Abstract
Objectives
To determine whether tenofovir disoproxil fumarate (TDF) is associated with renal dysfunction when used as part of an initial antiretroviral regimen and to assess the effect of ritonavir-boosted protease inhibitor (PI/r) coadministration on renal function in TDF-treated patients.
Design
Analysis from a prospective observational cohort.
Methods
We compared all antiretroviral-naive patients with an estimated glomerular filtration rate (eGFR) of more than 50 ml/min per 1.73 m2 (modification of diet in renal disease equation) who initiated either TDF (n = 201) or any alternative nucleoside reverse transcriptase inhibitor (NRTI) (n = 231) after 1 January 2002.
Results
Patients taking both TDF and NRTIs experienced an initial decline in eGFR during the first 180 days of therapy, but eGFR stabilized between 180 and 720 days. There was no difference between TDF and NRTI use in 25 or 50% decline in eGFR at 1 or 2 years or in change in eGFR at 6, 12, or 24 months. Those taking TDF and a PI/r had a greater median decline in eGFR than those taking TDF and a non-NRTI at 6 months (P = 0.01), with trends at 12 (P = 0.08) and 24 months (P = 0.08). There was no difference in median GFR decline between those on an NRTI and PI/r vs. an NRTI and non-NRTI.
Conclusion
Our data are consistent with results of clinical trials, which have shown no evidence of renal toxicity when TDF is used as part of an initial regimen. Our results support the use of TDF as a component of the initial antiretroviral regimen, and suggest that the eGFR should be monitored more closely when TDF is used with a PI/r.
Keywords: antiretroviral, kidney, nephrotoxicity, nucleoside analog reverse transcriptase inhibitor, protease inhibitor, renal, tenofovir
Introduction
The nucleotide reverse transcriptase inhibitor tenofovir disoproxil fumarate (TDF) is excreted renally via a combination of glomerular filtration and active tubular secretion. TDF has an excellent renal safety profile in clinical trials in antiretroviral-naive individuals [1–6]. However, there have been case reports of renal toxicity, including acute tubular necrosis [7] and Fanconi syndrome [8–10]. Some observational studies [11,12] have found evidence of a mild decrease in kidney function in TDF-treated patients. In a previous study [13] from the Johns Hopkins HIV Database, we found a significantly greater decline in estimated glomerular filtration rate (eGFR) in TDF-treated patients compared with those taking nucleoside analog reverse transcriptase inhibitors (NRTIs) without TDF, although the decline in eGFR was mild, and there was no difference between groups in rates of discontinuation because of renal insufficiency. A subsequent analysis [14] suggested that the decline in kidney function, which was observed in both TDF and NRTI-treated patients, was most pronounced in the first 6 months but was not progressive with continued dosing.
In a prior analysis [13] from the Johns Hopkins database, the use of ritonavir-boosted protease inhibitors (PI/r) was not associated with renal dysfunction by multivariate analysis. However, there are conflicting data on whether the use of PI/r-based regimens increases the risk of TDF-mediated renal toxicity. Some PI/r-containing regimens can increase tenofovir exposure by 20–30% [15,16], and it has been suggested that the use of TDF in PI/r-based regimens could increase the risk of nephrotoxicity [7]. Goicoechea and colleagues [17] found that patients treated with PI/r-based regimens had a significantly greater decline in renal function than those taking TDF with non-NRTIs (NNRTIs) or non-TDF-based regimens. In contrast, data from the HIV Outpatient Study (HOPS) found no such difference [18]. We wished to determine whether TDF is associated with renal dysfunction when part of an initial antiretroviral regimen in antiretroviral-naive patients in clinical practice, and to assess the effect of PI/r coadministration on renal function in TDF-treated patients.
Methods
The study sample consisted of HIV-infected patients receiving care in an urban HIV primary care clinic in Baltimore, Maryland who were antiretroviral naive, had an eGFR of more than 50 ml/min per 1.73 m2, and who initiated an antiretroviral regimen containing TDF or any NRTI after 1 January 2002. The methods of data collection for this observational longitudinal cohort have been described elsewhere [19]. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation [20]. Baseline eGFR was defined as the average of the two eGFRs obtained closest to and preceding the start of treatment by no more than 180 days. Change in eGFR was calculated from average of maximum serum creatinine measurements for each patient and the next creatinine obtained. If there was no on-treatment creatinine measurement after the maximum creatinine, then the maximum creatinine and the creatinine immediately prior to maximum were averaged. Two values were used to minimize regression to the mean.
We calculated change in eGFR in two ways. First, we calculated the time to a 25 and 50% decline in GFR from the baseline level (confirmed by two measures of creatinine) to a maximum of 2 years after starting TDF or an NRTI. We used right-censored Kaplan–Meier methods to determine time to eGFR decline. Censoring occurred at discontinuation of the baseline therapy, leaving care, or at 2 years. The log-rank test was used to determine statistical significance. Multivariate analyses were performed using Cox proportional hazards regression to assess therapy (TDF vs. alternative NRTI) and other demographic and clinical factors associated with eGFR decline. Second, we analyzed the absolute difference in eGFR from baseline by 6-month intervals after the start of therapy. For these analyses, the eGFR obtained closest in time to the interval threshold was used. If patients remained on their initial therapy and did not have eGFR measured in that interval, then the last measured value was carried forward.
Results
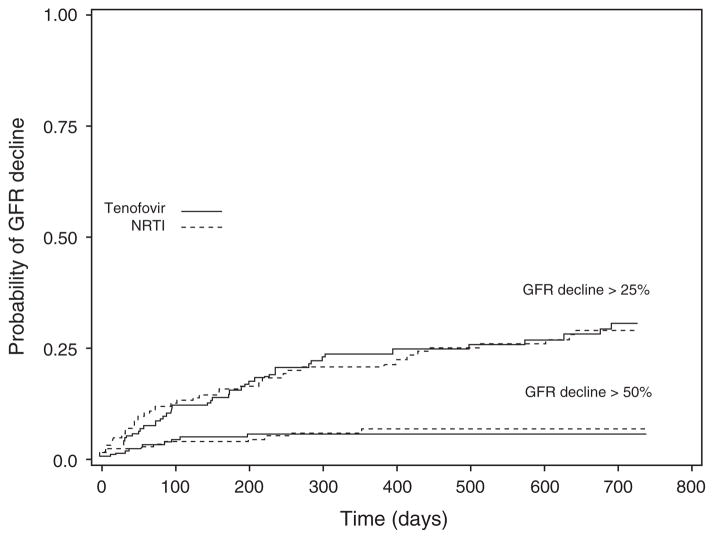
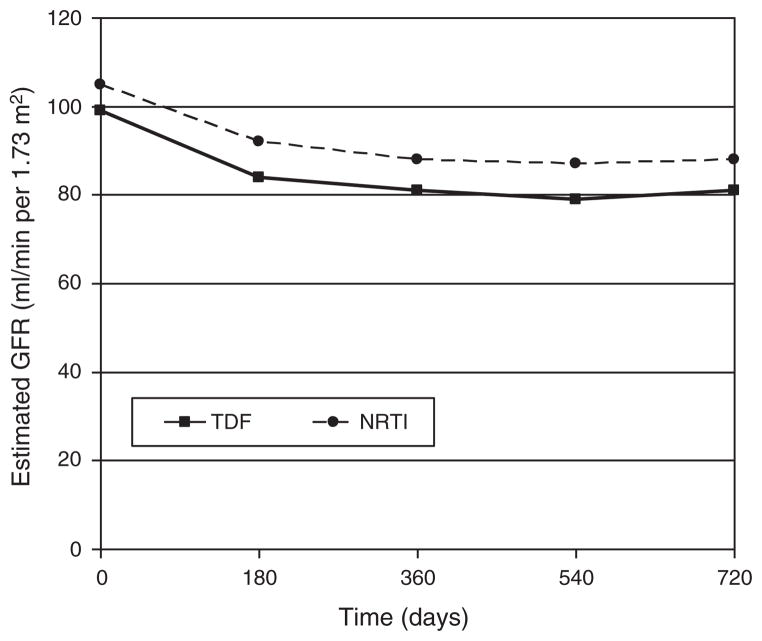
There were no significant differences between the TDF and NRTI groups in several demographic and clinical variables (Table 1). The majority of patients in the NRTI group were taking zidovudine (57%); abacavir was used by 37%, stavudine by 37%, and didanosine by 6%. Patients taking both TDF and NRTIs experienced an initial decline in eGFR during the first 180 days of therapy, but eGFR stabilized between 180 and 720 days. There was no difference by TDF vs. NRTI use in 25 or 50% decline in renal function by 2 years of follow-up (Fig. 1) and no significant difference between TDF and NRTI use in the change in eGFR from baseline value at 6, 12, and 24 months (Fig. 2).
Table 1.
Baseline characteristics of patients who received tenofovir disoproxil fumarate or a nucleoside reverse transcriptase inhibitor.
| TDF (n = 201) | NRTI (n = 231) | P | |
|---|---|---|---|
| Time on regimen, days (95% CI) | 438 (163–720a) | 410 (102–720a) | 0.27 |
| Baseline eGFR (ml/min per 1.73 m2) | 101 | 105 | 0.17 |
| Age (mean years) | 40 | 40 | 0.94 |
| Race (black) | 78% | 73% | 0.24 |
| Sex (male) | 59% | 61% | 0.77 |
| CD4 cell count, baseline (cells/μl) | 246 | 279 | 0.80 |
| HIV RNA, baseline (copies/ml) | 99 700 | 131 300 | 0.11 |
| Hypertension | 25% | 26% | 0.72 |
| Diabetes | 5% | 5% | 0.92 |
| Injection drug use as HIV transmission factor | 37% | 39% | 0.71 |
| Regimen cornerstone | |||
| NNRTI | 51% | 44% | 0.10 |
| PI/r | 48% | 50% | 0.74 |
| Protease inhibitor (unboosted) | 1% | 6% | 0.05 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; TDF, tenofovir disoproxil fumarate.
Patient data were censored after 720 days.
Fig. 1. Kaplan–Meier plots of estimated glomerular filtration rate decline greater than 25% and greater than 50% from baseline value stratified by tenofovir disoproxil fumarate vs. nucleoside reverse transcriptase inhibitor use.
There is no significant difference between TDF and NRTI use for a 25% decline (P = 0.59) nor for a 50% decline (P = 0.65, log-rank test). GFR, glomerular filtration rate; NRTI, nucleoside reverse transcriptase inhibitor; TDF, tenofovir disoproxil fumarate.
Fig. 2. Plots of the absolute estimated glomerular filtration rate over 2 years from start of therapy by tenofovir disoproxil fumarate vs. nucleoside reverse transcriptase inhibitor use.
There is no significant difference between TDF and NRTI use in the median change in eGFR from baseline value at 6 months (TDF, −14; NRTI, −12; P = 0.26, median scores test), at 12 months (TDF, −15; NRTI, −14; P = 0.76), or at 24 months (TDF, −16; NRTI, −15; P = 0.76). All eGFR values are expressed in ml/min per 1.73 m2. eGFR, estimated glomerular filtration rate; NRTI, nucleoside reverse transcriptase inhibitor; TDF, tenofovir disoproxil fumarate.
By multivariate analysis, there was no difference between the TDF and NRTI groups in 25% (P = 0.39) or 50% decline (P = 0.56) adjusting for age, race, baseline eGFR, and CD4 cell count, use of a PI/r vs. NNRTI-based regimen, concomitant diabetes, or hypertension (Table 2). Factors associated with greater than 25% decline in eGFR included age more than 45 years [hazard ratio 2.31, 95% confidence interval (CI) 1.44–3.69], baseline CD4 cell count less than 200 cells/μl (HR 2.66, 95% CI 1.65–4.29), hypertension (HR 1.56, 95% CI 1.00–2.45), and use of a PI/r (HR 2.14, 95% CI 1.37–3.34). Race, diabetes, and TDF vs. NRTI use were not associated with decline in eGFR after adjusting for the former variables.
Table 2.
Multivariate associations with 25% estimated glomerular filtration rate decline.
| Hazard ratio | 95% CI | |
|---|---|---|
| TDF vs. NRTI use | 1.04 | 0.68–1.59 |
| Age >45 years | 2.31 | 1.44–3.69 |
| Race (black) | 1.52 | 0.85–2.72 |
| CD4 cell count <200 cells/μl (baseline) | 2.66 | 1.65–4.29 |
| Hypertension | 1.56 | 1.00–2.45 |
| PI/r | 2.14 | 1.37–3.34 |
CI, confidence interval; NRTI, nucleoside reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; TDF, tenofovir disoproxil fumarate.
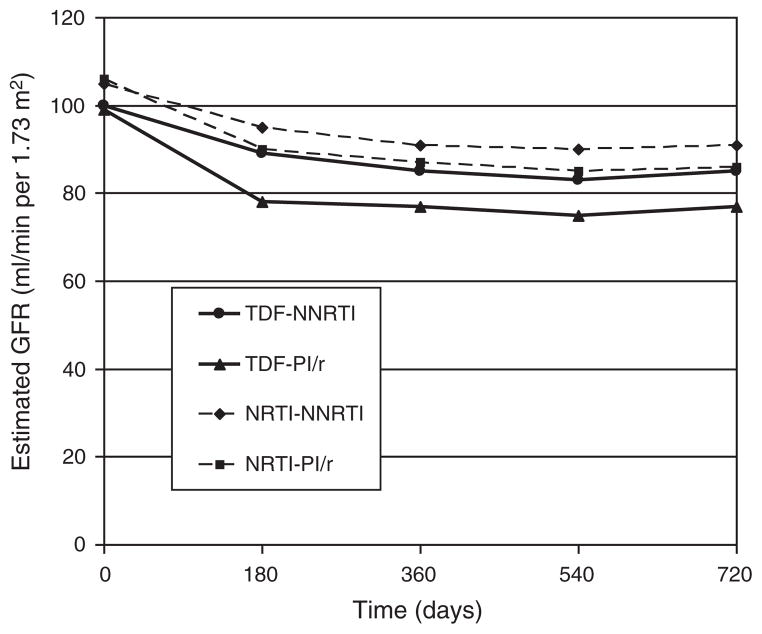
When analyzed by TDF vs. NRTI use, the group taking TDF and a PI/r had a greater decline in eGFR than those taking TDF and an NNRTI at 6 months (P = 0.01), with a trend observed at 12 months (P = 0.08) and 24 months (P = 0.08) (Fig. 3). In contrast, there were no significant differences between those taking NRTIs and a PI/r compared with those taking NRTIs and an NNRTI (P = 0.92, 0.81, and 0.85, respectively).
Fig. 3. Plots of the estimated glomerular filtration rate over two years from start of therapy stratified by tenofovir disoproxil fumarate–nucleoside reverse transcriptase inhibitor, tenofovir disoproxil fumarate–ritonavir-boosted protease inhibitor, nucleoside reverse transcriptase inhibitor–nonnucleoside reverse transcriptase inhibitor, and nucleoside reverse transcriptase inhibitor–ritonavir-boosted protease inhibitor.
For patients receiving TDF, there is a significant difference between concomitant use of PI/r vs. NNRTI in the median change in eGFR from baseline value at 6 months (PI/r, −18; NNRTI, −10; P = 0.01), with a trend at 12 months (PI/r, −18; NNRTI, −13; P = 0.08), and at 24 months (PI/r, −18; NNRTI, −13; P = 0.08). For patients receiving an NRTI, there was no significant difference between concomitant use of PI/r vs. NNRTI in the median change in eGFR from baseline at 6 months (PI/r, −13; NNRTI, −12; P = 0.92), 12 months (PI/r, −16; NNRTI, −14; P = 0.81), or 24 months (PI/r, −16; NNRTI, −14; P = 0.85). All eGFR values are expressed in ml/min per 1.73 m2. eGFR, estimated glomerular filtration rate; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; TDF, tenofovir disoproxil fumarate.
Discussion
We observed a modest initial decline in eGFR among antiretroviral-naive patients starting both TDF and alternative NRTIs as part of their initial antiretroviral regimen, with no significant difference between the two groups. These results emphasize the importance of always including a control group that receives an alternative NRTI when studying the effect of TDF on renal function, as the decline in renal function could have otherwise been attributed to TDF.
In contrast to our previous analyses, we found a significantly greater decline in eGFR between patients who took TDF with a PI/r compared with those who took TDF with an NNRTI. Data from other cohorts have been conflicting. No difference was observed in the HOPS, which included patients taking TDF both for initial and subsequent therapy [18]. In contrast, other studies or reports [7,17] have found evidence of a greater risk of renal toxicity in patients taking TDF with protease inhibitors. A study [17] from the California Collaborative Trials Group, which included both treatment-naive and experienced patients, found significantly greater declines in renal function among patients treated with TDF and a PI/r compared with those treated with TDF and an NNRTI. However, in contrast to the steady decline reported by these investigators, our results showed initial declines over the first 6 months, which subsequently plateaued over the 2-year observation period (Fig. 3).
The mechanism by which coadministration of protease inhibitors might increase the risk of TDF nephrotoxicity remains unclear. Coadministration of some protease inhibitors can increase tenofovir plasma exposure by 20–30% [16]. It has been debated whether this increase in plasma levels is due to a decrease in tenofovir renal clearance [21,22] or an increase in TDF oral absorption [23,24]. HIV drug accumulation in renal proximal tubule cells is determined in part by excretion into the urine by renal transporters [25]. It has been proposed that ritonavir could inhibit the active tubular secretion of tenofovir mediated by the multidrug resistance-associated protein (MRP)-2 transporter, leading to intracellular accumulation of the drug [26]. However, independent laboratories have reported that tenofovir is a substrate for MRP-4, a transporter not inhibited by ritonavir, and not MRP-2 [11,27,28]. Regardless of which mechanism is correct, the lack of progressive decline in renal function seen in our study, together with the lack of significant renal toxicity observed in clinical trials in which TDF was used in PI/r-based regimens [3–5], suggest that this is not a widespread problem.
Consistent with data from randomized clinical trials, our results support the use of tenofovir as part of an initial antiretroviral regimen, whether PI/r or NNRTI-based. Our data also suggest that the GFR should be monitored more closely in older patients, patients with hypertension, patients with baseline CD4 cell counts less than 200 cells/μl, and when a PI/r is used.
Acknowledgments
Financial support from National Institutes of Health grants #R01 DA11602 and K24 DA00432 and Gilead Sciences. Johns Hopkins University has received research support from Gilead, Merck, Pfizer, Roche, and Tibotec for research conducted by J.E.G. and from Gilead, Merck, and Pfizer for research conducted by R.D.M. J.E.G. has received consulting fees, honoraria, D.S.M.B. payments, or all from Abbott, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Pfizer, and Tibotec. R.D.M. has received consulting fees from Bristol-Myers Squibb and GlaxoSmithKline. Both authors (J.E.G. and R.D.M.) were involved in the design of this study, data analysis, and the preparation of the manuscript. Statistical analysis was conducted by R.D.M.
References
- 1.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JMAH, Miller MD, et al. Efficacy and safety of tenofovir DF vs.stavudine in combination therapy in antiretroviral-naïve patients: a randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 2.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz R, DeJesus E, Khanlou H, Veronin E, van Lunzen J, Andrade-Villanueva J, et al. Efficacy and safety of once-daily darunavir/ ritonavir versus lopinavir/ritonavir in treatment-naïve HIV-1-infected patients at 48 weeks. AIDS. 2008;22:1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 4.Molina JM, Podsadecki TJ, Johnson MA, Wilkin A, Domingo P, Myers R, et al. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is noninferior to a twice-daily regimen through 96 Weeks. AIDS Res Hum Retroviruses. 2007;23:1505–1514. doi: 10.1089/aid.2007.0107. [DOI] [PubMed] [Google Scholar]
- 5.Gathe J, da Silva BA, Cohen DE, Loutfy MR, Podzamczer D, Rubio R, et al. Once-daily lopinavir/ritonavir-based regimen is noninferior to twice-daily dosing and results in similar safety and tolerability in antiretroviral-naive subjects through 48 weeks. J Acquir Immune Defic Syndr. 2009;50:474– 481. doi: 10.1097/QAI.0b013e31819c2937. [DOI] [PubMed] [Google Scholar]
- 6.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20:743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 8.Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment: case report and review of literature. J Infect. 2005;51:E61–E65. doi: 10.1016/j.jinf.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mouquenot B, Girard PM, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40:1331–1333. doi: 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 10.Izzedine H, Isnard-Bagnis C, Hulot JS, Vittecoq D, Cheng A, Jais CK, et al. Renal safety of tenofovir in HIV treatment experienced patients. AIDS. 2004;18:1074–1076. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 11.Winston A, Amin J, Mallon P, Marriott D, Carr A, Cooper DA, et al. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med. 2006;7:105–111. doi: 10.1111/j.1468-1293.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 12.Mauss S, Berger F, Schmutz G. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS. 2005;19:93–95. doi: 10.1097/00002030-200501030-00012. [DOI] [PubMed] [Google Scholar]
- 13.Gallant JE, Parish MA, Keruly JD, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 14.Gallant JE, Parish MA, Keruly JC, Moore RD. Tenofovir and changes in renal function: reply to Gupta [letter] Clin Infect Dis. 2005;41:571. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 15.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fuma-rate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43:278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 16.Gilead Sciences. [Accessed 6 December 2008];Viread Medication Insert. http://www.gilead.com/pdf/viread_pi.pdf.
- 17.Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–108. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 18.Buchacz K, Young B, Baker RK, Moorman AC, Wood KC, Chmiel J, et al. Renal function in patients receiving tenofovir with ritonavir/lopinavir or ritonavir/atazanavir in the HIV Outpatient Study (HOPS) Cohort. J Acquir Immune Defic Syndr. 2006;43:626–628. doi: 10.1097/01.qai.0000242461.35768.45. [DOI] [PubMed] [Google Scholar]
- 19.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr. 1998;17 (Suppl 1):S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Jullien V, Tréluyer JM, Rey E, Jaffray P, Krivine A, Moachon L, et al. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob Agents Chemother. 2005;49:3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther. 2008;83:265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 23.Tong L, Phan T, Robinson K, Babusis D, Strab R, Bhoopathy S, et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother. 2007;51:3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray AS, Wright MR, Rhodes GR. Lack of evidence for an effect of lopinavir/ritonavir on tenofovir renal clearance. Clin Pharmacol Ther. 2008;84:660. doi: 10.1038/clpt.2008.140. [DOI] [PubMed] [Google Scholar]
- 25.Izzedine H, Launay-Vacher V, Deray G. Renal tubular transporters and antiviral drugs: an update. AIDS. 2005;19:455–462. doi: 10.1097/01.aids.0000162333.35686.4c. [DOI] [PubMed] [Google Scholar]
- 26.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–817. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. Mechanism of active renal tubular efflux of tenofovir. Anti-microb Agents Chemother. 2006;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71:619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]