Abstract
Objective:
To compare the early mortality pattern and causes of death among patients starting HAART in Brazil and the United States.
Methods:
We analyzed the combined data from two clinical cohorts followed at the Johns Hopkins AIDS Service in Baltimore, United States, and the Evandro Chagas Clinical Research Institute AIDS Clinic in Rio de Janeiro, Brazil. Participants included those who entered either cohort between 1999 and 2007 and were antiretroviral naive. Follow-up was at 1 year since HAART initiation. Cox proportional hazards regression analysis was used to assess the role of the city on the risk of death.
Results:
A total of 859 and 915 participants from Baltimore and Rio de Janeiro, respectively, were included. In Rio de Janeiro, 64.7% of deaths occurred within 90 days of HAART initiation; in Baltimore, 48.9% occurred between 180 and 365 days. AIDS-defining illness (61.8%) and non-AIDS-defining illness (55.6%) predominated as causes of death in Rio de Janeiro and Baltimore, respectively. Risk of death was similar in both cities (hazard ratio 1.04; P value=0.95) after adjusting for CD4+ T cell count, age, sex, HIV risk group, prior AIDS-defining illness, and Pneumocystis jirovecii pneumonia and Mycobacterium avium prophylaxis. Individuals with CD4+ T cell count less than or equal to 50 cells/μl (hazard ratio 4.36; P = 0.001) or older (hazard ratio, 1.03; P = 0.03) were more likely to die.
Conclusion:
Although late HIV diagnosis is a problem both in developed and developing countries, differences in the timing and causes of deaths clearly indicate that, besides interventions for early HIV diagnosis, different strategies to curb early mortality need to be tailored in each country.
Keywords: antiretroviral therapy, causes of death, cohort, early mortality, HIV/AIDS
Introduction
Development of HAART in the mid-1990s revolutionized the care of HIV-infected patients and led to a marked decline in morbidity and mortality associated with HIV [[1-[4]. The increasingly widespread use of HAART since 1996 has substantially improved the prognosis of HIV-infected patients who have access to these drugs [5,6]. Data from treatment cohorts have found that the efficacy of HAART, as reflected by rates of viral load suppression and CD4+ T-lymphocyte count recovery, is similar among patients treated in high-income and resource-limited settings [[7-[9].
Despite these positive findings, data from the resource-limited settings suggest that early mortality rates among adults in HAART programs are high [5,10], contributing to substantial losses in overall patient retention [11]. Moreover, early mortality has been shown to be severalfold higher among patients in these settings compared with that of patients treated in high-income settings, even after adjusting for baseline immune status [5]. Nevertheless, a comparative evaluation of the causes of death in high and low-income settings has not been performed.
Our objective was to compare the early mortality pattern and the causes of death among patients starting HAART in Brazil and the United States. This analysis could be performed because of the similar data collection methods used in the clinical cohorts of the Johns Hopkins AIDS Service in Baltimore, Maryland, and the Evandro Chagas Clinical Research Institute (IPEC) HIV Clinic, Oswaldo Cruz Foundation (FIOCRUZ), in Rio de Janeiro, Brazil, as previously reported [8].
Methods
Description of the clinical cohorts
The Johns Hopkins AIDS Service provides care for a large proportion of HIV-infected patients in Baltimore. An observational, longitudinal, clinical database has been maintained on patients receiving primary HIV care since 1990. In this longitudinal database, data are updated regularly using clinic and inpatient clinical documentation (from the Johns Hopkins AIDS Service and elsewhere), laboratory testing results, and pharmacy records. Prescription of antiretroviral therapy (drug, dates of use, and dose) is documented by the medical provider and support staff in the clinical records. Trained abstractors record all this information onto standardized forms for processing. Data on mortality came from the medical records, Maryland State vital statistics records, the National Death Index, and the national Social Security Death Index. Details of the methodology have been previously described [12].
The IPEC/FIOCRUZ AIDS Clinic has provided care to HIV-infected patients in Rio de Janeiro since 1986. An observational, longitudinal, clinical database has been maintained on patients receiving primary HIV care in the clinic since 1998. The data collection process was patterned after the process established at the Johns Hopkins AIDS Service. Data are updated regularly using clinic and inpatient clinical documentation, laboratory testing results, and pharmacy records. Since 1996, the Brazilian Ministry of Health initiated a program of providing HAART free of charge to all HIV-infected individuals. Locally produced antiretroviral drugs have been widely used in Brazil since then. Brazil now holds a unique position as a developing country with more than 200 000 patients receiving HAART. Prescription of antiretroviral therapy at IPEC–FIOCRUZ (drug, dates of use, and dose) is documented by the medical provider and support staff in the clinical records. Trained abstractors record all this information onto standardized forms for processing. Data on mortality came from the medical records and national death statistics. Details of the methodology have been previously described [13].
Study definitions
The outcome of interest was early mortality, defined as death occurring during the first year, after initiating HAART regimen in antiretroviral-naive patients. The primary cause of death was determined upon review of all available medical and vital records. Race/ethnicity is based on patient self-report in Baltimore and on provider report in Rio de Janeiro. HIV transmission risk group is defined by self-report in both locations. Prior AIDS-defining illness (ADI) was based on the occurrence of a 1993 Centers for Disease Control and Prevention (CDC)-defined ADI on or before the HAART initiation date. The CD4+ T-lymphocyte count measurements at HAART initiation and closest to the time of death were considered in this analysis. HAARTwas defined as two or more nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor combined with an NNRTI.
Statistical analysis
Given the compatible variable formatting, we combined data from Baltimore and Rio de Janeiro into a single database. For the present analysis, we included a followup period of 1 year for all individuals who used HAART as their first regimen initiated between January 1999 and December 2007. The outcome of interest was early mortality. We compared Rio de Janeiro and Baltimore subjected to the following points. Description of the causes of death according to specific periods after HAART initiation, as follows: within the first 30 days; between 31 and 90 days; from 91 to 180 days; from 181 to 360 days; the effect of CD4+ T-lymphocyte count and demographic factors on the risk and cause of death.
A Kaplan–Meier plot of early mortality stratified by CD4+ T-lymphocyte count (≤50, 51–200, 201–350, and >350 cells/μl) in Rio de Janeiro and in Baltimore was performed. Patients who were lost to follow-up before 1 year of follow-up were censored at the time of its occurrence. We fitted an unadjusted model and selected all covariables statistically significant at the 25% significance threshold. We used Cox proportional hazards regression analysis to assess the role of living in Rio de Janeiro or Baltimore while adjusting for CD4+ T-lymphocyte count and other factors on early mortality. The assumption of proportionality in Cox’s proportional hazard models was tested by including interactions between the variable ‘time’ and the other covariables [14]. We used the SAS software [15] to conduct the present analysis.
Results
A total of 859 and 915 patients from Baltimore and Rio de Janeiro, respectively, were included in the analysis. Demographic and clinical characteristics of the participating individuals are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study populations.
| Characteristic | Rio de Janeiro, n = 915 (%) | Baltimore, n = 859 (%) | P value |
|---|---|---|---|
| Agea | 41 (34, 48) | 39 (33, 45) | <0.001 |
| Sex | |||
| Male | 604 (66.0%) | 541 (63.0%) | 0.18 |
| Female | 311 (34.0%) | 318 (37.0%) | |
| HIV risk group | |||
| MSM | 266 (29.1%) | 166 (19.3%) | <0.001 |
| IDU | 11 (1.2%) | 311 (36.2%) | |
| Heterosexual | 468 (51.2%) | 331 (38.5%) | |
| Other/unknown | 170 (18.6%) | 51 (5.9%) | |
| HAART regimenb | |||
| PI | 356 (38.9%) | 384 (44.7%) | <0.01 |
| NNRTI | 624 (68.1%) | 516 (60.1%) | |
| CD4+count (cells/μl)a | 192 (82, 306) | 195 (49, 292) | 0.48 |
| Prior ADI | 159 (17.3%) | 126 (14.7%) | 0.12 |
| Prophylaxis | |||
| PCP | 629 (68.7%) | 598 (39.3%) | <0.001 |
| MAI | 87 (9.5%) | 524 (34.4%) | <0.001 |
ADI, AIDS-defining illness; IDU, injection drug user; MAI, Mycobacterium avium; MSM, men who have sex with men; NNRTI, nonnucleoside reverse transcriptase inhibitor; PCP, Pneumocystis jirovecii pneumonia; PI, protease inhibitor.
Median and Interquartile range.
Sums to more than 100% because some patients used both classes of HAART regimen.
There were significant differences between Rio de Janeiro and Baltimore in patient age and HIV risk group [51.2% heterosexual and 29.1% men who have sex with men (MSM) in Rio de Janeiro; 36.2% injection drug user (IDU) and 38.5% heterosexual in Baltimore; P value <0.001] (Table 1). In Rio de Janeiro, a higher proportion of patients received an NNRTI-based regimen (68.1%) than in Baltimore (60.1%) (P< 0.01). At HAART initiation, the median CD4+ T-lymphocyte count of patients from Rio de Janeiro (192 cells/μl) was not significantly different from that observed among those in Baltimore (195 cells/μl) and the proportion of patients who had prior ADI was similar in Baltimore (14.7%) and Rio de Janeiro (17.3%) (P = 0.12). In Rio de Janeiro, more patients were receiving cotrimoxazole prophylaxis for Pneumocystis jirovecii pneumonia (PCP) than in Baltimore (68.7 vs. 39.3%, respectively; P < 0.001), which had been initiated either before or at the same time as HAART. On the other hand, Mycobacterium avium (MAI) prophylaxis was more frequent in Baltimore (P < 0.001) (Table 1).
There were 34 (3.7%) deaths in Rio de Janeiro and 45 (5.2%) deaths in Baltimore within the first year after HAART initiation (Table 2). In Rio de Janeiro, early mortality occurred with greater intensity during the first 3 months (64.7%) compared with later months. The opposite was observed in Baltimore, where 13.3% of deaths occurred in the first 3 months; and 48.9% of these deaths were concentrated between 6 and 12 months after starting HAART.
Table 2.
Primary and secondary causes of death in Rio de Janeiro and Baltimore.
| Days after starting HAART |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0–30 |
31–90 |
91–180 |
181–360 |
|||||
| Rio de Janeiro | Baltimore | Rio de Janeiro | Baltimore | Rio de Janeiro | Baltimore | Rio de Janeiro | Baltimore | |
| Number of deaths | 11 | 6 | 11 | 7 | 4 | 10 | 8 | 22 |
| AIDS-defining | ||||||||
| Infection | 8 (4a) | 2 | 10 (5a) | 1 | 1 | 6 | 2 (2a) | 6 |
| Malignancy | 3 | 3 | 2 | 1 | 1 | |||
| Other | 1 | |||||||
| Non-AIDS-defining | ||||||||
| Infection | 4 | 1 | 4 | 3 | 3 | 2 | 8 | 3 |
| Malignancy | 1 | 2 | 1 | 2 | ||||
| Cardiovascular | 1 | 2 | 1 | 2 | 3 | |||
| Renal | 3 | 2 | 1 | 3 | ||||
| Hepatic/gastrointestinal | 1 | 1 | 3 | |||||
| Pulmonary | 1 | 2 | ||||||
| Trauma | 1 | 1 | 2 | |||||
| Overdose | 1 | 1 | 2 | |||||
| Seizure/neurological | 1 | |||||||
| Unknown | 1 | |||||||
Deaths due to tuberculosis.
Mortality rates per 1000 person-years in Rio de Janeiro were 143.3 [Poisson 95% confidence interval (CI), 120.9–169.7] up to 30 days, 75.4 (95% CI, 60.2–94.2) between 31 and 90 days, 18.6 (95% CI, 11.2–29.1) between 91 and 180 days and 18.5 (95% CI, 11.2–29.3) between 181 and 365 days. This decreasing trend was not observed for Baltimore, with mortality ranging from 97.2 (95% CI, 79.5–118.6) during the first 30 days, to 51.2 (95% CI, 38.0–67.6) between 31 and 90 days, 50.9 (95% CI, 37.8–67.2) between 91 and 180 days, and to 60.7 (95% CI, 46.7–78.9) between 180 and 365 days.
The most commonly occurring causes of death in Rio de Janeiro were ADI (61.8%), tuberculosis (TB) represented 32.4% of deaths, cryptoccocosis 11.8%, and disseminated Kaposi sarcoma 11.8% (Table 2). Sepsis was involved in 50% of deaths in Rio de Janeiro. In Baltimore, in contrast to Rio de Janeiro, the most commonly occurring causes of death were non-ADI (55.6% of patients) followed by 33.3% deaths due to ADI, but no cases of death occurred because of TB. In Baltimore, the most frequent ADI infections were cryptococcal meningitis, disseminated MAI, toxoplasmosis, and recurrent bacterial pneumonia. In addition, in this city, eight (17.8%) patients died from cardiovascular events, four (8.9%) from substance abuse-related events, and four (8.9%) from violence-related events (Table 2). Deaths due to malignancies related to HIV in Rio de Janeiro occurred in 17.6% of the patients whereas in Baltimore, malignancies contributed to 8.9% of deaths. Two (5.9%) and four (8.9%) patients died from non-AIDS-related malignancies in Rio de Janeiro and Baltimore, respectively (Table 2).
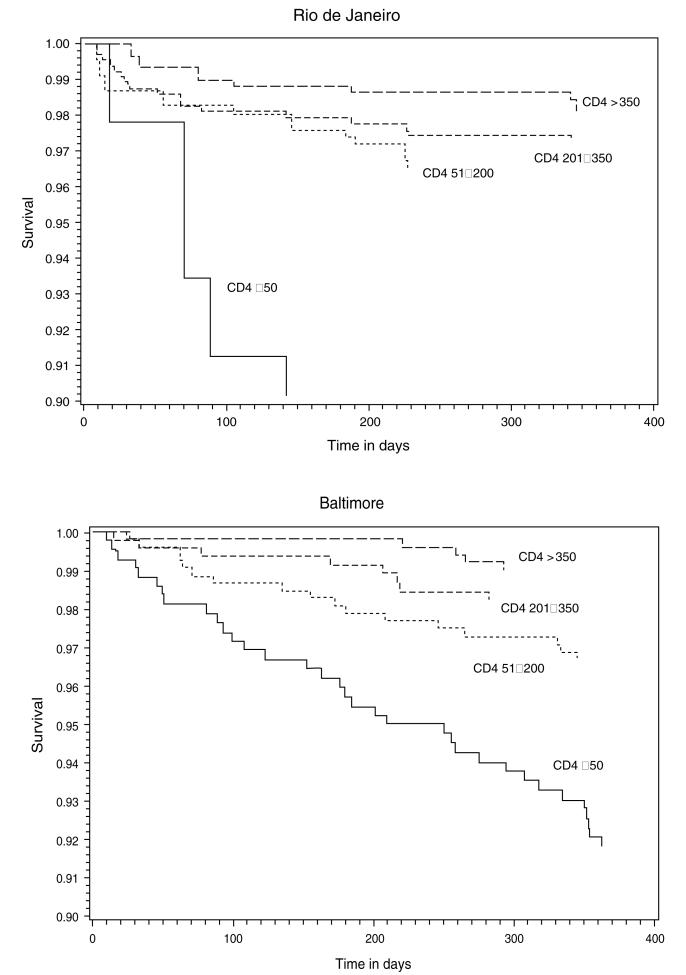
A Kaplan–Meier plot of mortality within 1 year of starting HAART stratified by CD4+ T-lymphocyte count (≤50, 51–200, 201–350, and >350 cells/μl) in Rio de Janeiro and in Baltimore is shown in Fig. 1. Patients from Rio de Janeiro contributed with 870 person-years (mean = 0.95 years per patient) and those from Baltimore contributed with 758 person-years (mean = 0.88 years per patient) of follow-up. There were significant differences in mortality between Baltimore (log rank P < 0.001) and Rio de Janeiro (log rank P = 0.001) for patients with CD4+ T-lymphocyte count of 50 or less, 201–350, and more than 350 cells/μl at HAART initiation. In Rio de Janeiro, we observed a higher early mortality occurring up to 100 days after starting HAART for patients with CD4+ T-lymphocyte count of 50 cells/μl or less. Less than 10% of patients in this category survived beyond 90 days. In Baltimore, early mortality for those severely immunosuppressed is also high but with a regular distribution within the 1-year follow-up.
Fig. 1.
Kaplan–Meier plots of the mortality within 1 year of starting HAART stratified by CD4+ T-lymphocyte count (50 or less, 51–200, 201–350, and at least 350 cells/μl) in Rio de Janeiro (top) and Baltimore (bottom).
The hazard ratio and its respective CI obtained from the unadjusted Cox proportional hazards regression are shown in Table 3. Participants from Rio de Janeiro Cohort had a 32% nonsignificant lower risk of dying within 1 year of starting HAART when compared with those from the Baltimore Cohort (hazard ratio 0.68; 95% CI 0.43–1.06; P value = 0.09). After adjusting for CD4+ T-lymphocyte count, age (per year), sex, HIV risk group, prior ADI, and PCP and MAI prophylaxis, participants from Rio de Janeiro had a risk of death within 1 year of starting HAART similar to participants from Baltimore (hazard ratio, 1.04; 95% CI, 0.59–1.78; P = 0.95) (Table 3). Patients with CD4+ T-lymphocyte count less than or equal to 50 cells/μl at HAART initiation were more likely to die within 1 year of starting HAART (hazard ratio, 4.36; 95% CI, 2.16–8.83; P =0.001). There was also a higher risk of death with older age (hazard ratio, 1.03; P = 0.03) and a borderline significant higher risk of death for those presenting with prior ADI (hazard ratio, 1.54; P = 0.07). There were no significant differences in mortality within 1 year of starting HAART according to sex, HIV risk group, and PCP and MAI prophylaxis. In a subgroup analysis considering only patients with CD4+ T-lymphocyte count less than or equal to 50 cells/μl, after adjusting for all covariates, participants from Rio de Janeiro had nonsignificantly higher risk of death within 1 year of starting HAART compared with participants from Baltimore (hazard ratio, 2.32; 95% CI, 0.84–6.45; P = 0.11). In addition, in this subgroup analysis, there was a higher risk of death with prior ADI (hazard ratio, 2.26; 95% CI, 1.09–4.67; P = 0.02).
Table 3.
Cox proportional hazards regression analysis of mortality within 1 year of starting HAART.
| Variable | Hazard ratio |
95% confidence interval |
P value |
|---|---|---|---|
| Univariate | |||
| Rio vs. Baltimore | 0.68 | 0.43, 1.06 | 0.09 |
| Multivariate | |||
| Rio vs. Baltimore | 1.04 | 0.59, 1.78 | 0.95 |
| CD4+ count (cells/μl) | |||
| <50 | 4.36 | 2.16, 8.83 | 0.001 |
| 51–200 | 1.41 | 0.73, 2.70 | 0.31 |
| 201–350 | 1.06 | 0.55, 2.02 | 0.88 |
| >350 | 1.0 | NA | |
| Age (per year) | 1.03 | 1.01, 1.05 | 0.03 |
| Sex – male | 0.87 | 0.55, 1.37 | 0.55 |
| HIV risk group | |||
| MSM | 1.0 | NA | |
| Heterosexual | 0.69 | 0.44, 1.11 | 0.12 |
| IDU | 1.02 | 0.53, 1.91 | 0.97 |
| Prior ADI | 1.54 | 0.97, 2.41 | 0.07 |
| Prophylaxis | |||
| PCP | 1.10 | 0.70, 1.99 | 0.54 |
| MAI | 1.17 | 0.67, 2.04 | 0.59 |
ADI, AIDS-defining illness; IDU, injection drug user; MAI, Mycobacterium avium; MSM, men who have sex with men; NA, not available; PCP, Pneumocystis jirovecii pneumonia.
Discussion
Our study comparing early mortality in Rio de Janeiro and Baltimore revealed a similar risk of death during the first year after initiating HAART in both cities but a striking difference in the timing and causes of deaths. In Rio de Janeiro, most of the deaths occurred within the first 90 days after HAART initiation, whereas in Baltimore, the vast majority occurred between 180 and 365 days. ADI predominated as primary or secondary causes of death in Rio de Janeiro, whereas in Baltimore, non-AIDS-related causes predominated. Although a higher mortality during the first months of treatment in developing countries compared with those in developed countries has already been demonstrated [5], to the best of our knowledge, this is the first direct comparison of causes of death. Our analysis is based on completely compatible databases, which allows for unbiased comparisons between the two cohorts.
Baltimore, in contrast with Rio de Janeiro, had a high proportion of IDU. Mortality in HIV-1-infected drug users has been found to be approximately two-fold to three-fold higher than among non-IDUs. Persistent or relapsed drug use directly affects adherence to both HAART and clinic appointments, contributing to clinical disease progression and higher mortality [16].
In Rio de Janeiro, infectious diseases were responsible for the majority of the early mortality. Deaths occurring in the first few weeks of HAART initiation are more likely to be caused by conditions that preexist at HAART initiation or new conditions that arise in the context of persisting immunodeficiency or immune reconstitution inflammatory syndrome. Indeed, the comorbidities present at the start of HAART, including TB, invasive bacterial, and fungal infections, have determined an increased mortality in resource-limited settings [17]. We found that infectious diseases determine an increased mortality to the HIV-infected population from Rio de Janeiro. Actually, beyond the HIV-infected, these diseases still pose a great burden among the general population.
Our data from Rio de Janeiro are in accordance with those of other authors who found TB as a leading cause of death in resource-limited settings [18–21]. Diagnosis of TB among HIV-infected individuals can be a particular challenge as 24–61% of those with pulmonary TB are smear negative [22]. Conventional solid media-based culture can take 6 weeks or longer for mycobacterial growth and may not be available at all in many of these settings. Effective strategies to screen for active TB at HAART initiation need to be developed, including detection of subclinical disease [23,24]. Prompt initiation of TB treatment may reduce patient mortality. In our study, the great majority of patients received TB treatment even before diagnosis confirmation. Therefore, the high TB-associated mortality cannot be attributed to lack of TB treatment.
The best timing of HAART initiation during TB therapy is still an unanswered question. Recent results from the Starting Antiretroviral therapy in three Points In Tuberculosis therapy (SAPIT) trial, conducted in South Africa, showed a 56% lower mortality with integrated TB/HIV treatment for patients with CD4 T-lymphocyte count less than 500 cells/μl [25]. Randomized controlled trials of early vs. delayed initiation of HAART for severely immunosuppressed patients are currently underway.
Cryptococcosis lethality is high in both HIV-infected and HIV-noninfected patients. It is one of the most common life-threatening opportunistic infections occurring in HIV-infected patients. In accordance with other studies, we also found cryptococcosis as an important cause of mortality in the Rio de Janeiro Cohort [18,26]. In much of the developing world, the most effective treatment options for cryptococosis are frequently not available. In Rio de Janeiro, though patients were treated with amphotericin B, fluocytosine was not used. The lack of fluocytosine, which remains the agent of choice for use in combination with amphotericin B during the initial therapy, has already been identified as a factor associated with poorer treatment outcomes [27]. Cryptococcus antigen screening for patients with CD4+ T-lymphocyte count up to 100 cells/μl has been shown to be highly effective for identifying those at risk of cryptococcal meningitis and death and might allow for the implementation of a targeted preemptive treatment strategy [28].
Bacterial sepsis was another important cause of death for patients from Rio de Janeiro when compared with those from Baltimore. Bacterial infections are more common among patients from developing countries than among those from developed countries. On the other hand, the significant difference of sepsis as a cause of death between the two cohorts can be attributed, at least in part, to the higher proportion of patients who initiated HAART during a hospitalization as a result of an ADI in Rio de Janeiro.
We found that non-AIDS malignancies were more common in patients from Baltimore when compared with those from Rio de Janeiro. Our results agree with those that have found causes of deaths to be mostly non-AIDS-related in developed countries and ADI in resource-limited countries. Although there has been a reduction in mortality since the introduction of HAART, the continued decline in AIDS-related mortality has been offset by an increase in non-AIDS-related mortality. The lack of competing risks as a result of HIV-infected patients not dying from ADI such as MAI or PCP is also a contributing factor in increasing rates of deaths from non-AIDS-related conditions.
We cannot rule out the participation of immune reconstitution inflammatory syndrome in some of the deaths included in this analysis. However, though deaths attributed to immune reconstitution inflammatory syndrome have been reported, most cases appear to be self-limiting [29].
Discernment of precise causes of death is always a challenge. We gathered cause of death information from meticulous review of medical records. The proportion of ‘unknown cause of death’ was small. In each city, the same institution was the primary source for inpatient and outpatient care. In addition, because most of the deaths occurred in the hospital, we were able to undertake systematic and careful review of inpatient as well as outpatient records, and our classification of causes of death may be more accurate than other studies relying on data reported to a central mortality database. Short of the routine performance of autopsies, we believe we thoroughly accessed available clinical data from existing sources. In addition, loss to follow-up was very low in both cohorts.
Late HIV diagnosis is a problem faced by both developed and developing countries. Indeed, this is a problem even in countries where access to antiretroviral therapy is free of charge, such as Brazil [30,31]. More attention should be given to strategies that lead to earlier diagnosis of HIV, especially among those at higher risk of infection, in order to widen the prescription of preventive treatments and to determine the appropriate timing of starting HAART, all with the aim of reducing infectious disease-related mortality.
In summary, our data showed no difference on early mortality after initiating HAART in Brazil and the United States. The differences on timing and pattern of disease causing deaths clearly indicate that, besides interventions to diagnose HIV infection earlier, other strategies to curb early mortality will need to be tailored to each country.
Acknowledgements
B.G. acknowledges funding from the National Counsel of Technological and Scientific Development (CNPq) and the Research Funding Agency of the State of Rio de Janeiro (FAPERJ). R.M. acknowledges funding from National Institutes of Health (NIH): R01 DA11602, R01 AA18693, K24 DA00432.
B.G., V.G.V. and R.D.M. conceived, designed, and coordinated the study, guided the discussion of the results, and drafted the manuscript. R.D.M. conducted the data analysis. D.P.C. and R.I.M. were responsible for data collection in Rio de Janeiro and participated in the discussion of the results. R.D.M. and J.C.K. were responsible for the data collection in Baltimore. R.K.F. and P.M.L. participated in the study design, discussion of the results, and in the writing of the manuscript. J.C.K., J.H.P. and S.W.C. participated in the study design and in the discussion of the results.
Footnotes
There was no conflict of interests.
References
- 1.Dore GJ, Li Y, McDonald A, Ree H, Kaldor JM. Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J Acquir Immune Defic Syndr. 2002;29:388–395. doi: 10.1097/00126334-200204010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, Montaner JS. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 4.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 5.Braitstein P, Brinkh of MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 6.Campos DP, Ribeiro SR, Grinsztejn B, Veloso VG, Valente JG, Bastos FI, et al. Survival of AIDS patients using two case definitions, Rio de Janeiro, Brazil, 1986–2003. AIDS. 2005;19(Suppl 4):S22–S26. doi: 10.1097/01.aids.0000191486.92285.1c. [DOI] [PubMed] [Google Scholar]
- 7.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 8.Grinsztejn B, Veloso VG, Pilotto JH, Campos DP, Keruly JC, Moore RD. Comparison of clinical response to initial highly active antiretroviral therapy in the patients in clinical care in the United States and Brazil. J Acquir Immune Defic Syndr. 2007;45:515–520. doi: 10.1097/QAI.0b013e3180decb6a. [DOI] [PubMed] [Google Scholar]
- 9.Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter TF, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 10.Tuboi SH, Schechter M, McGowan CC, Cesar C, Krolewiecki A, Cahn P, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181a44f0a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:1691–1701. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 13.Campos DP, Lisboa CSV, Matzenbacher L, Grinsztejn B, Veloso VG, Ribeiro SR, et al. Banco de dados de indivíduos HIV positivos para fins de pesquisa clínica: elaboração e atuali-zação. Presented at 10th Conference on Informatics in Health; Florianópolis. 2006. [Google Scholar]
- 14.Allison PD. Survival analysis using SAS: a practical guide. SAS Institute, Inc.; Cary, North Carolina, USA: 1995. [Google Scholar]
- 15.SAS . SAS Online Doc 9.1.3. SAS Institute, Inc.; Cary, North Carolina, USA: 2002–2005. [Google Scholar]
- 16.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 17.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 18.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 19.Kambugu A, Castelnuovo B, Wandera B, Ragga A, Kamia M. Abstracts of the 4th IAS conference on HIV Pathogenesis, Treatment and Prevention. International AIDS Society>; Sydney, Australia: 2007. Antiretroviral therapy in an urban African cohort does not pre-vent significant early mortality [abstract WEPEB055] [Google Scholar]
- 20.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococ-cocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 21.Saraceni V, King BS, Cavalcante SC, Golub JE, Lauria LM, Moulton LH, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–772. [PMC free article] [PubMed] [Google Scholar]
- 22.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 23.Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 24.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karim SA, Naidoo KJ, Glober A, Padayatchi N, Nair G, Bamber S, et al. Initiating ART during TB treatment significantly increases survival: results of a randomized controlled clinical trial in TB/HIV-co-infected patients in South Africa. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [abstract 36a] [Google Scholar]
- 26.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 27.Dromer F, Mathoulin-Pelissier S, Launay O, Lortholary O. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4:297–308. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 30.Girardi E, Sabin CA, Monforte AD. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(Suppl 1):S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 31.Souza-Jr PR, Szwarcwald CL, Castilho EA. Delay in introducing antiretroviral therapy in patients infected by HIV in Brazil, 2003–2006. Clinics. 2007;62:579–584. [PubMed] [Google Scholar]