Abstract
An ongoing new synthesis in evolutionary theory is expanding our view of the sources of heritable variation beyond point mutations of fixed phenotypic effects to include environmentally sensitive changes in gene regulation. This expansion of the paradigm is necessary given ample evidence for a heritable ability to alter gene expression in response to environmental cues. In consequence, single genotypes are often capable of adaptively expressing different phenotypes in different environments, i.e. are adaptively plastic. We present an individual-based heuristic model to compare the adaptive dynamics of populations composed of plastic or non-plastic genotypes under a wide range of scenarios where we modify environmental variation, mutation rate and costs of plasticity. The model shows that adaptive plasticity contributes to the maintenance of genetic variation within populations, reduces bottlenecks when facing rapid environmental changes and confers an overall faster rate of adaptation. In fluctuating environments, plasticity is favoured by selection and maintained in the population. However, if the environment stabilizes and costs of plasticity are high, plasticity is reduced by selection, leading to genetic assimilation, which could result in species diversification. More broadly, our model shows that adaptive plasticity is a common consequence of selection under environmental heterogeneity, and hence a potentially common phenomenon in nature. Thus, taking adaptive plasticity into account substantially extends our view of adaptive evolution.
Keywords: adaptation, developmental plasticity, genetic accommodation, heterogeneous environment, bottleneck, genetic variation
1. Introduction
Understanding the mechanisms of adaptation is the key to understand how life on the Earth has persisted over widely varying environmental conditions resulting in the observed biodiversity, and to understand how organisms would adapt to current global change. Adaptive evolution requires heritable phenotypic variation for selection to act upon, and the standing paradigm that emerged from Modern Synthesis argued that random genetic mutations of fixed phenotypic effects are the only source of heritable phenotypic variation fuelling adaptive evolution [1–3]. Under this scenario, mutations accumulate in populations through various combinations of recurrent mutation, drift, recombination, immigration and selection in heterogeneous environments [4–6]. Selection then acts on this standing genetic variation producing adaptations, and hence the environment acts merely as a sieve for phenotypes.
Nevertheless, there is now ample evidence showing that the environment can also act as a phenotypic inducer so that a single genotype is often capable of expressing alternative appropriate phenotypes in response to different environments [7–9]. This phenotypic plasticity is the consequence of environmentally induced changes in gene expression [10]. Plasticity is often heritable, and it evolves under selection if environmental cues are reliable and gene flow is high among subpopulations [11,12]. Conversely, local adaptation and reduced plasticity occur when dispersal is low [11] or environmental variation is unpredictable or negligible [13,14].
Extending the paradigm to include adaptive plasticity is a necessary step in evolutionary biology to extend our understanding of the mechanisms of adaptive evolution [15], and there has been a surge of interest in characterizing the evolutionary consequences of environmentally induced variation [16–18]. Previous theoretical studies have greatly contributed to our understanding of different aspects of the evolution of plasticity under particular scenarios, often using complex quantitative genetic models [19–22]. These models have shown that plasticity is advantageous in rapidly changing environments and that it may help colonizing new environments [22], although genetic correlations and costs of plasticity could limit these benefits of plasticity [23,24].
Adaptive plasticity can also result in evolutionary innovations [18]. If sister lineages evolve independently in different stable environments and ancestral plasticity is costly, divergent reaction norms are expected to evolve through selection on genetic modifiers available in the population [2,7,25]. This would lead to genetic accommodation of environmentally induced phenotypes, i.e. adaptive genetic changes in response to selection on the regulation and form of the phenotype [7]. Fixed-effect genes (i.e. not sensitive to environmental input) giving rise to phenotypes with increased fitness in the new environment will be positively selected, and the trait will become genetically assimilated, a particular case of genetic accommodation [7,26]. Thus, whether resulting in novel or canalized phenotypes, or simply in divergent reaction norms, developmental plasticity can foster speciation and diversification [17,27]. Genetic accommodation and assimilation of plasticity have been experimentally demonstrated [28–30] and also inferred from comparative analyses [31,32]. Plasticity is thus a common feature of organisms that is favoured by selection precisely under the same circumstances that maintain standing genetic variation, namely environmental heterogeneity and gene flow among subpopulations [11]. However, historically there has been some reluctance to recognize the importance of phenotypic plasticity in evolution [3,9,21,33,34]. Perhaps simple heuristic models may help illustrating the potential of plasticity in evolution while avoiding the so-often black-box feeling of complex models.
Here, we built and analysed a simple heuristic individual-based model comparing adaptive evolution in populations composed of either plastic or non-plastic genotypes. We examine how adaptive plasticity evolves under common scenarios assumed to maintain non-environmentally dependent standing genetic variation, and then examine how plasticity affects adaptive evolution because of the role of the environment as a phenotypic inducer. We simulated population dynamics under contrasting combinations of environmental stochasticity, occurrence of genetic changes, levels of plasticity and costs of plasticity. We specifically explored the conditions under which genetic assimilation occurs, and the relationship between plasticity and standing genetic variation. There is also evidence that in some organisms epigenetic marks allow induced phenotypes themselves (and not just the ability to produce them) to be inherited across multiple generations [35,36], but that is not the scope of this study. Here, we focus only on plastic genotypes that inherit the ability to produce different adaptive phenotypes according to perceived environmental cues.
We used the model to test the following predictions: (i) during rapid environmental change or when facing a novel environment, plasticity improves the persistence of populations and reduces the severity of bottlenecks; (ii) plasticity contributes to the maintenance of standing genetic variation within populations; (iii) by increasing population persistence and maintaining genetic variation, plasticity ‘buys time’ for appropriate genetic variants of fixed phenotypic effect to appear by mutation and (iv) costs of plasticity result in genetic assimilation (i.e. loss of plasticity) if heterogeneous environments stabilize.
2. The model
This model description follows the Overview, Design concepts and Details protocol for describing individual- and agent-based models [37–39]. The model is implemented in NetLogo v. 5.0.3 [40] (NetLogo is freely downloadable from http://ccl.northwestern.edu/netlogo/download.shtml) and available in the electronic supplementary material (Model.txt).
— Purpose. The main purpose of the model is to explore the consequences of phenotypic plasticity in adaptive evolution. This is done by simulating population persistence and genetic evolution under environmental change. Simulations are run separately for non-plastics and plastics. Non-plastics evolve by selection on random genotypic mutations with fixed phenotypic effects. Plastics evolve exactly in the same way, but also through selection on mutations conferring phenotypic plasticity (figure 1)
— Entities, state variables and scales. Environmental conditions are simulated by the variable environment. The entities of the model are asexual individuals of two kinds: either non-plastics or plastics. Each individual has a given genotype and a phenotype. Plastics also have a plasticity-range that allows them to improve their match with the environment. The match is an individual variable calculated as 1 - |phenotype - environment|, which shapes individual survival and reproduction (see below). The amount of plasticity-range used by the individual to improve its phenotypic match with the environment is the used-plasticity. For instance, a genotype of 0.7 in an environment of 0.8 with a plasticity-range of 0.2 would only need to use 0.1 of its plasticity-range to produce a perfectly matching phenotype (i.e. used-plasticity = 0.1). Thus, while plasticity-range is an inherited trait of the individual, plasticity-used is a value recorded by the model when the individual develops. One time step of the model corresponds to one generation, and generations are non-overlapping. See table 1 for variable definitions and range of parametrized values.
— Process overview and scheduling. See a schematic diagram in figure 1. At birth, individuals inherit from their parent's a genotype and (if plastics) a plasticity-range. Both genetic features mutate in the same way (see ‘mutation’ below). Non-plastics develop a phenotype equal to their genotype. Plastics, however, use their plasticity-range to fit their phenotype as much as possible to the environment (see ‘development’ below). Non-plastics and plastics have a mortality probability according to their realized match to the environment (see ‘die-by-mismatch?’ below). Subsequently, they can die by negative density-dependence (see ‘die-by-negative-density-dependence?’ below). Moreover, plastics could die by costs of maintaining a given plasticity-range and the costs of the plasticity-used (see ‘die-by-plasticity-costs?’ below). These two costs of plasticity are commonly identified in the literature on developmental plasticity as ‘maintenance costs’ and ‘production costs’ and correspond to the presumed costs of maintaining a sensory machinery and actually producing alterations on the phenotype, respectively [23,24]; see the electronic supplementary material. Surviving individuals reproduce (see ‘reproduction’ below) and die immediately after. The environment is updated before the new generation is born, starting the cycle again. The environment is thus updated between the death of generation t and the birth of generation t + 1 (see ‘environmental-change’ below). In this way, newborns can adjust (if plastics) their phenotype according to the environment where they will live until death; and this is the environment that will affect their survival and reproduction.
Figure 1.
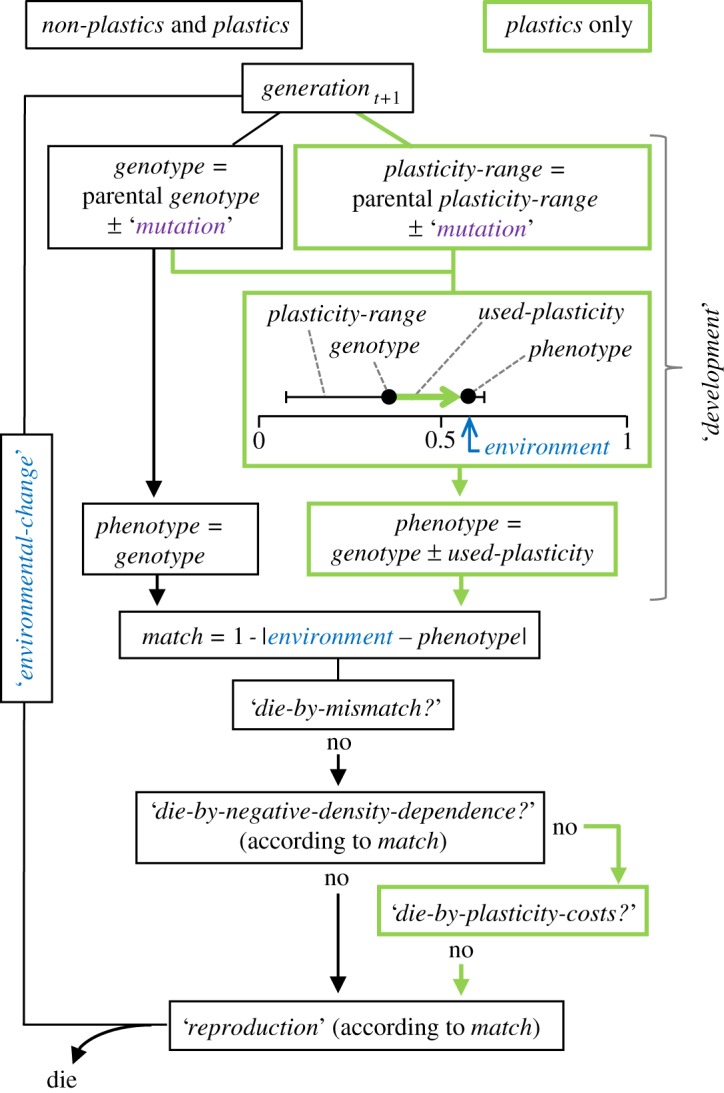
Schematic of the individual-based model comparing adaptive evolution in populations composed of plastic or non-plastic genotypes. They are all clonal organisms with no recombination so that non-plastic genotypes map directly into phenotypes and their odds of surviving and reproducing depend on the match with the environment. By contrast, plastic genotypes can respond to the environment modifying their phenotype to reduce the mismatch to the extent that their plasticity-range allows. In both cases, the environment acts as a selective factor, but for plastic genotypes it is also a phenotypic inducer. (Online version in colour.)
Table 1.
Variables and parametrization. All variables and parameters can take continuous values.
| initialization | constraints during simulations | description | |
|---|---|---|---|
| parameters | |||
| Std-Dev-environment-change | (0.04–1) | initialization value | determines the degree of environmental stochasticity environmentt + 1 = environmentt + N ∼ (0, Std-Dev-environment-change) |
| plasticity-costs | (0–1) | initialization value | determines whether plasticity carries a load reducing odds of survival and reproducing |
| mean-mutational-change | (0–0.002) | initialization value | determines both the probability of occurrence of genetic changes and their effect size on the phenotype |
| emergent values | |||
| environment | 0.5 | [0,1] | expresses the environmental conditions on a single dimension, the same one used to describe the phenotype, the genotype and the plasticity-range |
| non-plastics and plastics | |||
| phenotype | 0 | — | phenotypic value expressed in the same dimension as the environment |
| genotype | 0 | — | in the absence of plasticity, the phenotype = genotype |
| match | n.a. | — | absolute difference between the phenotypic value and the environmental value; the phenotype is optimized if match = 1 1 - |environment - phenotype| |
| plastics only | |||
| plasticity-range | 0 | — | the maximum phenotypic adjustment that a genotype is capable to increase match |
| used-plasticity | n.a. | 0 ≤ used-plasticity ≤ plasticity-range | amount of the plasticity-range that is actually used by an individual during development |
-
—
Design concepts. Evolution (changes in population mean/variance values of genotypes, either plastic or non-plastic, and plasticity-range) and other population dynamics (e.g. stability, bottlenecks and extinction) emerge from the combined effects of heredity, phenotypic plasticity (for plastics only), natural selection (differential survival and reproduction of individuals) and demographic (density-dependence) processes. Also, population genetic variability (either genotype or plasticity-range) is not imposed at initialization, but emerge during the first 100 generations when the population evolves under a mildly fluctuating environment (see ‘environmental-change’ below). Note that the genotype and the phenotype could potentially take any real value, but in simulations tended to remain between 0 and 1 because of the selection imposed by the environment and the initialization conditions (i.e. genotype = phenotype = 0.5; see figure 2 insets and figure 3c). Stochasticity affects environmental change, mutation, survival probability and reproduction. We recorded the number of individuals at the end of 300 generations (100 of them being the initialization generations). For illustrative purposes, we also recorded for some model runs longitudinal (e.g. environmental fluctuations, population size dynamics, mean population genotype, phenotype and plasticity-range) and transversal data (e.g. genotype of each individual) across and within generations, respectively.
Figure 2.
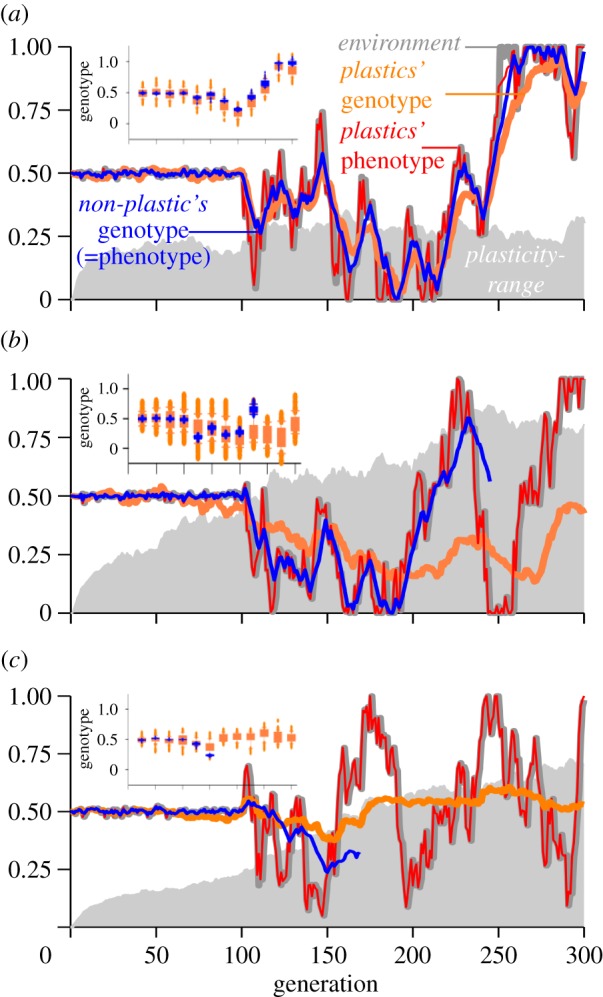
Examples of adaptive evolution of plastic and non-plastic populations under medium-low environmental fluctuations (Std-Dev-environment-change = 0.1) and different scenarios of mean-mutational-change and plasticity-costs. (a) At high mean-mutational-change and high plasticity-costs, plastics performed similar to non-plastics. Here, a high mean-mutational-change allowed both populations to closely track the environment. Plasticity-range was reduced compared with scenarios with lower costs but maintained owing to environmental fluctuations. (b) Under high mean-mutational-change but with low plasticity-costs, plasticity allowed a close phenotypic match to the environment and the persistence of the plastic population, but often non-plastics went extinct as shown in this example. (c) Under low mean-mutational-change and low plasticity-costs, plastic genotypes produced phenotypes that closely matched the environment while their genotypic values were intermediate across environmental fluctuations, and plasticity increased. Non-plastic genotypes could not adapt fast enough and quickly went extinct. At any given time and in all scenarios, genotypic variation was higher in the plastic population than in the non-plastic one. This is shown in inset boxplots in each panel, where blue boxes depict genetic variation of the non-plastic population and orange boxes that of the plastic population, sampled every 25 generations.
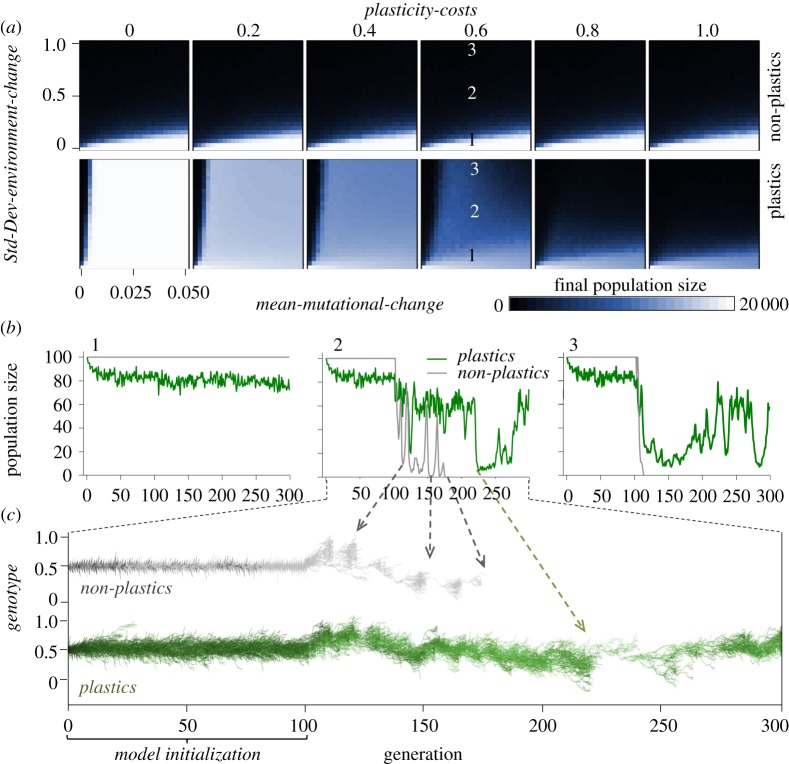
Figure 3.
(a) Results for population size for populations composed of either plastic or non-plastic genotypes from simulations sweeping over all parameter combinations of environmental stochasticity (Std-Dev-environment-change), mutation rate (mean-mutational-change) and plasticity-costs. Populations composed of plastic genotypes persisted over a much broader range of environmental stochasticity than populations of non-plastic genotypes, unless plasticity-costs were high, in which case they performed worse than non-plastic genotypes. (b) Examples of population dynamics for plastic and non-plastic populations at different levels of environmental stochasticity and mean-mutational-change = 0.04 and plasticity-costs = 0.6; panel numbers relate (a) and (b). (c) Example of clonal lineages trajectories (each line is a lineage) according to genotype and (for plastics) plasticity-range (lighter green colour depicts higher plasticity-range). Note that only very plastic lineages survived the strongest population bottleneck (as shown in corresponding (b) panel).
— Initialization. Simulations were initialized with environment = 0.5 and 100 individuals (either mutants or plastics). All individuals started with genotype = phenotype = 0.5. Plastics started with plasticity-range = 0.
— Input. The model does not have any external input; the environment is updated according to internal model rules.
- — Submodels
- — ‘environmental-change’. During the first 100 generations of a simulation, the environment tightly fluctuates around 0.5. This is achieved by changing the environment towards 0.5 by increasing (or decreasing) the environment by a pseudorandom number extracted from a normal distribution with mean = 0.5 and variance arbitrarily fixed at 0.01 to ensure small fluctuations of the environment around 0.5. For the next 200 generations, the environment fluctuates every generation according to the value of a pseudorandom number extracted from a normal distribution with zero mean and Std-Dev-environment-change variance. To test the adaptive response to rapid directional changes and the role of costs of plasticity in causing genetic assimilation, we also modelled a scenario in which the environment fluctuates during the first 100 generations as in the other simulations, but then rapidly drift upwards in steps of 0.015 from 0.5 to 1, then remaining at 1 for the rest of the simulation.
- — ‘reproduction’. Each individual produce match × 2 individuals, rounded to the nearest integer; i.e. they produce either 0, 1 or 2 individuals according to their match.
- — ‘mutation’. The genotype and the plasticity-range (if plastics) inherited from the parent mutate by extracting a pseudorandom number from an exponential decay distribution with mean mean-mutational-change (see the electronic supplementary material). This number is either added or extracted to the inherited trait with equal probability. In this way, we are jointly modelling the probability of mutation and the magnitude of its effect on the phenotype. Given the many sources and kinds of mutations, we preferred this approach over simply modelling a per base per generation substitution rate (see the electronic supplementary material).
- — ‘development’. Non-plastics develop a phenotype = genotype. Plastics, however, use their plasticity-range to produce a phenotype as close as possible (given their plasticity-range) to the environment. The amount of plasticity-range eventually used is called used-plasticity (i.e. 0 ≤ used-plasticity ≤ plasticity-range).
- — ‘die-by-mismatch?’. Individuals can die because of a low match with the environment. They do so with probability 1—match, i.e. extracting a pseudorandom number from a uniform distribution from 0 to 1, dying if this number is > match.
- — ‘die-by-negative-density-dependence?’. Plastics and non-plastics die because of negative density-dependence when (before reproduction) population size is above 100 individuals. The dying individuals are those with lower match with the environment (note that in any given model run all individuals are either plastics or non-plastics, so there is no competition between these types).
- — ‘die-by-plasticity-costs?’. With the same approach, plastics can also die first with probability = plasticity-range × plasticity-costs, and then also with probability = used-plasticity × plasticity-costs. That way, increased plasticity costs penalize separately plasticity maintenance and plasticity use. Maintenance is associated with the ability of being plastic, i.e. plasticity-range; the broader the range of possible phenotypes, the highest the cost. Production costs, however, are the costs incurred when actually altering the phenotype (i.e. used-plasticity; see the electronic supplementary material).
(a). Simulations
Simulations for non-plastics and plastics are run independently but using the same pseudorandom generator seed to make results fully comparable. For each group, we ran a total of 200 simulations for each of the 4056 combinations of 26 (equally spaced) values for Std-Dev-environment-change, 26 different values for mean-mutational-change and six values of plasticity-cost i.e. a total of 811 200 model runs (see table 1 for parameter details). For each of the 4056 parameter combinations, we calculated (separately for non-plastics and plastics) population size at the end of the simulations and the cumulated population size along the 200 generations after initialization. Note that we run 200 simulations for each of the 4056 parameter combinations for plastics and non-plastics although parametrizations only differing in the plasticity-cost value do not affect non-plastics. This way results from plastics were directly comparable with simulations (with same pseudorandom generation seeds) for non-plastics. To test hypothesis (iv) regarding genetic assimilation in a novel environment, we also modelled a scenario with an abrupt directional environmental change, which then stabilized (see above). This could represent either the colonization of a novel habitat, or a rapid environmental transformation such as those occurring as a consequence of global change across the world.
3. Results
During the first 100 generations of the model runs, the environment was forced to remain close to 0.5 and the initial generation had genotype = 0.5 and plasticity-range (if plastics) = 0. In all simulation runs, plastic and non-plastic populations survived these initial generations, generating standing genetic variation and (in plastics) variation in plasticity-range. As plasticity costs increased, population size during the first 100 generations of initialization was lower for plastics than for non-plastics (see examples for intermediate plasticity costs in figure 3b), indicating that under low environmental fluctuations, plasticity costs may outweigh the benefits of plasticity.
(a). Adapting to a fluctuating environment
Afterwards, when the environment was allowed to vary stochastically along 200 generations, the plastic and non-plastic populations began evolving to adapt to the changing environment. Both plastic and non-plastic populations were capable of persisting over simulated environmental fluctuations provided that the mean-mutational-change was high, but population viability was severely compromised as environmental fluctuations increased (figures 2 and 3). At low environmental fluctuations, plastics always performed slightly worse than non-plastics during the next 200 generations (figure 3a, and first panel of figure 3b). This also supports the idea that plasticity even at low plasticity costs has demographic consequences when occurring at low environmental fluctuations.
Selection favoured increased plasticity during bouts of rapid, recurrent or wide environmental shifts (figure 2 main panels), often being the most plastic genotypes the ones that persisted (see examples in figures 3c and 4a). Costs of plasticity reduced the effectiveness of the plastic response and when taken to the extreme ultimately made plastic genotypes evolve analogously to non-plastic ones (figure 3a). Except in such scenarios of extreme costs of plasticity, plastic genotypes always showed a better phenotypic match to the environment than non-plastic ones, even at high mean-mutational-change (figure 2 main panels).
Figure 4.
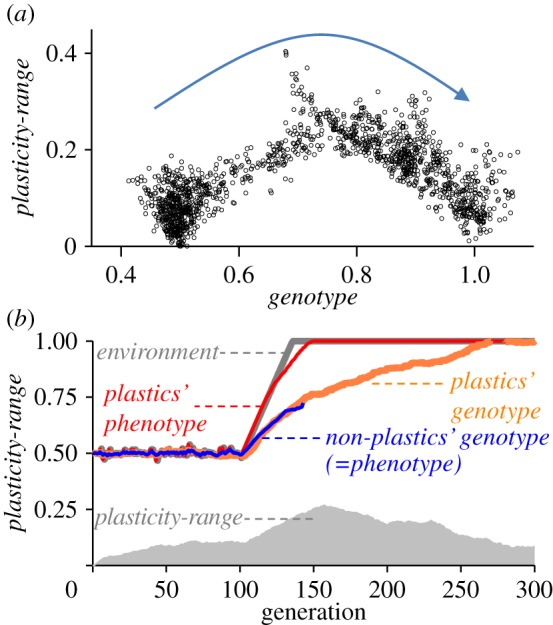
Example of model run for a scenario of directional environmental change, where environment changed abruptly from 0 to 1 and then stabilized at 1 with mean-mutational-change = 0.005 and plasticity-costs = 0.7. (a) Shows for plastic individuals their position in the genotype versus plasticity-range space. The arrow indicate the pass of time (in generations), beginning with all individuals with genotype = 0.5 and plasticity-range = 0 (initialization conditions) and ending at the end of the simulation with individuals with genotypes close to 1 and reduced plasticity-range. (b) Same as in figure 2. It is shown how plasticity increased temporarily under selection and the plastic population expressed well-matched phenotypes, allowing the population to persist over enough generations to allow genotypes to slowly evolve towards the new optimum. Once the environment stabilizes, plasticity is rapidly reduced owing to costs of plasticity, causing genetic assimilation.
At higher Std-Dev-environment-change, selective sweeps of poorly matched genotypes were more frequent and resulted in population bottlenecks (figure 3b), reducing the likelihood of persistence for both plastic and non-plastic genotypes (figure 3a). Population viability of non-plastics was restricted to low environmental fluctuations and high mean-mutational-change (figure 3a). Plastic genotypes, however, experienced attenuated population bottlenecks because a greater fraction of genotypes within the population were capable of expressing appropriate phenotypes, confirming our first prediction (figures 2 and 3). Plasticity allowed the persistence of populations even at low rates of mean-mutational-change and high environmental fluctuations, unless plasticity-costs were high (0.7 and above; figure 3a).
The maintenance of an average greater population size and alleviation of bottlenecks also contributed to increased genetic variation in the plastic populations (figure 2 insets). Moreover, because large plasticity-ranges allowed genotypes that would otherwise have had a poorly fitted phenotype to improve their match, the effect of selection was buffered and higher genotypic diversity within populations was retained in plastic populations at all times, confirming our second prediction. The strong genetic response to selection of non-plastics, however, resulted in a better match between average genotype and the environment for non-plastic than for plastic genotypes (figure 2). Consequently, in fluctuating environments, plasticity allowed the phenotype to closely match the environment while slowing down the genotypic response to selection (figure 2). At low plasticity-costs, the average genotypic value was maintained around the average value of the environmental conditions experienced throughout the simulations while at the same time retaining large genotypic variance (figure 2b,c). In consequence, low plasticity-costs allowed increased plasticity to evolve (figure 2b,c), leading to a higher genotype variance (figure 2b,c insets) and thus increasing the chances that appropriate genetic variants of fixed phenotypes arose by mutation.
(b). Environmental stabilization and genetic assimilation
To test the prediction that costs of plasticity result in loss of plasticity upon environment stabilization, we simulated a fast environmental transition from environment = 0.5–1, followed by environment stabilization at 1, such as it would occur for instance owing to human activity or if a population was to enter a distinct ecological region (figure 4). As in previous analyses (figures 2 and 3), our model exploration showed that adaptation to the novel environment in the non-plastic population depended on mean-mutational-change relative to environmental change (results not shown). Also, if the environment changed too abruptly given their mean-mutational-change, the non-plastic population failed to adapt and went extinct. Plastic genotypes, however, managed to persist even with a low mean-mutational-change and despite rapid transitions to the novel environment. It was possible because their plasticity-range allowed them to manifest phenotypes that better matched the environment at any given time. As shown in figure 4a, plasticity-range was strongly positively selected during the abrupt environmental change and only the most plastic genotypes survived the sharp environmental transition, because only very plastic genotypes were capable of producing extreme phenotypes. Nevertheless, plastic genotypes lagged substantially behind their phenotype (figure 4b). In other words, plasticity bought time for adaptive, fixed (i.e. non-environment sensitive) genetic changes to occur because individuals expressed the appropriate phenotype soon but it often still took the genotype many generations to match the environment (figure 4b). When costs of plasticity were high and the new environment remained stable, plasticity quickly decreased to background levels maintained by mutation, resulting in genetic assimilation of the environmentally induced phenotypes (figure 4a,b).
4. Discussion
With this simple heuristic model, we integrated adaptive plasticity into an explicit population genetic framework and examined some fundamental consequences of plasticity in adaptive evolution. We found that fluctuating or rapid directional environmental change strongly selected for plastic genotypes. This result is in accordance with previous modelling approaches [22,41,42], especially when environmental fluctuations are modelled to act after development but before selection [43]. In our model, increased plasticity allowed genotypes to produce phenotypes better matching the changing environmental conditions at each generation, hence showing a high potential for rapid adaptation to new environments. This relationship between plasticity and adaptive potential to novel environments has been suggested in some cases, as in invasive plant species having greater plasticity than non-invasive ones [44]; plasticity mediating rapid adaptation to introduced predators in zooplanktonic species [45]; or adaptations to climate change in birds [46].
Plasticity led to faster phenotypic modifications of whole populations because adaptive phenotypes were induced concurrently by environmental cues available to all individuals, instead of requiring the time for beneficial mutations to spread throughout the population by differential survival and reproduction [7]. This allowed populations composed of plastic genotypes to suffer fewer and lesser demographic bottlenecks despite steep fluctuations in the environment (figures 2 and 3).
An important result emerging from this model is that adaptive plasticity contributes to the maintenance of genetic variation within population (figure 2 insets) in two ways. First, plastic populations had higher genetic variation because plasticity shielded a broader range of genotypes from purifying selection by allowing them to express well-matched phenotypes. Second, plasticity reduced the effect of genetic drift as a consequence of maintaining greater population sizes (i.e. by reducing population bottlenecks). This result is supported by a very different modelling approach that has also recently proposed that plasticity tends to lead to populations with greater mutational and standing genetic variance [47].
It has often been debated whether plasticity fosters evolution by facilitating adaptation to novel environments or rather impede divergence by shielding genetic variation from divergent selection [17,48,49]. We show that plasticity allows phenotypically cryptic (or unexpressed) genetic variation to build up within populations by conferring similar fitness to distinct genotypic variants (see also [18,50]). Adaptive plasticity also allows otherwise imperilled populations to persist until appropriate genetic variants appear (figures 2 and 4). Moreover, the accumulated genetic variation can be rapidly released and manifested in the face of further environmental or mutational changes, enabling rapid adaptive divergences [6,17,51,52]. Our study suggests that plasticity facilitates adaptation to novel environments by allowing a synchronic phenotypic shift in response to the environment, while at the same time maintaining genetic variation that would otherwise be selected out (figure 2 insets), even though phenotypic plasticity slows down the response to selection (figures 2 and 4b).
Overall, shielding of genetic variation by plasticity may only be a transient effect of an otherwise rapid process of adaptation to divergent environments by genetic accommodation, as we found that plastic genotypes always showed a greater adaptive potential to a changing environment (figures 2–4). Congruently, there are many cases of rapidly diversifying groups of species where genetic accommodation of plasticity is likely to have been the main driver for divergence [53], as in sticklebacks [54,55], anole lizards [56] or arctic charrs [57]. Rapid adaptive transitions between environments are more easily achieved by plastic than non-plastic genotypes (figures 3 and 4), and we show that genetic assimilation of induced phenotypes and the associated loss of plasticity will occur if costs of plasticity are high and the environment stabilizes (figure 4).
Plasticity costs have been elusive and difficult to measure empirically [58–60], but there is evidence for plasticity costs from plants to invertebrates and vertebrates [61–63]. Moreover, patterns of evolution of plasticity are often congruent with theoretical expectations of the consequences of costs of plasticity, namely reduced plasticity under stable environmental conditions. American spadefoot toads, for instance, have evolved a canalized accelerated larval development with respect to the slow but plastic development ancestral to the group as a result of their adaptation to ephemeral desert ponds [31]. Accelerated development has become nearly genetically assimilated, and plasticity has been lost to a great extent in desert spadefoot toads so they are no longer capable of long larval periods [31,64]. Such translation of ancestral environmentally induced changes in development within populations into adaptive constitutive divergences among taxa is a clear path connecting micro- and macroevolution [2,7,31].
Because environmental variation is the rule in nature [65] and it often selects for adaptive plasticity [16,18,66], the evolutionary paradigm needs to be extended to include environmentally dependent regulation of gene expression as a heritable source of phenotypic variation, whether genetic or epigenetic [9,35,67–69]. Whether the incorporation of adaptive plasticity constitutes an extension of the paradigm emerged from the Modern Synthesis or a new paradigm, may ultimately be better evaluated retrospectively. To some extent, adaptive plasticity simply extends and strengthens the current paradigm, as it improves our understanding of the maintenance of genetic variation in populations, facilitates rapid adaptive shifts between adaptive peaks and helps explaining the adaptive radiations and recurrent parallel speciation. However, at the same time, accounting for adaptive plasticity expands the Modern Synthesis paradigm in several meaningful aspects that may warrant a new paradigm. Our model illustrates these aspects in a fairly simple and intuitive way. First, during organismal development, the environment acts as a phenotypic inducer in addition to its traditional role as a mere selective sieve. This is important because environmental induction may act simultaneously on most genotypes in a population inducing synchronous phenotypic shifts in the direction of the new local adaptive optimum. Second, plasticity increases the match of the phenotype to the environment, reducing bottlenecks and hence increasing population viability. Last, plasticity contributes to the maintenance of genetic variation within populations both by shielding many genetic variants from selection and by reducing genetic drift, and can become quickly accommodated between lineages evolving in divergent environments.
In this line of thought, our model shows the high relevance of plasticity to evolution and population ecology, while at the same time it shows that incorporating plasticity is conceptually as simple as acknowledging the fact that genotypes may have the potential to use environmental information to express better fit phenotypes. Other central tenets of mainstream evolutionary thought (i.e. random mutation and selection of phenotypes according to environmental conditions) evidently remain unchanged. The simple addition of environmentally sensitive adaptive gene regulation, however, provides a demonstrated mechanism for swift adaptation to rapidly changing environments that may have often lead to lineage diversification and evolutionary innovations.
Acknowledgements
We thank P. Edelaar, S. Sultan, J. Moyá and C. M. Herrera, and two anonymous reviewers for their comments and suggested improvements to the model.
Funding statement
This research was supported by the Ramón y Cajal Program of the Spanish Ministerio de Ciencia e Innovación (MICINN) to I.G.M. (RYC-2008-03519) and to R.J. (RYC-2009-03967).
References
- 1.Futuyma DJ. 2009. Evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Moczek AP. 2007. Developmental capacitance, genetic accommodation, and adaptive evolution. Evol. Dev. 9, 299–305 (doi:10.1111/j.1525-142X.2007.00162.x) [DOI] [PubMed] [Google Scholar]
- 3.Pigliucci M, Müller GB. 2010. Elements of an extended evolutionary synthesis. In Evolution: the extended synthesis (eds Pigliucci M, Müller GB.), pp. 3–17 Cambridge, MA: MIT Press [Google Scholar]
- 4.Gillespie JH, Turelli M. 1989. Genotype-environment interactions and the maintenance of polygenic variation. Genetics 121, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers DL. 2005. Evolution in heterogeneous environments and the potential of maintenance of genetic variation in traits of adaptive significance. Genetica 123, 107–124 (doi:10.1007/s10709-003-2721-5) [DOI] [PubMed] [Google Scholar]
- 6.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 7.West-Eberhard MJ. 2003. Developmental plasticity and evolution, p. 794 Oxford, UK: Oxford University Press [Google Scholar]
- 8.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture, p. 384 Baltimore, MA: Johns Hopkins University Press [Google Scholar]
- 9.Cabej NR. 2012. Epigenetic principles of evolution. London, UK: Elsevier [Google Scholar]
- 10.Aubin-Horth N, Renn SCP. 2009. Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol. Ecol. 18, 3763–3780 (doi:10.1111/j.1365-294X.2009.04313.x) [DOI] [PubMed] [Google Scholar]
- 11.Sultan SE, Spencer HG. 2002. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283 (doi:10.1086/341015) [DOI] [PubMed] [Google Scholar]
- 12.Scheiner SM, Barfield M, Holt RD. 2012. The genetics of phenotypic plasticity. XI. Joint evolution of plasticity and dispersal rate. Ecol. Evol. 2, 2027–2039 (doi:10.1002/ece3.327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallsson LR, Björklund M. 2012. Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity. J. Evol. Biol. 25, 1275–1290 (doi:10.1111/j.1420-9101.2012.02512.x) [DOI] [PubMed] [Google Scholar]
- 14.Roff DA. 1997. Evolutionary quantitative genetics. New York, NY: Chapman & Hall [Google Scholar]
- 15.Losos JB, et al. 2013. Evolutionary Biology for the 21st Century. PLoS Biol. 11, e1001466 (doi:10.1371/journal.pbio.1001466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West-Eberhard MJ. 2002. Development and selection in adaptive evolution. Trends Ecol. Evol. 17, 65 (doi:10.1016/S0169-5347(01)02413-2) [Google Scholar]
- 17.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol. Evol. 25, 459–467 (doi:10.1016/j.tree.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 18.Moczek AP, Sultan S, Foster S, Ledon-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW. 2011. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 278, 2705–2713 (doi:10.1098/rspb.2011.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancel LW. 1999. A quantitative model of the Simpson–Baldwin effect. J. Theor. Biol. 196, 197–209 (doi:10.1006/jtbi.1998.0833) [DOI] [PubMed] [Google Scholar]
- 20.Berrigan D, Scheiner SM. 2004. Modeling the evolution of phenotypic plasticity. In Phenotypic plasticity (eds DeWitt TJ, Scheiner SM.), pp. 82–97 Oxford, UK: Oxford University Press [Google Scholar]
- 21.de Jong G. 2005. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytol. 166, 101–118 (doi:10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 22.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 (doi:10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 23.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 24.Auld JR, Agrawal AA, Relyea RA. 2009. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pigliucci M, Murren CJ. 2003. Genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57, 1455–1464 [DOI] [PubMed] [Google Scholar]
- 26.Crispo E. 2007. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479 (doi:10.1111/j.1558-5646.2007.00203.x) [DOI] [PubMed] [Google Scholar]
- 27.Foster SA, Wund MA. 2011. Epigenetic contributions to adaptive radiations: insights from threespine sticklebacks. In Epigenetics linking genotype and phenotype in development and evolution (eds Hallgrimsson B, Hall BK.), pp. 317–336 Berkeley, CA: University of California Press [Google Scholar]
- 28.Waddington CH. 1952. Selection of the genetic basis for an acquired character. Nature 169, 278 (doi:10.1038/169278a0) [DOI] [PubMed] [Google Scholar]
- 29.Waddington CH. 1959. Canalization of development and the inheritance of acquired characters. Nature 183, 1654–1655 (doi:10.1038/1831654a0) [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Nijhout HF. 2006. Evolution of a polyphenism by genetic accommodation. Science 311, 650–652 (doi:10.1126/science.1118888) [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Mestre I, Buchholz DR. 2006. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl Acad. Sci. USA 103, 19 021–19 026 (doi:10.1073/pnas.0603562103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledon-Rettig CC, Pfennig DW, Nascone-Yoder N. 2008. Ancestral variation and the potential for genetic accommodation in larval amphibians: implications for the evolution of novel feeding strategies. Evol. Dev. 10, 316–325 (doi:10.1111/j.1525-142X.2008.00240.x) [DOI] [PubMed] [Google Scholar]
- 33.Simpson GG. 1953. The Baldwin effect. Evolution 7, 110–117 (doi:10.2307/2405746) [Google Scholar]
- 34.Orr HA. 1999. Evolutionary biology: an evolutionary dead end? Science 285, 343–344 (doi:10.1126/science.285.5426.343) [Google Scholar]
- 35.Jablonka E, Lamb MJ. 2010. Transgenerational epigenetic inheritance. In Evolution: the extended synthesis (eds Pigliucci M, Müller GB.), pp. 137–174 Cambridge, MA: The MIT Press [Google Scholar]
- 36.Holeski LM, Jander G, Agrawal AA. 2012. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27, 618–626 (doi:10.1016/j.tree.2012.07.011) [DOI] [PubMed] [Google Scholar]
- 37.Grimm V, Railsback SF. 2005. Individual-based modeling and ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 38.Grimm V, et al. 2006. A standard protocol for describing individual-based and agent-based models. Ecol. Model. 198, 115–126 (doi:10.1016/j.ecolmodel.2006.04.023) [Google Scholar]
- 39.Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J, Railsback SF. 2010. The ODD protocol: a review and first update. Ecol. Model. 221, 2760–2768 (doi:10.1016/j.ecolmodel.2010.08.019) [Google Scholar]
- 40.Wilensky U. 1999. NetLogo. Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL, USA See http://ccl.northwestern.edu/netlogo
- 41.Scheiner SM. 1998. The genetics of phenotypic plasticity. VII. Evolution in a spatially-structured environment. J. Evol. Biol. 11, 303–320 [Google Scholar]
- 42.Chevin LM, Lande R. 2011. Adaptation to marginal habitats by evolution of increased phenotypic plasticity. J. Evol. Biol. 24, 1462–1476 (doi:10.1111/j.1420-9101.2011.02279.x) [DOI] [PubMed] [Google Scholar]
- 43.Scheiner SM, Holt RD. 2012. The genetics of phenotypic plasticity. X. Variation versus uncertainty. Ecol. Evol. 2, 751–767 (doi:10.1002/ece3.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 14, 419–431 (doi:10.1111/j.1461-0248.2011.01596.x) [DOI] [PubMed] [Google Scholar]
- 45.Scoville AG, Pfrender ME. 2010. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl Acad. Sci. USA 107, 4260–4263 (doi:10.1073/pnas.0912748107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 47.Draghi JA, Whitlock MC. 2012. Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution 66, 2891–2902 (doi:10.1111/j.1558-5646.2012.01649.x) [DOI] [PubMed] [Google Scholar]
- 48.Price TD, Qvarnstrom A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440 (doi:10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (doi:10.1111/j.1365-2435.2007.01283.x) [Google Scholar]
- 50.Flatt T. 2005. The evolutionary genetics of canalization. Q. Rev. Biol. 80, 287–316 (doi:10.1086/432265) [DOI] [PubMed] [Google Scholar]
- 51.Gibson G, Dworkin I. 2004. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5, 681–690 (doi:10.1038/nrg1426) [DOI] [PubMed] [Google Scholar]
- 52.Le Rouzic A, Carlborg N. 2008. Evolutionary potential of hidden genetic variation. Trends Ecol. Evol. 23, 33–37 (doi:10.1016/j.tree.2007.09.014) [DOI] [PubMed] [Google Scholar]
- 53.West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (doi:10.1146/annurev.es.20.110189.001341) [Google Scholar]
- 54.Wund M, Baker JA, Clancy B, Golub JL, Foster SA. 2008. A test of the ‘Flexible Stem’ model of evolution: ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. Am. Nat. 172, 449–462 (doi:10.1086/590966) [DOI] [PubMed] [Google Scholar]
- 55.Wund MA, Valena S, Wood S, Baker JA. 2012. Ancestral plasticity and allometry in threespine stickleback reveal phenotypes associated with derived, freshwater ecotypes. Biol. J. Linn. Soc. 105, 573–583 (doi:10.1111/j.1095-8312.2011.01815.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. 2000. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54, 301–305 [DOI] [PubMed] [Google Scholar]
- 57.Adams CE, Huntingford FA. 2004. Incipient speciation driven by phenotypic plasticity? Evidence from sympatric populations of Arctic charr. Biol. J. Linn. Soc. 81, 611–618 (doi:10.1111/j.1095-8312.2004.00314.x) [Google Scholar]
- 58.Scheiner SM, Berrigan D. 1998. The genetics of phenotypic plasticity. VIII. The cost of plasticity in Daphnia pulex. Evolution 52, 368–378 (doi:10.2307/2411074) [DOI] [PubMed] [Google Scholar]
- 59.Johansson F. 2002. Reaction norms and production costs of predator-induced morphological defences in a larval dragonfly (Leucorrhinia dubia : Odonata). Can. J. Zool. 80, 944–950 (doi:10.1139/z02-073) [Google Scholar]
- 60.Van Buskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860 (doi:10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 61.DeWitt TJ. 1998. Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J. Evol. Biol. 11, 465–480 (doi:10.1007/s000360050100) [Google Scholar]
- 62.Agrawal AA, Conner JK, Johnson MTJ, Wallsgrove R. 2002. Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution 56, 2206–2213 [DOI] [PubMed] [Google Scholar]
- 63.Relyea RA. 2002. Costs of phenotypic plasticity. Am. Nat. 159, 272–282 (doi:10.1086/338540) [DOI] [PubMed] [Google Scholar]
- 64.Kulkarni SS, Gomez-Mestre I, Moskalik CL, Storz BL, Buchholz DR. 2011. Evolutionary reduction of developmental plasticity in desert spadefoot toads. J. Evol. Biol. 24, 2445–2455 (doi:10.1111/j.1420-9101.2011.02370.x) [DOI] [PubMed] [Google Scholar]
- 65.Ricklefs RE, Schluter D. 1993. Species diversity in ecological communities: historical and geographical perspectives. Chicago, IL: University of Chicago Press [Google Scholar]
- 66.Scheiner SM. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68 (doi:10.1146/annurev.es.24.110193.000343) [Google Scholar]
- 67.Bossdorf O, Richards CL, Pigliucci M. 2008. Epigenetics for ecologists. Ecol. Lett. 11, 106–115 [DOI] [PubMed] [Google Scholar]
- 68.Carroll SB. 2008. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36 (doi:10.1016/j.cell.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 69.Richards CL, Bossdorf O, Pigliucci M. 2010. What role does heritable epigenetic variation play in phenotypic evolution? BioScience 60, 232–237 (doi:10.1525/bio.2010.60.3.9) [Google Scholar]