Abstract
BACKGROUND
Immunoneutralization of elevated circulating levels of endogenous digitalis-like Na/K-ATPase inhibitors (i.e. cardiotonic steroids (CTS)) represents a novel approach in the treatment of preeclampsia (PE). Recently we demonstrated that DigiFab (Fab fragments of affinity-purified ovine digoxin antibody) restores PE-induced inhibition of Na/K-ATPase in erythrocytes ex vivo. Previously magnesium ions were shown to antagonize digitalis-induced toxicity, which is mediated by Na/K-ATPase inhibition. We hypothesized that magnesium sulfate would potentiate the effect of DigiFab in the reversal of CTS-induced Na/K-ATPase inhibition.
METHODS
To test this hypothesis, we studied the ex vivo effect of DigiFab on Na/K-ATPase activity in erythrocytes from patients with PE in the absence and in the presence of 3 mmol/L magnesium sulfate.
RESULTS
Compared with 11 normotensive pregnant subjects (29±1 years; gestational age = 39.0±0.2 weeks; blood pressure = 111±2/73±2mm Hg), the 12 patients with PE (30±1 years; gestational age = 37.9±0.3 weeks; blood pressure = 159±5/99±3mm Hg) had plasma levels of marino bufagenin increased 3-fold (1.38±0.40 vs. 0.38±0.10 nmol/L; P < 0.01) and activity of Na/K-ATPase in erythrocytes was inhibited (1.16±0.11 vs. 2.80±0.20 μmol Pi/ml/h; P < 0.01). Ex vivo, DigiFab (1 µg/ml) restored erythrocyte Na/K-ATPase activity (1.72±0.13 µmol Pi/ml/h; P < 0.01), and 3 mmol magnesium sulfate potentiated the effect of DigiFab (2.30±0.20 µmol Pi/ml/h; P < 0.01).
CONCLUSIONS
Magnesium is capable of increasing the efficacy of immunoneutralization of marinobufagenin-induced Na/K-ATPase inhibition.
Keywords: blood pressure, cardiotonic steroids, digifab, digitalis-like factors, hypertension, immunotherapy, magnesium, marinobufagenin, NA/K-ATPase, preeclampsia.
Although preeclampsia (PE) is a serious complication of pregnancy leading to maternal and fetal morbidity and mortality, mechanisms of its pathogenesis are not well understood, and there is no effective therapy for this syndrome.1 One of the factors implicated in pathogenesis of PE is the presence of endogenous digitalis-like Na/K-ATPase inhibitors (i.e., cardiotonic steroids (CTSs)).2 , 3 CTSs, including a bufadienolide marinobufagenin (MBG), contribute to the pathogenesis of PE by induction of vasoconstriction,3 , 4 vascular fibrosis,5 and impairment of invasion of the cytotrophoblast.6
Immunoneutralization of CTSs is a novel approach in the treatment of PE, and several successful cases of the treatment of PE by Digibind have been reported.7 , 8 Digibind, the affinity-purified Fab fragments of sheep antidigoxin antibody, because of cross-reactivity with digitalis-like compounds, is capable of reversing the effects of endogenous CTSs in vivo.4 , 7 , 8 The effect of Digibind in PE is based on the binding of CTSs and reversal of the vasoconstriction, which is associated with restoration of activity of erythrocyte Na/K-ATPase, a target enzyme for CTSs.7 , 13 DigiFab, which recently replaced Digibind on the market, is similar to Digibind in its ability to neutralize CTSs ex vivo and to restore PE-induced inhibition of erythrocyte Na/K-ATPase.14
Interactions between digitalis/CTSs and its receptor on the Na/K-ATPase are modulated by many factors, including magnesium (Mg) ions.10 , 11 Notably, in vivo Mg ions antagonize digitalis-induced toxicity, which is mediated by Na/K-ATPase inhibition.12 Mg deficiency, on the contrary, sensitizes myocardium to the proarrhythmic action of digitalis.13 Because of the above evidence and because Mg sulfate (MgSO4) exerts beneficial effects in PE,1 we hypothesized that MgSO4 would potentiate the effect of DigiFab with respect to Na/K-ATPase restoration and reduction of blood pressure in PE. To test this hypothesis, in erythrocytes from patients with PE we studied the effect of DigiFab on Na/K-ATPase activity in the absence and in the presence of 3 mmol/L MgSO4 ex vivo.
METHODS
The protocol for this study was approved by the ethical committee of Almazov Federal Heart, Blood and Endocrinology Center, and by the institutional review board of Medstar Research Institute, Washington, DC. Twelve participants who were admitted to the Institute of Neonatology and Pediatrics, Almazov Federal Heart, Blood and Endocrinology Center in St Petersburg, Russia, were enrolled in the study after giving informed consent. Diagnosis of mild PE in 7 patients was based on the criteria of the American Congress of Obstetrics and Gynecology.14 This definition includes systolic blood pressure of at least 140mm Hg or diastolic blood pressure of at least 90mm Hg on at least 2 occasions 6 hours apart and new onset proteinuria (urinary protein excretion >0.3g/24 hours or a urinary protein concentration of >1g/L in at least 2 random urine specimens collected ≥6 hours apart) in a pregnancy after the 20th week of gestation. Exclusion criteria included patients with a clinical need for digitalis drugs, antecedent history of essential hypertension, and chronic cardiovascular, renal, hepatic, or endocrine disorders. We specifically enrolled 11 age-matched normotensive women with uncomplicated pregnancies to serve as the control group.
Ten milliliters of venous blood were collected. Four milliliters of blood were centrifuged at 1,500g for 15 minutes, and plasma samples were kept at −80 °C for measurement of MBG. Each plasma sample was extracted on C18 SepPak cartridges, dried, reconstituted in 10% acetonitrile, and fractionated on Agilent 1,100 series high performance liquid chromatography system using Agilent Zorbax Eclipse XDB-C18 (Agilent Technologies, Palo Alto, CA), 4.6×150mm, 5 μm particle size, 80 Å column, flow rate 1ml/minute, in linear (10%–85.5%) gradient of acetonitrile against 0.1% trifluoroacetic acid for 45 minutes. Levels of MBG were determined in chromatographic fractions coeluting with authentic MBG (16 minutes) using fluoroimmunoassay (Dissociation Enhanced FluoroImmunoAssay (DELFIA)) based on a murine anti-MBG 4G4 monoclonal antibody recently described in detail.4 This assay is based on competition between immobilized antigen (MBG-glycoside-thyroglobulin) and MBG, other cross-reactants, or endogenous CTSs within the sample for a limited number of binding sites on an anti-MBG monoclonal antibodies. Secondary (goat antimouse) antibody labeled with nonradioactive Europium was obtained from Perkin-Elmer (Waltham, MA). The sensitivity of this MBG DELFIA is 0.05 nmol/L, and the cross-reactivity of 4G4 monoclonal antibody used in this assay with other steroids is as follows: MBG: 100%; marinobufotoxin: 43%; cinobufotalin: 40%; telocinobufagin: 14%; resibufagenin: 0.5%; bufalin: 0.08%; cinobufagin: 0.07%; digoxin: 0.03%; ouabain: 0.005%; digoxigenin: 0.004%; proscillaridin A, digitoxin, aldosterone, progesterone, prednisone, corticosterone, and thyroglobulin: <0.001%.
Six milliliters of blood were used for the measurement of erythrocyte Na/K-ATPase activity in the presence and in the absence of DigiFab, 3 mmol/L MgSO4, and their combination as reported previously in detail.4 The amount of DigiFab for the ex vivo incubation with blood was 1 μg/ml, which corresponds to our recent observation of ex vivo activity of DigiFab9 and to the dose of Digibind used clinically in PE.8 In a separate experiment in erythrocytes obtained from normotensive pregnant subjects, we studied the effect of MgSO4 on MBG-induced Na/K-ATPase inhibition. For that, aliquots of the whole blood (0.5ml) were preincubated at room temperature for 30 minutes with MBG in the absence and in the presence of MgSO4. Erythrocytes were washed 3 times in an isotonic medium (145 mmol/L sodium chloride in 20 mmol/L Tris buffer; pH = 7.6, 4 °C). Activity of Na/K-ATPase was determined, as reported previously in detail.4 Erythrocytes were preincubated with Tween-20 (0.5%) in sucrose (250 mmol/L) and Tris buffer (20 mmol/L; pH = 7.4, 37 °C) for 30 minutes and were incubated for 30 minutes in the medium: sodium chloride 100 mmol/L, potassium chloride 10 mmol/L, magnesium chloride 3 mmol/L, ethylenediaminetetraacetic acid 0.5 mmol/L, Tris 50 mmol/L, ATP 2 mmol/L (pH = 7.4, 37 °C) in the final dilution 1:40. The reaction was stopped by the addition of trichloracetic acid to final concentration 7%. Total ATPase activity was measured by the production of inorganic phosphate (Pi), and Na/K-ATPase activity was estimated by the difference between ATPase activity in the presence and in the absence of 5 mmol/L ouabain.
All chemicals were from Sigma-Aldrich (St. Louis, MO). DigiFab was obtained from BTG International (London, UK). MBG (>98% purity by high performance liquid chromatography (HPLC)) was purified from the secretion from parotid glands of Bufo marinus toads, and 4G4 monoclonal antibody were developed as reported recently in detail.7
The results are presented as mean ± SEM. Data were analyzed using 1-way analysis of variance (ANOVA) (intergroup analysis) or by repeated measures ANOVA (intragroup analysis) followed by Newman–Keuls test, and by 2-tailed t test when applicable (Graph Pad Prism Software, San Diego, CA). A 2-sided P < 0.05 was considered to be statistically significant.
RESULTS
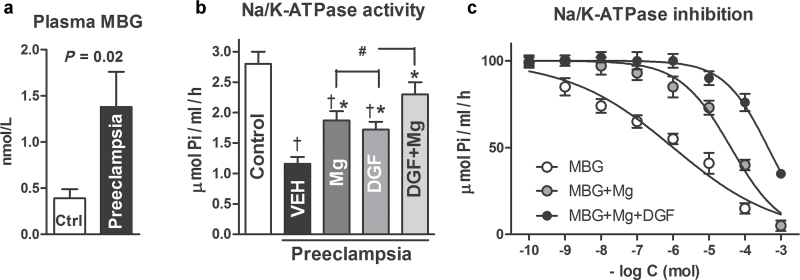
Maternal demographics and data on levels of blood pressure are presented in Table 1. Data on levels of plasma MBG and erythrocyte Na/K-ATPase activity in patients with PE and in control subjects are summarized in Figure 1. In patients with PE, elevated blood pressure was accompanied by a 4-fold increase in plasma MBG concentration (Figure 1a) and a concomitant 61% inhibition of Na/K-ATPase acti vity in erythrocytes (Figure 1b). Ex vivo incubation of erythrocytes in the presence of 1 μg/ml DigiFab or 3 mmol/L MgSO4 produced a comparable increase in Na/K-ATPase activity. Treatment of erythrocytes with a combination of DigiFab and MgSO4 produced a more substantial increase in Na/K-ATPase activity, which exceeded the effect of the each individual treatment.
Table 1.
Characteristics of study subjects and levels of blood pressure
| Characteristic | Normal pregnancy (n = 11) | Preeclampsia (n = 12) |
|---|---|---|
| Maternal age, y | 29±1 | 30±1 |
| Gestational weeks at delivery | 39±0.2 | 37.9±0.6 |
| No. of primigravida (%) | 5 (45%) | 5 (42%) |
| Cesarean section, No. | 4 | 7 |
| Vaginal delivery, No. | 7 | 5 |
| Infant birth weight, g | 3796±121 | 3404±127* |
| Systolic blood pressure, mm Hg | 111±2 | 159±5** |
| Diastolic blood pressure, mm Hg | 73±2 | 99±3** |
Data are given as means ± SEMs unless otherwise noted.
*P < 0.05, **P < 0.01 versus normal pregnant subjects, 2-tailed t test.
Figure 1.
Plasma marinobufagenin, erythrocyte Na/K-ATPase activity, and Na/K-ATPase inhibition. (a) Plasma levels of marinobufagenin (MBG) in 11 subjects with noncomplicated pregnancy (control) and in 12 patients with preeclampsia (two-tailed t test). (b) Activity of sodium (Na)/potassium (K)–ATPase in erythrocytes from 11 subjects with noncomplicated pregnancy (control) and in 12 patients with preeclampsia in the presence of vehicle (VEH), DigiFab (DGF, 1 ug/ml), magnesium sulfate (Mg, 3 mmol/L), and their combination (DGF + Mg). Means ± SEMs. By 1-way analysis of variance (ANOVA) and Newman–Keuls test: † P < 0.01 vs. control. By repeated measures ANOVA and Newman–Keuls test: *P < 0.01 vs. VEH and # P < 0.05 vs. DGF and Mg. (c) Inhibition of Na/K-ATPase in the erythrocytes from healthy pregnant subjects by MBG in the absence and in the presence of 3 mmol/L magnesium sulfate. Means +/-SEM. By repeated measures ANOVA and Newman-Keuls test: MBG vs. MBG + Mg and MBG + Mg + DGF: P < 0.01; MBG + Mg vs. MBG + Mg + DGF: P < 0.05.
As presented in Figure 1c, MBG inhibited activity of Na/K-ATPase in erythrocyte from control subjects in a concentration-dependent manner. MgSO4 did not affect the activity of the Na/K-ATPase at baseline, but the addition of 3 mmol/L MgSO4 to the incubation medium markedly reduced sensitivity of Na/K-ATPase to MBG. Thus, the median inhibitory concentration (IC50) to the Na/ K-ATPase inhibitory effect of MBG in the absence and in the presence of MgSO4 was 1.1±0.2 μmol/L and 40±10 μmol/L, respectively. Addition of 1.0 μmol/L DigiFab to the incubation medium further desensitized Na/K-ATPase to MBG (IC50=290±49 μmol/L).
DISCUSSION
The main observation of this study is that MgSO4 potentiates the effect of DigiFab with respect to reversal of PE-induced inhibition of Na/K-ATPase by an endogenous CTS, MBG. In PE, elevated plasma levels of MBG exhibit prohypertensive effect by inhibition of vascular Na/K-ATPase.3 Erythrocyte Na/K-ATPase is sensitive to endogenous CTSs, and changes in its activity could serve as a marker characterizing magnitude to CTS-dependent effects.4 , 9 Accordingly, in this study heightened levels of MBG in patients with PE were associated with substantial inhibition of erythrocyte Na/K-ATPase, whereas ex vivo incubation of PE blood in the presence of the CTS-neutralizing antibody DigiFab restored Na/K-ATPase activity. In this study DigiFab restored Na/K-ATPase activity ex vivo, and an increase in levels of Mg2+ ions in incubation medium potentiated the effect of DigiFab. Accordingly, in vitro, MBG potently inhibited Na/K-ATPase in erythrocytes from control subjects and Mg2+ desensitized Na/K-ATPase to MBG. These findings agree with previous clinical data demonstrating that MgSO4 is capable of offsetting the deleterious cardiac effects of digitalis overdose12 and that Mg2+ ions are capable of changing Na/K-ATPase conformation.10 Our observations suggest that MgSO4 is capable of increasing the efficacy of DigiFab-mediated immuno neutralization of MBG-induced Na/K-ATPase inhibition in pathological states in which levels of CTSs are elevated.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
This work was supported by Intramural Research Program, National Institute on Aging, National Institutes of Health (O.V.F. and A.Y.B.); by a grant from Ministry of Science and Education of Russian Federation Nr. 14.740.11.0928 (I.E.Z., V.V.I., E.V.F., N.G.S.); and by Glenveigh Pharmaceuticals, Chattanooga, Tennessee (C.D.A.). We are grateful to Brigit Sullivan, NIH Library, for editing assistance.
REFERENCES
- 1. Karumanchi SA, Lindheimer MD. Advances in the understanding of eclampsia. Curr Hypertens Rep 2008; 10:305–312 [DOI] [PubMed] [Google Scholar]
- 2. Gusdon JP, Jr, Buckalew VM, Jr, Hennessy JF. A digoxin-like immunoreactive substance in preeclampsia. Am J ObstetGynecol 1984; 150:83–85 [DOI] [PubMed] [Google Scholar]
- 3. Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertension 1999; 17:1179–1187 [DOI] [PubMed] [Google Scholar]
- 4. Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, Tapilskaya NI, Bzhelyansky A, Reznik VA, Frolova EV, Nikitina ER, Budny GV, Longo DL, Lakatta EG, Bagrov AY. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens 2008; 26:2414–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nikitina ER, Mikhailov AV, Nikandrova ES, Frolova EV, Fadeev AV, Shman VV, Shilova VY, Tapilskaya NI, Shapiro JI, Fedorova OV, Bagrov AY. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J Hypertens 2011; 29:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem 2008; 283:17946–17953 [DOI] [PubMed] [Google Scholar]
- 7. Goodlin RC. Antidigoxin antibodies in eclampsia. N Engl J Med 1988; 318:518–559 [DOI] [PubMed] [Google Scholar]
- 8. Adair CD, Buckalew VM, Graves SW, Lam GK, Johnson DD, Saade G, Lewis DF, Robinson C, Danoff TM, Chauhan N, Hopoate-Sitake M, Porter KB, Humphrey RG, Trofatter KF, Amon E, Ward S, Kennedy L, Mason L, Johnston JA. Digoxin immune fab treatment for severe preeclampsia. Am J Perinatol 2010; 27:655–662 [DOI] [PubMed] [Google Scholar]
- 9. Ishkaraeva-Yakovleva VV, Fedorova OV, Solodovnikova NG, Frolova EV, Bzhelyansky AM, Emelyanov IV, Adair CD, Zazerskaya IE, Bagrov AY. DigiFab interacts with endogenous cardiotonic steroids and reverses preeclampsia-induced Na/K-ATPase inhibition. Reprod Sci 2012; 19:1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skou JC, Butler KW, Hansen O. The effect of magnesium, ATP, P i, and sodium on the inhibition of the (Na + + K +)-activated enzyme system by g-strophanthin. Biochim Biophys Acta 1971; 241:443–461 [DOI] [PubMed] [Google Scholar]
- 11. Akera T, Brody TM, So RH, Tobin T, Baskin SI. Factors and agents that influence cardiac glycoside-Na+, K+-ATPase interaction. Ann N Y Acad Sci 1974; 242:617–634 [DOI] [PubMed] [Google Scholar]
- 12. Reisdorff EJ, Clark MR, Walters BL. Acute digitalis poisoning: the role of intravenous magnesium sulfate. J Emerg Med 1986; 4:463–469 [DOI] [PubMed] [Google Scholar]
- 13. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol 1992; 69:108G–118G [DOI] [PubMed] [Google Scholar]
- 14. National Institutes of Health Working Group on Hypertension in Pregnancy Classification of Hypertensive Disorders of Pregnancy. US Department of Health and Human Services: Bethesda, MD, 1991. [Google Scholar]