Abstract
This study aimed to elucidate the genetic causes underlying early-onset parkinsonism (EOP) in a consanguineous Iranian family. To attain this, homozygosity mapping and whole-exome sequencing were performed. As a result, a homozygous mutation (c.773G>A; p.Arg258Gln) lying within the NH2-terminal Sac1-like inositol phosphatase domain of polyphosphoinositide phosphatase synaptojanin 1 (SYNJ1), which has been implicated in the regulation of endocytic traffic at synapses, was identified as the disease-segregating mutation. This mutation impaired the phosphatase activity SYNJ1 against its Sac1 domain substrates in vitro. We concluded that the SYNJ1 mutation identified here is responsible for the EOP phenotype seen in our patients probably due to deficiencies in its phosphatase activity and consequent impairment of its synaptic functions. Our finding not only opens new avenues of investigation in the synaptic dysfunction mechanisms associated with parkinsonism, but also suggests phosphoinositide metabolism as a novel therapeutic target for parkinsonism.
Keywords: Homozygosity mapping, whole exome sequencing, SYNJ1, autosomal recessive parkinsonism
Introduction
Early-onset parkinsonism (EOP) is characterized by the presentation at a young age of tremor, hypokinesia, muscular rigidity, and postural instability (Jankovic, 2008). Parkinsonism may have many etiologies, including metabolic or infectious diseases, pharmacotherapy, or genetic causes. During the past years a number of inherited forms, which are usually characterized by juvenile onset, L-dopa responsiveness, and recessive pattern of inheritance, have been described (Martin, et al., 2011; Paisan-Ruiz, et al., 2010). To date, there are at least six different genes associated with autosomal recessive early-onset parkinsonism (AR-EOP): PARK2 (PRKN; MIM# 600116), PARK6 (PINK1; MIM# 605909), PARK7 (DJ-1; MIM# 602533), PARK9 (ATP13A2; MIM# 606693), PARK14 (PLA2G6; MIM# 612953), and PARK15 (FBXO7; MIM# 260300) (Hardy, et al., 2009). Mutations in PRKN, PINK1, and DJ-1 cause more typical parkinsonism while ATP13A2, PLA2G6, and FBXO7 mutations are responsible for complex syndromes, with pyramidal signs, cognitive decline, as well as other neurological and psychiatric symptoms, and may become non-responsive to levodopa treatment with the disease progression (Paisan-Ruiz, et al., 2010). Additionally, PRKRA (DYT16; MIM# 612067) and Spactasin (SPG11; MIM# 610844) mutations have also been reported in juvenile atypical parkinsonism forms and SLC6A3 mutations are known to cause infantile dystonia-parkinsonism syndrome (PKDYS; MIM# 613135) (Camargos, et al., 2008; Kurian, et al., 2009; Paisan-Ruiz, et al., 2010). More recently, mutations within DNAJC6 (MIM# 608375) encoding auxilin, a protein implicated in clathrin-mediated endocytosis, have been identified in families featuring autosomal recessive juvenile parkinsonism (Edvardson, et al., 2012; Koroglu, et al., 2013).
In the current study we aimed to identify the genetic causes underlying disease in a consanguineous family of Iranian ancestry featuring early-onset parkinsonism with generalized seizures. Whole exome sequencing (WES), particularly in conjunction with other methods like SNPs-based arrays, has become a fruitful strategy for gene identification in both complex and Mendelian traits and as such is an ideal approach to identify disease causal alleles (Krebs and Paisan-Ruiz, 2012; Singleton, 2011). Therefore, HM through high-throughput SNP genotyping followed by WES and disease-segregation analyses was performed for disease-locus and gene identification. As a result, we identified a disease-segregating mutation, c.773G>A causing p.Arg258Gln, within the NH2-terminal SAC1-like inositol phosphatase (Sac1) domain of the polyphosphoinositide phoshatase SYNJ1 (MIM# 604297), which also contains an inositol 5-phosphatase domain and a COOH-terminal proline-rich region that interacts with a variety of SH3 domain-containing endocytic factors, such as endophilin and amphiphysin (Dittman and Ryan, 2009). We later evaluated the effects of the p.Arg258Gln mutation on the enzymatic properties of SYNJ1 and concluded that the SYNJ1 p.Arg258Gln mutation impairs the Sac1-like phosphatase activity. Our current finding not only opens a new avenue of investigation in the field of Parkinson research, but also adds new evidence that defects in membrane traffic at the synapse may be key factors in the development of both Parkinson disease and parkinsonism.
Methods
Subjects
A consanguineous family featuring early-onset parkinsonism was clinically examined. The family consisted of healthy parents, who were first-degree relatives, two affected and three unaffected siblings (Figure 1). The local ethics committee at Tehran University of Medical Sciences approved this study and informed consent was obtained from all participants. DNA samples from all members were isolated from whole blood using standard procedures.
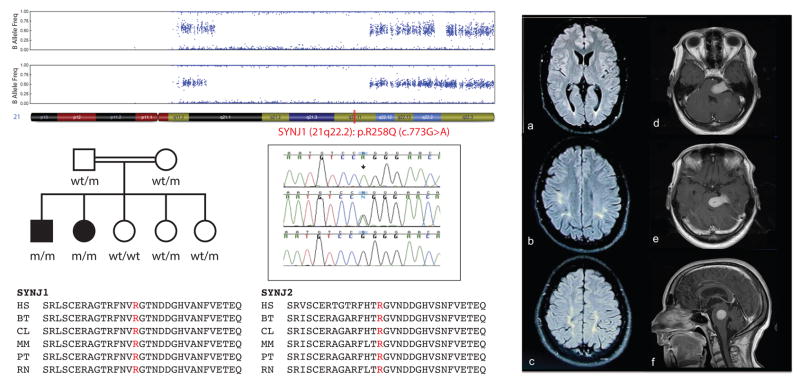
Figure 1.
Left side Upper panel: B allele frequency plots from Genome studio software (Illumina) showing the loss of heterozygosity area (LOH (blank areas); 21q21.1-22.2) shared by both affected siblings; the location of SYNJ1 within the disease-associated LOH area is highlighted in red. Middle panel: The pedigree structure of the Iranian family with EOP is shown in the left side while the Sanger chromatograms of the human reference sequence (bottom) as well as both heterozygous (middle) and homozygous (top) mutant sequences are shown in the right side; a black arrow highlights the pathogenic mutation. The p.Arg258Gln mutation is represented as p.R258Q. Bottom panel shows the conservation of R258 amino-acid (highlighted in red) within different orthologs in both SYNJ1 and SYNJ2 proteins. HS: Homo sapiens; BT: Bos Taurus; CL: Canis lupus; MM: Mus musculus; PT: Pan troglodytes; RN: Rattus norvegicus. Right side: Brain FLAIR magnetic resonance images (MRIs) of patient 1 showing bilateral periventricular (a) and subcortical (b and c) white matter hyperintense signals predominantly in parietal-occipital regions. Axial (e & e) and sagittal (f) post-gadolinium brain MR images of patient 2 show an intensely enhancing meningioma with compressing the left pons and lower midbrain. There was no evidence of obstructive hydrocephalus.
Genetic variants identified within disease-associated loci (Tables 1 and 2) were additionally tested by direct Sanger sequencing in 96 DNA samples belonging to controls individuals of Iranian ancestry from the same geographical region of our family and 92 DNA samples of Caucasian neurologically normal individuals available to purchase at Coriell Cell Repository (NDPT093; http://www.coriell.org/).
Table 1.
Homozygous segments found only present in both affected sibling by homozygosity mapping through genome-wide SNP genotyping
| CHR | SNP1 | SNP2 | Position 1 (bp) | Position 2 (bp) | Size (Kb) | N° SNP |
|---|---|---|---|---|---|---|
| 3 | rs9871790 | rs2046037 | 0 | 2,600,804 | 2600.8 | 1039 |
| 8 | rs11993658 | rs16938809 | 46,886,735 | 75,062,690 | 28176 | 6097 |
| 21 | rs171477 | rs2834280 | 19,150,133 | 35,228,502 | 16078.4 | 4600 |
Highlighted in bold is the genomic region turned out to be the disease-associated locus.
Table 2.
Homozygous SNPs identified in both affected siblings after performing whole exome sequencing followed by adequate filtering
| Chr | Position (bp) | Gene | MIM (#) | Nucleotide change | Protein change | Pathogenicity’s Prediction# | Control data | Brain expression& | Function/Associated disease |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 48,877,146 | MCM4 | 602638 | c.706A>G | p.Thr236Ala | 0.493/Neutral | 0/376 | Cerebral cortex, cerebellum, hippocampus | Adrenal insufficiency, short stature, NK cell deficiency, and FGD |
| 9 | 39,357,781 | SPATA31A1* | N.A | c.274C>G | p.Gln92Glu | 0.213/Neutral | Not examined | No Information | Unknown |
| 21 | 34,067,416 | SYNJ1 | 604297 | c.773G>A | p.Arg258gln | 0.877/Disease | 0/376 | Cerebral cortex, cerebellum, hippocampus | Synaptic vesicle recycling |
Chromosome position, affected gene, nucleotide and protein changes, expression data, as well as function or associated disease are shown for each SNP identified. The nt numbering reflects cDNA with +1 as the A of the ATG initiation codon in the reference sequence (www.hgvs.org/mutnomen). The initiation codon is codon 1. Highlighted in bold are the disease-segregating mutations also found present in www.hgvs.org/mutnomen previously shown disease-associated loci (Table 1). NK: natural killer. FGD: Familial glucocorticoid deficiency P.Gln92Glu mutation did not segregate with disease.
Predictions are based on MutPred and SNPs&GO computational methods.
Previous symbol, FAM75A1.
20 DNA samples of affected individuals with early-onset parkinsonism were also tested for the entire coding region of SYNJ1. Although seizures were not present in any of these patients, all presented with complex early-onset parkinsonism, including parkinsonism, spasticity, and dystonia as common phenotypic features.
Genome-wide SNP Genotyping and Homozygosity Mapping
SNP genotyping was performed in all available family members (n=7) using the HumanOmniExpress beadchips and HiScanSQ system (Illumina Inc., San Diego, CA, USA). Genotyping quality assessments were undertaken according to the appropriate options within the Genome Studio program (GS; Illumina). PLINK input reports were generated within the GS and uploaded to PLINK v1.07 program (Purcell, et al., 2007). Homozygous segments were identified using the ROH tool (Runs of homozygosity) within PLINK, where a minimum physical size threshold of 1 Mb and at least 100 homozygous adjacent markers in length, including no more than two SNPs with missing genotypes and only one possible heterozygous genotype, were used as inclusion criteria. Subsequently, overlapping and potentially matching segments were also identified in PLINK using an allelic matching of 0.99 as threshold. Homozygous segments were also visualized using the Illumina genome viewer (IGV) within the GS program.
Whole Exome Sequencing
Whole exome sequencing was performed in both affected siblings. The SureSelect Human All exon 50Mb exon-capture kit was used for library enrichment (Agilent Technologies Inc., Santa Clara, CA, USA). The captured exome libraries were then sequenced on a HiSeq2000 according to the manufacturer’s instructions for paired-end 100-bp reads (Illumina Inc, San Diego, CA, USA) and on a single flow cell lane. After sequencing, data were put through a computational pipeline for WES data processing and analysis following the general workflow adopted by the 1000 genomes project (DePristo, et al., 2011). First, the alignment of raw sequence reads to the human reference genome sequence (NCBI GRCh37) was performed using a fast lightweight Burrows-Wheeler Alignment Tool (BWA) (Li and Durbin, 2009). The Genome Analysis Toolkit (GATK v1.5-16-g58245bf) was then used for base-quality recalibration and local realignment to minimize base calling error and mapping error, respectively. Lastly, the GATK Unified Genotyper tool was employed to call single-nucleotide substitutions (SNP/SNV) and short insertions/deletions (INDEL). Only passing variants were included in the final variant set. Calls were filtered based on the mapping quality (q30 or higher) and depth of coverage (d10 or higher). Resulting calls were annotated with AnnTools, an exhaustive genome annotation toolkit (Makarov, et al., 2011).
Filtering of Common Genetic Variation
Any potential mutation observed as common variation (frequency > 5%) in the dbSNP137 or 1000 Genomes Project Phase 1 was removed for further analyses. Genetic variants mapping to intra-genic, intronic, and non-coding exonic regions, with the exception of those variants mapping close to splice sites, were also removed since they are unlikely to be causative. It is worth noting that in the 1,000 Genome project five major populations, including West African, European, American, and East and South Asian, are targeted. Then, a second filter, which consisted of removing common variation (frequency > 5%) present in other public databases, such as the Exome Variant Server of the National Heart, Lung, and Blood Institute (NHLBI) Exome Sequencing Project (http://evs.gs.washington.edu/EVS/)(Exome Variant Server, 2012), exomes generated in house from unaffected members of families with various phenotypes (n=40), and exome data generated in 500 Caucasian control samples that were obtained as VCF files from a large collection of unaffected Caucasian individuals that have been sequenced as part of the ARRA Autism Sequencing Collaboration (AASC) (MH089025, Mark Daly, communicating PI, Joseph D Buxbaum, Bernie Devlin, Richard Gibbs, Gerard Schellenberg, James Sutcliffe, collaborating PI’s), was applied to all novel coding genetic mutations found to be present in both siblings. Only variants that we know for certain that were not pathogenic were included in this second filter. This filter was done last because low frequency genetic alleles could not have been removed, since, for example, low frequency heterozygous alleles may cause a disease when present in the homozygous state.
Prediction of Mutation Pathogenicity
To assist in causative gene identification, the pathogenicity of each novel mutation identified present in both siblings and absent in large number of control individuals was predicted by two computational methods previously evaluated as most efficient (Thusberg, et al., 2011): MutPred (http://mutpred.mutdb.org/) and SNPs&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go/). All sequence variant descriptions were also examined using the Mutalyzer program (http://www.LOVD.nl/mutalyzer/). The HomoloGene database from NCBI web site was also used to examine the conservation of both disease-segregating mutations in different species (http://www.ncbi.nlm.nih.gov/homologene). Clustalw2 was also used to align Synaptojanin 1 with other human proteins containing Sac1 domains (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Gene Screening Analyses
Genomic primers for PCR amplifications were designed using a primer design public website (http://ihg.gsf.de/ihg/ExonPrimer.html). Primers were used to amplify exon 7 of MCM4, exon 3 of SPATA31A1 (previous symbol, FAM75A1), and all coding exons of SYNJ1 (primer sequences available upon request). PCR amplifications were performed as previously described (Karkheiran, et al., 2012). All purified PCR products were then sequenced in both forward and reverse directions with Applied Biosystems BigDye terminator v3.1 sequencing chemistry as per the manufacturer’s instructions. The resulting sequencing reactions were resolved on an ABI3130 genetic analyzer (Applied Biosytems, Foster city, CA, USA) and analyzed using Sequencer 5.0 software (Gene Codes Corporation, Ann Arbor, MI, USA).
Mutagenesis and Protein Expression and Purification
Direct mutagenesis was done using the QuikChange Lightning Site-Directed Mutagenesis kit (Stratagene, Agilent Technologies Inc., Santa Clara, CA, USA), both pcDNA3-FLAG-human-Synaptojanin 1-145 and pEGFPC1-FLAG-human-Synaptojanin 1-145 (Addgene: http://www.addgene.org/22291/ and http://www.addgene.org/22293/, respectively) and corresponding primers (Primer sequences available upon request). Plasmids were purified using the Plasmid Purification Maxi kit for high-copy plasmids. The tagged human SYNJ1 wild-type and mutants were transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen, Life Technologies, Grand Island, NY, USA) and purified by immunoprecipitation on anti-FLAG M2 bead. Protein concentration of affinity purified SYNJ1 was determined by loading BSA protein standards alongside purified SYNJ1 and performing SDS-PAGE followed by coomassie blue staining (Invitrogen, Life Technologies, Grand Island, NY, USA). Western Blot was performed using anti-FLAG M2 antibody (Sigma Aldrich, St. Louis, MO, USA) to show equal amounts of affinity purified wild-type and mutant SYNJ1. Anti β-Actin antibody (8H10D10) (Cell Signaling Technology, Danvers, MA, USA) was used as loading control.
Malachite Green-Based Phosphatase Assay
Enzymatic activity of wild-type and mutants SYNJ1 was measured by malachite-green based assays. The final assay mixture (25μL) contained either 1μg of PI(4,5)P2, 10μg of PI3P or PI4P, and 250ng enzyme with 1μg substrate or 500ng enzyme with 10μg substrate. Reactions were incubated for 30 min at 37°C and the supernatants were added to 80 μL of malachite green solution (Echelon Biosciences Inc, Salt Lake City, UT, USA) and allowed to incubate for 5 min at 37°C, and then at room temperature for another 5 min. The absorbance was measured by plate reader at a wavelength of 620 nm and normalized to the wild-type value.
Statistical Analyses
All the data are expressed as the mean ± SEM (Standard Error of the Mean). Graphics represent means and SEM of at least nine independent experiments. The statistical differences between wild-type and both mutants synaptojanin 1 were determined by a non-parametric test like Mann-Whitney U test, in which p values of p≤0.001 were considered highly significant (***).
Reporting pathogenic genetic variants
The genetic variant identified in this study as pathogenic is now included in an existing locus-specific database (LSDB) at http://databases.lovd.nl/shared/genes/SYNJ1.
Results
We report two affected siblings suffering from early-onset parkinsonism and generalized seizures. The main clinical features of both patients are described below.
Patients’ Clinical Details
Patient I
This 29-year-old man was born to consanguineous parents. At the age of 3 years he developed generalized seizures, which responded to phenobarbital. At the age of 20 he developed a right hand tremor and progressive bradykinesia. Within two years, he developed severe bradykinesia, bilateral hand (R>L) and chin tremor, apraxia of eyelid opening (ALO), and dysarthria. He was treated with trihexyphenidyl and l-dopa/carbidopa with improvement in both tremor and bradykinesia but soon developed l-dopa-induced dyskinesias at very low doses (50mg). Due to severity of dyskinesia l-dopa was stopped and he was treated with the dopamine agonist bromocriptine up to 15 mg/day with modest improvement of all parkinsonian symptoms and a reduction in dyskinesia. There was no sign of cognitive impairment. He continued taking phenobarbital for over 20 years with acceptable seizure control until three years ago; he now only has one to two seizures per year.
On physical examination at the age of 29, he had generalized bradykinesia with bilateral limb rigidity, right side predominant resting tremor, chin tremor, and inability to open his eyelids spontaneously. He had jerky saccades and saccadic pursuits. His speech was unintelligible due to severe hypophonia and his gait was shuffling with postural instability. Off medication Unified Parkinson Disease Rating Scale part III (UPDRS-III) score was 38 (maximum score = 56) with most scores related to bradykinesia and rigidity. Muscle strength, deep tendon reflexes, cerebellar, and sensory functions were all normal. Administration of 25mg/day of l-dopa completely alleviated the ALO and improved bradykinesia, rigidity, oculomotor abnormalities, and shuffling gait. One hour after medication administration, his gait was normal, his speech became more intelligible, but postural instability was unchanged. Wearing off started to appear 2 hours after drug administration (The UPDRS-III scores were 28 and 18 at 30 minutes and 1 hour respectively after taking levodopa, the latter being mostly due to rest tremor). With doses greater than 25 mg of levodopa, in addition to dyskinesias, the patient became euphoric and talkative. There was no evidence of hyposmia, urinary urgency, incontinence, erectile dysfunction, constipation, or orthostatic hypotension. During the “on” period, cognitive functioning was intact with moderate bradyphrenia. Brain MRI showed mild cortical atrophy and bilateral symmetric T2 hyperintensities in the white matter, mostly posteriorly (Figure 1a, b, c).
Patient II
This 39-year-old woman had a febrile convulsion in infancy followed by unprovoked generalized tonic-clonic seizures for which she was treated with phenobarbital. Developmental milestones were normal. In her early twenties she developed a right hand tremor, followed by bradykinesia, severe jaw tremor, and eyelid twitching. The disease progression was similar to that of her brother. She could walk unassisted until the age of 32 years, but subsequently required assistance. She had severe dyskinesia with small doses of levodopa (25 mg) and non-ergot dopamine agonists, and only tolerated anticholinergics and bromocriptine, which gave her good tremor control and moderate improvement in bradykinesia. Cognitive function was grossly normal. Brain MRI performed at 37 years old showed a large foramen magnum meningioma (Figure 1d, e, f). After surgery to remove this tumor, the patient lost her ability to walk and became bedbound. During her last visit, at the age of 39, she was anarthric and in fixed “quadriplegia in flexion” posture, but she correctly responded to “open and close your eyes” and “make a sound” commands. Mass lesions compressing the brainstem may cause parkinsonism; however, the SYNJ1 mutation, shared with her brother, and not this lesion, are likely to be responsible for our patient’s clinical syndrome. The absence of localizing corticospinal signs and the lack of surrounding edema seen on MRI indicate that the tumor grew extremely slowly, displacing the brainstem without causing significant neuronal dysfunction. The patient’s seizures could not be attributed to this tumor. Although not illustrated here, other views of the brain MRI clearly show that there was no evidence of obstruction of cerebrospinal fluid flow through the aqueduct and the lateral ventricles were of normal size. In addition, the features of parkinsonism seen here are not observed in ventricular obstruction.
Molecular Analyses
All available family members (n=7) were subject to genome-wide SNP genotyped analyses and HM, which revealed three potential disease-associated loci. These loci, only shared by the two affected siblings, were located at chromosomes 3, 8, and 21 (Table 1). No shared region of homozygosity or copy number variation (CNV) was identified within the known PD loci. The WES performed in the two affected siblings captured 95.18% and 95.88% of the target exome at 20-fold coverage or higher for patients I and II, respectively. This lead us to the high-quality identification of 61,286 SNVs and 9,836 indels for Patient I and 67,337 SNVs and 13,061 indels for Patient II. All known PD genes were well covered by our WES approaches and excluded. After an adequate filtering (see methods), 832 non-synonymous, 11 nonsense, and 459 synonymous genetic variants were identified for Patient I, while 982 non-synonymous, 10 nonsense, and 463 synonymous genetic variants were identified for Patient II. None of the nonsense genetic variants were present in both patients and only 223 non-synonymous genetic variants (187 heterozygous and 36 homozygous) were common to both affected siblings. Further investigation of the 223 novel genetic variants in the dbSNP137, in other public databases, and exomes generated in house (see Methods) left us with only three novel genetic variants present in both siblings (Table 2). Two of these, c.706A>G (p.Thr236Ala) within minichromosome maintenance complex component 4 (MCM4; 8q11.2) and c.773G>A (p.Arg258Gln) within synaptojanin 1 (SYNJ1; 21q22.2), were located in genomic regions previously found associated with disease through homozygosity mapping (Tables 1 & 2). The genotyping area harboring the third variant (SPATA31A1 locus) was additionally inspected, but only a small homozygous area of 91kb also shared by unaffected individual was identified. As expected, subsequent sequencing of this variant in all family members revealed that it does not segregate with disease. Both variants located within the disease-associated loci were subsequently validated through Sanger sequencing and examined in the remaining family members: both mutations were present in the homozygous state in both affected siblings while both parents where heterozygous mutation carriers and unaffected members were either homozygous carriers for the wild-type allele or heterozygous mutation carriers, clearly consistent with a disease-segregation status. Further testing of both mutations in 376 control chromosomes (184 Caucasians and 192 Iranians) failed to identify any additional mutation carrier. However it is very unlikely that the phenotype observed in our patients is due to the p.Thr236Ala mutation in MCM4, which is essential for the initiation of eukaryotic genome replication, since it was predicted to be non-causative and MCM4 mutations have recently been associated with clinical features, such as autosomal recessive adrenal insufficiency, short stature, natural killer cell deficiency, and familial glucocorticoid deficiency (Casey, et al., 2013; Hughes, et al., 2012; Yamaguchi, et al., 2013), not present in our patients (Table 2). Moreover, none of the patients already described with pathogenic MCM4 mutations showed signs of parkinsonism; and although none of the MCM4 mutations identified, including the one described here, lie within a functional domain, they all seem to be located throughout the protein, ruling out the possibility that MCM4-associated phenotypes are determined by the localization of the mutation within the protein. By contrast, the p.Arg258Gln mutation in SYNJ1, a highly brain enriched polyphosphoinositide phoshatase involved in synaptic vesicle recycling (Cremona, et al., 1999; McPherson, et al., 1996), was predicted to be pathogenic. This amino-acid change results from a G>A mutation at position 258 for transcripts 1 (NM_003895.3) and 2 (NM_203446.2) and 219 for transcripts 3 (NM_001160302.1) and 4 (NM_001160306.1). SYNJ1 comprises two inositol phosphatase domains arranged in tandem – an N-terminal Sac1 inositol phosphatase domain and a central inositol 5-phosphatase domain – followed by a COOH-terminal proline-rich region that interacts with a variety of protein factors implicated in signaling, endocytosis and actin nucleation (McPherson, et al., 1996; Slepnev and De Camilli, 2000). The Sac 1 domain dephosphorylates PI3P, PI4P, and PI(3,5)P2 (Guo, et al., 1999), while the preferred substrates of the 5-phosphatase domain are PI(4,5)P2 and PI(3,4,5)P3 (Cremona, et al., 1999). The p.R219/258 amino-acid is localized in the Sac1 domain and is highly conserved among 18 different orthologs and in the SYNJ2 protein (NM_003889), which has a similar domain structure but more widespread tissue distribution than SYNJ1(Nemoto, et al., 1997) (Figure 1); it is also conserved among three other human proteins containing Sac1 domains, including SACM1L (SAC1, NM_014016.3), INPP5F (SAC2, NM_014937), and FIG4 (SAC3, NM_014845) proteins (data not shown). Taking into account all public SNPs databases, exomes generated in house, and DNA control samples testing by direct Sanger sequencing, p.Arg258Gln was absent in over 10,000 control chromosomes, likely supporting its pathogenicity.
Although 20 DNA samples of affected individuals with complex early-onset parkinsonism were additionally tested for the entire coding region of SYNJ1, we failed to identify any other mutation carrier.
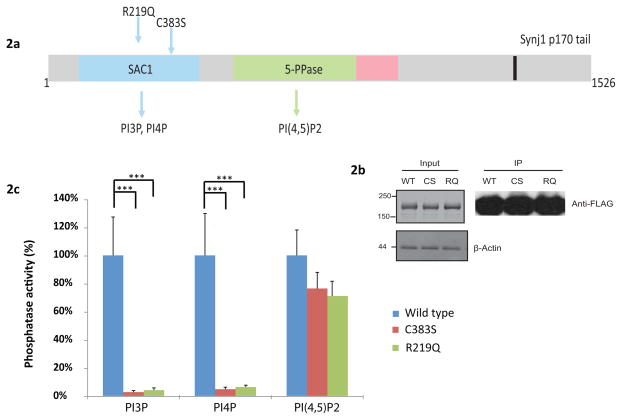
We thus tested whether the p.Arg258Gln (p.Arg219Gln) mutation impairs the phosphoinositide phosphatase activity of the Sac1 domain. The c.1149T>A/c.1264T>A (p.Cys383Ser/p.Cys422Ser) mutation in the Sac1 domain of human SYNJ1 domain was used as a control, based on previous work showing that p.Cys383Ser impairs the Sac1-like phosphatase activity towards PI3P and PI4P (Mani, et al., 2007). This analysis showed that while p.Cys383Ser (p.Cys422Ser) and p.Arg219Gln (p.Arg258Gln) mutations did not affect in a significant way the phosphatase activity against PI(4,5)P2, which is known to be accounted by the 5-phosphatase domain of SYNJ1, they drastically inhibited the phosphatase activity against PI3P and PI4P (Figure 2c), as expected for mutations that impair the Sac1-like phosphatase activity of this enzyme (Guo, et al., 1999).
Figure 2.
2a: Domain organization of SYNJ1-145 (3) and SYNJ1-170 (4) isoforms (Haffner, et al., 1997; Perera, et al., 2006). SYNJ1-145 is highly expressed in the nervous system, specifically in mature brain and synapses, while the alternatively spliced isoform SYNJ1-170, which has a longer C-terminal, is ubiquitously expressed but at lower levels. The domain organization was drawn based on published reports and SMART predictions (http://smart.embl-heidelberg.de/) (Guo, et al., 1999; Jha, et al., 2004) and includes the two polyphosphoinositide phosphatase regions and the C-terminal region, which is responsible for protein-protein interactions. The extended C-terminal tail of SYNJ1-170 contains binding sites for proteins of the endocytic machinery. Both p.Arg219Gln (R219Q) and p.Cys383Ser (C383S) mutations lying in the Sac1-like region are shown. 2b: Anti-Flag Western-Blot showing equal amounts of affinity purified Flag-tagged wild-type and mutant SYNJ1. 2c: Wild-type and mutants SYNJ1 were expressed in HEK 293T cells and purified by immunoprecipitation. The enzymatic activity was measured by malachite-green based assays. Error bars are SEM. Graphics represent means and SEM of at least nine independent experiments. (***) indicates p values of p≤0.001.
Discussion
We report on a consanguineous family with autosomal recessive early-onset parkinsonism with generalized seizures. Two siblings developed seizures in early childhood, followed by resting hand tremor and bradykinesia in their early 20’s, and progressing to chin tremor and apraxia of eyelid opening. HM, WES, and gene screening analyses led us to the identification of three disease-associated loci and two disease-segregating genetic variants (Tables 1 and 2). Both disease-segregating variants, p.Thr236Ala and p.Arg258Gln, were absent in controls, including some of Iranian ancestry. Although the Iranian control population tested here was not large, given the high rate of consanguinity extant in the Iranian population (Akrami and Osati, 2007), the prevalence of a rare private allele in this population is much higher than the generally expected (Bittles and Black, 2010). Moreover, our follow up investigations in the most likely disease-causing mutation and the only one predicted to be pathogenic, provided additional evidences to support the p.Arg258Cys mutation as the disease-causing mutation. Previous studies demonstrated that the synaptic function of SYNJ1 depends on its intact dual phosphatase activity (Mani, et al., 2007). Here we showed that the disease-linked mutation almost entirely eliminated both 3-phosphatase and 4-phosphatase activities (Figure 2c), which also suggests that the patients carrying this mutation may have increased levels of SYNJ1 substrates. The inhibition of the Sac-1 activity is expected to impair synaptic vesicle endocytosis and reavailability in nerve terminals, as suggested by previous reports and studies done in mice (Cremona, et al., 1999; Mani, et al., 2007).
The identification of a SYNJ1 mutation linked to parkisonism is interesting because this lipid metabolizing enzyme is a key regulator of phosphoinositide metabolism in the nervous system and has been shown to regulate key synaptic processes, such as the recycling of synaptic vesicles, the internalization of AMPA receptors, as well as actin dynamics (Frere, et al., 2012; Gad, et al., 2000; Gong and De Camilli, 2008; Harris, et al., 2000; Verstreken, et al., 2003). Although SYNJ1 mutations have not been previously associated with a human neurological disease, the overexpression of this protein in Down syndrome mouse models causes a deficiency of PI(4,5)P2 as well as learning deficits (Voronov, et al., 2008), while Synj1 haploinsufficiency is protective against the synaptotoxic action of beta-amyloid in vitro and in mouse models of Alzheimer disease (Berman, et al., 2008; McIntire, et al., 2012). Additionally, Synj1 knockout mice fail to thrive and rapidly develop severe neurological manifestations including severe weakness, ataxia, spontaneous epileptic seizures, and poor motor coordination (Cremona, et al., 1999); and loss of SYNJ function in C. elegans, zebrafish and drosophila results in severe nervous system defects mutants, such as abnormal balance, posture, and locomotion (Harris, et al., 2000; Minagawa, et al., 2001; Stefan, et al., 2002; Van Epps, et al., 2004; Verstreken, et al., 2003).
There is mounting evidence that synaptic vesicle trafficking pathways are implicated in neurodegeneration (Esposito, et al., 2012). Alpha-synuclein has been implicated in synaptic vesicle exocytosis and in synaptic vesicle recycling (Burre, et al., 2010; Nemani, et al., 2010). LRRK2 mutations also cause a defect in synaptic vesicle endocytosis, which can be rescued by co-expression of Rab5b (Heo, et al., 2010). LRRK2 has also been identified as regulator of endophilin-A (Matta, et al., 2012), which is a major binding partner of SYNJ1 and interacts and participates with Parkin in the ubiquitination of proteins within synaptic endophilin-A complexes (Trempe, et al., 2009). And lastly, disruption of the SYNJ1/endophilin interaction by Cdk5, which phosphorylates parkin (Rubio de la Torre, et al., 2009; Yamamoto, et al., 2005) and SYNJ1 (Tan, et al., 2003), has been suggested to be important for synaptic vesicles recycling (Lee, et al., 2004).
These findings add further support to the evidence that many parkinsonism-associated proteins, including alpha-synuclein, parkin, dardarin (LRRK2), auxilin, and now also SYNJ1, act as important regulators of synaptic vesicle trafficking pathways at synapses. All together they foster the hypothesis that aberrant functions of proteins implicated in the SV recycling may be responsible for at least part of the neuronal cell loss seen in Parkinson disease and other neurodegenerative diseases.
In conclusion, our data support the disease-segregating p.Arg258Gln (p.Arg219Gln) mutation, which is absent in over 10,000 control chromosomes and impairs the Sac1-like domain activity, as the genetic cause responsible for autosomal recessive parkinsonism with generalized seizures in our patients. Although further studies are warranted to gain insights into the mechanisms by which dysfunction of phosphoinositide phosphatases, such as synaptojanin 1, results in neurological dysfunction and likely cell death, our current finding opens a new avenue of investigation in the field of Parkinson research, adds further evidence that defects in membrane trafficking at the synapse may be key factors in the development of both Parkinson disease and parkinsonism, and suggests phosphoinositide metabolism as a novel therapeutic target for parkinsonism.
Acknowledgments
Grant Support: The Parkinson’s Disease Foundation (CPR), the Ellison Foundation (PDC), and the Michael J. Fox Foundation (ZY).
NIH/NINDS: R01NS060809 and R01NS072359 to ZY; R37NS036251 to PDC; R01NS079388 to CPR
We thank the patients and their families for participating in this study and the Parkinson’s Disease Foundation (Lucien Côté Early Investigator Award given to C.P-R) for support. This work was also supported in part by grants from the NIH/NINDS (R01NS060809 and R01NS072359; ZY), the Michael J. Fox Foundation (ZY), the NIMH R01MH095797 (Luliana Ionita-Laza (VM), the NIH/NINDS (R37NS036251; PDC), the Ellison Foundation (PDC), and the NIH/NINDS (R01NS079388; CPR). Professor Gareth R. John from the Department of Neurology of the Icahn School of Medicine at Mount Sinai is thanked for letting us use his microplate reader and software.
Footnotes
Disclosure statement: The authors declare no conflict of interest
References
- Akrami SM, Osati Z. Is consanguineous marriage religiously encouraged? Islamic and Iranian considerations. J Biosoc Sci. 2007;39(2):313–316. doi: 10.1017/S0021932006001684. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dall’Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G. Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci. 2008;11(5):547–554. doi: 10.1038/nn.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittles AH, Black ML. Evolution in health and medicine Sackler colloquium: Consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1779–1786. doi: 10.1073/pnas.0906079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7(3):207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Casey JP, Nobbs M, McGettigan P, Lynch S, Ennis S. Recessive mutations in MCM4/PRKDC cause a novel syndrome involving a primary immunodeficiency and a disorder of DNA repair. J Med Genet. 2013;49(4):242–245. doi: 10.1136/jmedgenet-2012-100803. [DOI] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99(2):179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One. 2012;7(5):e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Ana Clara F, Verstreken P. Synaptic vesicle trafficking and Parkinson’s disease. Dev Neurobiol. 2012;72(1):134–144. doi: 10.1002/dneu.20916. [DOI] [PubMed] [Google Scholar]
- Exome Variant Server. NHLBI Exome Sequencing Project (ESP) Seattle, WA: 2012. (URL: http://evs.gs.washington.edu/EVS/) [05/2013] [Google Scholar]
- Frere SG, Chang-Ileto B, Di Paolo G. Role of phosphoinositides at the neuronal synapse. Subcell Biochem. 2012;59:131–175. doi: 10.1007/978-94-007-3015-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27(2):301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc Natl Acad Sci U S A. 2008;105(45):17561–17566. doi: 10.1073/pnas.0809221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274(19):12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Haffner C, Takei K, Chen H, Ringstad N, Hudson A, Butler MH, Salcini AE, Di Fiore PP, De Camilli P. Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419(2–3):175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C. The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev. 2009;19(3):254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150(3):589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo HY, Kim KS, Seol W. Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5. Exp Neurobiol. 2010;19(2):97–105. doi: 10.5607/en.2010.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, Costigan C, Clark AJ, Metherell LA. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jha A, Agostinelli NR, Mishra SK, Keyel PA, Hawryluk MJ, Traub LM. A novel AP-2 adaptor interaction motif initially identified in the long-splice isoform of synaptojanin 1, SJ170. J Biol Chem. 2004;279(3):2281–2290. doi: 10.1074/jbc.M305644200. [DOI] [PubMed] [Google Scholar]
- Karkheiran S, Krebs CE, Makarov V, Nilipour Y, Hubert B, Darvish H, Frucht S, Shahidi GA, Buxbaum JD, Paisan-Ruiz C. Identification of COL6A2 mutations in progressive myoclonus epilepsy syndrome. Hum Genet. 2012;132(3):275–283. doi: 10.1007/s00439-012-1248-1. [DOI] [PubMed] [Google Scholar]
- Koroglu C, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord. 2013;19(3):320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Krebs CE, Paisan-Ruiz C. The use of next-generation sequencing in movement disorders. Front Genet. 2012;3:75. doi: 10.3389/fgene.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest. 2009;119(6):1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101(2):546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V, O’Grady T, Cai G, Lihm J, Buxbaum JD, Yoon S. AnnTools: a comprehensive and versatile annotation toolkit for genomic variants. Bioinformatics. 2011;28(5):724–725. doi: 10.1093/bioinformatics/bts032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P, Ryan TA. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron. 2007;56(6):1004–1018. doi: 10.1016/j.neuron.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson’s disease. Annu Rev Genomics Hum Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, De Bock PJ, Morais VA, Vilain S, Haddad D, et al. LRRK2 Controls an EndoA Phosphorylation Cycle in Synaptic Endocytosis. Neuron. 2012;75(6):1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- McIntire LB, Berman DE, Myaeng J, Staniszewski A, Arancio O, Di Paolo G, Kim TW. Reduction of synaptojanin 1 ameliorates synaptic and behavioral impairments in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32(44):15271–15276. doi: 10.1523/JNEUROSCI.2034-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379(6563):353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Minagawa T, Ijuin T, Mochizuki Y, Takenawa T. Identification and characterization of a sac domain-containing phosphoinositide 5-phosphatase. J Biol Chem. 2001;276(25):22011–22015. doi: 10.1074/jbc.M101579200. [DOI] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y, Arribas M, Haffner C, DeCamilli P. Synaptojanin 2, a novel synaptojanin isoform with a distinct targeting domain and expression pattern. J Biol Chem. 1997;272(49):30817–30821. doi: 10.1074/jbc.272.49.30817. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P, Bajaj N, Emre M, et al. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord. 2010;25(12):1791–1800. doi: 10.1002/mds.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci U S A. 2006;103(51):19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio de la Torre E, Luzon-Toro B, Forte-Lago I, Minguez-Castellanos A, Ferrer I, Hilfiker S. Combined kinase inhibition modulates parkin inactivation. Hum Mol Genet. 2009;18(5):809–823. doi: 10.1093/hmg/ddn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB. Exome sequencing: a transformative technology. Lancet Neurol. 2011;10(10):942–946. doi: 10.1016/S1474-4422(11)70196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1(3):161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol Biol Cell. 2002;13(2):542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5(8):701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Thusberg J, Olatubosun A, Vihinen M. Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat. 2011;32(4):358–368. doi: 10.1002/humu.21445. [DOI] [PubMed] [Google Scholar]
- Trempe JF, Chen CX, Grenier K, Camacho EM, Kozlov G, McPherson PS, Gehring K, Fon EA. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell. 2009;36(6):1034–1047. doi: 10.1016/j.molcel.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24(40):8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40(4):733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc Natl Acad Sci U S A. 2008;105(27):9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Kato F, Hasegawa T, Katsumata N, Fukami M, Matsui T, Nagasaki K, Ogata T. A novel homozygous mutation of the nicotinamide nucleotide transhydrogenase gene in a Japanese patient with familial glucocorticoid deficiency. Endocr J. 2013 doi: 10.1507/endocrj.EJ13-0024. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Friedlein A, Imai Y, Takahashi R, Kahle PJ, Haass C. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J Biol Chem. 2005;280(5):3390–3399. doi: 10.1074/jbc.M407724200. [DOI] [PubMed] [Google Scholar]