Abstract
Prey organisms do not tolerate predator attack passively but react with a multitude of inducible defensive strategies. Although inducible defence strategies are well known in plants attacked by herbivorous insects, induced resistance of fungi against fungivorous animals is largely unknown. Resistance to fungivory is thought to be mediated by chemical properties of fungal tissue, i.e. by production of toxic secondary metabolites. However, whether fungi change their secondary metabolite composition to increase resistance against arthropod fungivory is unknown. We demonstrate that grazing by a soil arthropod, Folsomia candida, on the filamentous fungus Aspergillus nidulans induces a phenotype that repels future fungivores and retards fungivore growth. Arthropod-exposed colonies produced significantly higher amounts of toxic secondary metabolites and invested more in sexual reproduction relative to unchallenged fungi. Compared with vegetative tissue and asexual conidiospores, sexual fruiting bodies turned out to be highly resistant against fungivory in facultative sexual A. nidulans. This indicates that fungivore grazing triggers co-regulated allocation of resources to sexual reproduction and chemical defence in A. nidulans. Plastic investment in facultative sex and chemical defence may have evolved as a fungal strategy to escape from predation.
Keywords: insect–fungus ecology, chemical defence, induced resistance, facultative sexuality, mycotoxins
1. Introduction
The nature of predator–prey interactions, i.e. consumption of one living organism by another organism, is of utmost importance for understanding the dynamics of predator and prey populations and hence community structure and diversity [1,2]. Grazers form a large functional class of predators that do not kill and consume their prey entirely, but remove only parts of each prey individual, which is rarely lethal in the short term. Herbivore–plant interactions are a prime example where the grazing-type of predator–prey interaction is predominant [3]. Notwithstanding the ecosystem relevance of interactions between plants and herbivorous animals and the consequences for functional character (co-)evolution [4], a huge diversity of vertebrates, molluscs, nematodes and arthropods extract essential nutrients by facultative or obligatory grazing on fungal organisms [5–7]. Like plants, fungi are immobile organisms unable to escape from such predator attacks. Therefore, in analogy to plant adaptations to herbivore attack [8], fungal secondary metabolites are increasingly recognized as candidate characters mediating resistance against fungivore grazing [9,10]; yet, no evidence exists whether fungi are able to change the proposed secondary metabolite shield in response to fungivore grazing.
In species of the wide-spread fungal genus Aspergillus, highly toxic polyketides are prime candidate compounds for direct chemical defence against generalist fungivores, as ingestion of these mycotoxins causes dose-dependent impairment of arthropod fitness [11]. Knocking out genetically the mechanisms that control the regulation of cytotoxic and carcinogenic sterigmatocystin (ST) and other secondary metabolites in the mould Aspergillus nidulans [12] leads to a striking preference of fungivorous collembolans, Folsomia candida, for the chemical-deficient mutant strain [13]. Relative to the toxin-producing wild-type, this preference is accompanied by enhanced fungivore feeding rates, a higher fitness gain and reduced expression of stress-related genes in the collembolans [14,15]. In the same system, constitutive in vivo activation of the ST biosynthetic pathway results in a massive over-production of this mycotoxin and in a distinct avoidance reaction of the fungivores [16]. Fungivorous arthropods, therefore, exploit fungal-borne cues to minimize intake of toxic fungal metabolites. Consequently, arthropod behavioural and fitness responses are crucial indicators for revealing variation in the ability of fungi to resist fungivory.
Biosynthesis of secondary metabolites is genetically co-regulated with developmental differentiation processes in fungi, such as a facultative switch from asexual conidiospore production to sexual reproduction and hence sexual fruiting body formation [17]. Generally, facultative sexual organisms tend to invest more in costly sex when individuals face a decline in fitness due to degrading environmental conditions, such as nutrient depletion, competitive or abiotic stress (summarized in [18]). A negative relationship between investment in sexual reproduction and fitness gain has recently been demonstrated for A. nidulans, however, the adaptive value of this response remains elusive [19]. Unlike the production of myriads of asexual conidia that would allow efficient aerial dispersal, ascospores developing within the capsule of the sexual fruiting body (cleistothecium) (see electronic supplementary material, figure S1) are limited in their ability to escape from unfavourable conditions [19]. One potential long-term advantage is that sexually generated ascospores harbour novel genotypes giving rise to offspring colonies probably better adapted to the changing environment than the parental genotype [20]. An alternative possibility is that tightly co-regulated facultative sexual development and induction of anti-fungivore secondary metabolites may represent a combined investment in defence that provides a flexible means to escape directly from fungivore feeding attack. Showing that fungi use co-regulation of morphological and chemical changes as a strategy to defend against fungivores requires demonstrating that fungivory induces a more arthropod resistant fungal phenotype and that acquired resistance is characterized by enhanced levels of secondary metabolite production and induction of sexual development. To the best of our knowledge, neither induced resistance (increased repellence of fungivores) nor its relationship to plasticity in secondary metabolite biosynthesis and/or sexual development has been demonstrated in any fungus.
To test this idea, we examined the food choice behaviour of F. candida in relation to A. nidulans colonies (see electronic supplementary material, figure S1) that were treated in different ways: preceding grazing by conspecific fungivores, artificial wounding and unchallenged. In addition, we compared quantitatively secondary metabolite profiles of fungivory-challenged and unchallenged A. nidulans colonies and followed the development of sexual fruiting bodies. Our results provide evidence that arthropod grazing induces a fungal phenotype that shows enhanced resistance to fungivory and that induced resistance coincides with elevated levels of secondary metabolites and formation of sexual fruiting bodies. Collembolans avoid consumption of sexual fruiting bodies, which suggests that predatory pressure may have contributed to adaptive regulation of the fungal chemical and morphological phenotype.
2. Material and methods
(a). Organisms and general experimental conditions
Cultivation of both F. candida (‘Berlin’ strain) and A. nidulans (strain RDIT2.3; veA1) as well as the generation of experimental animals and fungal conidiospores followed standard protocols [15]. Confrontation experiments with F. candida were done in constant darkness at 20 ± 0.5°C.
(b). Food choice assays
Autoclaved glass fibre filters discs (1.0 cm in diameter, MN85/70 BF, Macherey-Nagel GmbH & Co. KG, Germany) were soaked in melted malt extract agar [16]. After hardening, each disc was inoculated with 2 µl of a conidial suspension (1000 conidia per µl). After 72 ± 1 h, filter discs with fungal colonies were placed individually in autoclaved 13 ml polyethylene vials (Bock Kunstofftechnik, Germany) and were randomly assigned to the treatments. The primary fungivory treatment (+PF) was generated by adding 50 ± 5 F. candida individuals to each vial. For wounding fungal colonies (WO), approx. 25% of each colony was removed with a sterile scalpel. For the control (−PF) treatment, colonies were left untouched. To test whether collembolans deposit during foraging any chemical cue that may affect the food choice behaviour of future fungivores, we conditioned fungal-free patches with F. candida. After 20 ± 0.5 h, all animals were removed from the +PF treatments. To account for time-dependent changes of fungal patches, e.g. due to recovery of fungal colonies, lag of induced response and evaporation/degradation of conspecific signals/cues, we included post fungivory periods (PFP) of 0, 6 and 24 h (see electronic supplementary material, figure S2).
For the food choice assays, two experimental patches were placed at a distance of approximately 6 cm in sterile Petri dishes (9 cm diameter). The patches were connected with a wet piece of filter paper (length, 7 cm; width, 1.5 cm). Both the position of the two patches relative to one another (left/right) in each arena and the position of the arenas in a 6 × 10 grid were randomized. Twenty-five 14-day-old F. candida, which had previously received no food for 2 days, were released in the centre of the filter paper. Subsequently, collembolans that stayed on the experimental patches were counted 1.5, 20 and 44 h after they were released into the arenas. Patch preferences were determined by subtracting the proportion of individuals on −PF colonies from the proportion of individuals on +PF colonies resulting in Δ-values ranging from +1 (full preference for PF) to −1 (full preference for −PF). Δ-values not significantly different from zero indicate no preference. In total, we conducted three independent F. candida choice experiments to test for the influence of (i) A. nidulans previously challenged with con-specific collembolans, i.e. +PF versus −PF, (ii) artificially wounded colonies and (iii) con-specific cues on fungivore feeding decisions.
We separated asexual conidia from sexual fruiting bodies to test whether F. candida displays a feeding preference for either fungal reproductive structure. For this, approximately 10 ml Ringer solution were added to three independent 10-day-old A. nidulans colonies on malt-extract agar (9 cm Petri dishes). Using a scalpel, both conidiophores and cleistothecia were scraped off the colonies. In order to separate the two tissue types, the suspension was washed and gently centrifuged three times with Ringer solution. Cleistothecia were removed with a pipette and washed once again. The remaining conidia were filtered through Miracloth to remove vegetative hyphae. This protocol enabled us to obtain intact cleistothecia that were still carrying the surrounding layer of Hülle cells. For the F. candida food choice experiment (see above), we added the same fresh weight (approx. 10 mg) of cleistothecia and conidia to 1 cm glass fibre discs in the same volume of Ringer solution (80 µl).
(c). Fungivore fitness and induction of sexual fruiting bodies
Groups of five F. candida individuals were added to vials containing one −PF or one +PF fungal patch, which were allowed to recover from primary fungivore grazing for 24 h (equivalent to a PFP of 24 h, see above). With the aid of a stereomicroscope (Discovery V8, Zeiss, Germany) equipped with a digital camera (AxioCam Icc1, Zeiss, Germany), insect body length, which served as a fitness proxy, was determined at the beginning of the experiment and 6 days later. Total body length was measured for all animals per arena using ImageJ (http://rsbweb.nih.gov/ij/). Body lengths were averaged for each arena to obtain one measurement per arena. Additionally, we counted the number faecal pellets to provide a measure of feeding intensity [16]. The same setup was used for quantifying cleistothecia on A. nidulans colonies that were exposed to groups of three F. candida for 6 days. Also, we exposed initially 3-day old A. nidulans colonies growing on malt extract agar (9 cm Petri dishes) to a three-week grazing pressure by 50 F. candida (during the course of the experiment collembolans deposited eggs and after three weeks numerous juveniles populated the plates). In parallel, we set up unchallenged colonies; at the end of the experiment we counted the number of cleistothecia in all plates.
(d). Metabolic profiling
To obtain pure fungal tissue for the secondary metabolite analysis, A. nidulans was inoculated onto KOH-treated, sterile cellophane sheets placed on malt extract agar. Following a pre-incubation period of 3 days at 20°C, 25 F. candida were added and allowed to graze on the colonies for 24 h. Ten days after inoculation fungal tissue was thoroughly removed with a scalpel, shock-frozen in liquid nitrogen and lyophilized. The tissue of five randomly chosen colonies was pooled to generate one biological replicate (in total six replicates per treatment) and thoroughly grinded using a porcelain mortar. Fungal metabolites were extracted by adding 1 ml of acetonitrile/water (84/16, v/v) to 100 mg of pulverized tissue. For targeted and non-targeted metabolite analysis and MS fragmentation we used a reverse-phase HPLC system coupled with an ion trap mass spectrometer (500-MS, Varian) equipped with an electrospray ionization (ESI) source [21]. Data processing encompassed background reduction, peak identification, peak alignment and normalization, which followed standard protocols [22]. For the identification of metabolites that appeared consistently in all biological replicates, collision-induced fragmentation (MS2) was used, and the spectra were compared with published data [23,24]. The peak area of one compound-specific fragment ion was monitored to provide a quantitative measure of the metabolite production. HPLC-MS/MS analysis was also used for the quantification of ST using mass transition m/z 325 > 281 and m/z 325 > 310 and a calibration curve constructed with a certified analytical standard for ST (S3255, Sigma Aldrich).
(e). Statistical analysis
Statistical analyses were performed using SAS v. 9.2 software. For time-dependent food choice we applied mixed repeated measurement models as described previously [15,16]. Depending on data properties parametric one-way ANOVA (measure of metabolite formation, fungivore body size, faecal pellets), one-sample t-tests (post hoc analysis of fungivore food choice) or non-parametric tests (cleistothecia count) were used.
3. Results and discussion
(a). Arthropod grazing enhances resistance to fungivores
To clarify whether arthropod grazing induces an increase in resistance to fungivory, we subjected proliferating 3-day-old A. nidulans colonies to two treatments, primary fungivores, F. candida (+PF) and untouched controls (−PF). Subsequently, +PF and −PF colonies were offered in fungivore food choice experiments. Because we assumed a time lag between the challenge by fungivory and the onset of a defence response affecting resistance to fungivores, we included a post-fungivory period (PFP) of 0, 6 and 24 h. Different PFPs were included to allow fungi to recover from the initial feeding attack before they were used in the food choice assays. In a first experiment, choice of fungal food patches (+PF versus −PF) by F. candida (those added after the PFP) was significantly affected by both the observation period (1.5, 20 or 44 h) and the PFP (figure 1). While experimental fungivores after 1.5 h in the food choice situation displayed a striking preference for +PF colonies with PFPs of 0 and 6 hours, they tended to avoid the +PF colonies with a PFP of 24 h. With increasing observation period, however, fungivore foraging behaviour changed drastically in all PFP treatments, finally resulting in a strong avoidance of +PF colonies (figure 1). This behavioural response suggests that feeding on +PF colonies had a negative effect on fungivore fitness. Consistent with this hypothesis, we found that F. candida suffered reduced growth rate on +PF colonies compared with con-specifics on −PF colonies (figure 2a). Because F. candida feeding activity was significantly reduced in the +PF treatment (figure 2b) impaired fungivore growth was mainly due to reduced food intake rather than a growth inhibiting effect of ingested fungal tissue.
Figure 1.
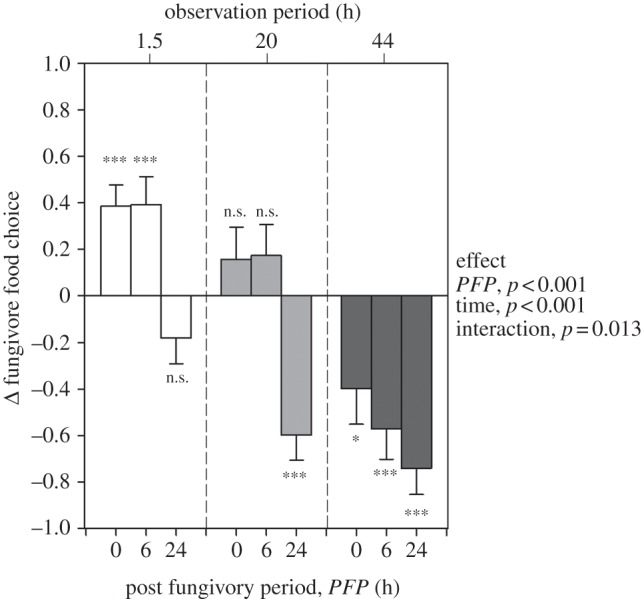
Folsomia candida food choice. Food choice (+PF, fungivore-challenged versus –PF, unchallenged) of F. candida as a function of increasing post-fungivory period (PFP) and observation period (1.5, 20 and 44 h). Fungivore preference was measured by subtracting the proportion of individuals on −PF colonies from the proportion of individuals on +PF colonies resulting in Δ-values ranging from +1 (full preference for PF) to −1 (full preference for −PF). Δ-values not significantly different from zero indicate no preference (n = 20 per treatment, see electronic supplementary material, table S1 for statistical details). (*p < 0.05, ***p < 0.001, n.s. not significantly different from zero, error bars are s.e.m.).
Figure 2.
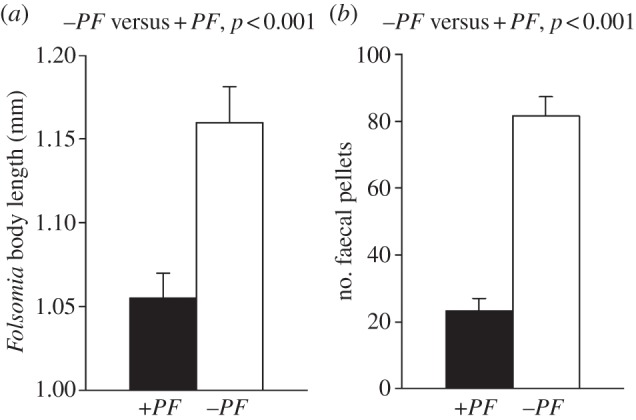
Folsomia candida feeding and fitness responses. (a) Mean body length of F. candida after feeding for 7 days (on −PF or +PF colonies (n = 20 per treatment). (b) Mean number of faecal pellets produced by experimental F. candida after feeding for 7 days on −PF or +PF colonies (n = 20 per treatment, error bars are s.e.m.).
Two mutually non-exclusive explanations may hold for the observed attractiveness of +PF colonies to fungivores with PFPs of 0 and 6 h: (i) Attack by primary fungivores triggered a fungal wounding response, e.g. emission of volatile compounds, that were attractive to the secondary F. candida [25]; (ii) experimental F. candida exploit cues left by con-specifics, such as aggregation pheromones, to find suitable feeding sites [26]. To test the first hypothesis, we artificially wounded A. nidulans colonies (WO) and offered them F. candida together with untouched control colonies in a choice experiment. We found that WO colonies tended to be attractive directly after wounding (equivalent to a PFP of 0 h). This behavioural trend completely vanished, however, when the fungi were given the opportunity to recover for 24 h, with arthropods showing preference for neither experimental patch type (see electronic supplementary material, figure S3). To test the second hypothesis, we conditioned patches free of the fungus with con-specifics and gave F. candida the choice between fungal free +PF and −PF patches. +PF patches strongly attracted F. candida, an effect that did not decline for up to the 24 h observation period (see electronic supplementary material, figure S4).
These data suggest that, when sufficient time elapses after primary fungivory (PFP ≥ 24 h), fungal-borne cues superimpose the effect of F. candida ‘aggregation pheromones’. Moreover, the conversion of initially highly attractive arthropod-challenged A. nidulans to a strongly repellent phenotype suggests that the change in resistance to fungivores is triggered by arthropod-specific cues rather than by simple wounding. The ability to distinguish between fungivore feeding and mere wounding is analogous to plant–herbivore interactions, in which insect-specific signals have been implicated in the ability of plants to increase herbivore resistance by recognizing herbivory-associated molecular patterns in insect oral secretions [27]. How fungi perceive fungivore grazing is unknown. In analogy to plant–herbivore systems, perception of fungivore-borne elicitors and hence the induction of local and systemic defence responses may involve activation of mitogen-activated protein kinases (MAPK) [28]. Recent efforts to disentangle the molecular network that integrate development and secondary metabolites biosynthesis in Aspergillus demonstrate the existence of a MAPK signalling pathway required for both sexual differentiation and mycotoxin formation [29]. Notably, control of A. nidulans secondary metabolites by MAPKs is connected with to the expression of LaeA [30]. LaeA is part of an epigenetic regulatory machinery that is involved in the biosynthesis of secondary metabolites [12], the maturation of sexual fruiting bodies [31] and resistance to fungivores [15].
(b). Arthropod grazing triggers formation of sexual fruiting bodies well protected from fungivory
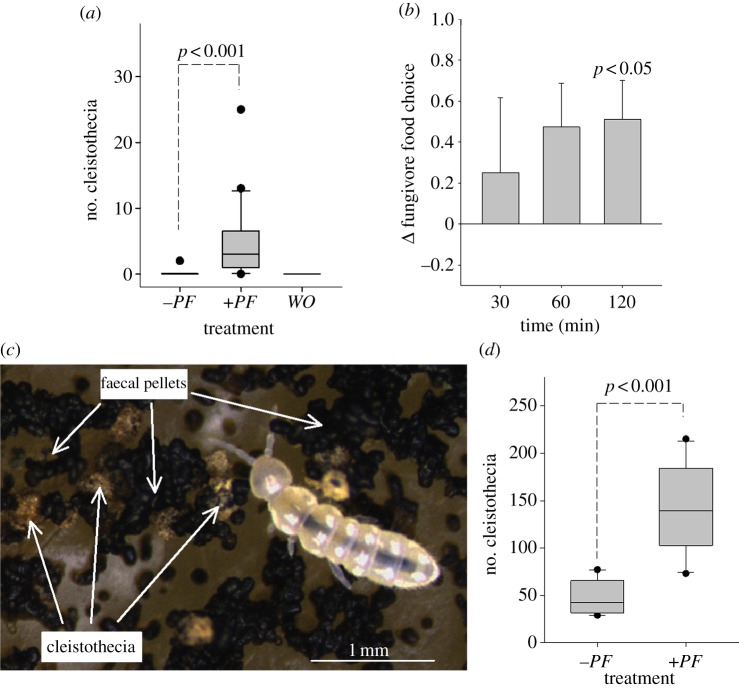
To determine whether the observed induction of resistance to fungivores is linked to enhanced investment in sexual reproduction, we exposed A. nidulans to moderate feeding pressure by F. candida and recorded the number of cleistothecia. We found that arthropod grazing induced a markedly premature formation of sexual fruiting bodies relative to −PF or WO colonies (figure 3a). We then considered whether fungivores display a food preference when they are given the choice between asexual and sexual fruiting tissue. We offered isolated asexual conidia and cleistothecia to F. candida and observed significant avoidance of cleistothecia (figure 3b), which indicates better protection of sexual ascospores from being consumed by collembolans compared with asexual conidia. The result of this short-term food choice experiment is supported by data from a long-term feeding assay, in which cleistothecia remained intact when exposed to F. candida for three weeks, but vegetative hyphae and conidia were almost entirely consumed (figure 3c). We again found a significantly larger number of cleistothecia produced by +PF as opposed to −PF colonies (figure 3d), which suggests that A. nidulans not only responded to fungivore grazing with accelerated ascospore production, but with enhanced investment in sexually generated progeny. Altogether, these results indicate that the advantage of the induced response leading to the repellence of fungivores from the whole colony, including hyphae and conidia, only accrues when fungivores have the opportunity to seek out alternative food sources, as in our choice experiments (a). Without the opportunity to exploit alternative food patches, fungivores exert severe grazing pressure on both vegetative hyphae and conidia, and long-term protection of fungal tissue is restricted to sexual fruiting bodies. The successful exploitation of (induced) hyphae and conidia by F. candida in the long-term feeding assay may be due to physiological adaption that involves activation of collembolan stress-response pathways [14].
Figure 3.
Induction of sexual fruiting bodies. (a) Box-plots depicting the number of cleistothecia produced in response to untouched control colonies (−PF), arthropod feeding (+PF) or artificial wounding (WO) (n = 20 per treatment). (b) Food choice of F. candida when offered asexual conidia and sexual cleistothecia. Positive Δ-values indicate preference for the conidia (n = 8; error bars are s.e.m.). (c) Representative image of the effect of long-term (three weeks) F. candida grazing on A. nidulans. Both the entire vegetative and asexual reproductive tissue has been consumed and the whole arena is covered with faecal pellets. Only the sexual fruiting bodies remain intact. (d) Box-plots depicting the number of cleistothecia found in A. nidulans exposed to long-term fungivory (+PF) compared with the number of cleistothecia formed by undisturbed control colonies (−PF). (n = 10 per treatment).
(c). Arthropod grazing enhances secondary metabolite production
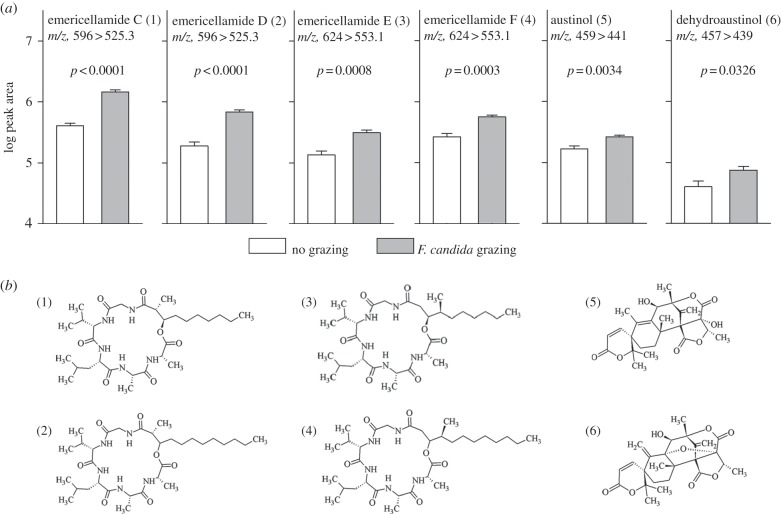
The previous results support the recent conjecture that F. candida spreads its feeding activity evenly across multiple wild-type A. nidulans colonies because single colonies enhance their capacity to repel collembolans in response to fungivore grazing [15]. By contrast, chemical deficient A. nidulans colonies were exploited unevenly [15], which prompted us to hypothesize that the induced change in resistance to fungivores is linked to dynamics in secondary metabolite biosynthesis, in particular ST [16]. To test this we directly quantified ST by HPLC-MS/MS in 10-day-old +PF and −PF colonies and revealed an almost threefold higher amount of ST in +PF colonies relative to −PF colonies (200.95 µg ± 9.42 versus 71.61 µg ± 10.50 ST per milligram fungal tissue; t-test on square-root-transformed data, t = −8.49, d.f. = 10, p < 0.001, n = 6 per treatment). We then used non-targeted metabolite profiling (HPLC-ESI-MS) to elucidate arthropod-mediated changes in the secondary metabolite composition of A. nidulans. Several signals enhanced by the exposure to F. candida grazing were identified (figure 4a and electronic supplementary material, figure S5). Fragmentation analysis (see electronic supplementary material, figure S6) revealed that the signals originated from emericellamides (mixed polyketides/peptides) [23] and austinol/dehydroaustinol (meroterpenoids of mixed polyketide/terpenoid origin) [24] (figure 4b). Some emericellamides have antibiotic activity [32] and fungal meroterpenoids exhibit neurotoxicity to insects [33], however, their explicit role in providing resistance to F. candida remains to be determined. Moreover, we found various unknown compounds exclusively expressed in challenged fungi (see electronic supplementary material, table S3). These data show that the induction of resistance to fungivore grazing in A. nidulans is accompanied by a fundamental shift in its secondary metabolite profile, in addition to the predicted up-regulation of ST. We hypothesize that synergistic effects of various secondary metabolites on fungivore performance [34] may be the driving force that favours the induction of a combination of secondary metabolites rather than investment in one single defensive compound. Furthermore, the results of the current study tend to demonstrate that the arthropod-mediated change in the secondary metabolite profile depends on the fungal developmental stage and is tissue-specific. In particular, striking avoidance of feeding on cleistothecia implies a heterogeneous distribution of anti-fungivore defence within a colony and possibly tissue-specific allocation of chemical defence in proportion to the tissue's value in fungal fitness, e.g. in Hülle cells proposed to protect the cleistothecium [31], as it is predicted by the optimal defence theory [35].
Figure 4.
Quantification of A. nidulans secondary metabolites by HPLC-MS/MS. (a) Peak area of ion chromatograms of secondary metabolites that were identified by using published mass spectra (see electronic supplementary material, figure S4). m/z values represent the transition of mass-to-charge ratios at which the HPLC peak areas were quantified. (n = 6 per treatment, error bars are s.e.m.) (b) Chemical structures of secondary metabolites enhanced in response to F. candida grazing.
4. Conclusion
The molecular machinery underlying co-regulation of fungal development and secondary metabolite production, the evolutionary biology of sexual reproduction and the ecology of fungus–fungivore interactions have been disparate research topics. We have shown here that fungivore feeding induces changes in the developmental program along with the secondary metabolite profile of A. nidulans, which benefits the fungus in the face of recurrent and long-term fungivory. To our knowledge, these data are the first to demonstrate that a fungus is able to adjust its morphological and chemical phenotype to fungivore grazing, and that flexible investment in sexual reproduction may allow facultative sexual fungi to compensate directly for fitness loss in fungivore-rich niches. The arthropod-induced chemical defence response appears to comprise more metabolites than previously thought. Deciphering the cryptic diversity of both induced and constitutively formed metabolites coupled with a mode of action analysis in the affected fungivore may help us understanding how fungal secondary metabolism has been shaped by fungivore grazing and has driven fungivore physiological and behavioural counter-adaptations [36]. A further consequence will be to conceptually integrate fungal resistance and metabolic plasticity into multitrophic interactions beyond a plant-centred perspective on inducible defences [37].
Acknowledgements
We thank Nancy P. Keller (University of Wisconsin) for providing the A. nidulans strain. Clay C.C. Wang (University of Southern California) is acknowledged for providing austinol/dehydroaustinol MS/MS data.
Funding
The study was supported by a German Research Foundation (DFG) grant to M.R. (RO3523/3-1). K.D., S.C. and P.K. established secondary metabolite extraction and metabolic profiling protocols. M.R. and S.S. designed the experiments. M.R. conducted the behavioural experiments. K.D., S.C. and M.R. analysed the data and wrote the manuscript. The authors declare no competing interests.
References
- 1.Begon ME, Townsend CR, Harper JL. 2006. Ecology: From Individuals to Ecosystems. Oxford, UK: Blackwell Publishing [Google Scholar]
- 2.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692 (doi:10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 3.Strong DR, Lawton JH, Southwood R. 1984. Insects on plants—community patterns and mechanisms. Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Johnson MTJ. 2011. Evolutionary ecology of plant defences against herbivores. Funct. Ecol. 25, 305–311 (doi:10.1111/j.1365-2435.2011.01838.x) [Google Scholar]
- 5.Boddy L, Jones TH. 2008. Interactions between basidiomycota and invertebrates. In Ecology of saprotrophic basidiomycetes (eds Boddy L, Frankland JC, van West P.), pp. 155–179 New York, NY: Academic Press [Google Scholar]
- 6.Ruess L, Lussenhop J. 2005. Trophic interactions of fungi and animals. In The fungal community—its organization and role in the ecosystem (eds Dighton J, White JK, Oudemans P.), pp. 581–598 Boca Raton, FL: CRC Press [Google Scholar]
- 7.Wilding N, Collins NM, Hammond PM, Webber JF. 1989. Insect–fungus interactions. London, UK: Academic Press [Google Scholar]
- 8.Mithöfer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annu. Rev. Plant Biol. 63, 431–450 (doi:10.1146/annurev-arplant-042110-103854) [DOI] [PubMed] [Google Scholar]
- 9.Spiteller P. 2008. Chemical defence strategies of higher fungi. Chemistry Eur. J. 14, 9100–9110 (doi:10.1002/chem.200800292) [DOI] [PubMed] [Google Scholar]
- 10.Rohlfs M, Churchill ACL. 2011. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fung. Genet. Biol. 48, 23–34 (doi:10.1016/j.fgb.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 11.Rohlfs M, Obmann B. 2009. Species-specific responses of dew fly larvae to mycotoxins. Mycotoxin Res. 25, 103–112 (doi:10.1007/s12550-009-0015-1) [DOI] [PubMed] [Google Scholar]
- 12.Bok JW, Keller NP. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukary. Cell 3, 527–535 (doi:10.1128/EC.3.2.527-535.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staaden S, Milcu A, Rohlfs M, Scheu S. 2011. Olfactory cues associated with fungal grazing intensity and secondary metabolite pathway modulate Collembola foraging behaviour. Soil Biol. Biochem. 43, 1411–1416 (doi:10.1016/j.soilbio.2010.10.002) [Google Scholar]
- 14.Janssens TKS, Staaden S, Scheu S, Mariën J, Ylstra B, Roelofs D. 2010. Transcriptional responses of Folsomia candida upon exposure to Aspergillus nidulans secondary metabolites in single and mixed diets. Pedobiologia 54, 45–52 (doi:10.1016/j.pedobi.2010.09.002) [Google Scholar]
- 15.Stötefeld L, Scheu S, Rohlfs M. 2012. Fungal chemical defense alters density-dependent foraging behavior and success in a fungivorous soil arthropod. Ecol. Entomol. 37, 323–329 (doi:10.1111/j.1365-2311.2012.01373.x) [Google Scholar]
- 16.Yin W-B, Amaike S, Wohlbach DJ, Gasch AP, Chiang Y-M, Wang CCC, Bok JW, Rohlfs M, Keller NP. 2012. An Aspergillus nidulans bZIP response pathway hardwired for defensive secondary metabolism operates through aflR. Mol. Microbiol. 83, 1024–1034 (doi:10.1111/j.1365-2958.2012.07986.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayram Ö, Braus GH. 2012. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36, 1–24 (doi:10.1111/j.1574-6976.2011.00285.x) [DOI] [PubMed] [Google Scholar]
- 18.Hadany L, Otto SP. 2007. The evolution of condition-dependent sex in the face of high costs. Genetics 176, 1713–1727 (doi:10.1534/genetics.107.074203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoustra S, Rundle H, Dali R, Kassen R. 2010. Fitness-associated sexual reproduction in a filamentous fungus. Curr. Biol. 20, 1350–1355 (doi:10.1016/j.cub.2010.05.060) [DOI] [PubMed] [Google Scholar]
- 20.Jokela J, Dybdahl MF, Lively CM. 2009. The maintenance of sex, clonal dynamics, and host-parasite coevolution in a mixed population of sexual and asexual snails. Am. Nat. 174, S43–S53 (doi:10.1086/599080) [DOI] [PubMed] [Google Scholar]
- 21.Ratzinger A, Riediger N, von Tiedemann A, Karlovsky P. 2009. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. J. Plant Res. 122, 571–579 (doi:10.1007/s10265-009-0237-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurentin H, Ratzinger A, Karlovsky P. 2008. Relationship between metabolic and genomic diversity in sesame (Sesamum indicum L.). BMC Genom. 9, 250 (doi:10.1186/1471-2164-9-250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang Y-M, et al. 2008. Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the emericellamide biosynthetic pathway. Chem. Biol. 15, 527–532 (doi:10.1016/j.chembiol.2008.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo H-C, Entwistle R, Guo C-J, Ahuja M, Szewczyk E, Hung J-H, Chiang Y-M, Oakley BR, Wang CCC. 2012. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids, austinol and dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 134, 4709–4720 (doi:10.1021/ja209809t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morath SU, Hung R, Bennett JW. 2012. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fung. Biol. Rev. 26, 73–83 (doi:10.1016/j.fbr.2012.07.001) [Google Scholar]
- 26.Wertheim B, van Baalen E-JA, Dicke M, Vet LEM. 2005. Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu. Rev. Entomol. 50, 321–346 (doi:10.1146/annurev.ento.49.061802.123329) [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Baldwin IT. 2009. Herbivory-induced signalling in plants: perception and action. Plant, Cell Environ. 32, 1161–1174 (doi:10.1111/j.1365-3040.2009.01943.x) [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Hettenhausen C, Meldau S, Baldwin IT. 2007. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19, 1096–1122 (doi:10.1105/tpc.106.049353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayram Ö, Sarikaya Bayram Ö, Ahmed YL, Maruyama J-I, Valerius O, Rizzoli SO, Ficner R, Irniger S, Braus GH. 2012. The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 8, e1002816 (doi:10.1371/journal.pgen.1002816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atoui A, Bao D, Kaur N, Grayburn WS, Calvo AM. 2008. Aspergillus nidulans natural product biosynthesis is regulated by MpkB, a putative pheromone response mitogen-activated protein kinase. Appl. Environ. Microbiol. 74, 3596–3600 (doi:10.1128/aem.02842-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarikaya Bayram Ö, et al. 2010. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet. 6, e1001226 (doi:10.1371/journal.pgen.1001226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez JF, Somoza AD, Keller NP, Wang CCC. 2012. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 29, 351–371 (doi:10.1039/c2np00084a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataoka S, Furutani S, Hirata K, Hayashi H, Matsuda K. 2011. Three austin family compounds from Penicillium brasilianum exhibit selective blocking action on cockroach nicotinic acetylcholine receptors. Neurotoxicology 32, 123–129 (doi:10.1016/j.neuro.2010.10.003) [DOI] [PubMed] [Google Scholar]
- 34.Dowd PF. 1988. Synergism of aflatoxin B1 toxicity with the co-occurring fungal metabolite kojic acid to two caterpillars. Entomol. Exp. Appl. 47, 69–71 (doi:10.1007/bf00186717) [Google Scholar]
- 35.Rhoades DF. 1979. Evolution of plant chemical defense against herbivores. In Herbivores—their interaction with secondary plant metabolites (eds Rosenthal GA, Janzen DH.), pp. 3–54 New York, NY: Academic Press [Google Scholar]
- 36.Després L, David J-P, Gallet C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22, 298–307 (doi:10.1016/j.tree.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 37.Kaplan I. 2012. Trophic complexity and the adaptive value of damage-induced plant volatiles. PLoS Biol. 10, e1001437 (doi:10.1371/journal.pbio.1001437) [DOI] [PMC free article] [PubMed] [Google Scholar]