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813024975/sj5349Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




