Abstract
Restricted reproduction is traditionally posited as the defining feature of eusocial insect workers. The discovery of worker reproduction in foreign colonies challenges this view and suggests that workers’ potential to pursue selfish interests may be higher than previously believed. However, whether such reproductive behaviour truly relies on a reproductive decision is still unknown. Workers’ reproductive decisions thus need to be investigated to assess the extent of workers’ reproductive options. Here, we show in the bumblebee Bombus terrestris that drifting is a distinct strategy by which fertile workers circumvent competition in their nest and reproduce in foreign colonies. By monitoring workers’ movements between colonies, we show that drifting is a remarkably dynamic behaviour, widely expressed by both fertile and infertile workers. We demonstrate that a high fertility is, however, central in determining the propensity of workers to enter foreign colonies as well as their subsequent reproduction in host colonies. Moreover, our study shows that the drifting of fertile workers reflects complex decision-making processes associated with in-nest reproductive competition. This novel finding therefore adds to our modern conception of cooperation by showing the previously overlooked importance of alternative strategies which enable workers to assert their reproductive interests.
Keywords: drifting, intraspecific parasitism, cooperation, reproductive strategy, evolution of sociality, semi-natural conditions
1. Introduction
Nest-site fidelity and nest-site defence are common characteristics of social species. This ensures social cohesion and that altruistic acts are directed towards group members. Drifting, that is leaving the nest to enter a foreign one, is, however, a surprisingly widespread phenomenon among flying social insects [1]. The initial view of drifting as a purely accidental behaviour [2–4] has recently been questioned by the discovery that some drifters actively engage in reproduction in their host colony [5–10] thus suggesting that drifting may constitute a distinct strategy by which workers enhance their fitness [11]. However, while the departure from the nest and the entry in a foreign colony may actually arise from a deliberate reproductive strategy, drifting may alternatively be a random phenomenon ending with an opportunistic reproduction of some workers, as pointed out in previous studies [5,12]. More knowledge about drifting behaviour and its underlying mechanisms is therefore necessary to assess the true extent of reproductive options available to workers, and thus get a better understanding of the factors involved in the extreme cooperation characterizing insect societies.
Owing to the existence of an expressed reproductive conflict between the queen and workers, Bombus terrestris is a widely used model system in the study of worker reproduction. Its annual colony life cycle consists of two contrasting phases with respect to intracolonial worker reproduction: some workers have activated ovaries in the early developmental stages of the colony (‘social phase’) but refrain from reproducing up to the end of the colony cycle (‘competition phase’) where they start to intensively compete with each other for male production [13–17]. Besides the well-studied occurrence of intracolonial reproduction, cases of heterocolonial reproduction by workers have been described [5,9]. However, little is known about the causative determinants of this type of reproduction. We recently showed that a particular physiological state may be a pre-requisite for the expression of heterocolonial reproductive behaviour. Through experimental introduction of workers into foreign colonies, we indeed discovered that only workers with already activated ovaries develop a reproductive phenotype in host colonies [18]. Workers’ fertility level could thus be a key parameter in the decision-making process leading workers to leave their nest and engage in heterocolonial reproduction.
In this study, we investigated in semi-natural conditions whether B. terrestris drifting behaviour is a genuine reproductive strategy by closely examining both drifting behaviour and worker reproduction in workers of differing fertility level. In accordance with kin-selection theory, we predicted that the expression of drifting would be affected by workers’ reproductive potential. While fertile bees could assert their fitness interest by reproducing in a foreign colony, we predicted that infertile bees would remain in their own nest, thus enhancing their indirect fitness benefits by favouring reproduction of their close relatives. Furthermore, to get insights into the proximate and ultimate factors at the basis of B. terrestris workers’ reproductive decisions, we also examined the influence of the colony phase (i.e. social and competition phases) on the departure of workers. As the colony developmental stage determines the initiation of worker reproductive competition [19,20], we predicted colony phase to be a key factor affecting the decision of workers to engage in heterocolonial reproduction.
2. Material and methods
(a). Experimental colonies
The bumblebee B. terrestris forms annual colonies headed by a single, singly mated queen [21]. Colonies at two different developmental stages were obtained from a commercial supplier (GTICO SARL, Villeneuve l'Archevêque, France): a few days after the emergence of the first workers (‘social phase’ (SP); n = 22 colonies) and a few days after the first worker oviposition (‘competition phase’ (CP); n = 22 colonies). Each experimental colony had a queen and brood at every developmental stage. Colonies were reared in laboratory conditions without outside foraging experience before the experiment started. They were maintained in wooden boxes (17.5 × 26 × 15 cm) in a dark room at a temperature of 28 ± 2°C and a relative humidity of 55 ± 5%. Colonies were provided ad libitum with sugar syrup and fresh pollen into the wooden boxes. Moreover, each rearing box was connected to a wooden board (in a cage/plastic box) where fresh pollen was also present in order to stimulate foraging behaviour as well as colonial marking of the board [22]. Daily observations of the colonies were performed to detect the initiation of the competition phase (when at least one of these characteristics occurs: two egg-cells or more opened for at least two consecutive days, worker oviposition, egg-eating and clear signs of cell destruction [14,23]).
(b). Colony preparation
Upon colony reception, all adult bees were marked with a colony-specific colour pattern. During the following days, newly emerged workers (n = 24 in SP colonies; n = 40 in CP colonies) were individually labelled with coloured and numbered tags (Opalith Plättchen, Friedrich Wienold, Germany). These workers were reintroduced into their native colony where they stayed at least for 3 days to allow their social integration and the learning of their colony odour. After this period, 75% of the labelled bees were isolated in trios for 7 days (n = 6 trios and n = 10 trios for each SP colony and each CP colony, respectively). Each trio comprised one bee 1 or 2 days older than the two other bees. When isolated from the queen and placed in trio, a dominance hierarchy establishes between the workers, with the oldest worker always becoming a laying-bee with fully developed ovaries and the two others becoming subordinates with moderately developed ovaries [16,18]. The laying (‘F+’) and one of the subordinate non-laying (‘F’) bees were then reintroduced into their mother colony. After the reintroduction, each colony was then composed of the same number of workers with developed (F+), moderately developed (F) and undeveloped ovaries (the 25% of the labelled bees that remained in their natal nest, ‘F−’). Each worker group was composed of six workers in SP colonies and of 10 workers in CP colonies. The number of experimental workers was higher in CP than in SP colonies to keep similar the respective proportion of experimental workers in relation with the number of workers in colonies. The whole experiment comprised three sessions that were performed in summer 2011. We used 10 experimental colonies in the first session (SP, n = 5; CP, n = 5), 12 in the second session (SP, n = 6; CP, n = 6) and six in the third session (SP, n = 3; CP, n = 3). Non-manipulated colonies were added to reach a total of 12 colonies in each session (comprising an equal number of SP and CP colonies).
(c). Experimental set-up and behavioural observations
On the day the manipulated bees were reintroduced into their nest, the wooden boxes containing the colonies were placed into protective boxes to shelter the colonies from the weather conditions (i.e. temperature, wind and rain). These colonies were then placed in an open environment on the roof of the laboratory where they were equally distributed in two rows 9.5 m apart. Within rows, inter-colony distance was 7.18 ± 1.22 m (mean ± s.d.), in accordance with natural conditions because wild bumblebee colonies can be found within 2 m of distance [9,24]. Each protective box bore above the nest entrance a 15 × 15 cm distinct visual pattern designed to maximize visual nest recognition by bumblebees (following [25]) and therefore minimize the possibilities of orientation mistakes during the experiment. Moreover, the marked wooden boards (see Experimental colonies) were placed at the entrance of their respective colony to provide workers with olfactory-cues on the colonial identity of the nest [22,26,27]. External cues (chimneys and buildings) added potential landmarks for bumblebee orientation.
From the next morning and for 7 days, the entrances of the colonies were opened so that workers were free to exit outside. Video cameras were positioned above the boards on all the colonies to monitor the labelled workers as they exited or entered the colonies during the first 4 days. In the subsequent video analyses, we noted for each bee the identity (individual and colonial), the direction of movement (exit or enter the nest), the time, the presence of pollen as well as the performance of orientation flights. In addition, daily scans were performed in each nest during the night to record the identity of non-natal workers. Data from all colonies were thereafter combined to trace the movements of each worker between nests.
In the first session of the experiment, egg-laying activity of workers was monitored in each SP colony (n = 6) during each night of the observation period. For each monitored colony, the top part of the protective box was removed so that a night shot video camera placed above the colony could record the behavioural activity in the nest through the glass lid. Workers were characterized as egg-layers if they performed the typical ovipositing behaviour that consists in the insertion of the abdomen into an open egg cup for a few minutes with regular movements of the abdomen and legs [23].
In the second and third sessions of the experiment, colour-marked drifters were recovered daily and the labelled bees were collected in each nest at the end of the observation period (7 days after the bees’ introduction) for subsequent ovary dissections.
(d). Fertility measurement
The collected bees were dissected and the mean size of the eight terminal oocytes was taken as a measure of the ovarian development. The bees which had never been seen in a foreign nest were considered as ‘non-drifting’ workers, whereas those that were seen at least once in a foreign host colony were considered as ‘drifting’ workers.
To assess the dynamic of workers’ ovarian activity, control groups of workers (F+, F and F−) were created from additional colonies (n = 8) in the same conditions as described in the ‘colony preparation’ section. Workers were collected after a 7-day isolation period in trios to assess the workers’ ovarian development at the beginning of the experiment. The dissections of control workers confirmed that the different worker groups had different levels of ovary activation following the isolation procedure (mean ± s.e.: F+, 2.60 ± 0.11 mm, n = 36; F, 1.59 ± 0.08 mm, n = 55; F−, 1.09 ± 0.09 mm, n = 39; generalized linear mixed-effect model (GLMM) χ2 = 114.43, d.f. = 2, p < 0.0001, post hoc all values of p < 0.0001).
(e). Statistical analyses
GLMMs and generalized linear models (GLMs) were fitted to the data using the statistical software R v. 2.15.0 [28] with the package lme4 [29]. Post hoc tests were corrected for multiple comparisons with the sequential Bonferroni–Holm correction procedure [30] when necessary, and adjusted p-values are noted p′ (they should be compared to the standard 0.05 significance threshold). Data analyses are described in the electronic supplementary material.
3. Results and discussion
Of the 665 labelled workers, 373 exited their nest at least once, among which 220 (58.9%) visited at least one foreign nest. This drifting rate is remarkably high compared with most of previous experimental records of drifting, including experiments conceived to favour drifting (reviewed in [1]). It is, however, similar to the drifting rate found in the two other studies that exhaustively monitored movements between nests in social insect workers (Polistes canadensis: 56.1% [1]; B. terrestris: at least 52% [31]). At least 73.2% of the drifters did not stay permanently in the first foreign colony they visited, and were seen entering other colonies later (38.8% of the drifters visited at least two foreign nests) and/or coming back to their natal nest (64.8%). In accordance with an earlier report [31], we thus found that drifting did not always lead workers to take up permanent residence in the host colony, providing a much more dynamic picture of this behaviour and possibly, of intercolonial interactions within bumblebee populations. This result emphasizes that drifting rate in social insects may have been underestimated in previous studies owing to the use of sampling methods that are unsuitable to detect rapid changes in workers’ location [1]. It must be noted however that the observed drifting rate could not exactly reflect what occurs in wild bumblebee populations [9]. Nest-density in our experimental population, combined to the use of commercially reared colonies with possibly decreased chemical diversity, could indeed have favoured a higher level of drifting [2,32–34]. We observed no direct aggressions at colony entrances, where non-nest-mate rejection is most likely to occur [35,36]. Neither did we observe any rise in the mortality rate of drifters (only six drifters were found dead in their host colony compared with 26 workers of similar age found dead in their natal colony, that is, respectively, 2.7% of the drifters versus 5.8% of the residents). Whether drifters are tolerated to the same extent in wild colonies should be assessed in further studies. A similar level of social tolerance was, however, found in colonies headed by wild-caught queens [5]. In our semi-natural conditions, drifting of B. terrestris workers was a remarkably dynamic and widely expressed behaviour relying on both the high propensity of workers to visit foreign nests and the high tolerance of colonies.
(a). Drifting as a fertility-dependent reproductive strategy
Manipulating workers’ fertility strongly affected the performance of drifting behaviour (figure 1). The drifting rate of workers from the highly fertile F+ group was indeed significantly higher than the drifting rate of workers from both the F and F− groups (figure 1a, post hoc p′ = 0.008 and p′ = 0.004, respectively). While our procedure to manipulate workers’ fertility led to slight age differences between the worker groups, we did not find any significant influence of age on the behaviour of workers, including the drifting rate (see the electronic supplementary material). The likelihood to drift opportunistically or by mistake is likely to be higher in workers performing frequent flying trips because of a greater probability of encountering foreign nests. The higher drifting rate of fertile workers could thus be simply related to a higher exiting rate. However, we found that F+ drifters performed significantly less flying trips before entering a foreign nest than both F and F− drifters (figure 1b, post hoc both p′ < 0.001). At least 67.8% of the F+ drifters entered a foreign colony from their first or second exit compared with 53.1% of the F and 29.3% of the F− drifters which drifted as rapidly. Furthermore, the first drifting flight of highly fertile F+ workers was significantly shorter than that of infertile F− workers (figure 1c, post hoc p′ = 0.0023). Therefore, drifting here appears to be a plastic behaviour [37,38] whose expression is directly affected by workers’ physiological state rather than a mere stochastic phenomenon. Although drifting occurred in workers of all fertility levels, our results indeed show that fertile workers did not only display the highest propensity to drift, as predicted, but also performed highly specific drifting behaviour.
Figure 1.
Comparison of the drifting behaviour performed by experimental bees. For details, see Material and methods and the electronic supplementary material. The different letters denote statistical differences between the experimental groups. Data are represented as mean ± s.e. (a) Comparison of drifting rate. Drifting rate is defined as the proportion of bees that drifted relative to the number of workers that exited at least once their natal nest (GLMM, χ2 = 9.84, d.f. = 2, p = 0.0073, n = 373). (b) Comparison of the number of flights before the first entry into a foreign colony (GLMM, χ2 = 73.74, d.f. = 2, p < 0.0001, n = 205). (c) Comparison of the flight duration for the first entry in a foreign colony (GLMM, χ2 = 9.64, d.f. = 2, p = 0.008; n = 180). (d) Comparison of the proportion of reproducing drifters (GLM, χ2 = 7.77, d.f. = 2, p = 0.02, n = 40).
About a third (32.5%) of the monitored drifters (n = 40, see Experimental procedure) were seen laying eggs in host colonies thus confirming that at least some B. terrestris workers engage in reproduction after drifting in a foreign colony [5,9]. Egg-laying drifters mainly belonged to the F+ worker group (figure 1d). There were significantly more reproducing drifters in the F+ group than in the F− group and marginally more than in the F group (figure 1d, post hoc p′ = 0.05 and p′ = 0.084, respectively). Because the F and F− groups included some fertile workers, we do not know whether all the egg-laying drifters had activated ovaries prior to their departure from their natal nest. In laboratory conditions, only workers with pre-developed ovaries achieve a reproductive phenotype in a foreign colony [18]. Here, drifters had actively flown to the foreign colonies and could thus have experienced some physiological changes potentiating their ovarian development [39,40]. However, drifters from the F− worker group that remained for at least 6 days in the host colonies (n = 16) displayed a low ovarian development (mean ± s.e.: 0.94 ± 0.22 mm), similar to the one F− workers had when the experiment started (1.08 ± 0.17 mm, GLMM, χ2 = 1.18, d.f. = 1, p = 0.27, n = 32), thus confirming that infertile workers do not activate their ovaries despite having actively flown to a foreign colony. This finding thus discards the hypothesis of drifting as a hopeful reproductive strategy for infertile workers, and emphasizes the fact that bumblebee workers need to possess active ovaries before drifting to reproduce in foreign colonies.
Because most fertile bees that exited their nest entered foreign colonies and engaged in reproduction in host colonies, our findings strongly support the hypothesis that drifting constitutes a distinct reproductive strategy for workers with active ovaries. Interestingly, however, drifting did not only occur in potentially reproductive individuals but also in a large proportion of infertile workers. Rather than activating their ovaries and laying eggs, infertile drifters performed foraging trips for host colonies (78.9% of them returned to a host colony with pollen loads). Foraging behaviour of B. terrestris drifters has already been described in earlier reports [5,31]. The finding that infertile drifters perform this behaviour is, however, highly surprising. While foraging could be functionally seen as a pay-to-stay strategy [41] in the hope of direct reproduction in fertile drifters, this could not be the case in infertile workers that do not reproduce. According to kin-selection theory, non-reproductive workers should favour reproduction of their close relatives rather than working in foreign colonies. Confronted to similar findings in a wild population of the paper wasp P. canadensis, Sumner et al. [1] concluded that, in case of high constraints on nests survival and of a viscous genetic structure of population, drifting can be selected for even in non-reproductive individuals because of the indirect fitness benefits it provides. Further studies in wild B. terrestris colonies are needed to assess how frequent drifting is in infertile bees and whether these workers genuinely contribute to the host colony functioning. Along with genetic data on wild bumblebee populations, this could shed some lights on the origin of drifting in B. terrestris societies. In accordance with recent reports [1,31], our observations lend thus credence to the idea that drifting may be a much more complex phenomenon than previously thought. Because it possibly increases the host's fitness through an increase in working force, the non-null background rate of drifting in infertile workers could explain the commonly described high social tolerance of bumblebee colonies [5,32,33]. Such an open colony structure would have provided the ideal ground for the evolution of drifting for direct reproduction in fertile bumblebees. Whether the latter are not rejected because their high reproductive potential is not detected or whether they are recognized (see [42]) but tolerated (with the resulting fitness variation for the host colony still to be determined, see [9,18]) remains to be investigated.
(b). Drifting as a context-dependent reproductive decision
To examine the dynamic of drifting during the colony cycle, we assessed the influence of the colony phase (social or competition) on the departure of workers (figure 2). Interestingly, the proportion of F− workers that exited their nest was high and similar in both colony conditions while F and F+ workers exited mainly in competition phase colonies (figure 2, GLMMs, χ² = 0.81, d.f. = 1, p = 0.37; χ² = 24.78, d.f. = 1, p < 0.0001; χ² = 27.98, d.f. = 1, p < 0.0001, respectively). The F+, F and F− groups had, respectively, a low, intermediate and high number of exiting workers in social phase colonies (figure 2, GLMM, χ² = 41.83, d.f. = 2, p < 0.0001, post hoc all p′ < 0.048), whereas the number of exiting workers was high in all groups in competition phase colonies even if slightly lower in the F+ group (figure 2, GLMM, χ² = 7.46, d.f. = 2, p = 0.024; post hoc F+/F: p′ = 0.036, F+/F−: p′ = 0.036, F/F−: p′ = 0.88). Therefore, contrary to infertile workers who exited their nest frequently and regardless of the colony phase, fertile workers displayed a highly context-dependent behaviour, mostly remaining in their nest when their colony was in the social phase. The onset of the competition phase thus appears to play a key role in the decision making of fertile bees to exit the nest. Along with the finding that most of the fertile workers that exited their nest entered a foreign colony (figure 1a), these results indicate that the competition for reproduction between workers could be the critical event that induces drifting in fertile bumblebees.
Figure 2.
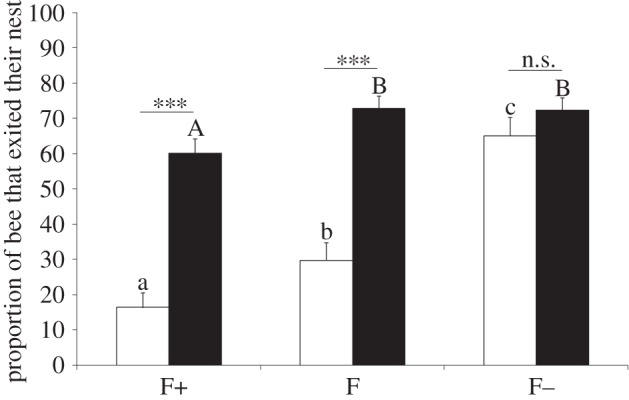
Proportions of experimental bees in social and competition colonies that exited their nest. For details, see Material and methods and the electronic supplementary material. White and black bars indicate social and competition phase colonies, respectively. The different letters denote statistical differences between the worker groups within the social phase (lowercase letters) and within the competition phase (uppercase letters). n.s., not significant; ***p < 0.001. Data are represented as mean ± s.e.
Within-group reproductive competition is known to be a key factor in triggering the expression of alternative reproductive strategies in social species. Leaving the group may allow potentially reproductive individuals to avoid costs such as reproductive inhibition resulting from competition [43,44]. To explore this hypothesis, we assessed the impact of colony phase on the fertility of bees that remained in their natal nest during the experiment (see Fertility measurement). No ovarian regression could be detected among fertile F+ individuals in social phase colonies (figure 3, post hoc p′ = 0.57). Conversely, the reproductive potential of fertile F+ bees was significantly reduced in competition phase colonies (figure 3, post hoc p′ = 0.0457). This effect is also present in F and F− workers who developed their ovaries in social (figure 3, post hoc both p′ < 0.002) but not in competition phase colonies (figure 3, post hoc both p′ > 0.65). As ovarian regression is a well-known consequence of aggressive interactions in social insects [45], dominance behaviour specifically expressed against bees with active ovaries during the competition phase [23,46] may account for the ovary regression that occurred in fertile workers. In any case, it appears that remaining in the natal nest when the colony enters the competition phase could negatively impact the potential reproductive success of some fertile bees. Engaging in drifting at this time of the colony cycle may thus allow fertile workers to avoid the detrimental effects caused by in-nest reproductive competition. Whether or not drifters are affected by the competition in host colonies remains to be investigated. Previous studies have, however, shown that drifters adjust their reproductive behaviour to the heterocolonial environment by not responding to colony cues mediating reproductive self-restraint [18] and by behaving more aggressively [5]. This behavioural adjustment according to the experienced environment is thus likely to confer a reproductive advantage for drifters over host workers.
Figure 3.

Ovarian development of control (white bars) and experimental bees (grey bars, social; black bars, competition) that stayed in their natal colonies. For results and details, see Material and methods and the electronic supplementary material. The different letters denote statistical differences within the worker groups (lowercase letters) and between the control bees of the different groups (uppercase letters). Numbers indicate the sample sizes. Data are represented as mean ± s.e.
4. Conclusion
Overall, our study reveals the high reproductive flexibility of B. terrestris workers. In addition to the well-studied possibility for workers to reproduce in their own nest, we found direct empirical evidence that drifting constitutes an alternative reproductive strategy for fertile workers. Reproducing either in its own nest or in a foreign colony appears to be alternative phenotypes whose expression depends on the interplay between the individual's status and the social context. During the early colony developmental stages, workers with activated ovaries cooperate while waiting for reproductive opportunities. At the end of the colony cycle, as soon as highly related gynes (future queens) have been produced, fertile workers have no more incentive to restrain themselves from reproducing, and thus enter in the competition for male production [19,20]. While some workers gain direct reproduction in their nest owing to their dominant position, a significant fraction of the remaining fertile workers engage in heterocolonial reproduction. Two key conditions are thus likely to have favoured the evolution of drifting as a reproductive strategy in B. terrestris societies: the harsh reproductive competition among fertile workers deriving from the expression of their kin-selected interests and a high tolerance of colonies towards intruders, possibly linked to the contribution of infertile foreign workers to the colony workforce.
Restricted reproduction is traditionally posited as the defining feature of workers in eusocial insects. Even when intracolony relatedness is high, it is assumed that workers are enforced to behave altruistically [47,48] because of coercive mechanisms that restrict access to and the benefits of direct reproduction. We here reveal that workers’ potential to evade coercion can be much higher than previously considered. This potentially widespread [11] but little studied alternative possibility for workers to assert their own interest will have to be carefully taken into consideration when examining levels of worker reproduction and cooperation. The possibility to desist cooperation probably has important consequences on the expression of both reproductive and coercive phenotypes and therefore must have played a great role in the evolution of the reproductive division of labour and its associated regulation mechanisms in social species.
Acknowledgements
We thank L. Boreggio for help with the bee husbandry, R. Gouttefarde for video analysis and M. Perez for useful advice for statistical analysis. We are very grateful to A. Maille, A. Wystrach, G. Gheusi and two anonymous referees for helpful suggestions on the manuscript.
Funding statement
This work was supported by the French National Research Agency (ANR-09-JCJC-0031-01 SEUILS).
References
- 1.Sumner S, Lucas E, Barker J, Isaac N. 2007. Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr. Biol. 17, 140–145 (doi:10.1016/j.cub.2006.11.064) [DOI] [PubMed] [Google Scholar]
- 2.Jay SC. 1965. Drifting of honeybees in commercial apiaries. I. Effect of various environmental factors. J. Apic. Res. 4, 167–175 [Google Scholar]
- 3.Pfeiffer KJ, Crailsheim K. 1998. Drifting of honeybees. Insectes Soc. 45, 151–167 (doi:10.1007/s000400050076) [Google Scholar]
- 4.Paar J, Oldroyd BP, Huettinger E, Kastberger G. 2002. Drifting of workers in nest aggregations of the giant honeybee Apis dorsata. Apidologie 33, 553–561 (doi:10.1051/apido:2002040) [Google Scholar]
- 5.Lopez-Vaamonde C, Koning JW, Brown RM, Jordan WC, Bourke AFG. 2004. Social parasitism by male-producing reproductive workers in a eusocial insect. Nature 430, 557–560 (doi:10.1038/nature02769) [DOI] [PubMed] [Google Scholar]
- 6.Nanork P, Paar J, Chapman NC, Wongsiri S, Oldroyd BP. 2005. Asian honeybees parasitize the future dead. Nature 437, 829 (doi:10.1038/437829a) [DOI] [PubMed] [Google Scholar]
- 7.Nanork P, Chapman NC, Wongsiri S, Lim J, Gloag RS, Oldroyd BP. 2007. Social parasitism by workers in queenless and queenright Apis cerana colonies. Mol. Ecol. 16, 1107–1114 (doi:10.1111/j.1365-294X.2006.03207.x) [DOI] [PubMed] [Google Scholar]
- 8.Takahashi J, Martin SJ, Ono M, Shimizu I. 2010. Male production by non-natal workers in the bumblebee Bombus deuteronymus (Hymenoptera: Apidae). J. Ethol. 28, 61–66 (doi:10.1007/s10164-009-0155-y) [Google Scholar]
- 9.O'Connor S, Park KJ, Goulson D. 2013. Worker drift and egg dumping by queens in wild Bombus terrestris colonies. Behav. Ecol. Sociobiol. 67, 621–627 (doi:10.1007/s00265-013-1481-1) [Google Scholar]
- 10.Chapman NC, Beekman M, Oldroyd BP. 2009. Worker reproductive parasitism and drift in the Western honeybee Apis mellifera. Behav. Ecol. Sociobiol. 64, 419–427 (doi:10.1007/s00265-009-0858-7) [Google Scholar]
- 11.Beekman M, Oldroyd BP. 2008. When workers disunite: intraspecific parasitism by eusocial bees. Annu. Rev. Entomol. 53, 19–37 (doi:10.1146/annurev.ento.53.103106.093515) [DOI] [PubMed] [Google Scholar]
- 12.Beekman M, Wossler TC, Martin SJ, Ratnieks FLW. 2002. Parasitic Cape honey bee workers (Apis mellifera capensis) are not given differential treatment by African guards (A. m. scutellata). Insectes soc. 49, 216–220 (doi:10.1007/s00040-002-8304-0) [Google Scholar]
- 13.Honk CGJ, Van Röseler PF, Velthuis HHW, Hoogeveen JC. 1981. Factors influencing the egg laying of workers in a Captive Bombus terrestris colony. Behav. Ecol. Sociobiol. 9, 9–14 (doi:10.1007/BF00299847) [Google Scholar]
- 14.Duchateau MJ, Velthuis HHW. 1988. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 107, 186–207 (doi:10.1163/156853988X00340) [Google Scholar]
- 15.Bloch G. 1999. Regulation of queen-worker conflict in bumblebee (Bombus terrestris) colonies. Proc. R. Soc. Lond. B 266, 2465–2469 (doi:10.1098/rspb.1999.0947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alaux C, Boutot M, Jaisson P, Hefetz A. 2007. Reproductive plasticity in bumblebee workers (Bombus terrestris): reversion from fertility to sterility under queen influence. Behav. Ecol. Sociobiol. 62, 213–222 (doi:10.1007/s00265-007-0455-6) [Google Scholar]
- 17.Zanette LRS, Miller SDL, Faria CMA, Almond EJ, Huggins TJ, Jordan WC, Bourke AFG. 2012. Reproductive conflict in bumblebees and the evolution of worker policing. Evolution 66, 3765–3777 (doi:10.1111/j.1558-5646.2012.01709.x) [DOI] [PubMed] [Google Scholar]
- 18.Yagound B, Blacher P, Chameron S, Châline N. 2012. Social context and reproductive potential affect worker reproductive decisions in a eusocial insect. PLoS ONE 7, e52217 (doi:10.1371/journal.pone.0052217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alaux C, Jaisson P, Hefetz A. 2005. Reproductive decision-making in semelparous colonies of the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 59, 270–277 (doi:10.1007/s00265-005-0035-6) [Google Scholar]
- 20.Alaux C, Jaisson P, Hefetz A. 2006. Regulation of worker reproduction in bumblebees (Bombus terrestris): workers eavesdrop on a queen signal. Behav. Ecol. Sociobiol. 60, 439–446 (doi:10.1007/s00265-006-0184-2) [Google Scholar]
- 21.Schmid-Hempel R, Schmid-Hempel P. 2000. Female mating frequencies in Bombus spp. from Central Europe. Insectes Soc. 47, 36–41 (doi:10.1007/s000400050006) [Google Scholar]
- 22.Kreuter K, Twele R, Francke W, Ayasse M. 2010. Specialist Bombus vestalis and generalist Bombus bohemicus use different odour cues to find their host Bombus terrestris. Anim. Behav. 80, 297–302 (doi:10.1016/j.anbehav.2010.05.010) [Google Scholar]
- 23.Bloch G, Hefetz A. 1999. Regulation of reproduction by dominant workers in bumblebee (Bombus terrestris) queenright colonies. Behav. Ecol. Sociobiol. 45, 125–135 (doi:10.1007/s002650050546) [Google Scholar]
- 24.Harder LD. 1986. Influences on the density and dispersion of bumble bee nests (Hymenoptera: Apidae). Hol. Ecol. 9, 99–103 [Google Scholar]
- 25.Fauria K, Colborn M, Collett TS. 2000. The binding of visual patterns in bumblebees. Curr. Biol. 10, 935–938 (doi:10.1016/S0960-9822(00)00623-0) [DOI] [PubMed] [Google Scholar]
- 26.Cederberg B. 1977. Evidence for trail marking in Bombus terrestris workers (Hymenoptera, Apidae). Zoon 5, 143–146 [Google Scholar]
- 27.Foster RL, Gamboa GJ. 1989. Nest entrance marking with colony specific odors by the bumble bee Bombus occidentalis (Hymenoptera: Apidae). Ethology 81, 273–278 (doi:10.1111/j.1439-0310.1989.tb00773.x) [Google Scholar]
- 28.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 29.Bates DM, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package v. 0.999375-39. See http://cran.r-project.org/web/packages/lme4/index.html.
- 30.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- 31.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108 (doi:10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birmingham AL, Hoover SE, Winston ML, Ydenberg RC. 2004. Drifting bumble bee (Hymenoptera: Apidae) workers in commercial greenhouses may be social parasites. Can. J. Zool. 82, 1843–1853 (doi:10.1139/z04-181) [Google Scholar]
- 33.Birmingham AL, Winston ML. 2004. Orientation and drifting behaviour of bumblebees (Hymenoptera: Apidae) in commercial tomato greenhouses. Can. J. Zool. 82, 52–59 (doi:10.1139/z03-201) [Google Scholar]
- 34.Jay SC. 1966. Drifting of honeybees in commercial apiaries. II. Effect of various factors when hives are arranged in rows. J. Apic. Res. 5, 103–112 [Google Scholar]
- 35.d'Ettorre P, Lenoir A. 2010. Nestmate recognition in ants. In Ant ecology, Lori Lach (eds Parr Catherine, Abbott Kirsti.), pp. 194–209 Oxford, UK: Oxford University Press [Google Scholar]
- 36.Sturgis SJ, Gordon DM. 2012. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 16, 101–110 [Google Scholar]
- 37.Duckworth RA. 2009. The role of behavior in evolution: a search for mechanism. Evol. Ecol. 23, 513–531 (doi:10.1007/s10682-008-9252-6) [Google Scholar]
- 38.Houston AI, McNamara JM. 1992. Phenotypic plasticity as a state-dependent life-history decision. Evol. Ecol. 6, 243–253 (doi:10.1007/BF02214164) [Google Scholar]
- 39.Withgott JH, Abbot DK, Moran NA. 1997. Maternal death relaxes developmental inhibition in nymphal aphid defenders. Proc. R. Soc. Lond. B 264, 1197–1202 (doi:10.1098/rspb.1997.0165) [Google Scholar]
- 40.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. 2005. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441 (doi:10.1523/JNEUROSCI.4258-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmuller R, Johnstone R, Russell A, Bshary R. 2007. Integrating cooperative breeding into theoretical concepts. Behav. Processes 76, 61–72 (doi:10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 42.Chapman NC, Makinson J, Beekman M, Oldroyd BP. 2009. Honey bee, Apis mellifera, guards use adaptive acceptance thresholds to limit worker reproductive parasitism. Anim. Behav. 78, 1205–1211 (doi:10.1016/j.anbehav.2009.08.007) [Google Scholar]
- 43.Schradin C, Lindholm AK, Johannesen J, Schoepf I, Yuen CH, Koenig B, Pillay N. 2011. Social flexibility as a tool to understand social evolution in mammals: the case of the African striped mouse (Rhabdomys pumilio). Mol. Ecol. 21, 541–553 (doi:10.1111/j.1365-294X.2011.05256.x) [DOI] [PubMed] [Google Scholar]
- 44.Schoepf I, Schradin C. 2012. Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J. Anim. Ecol. 81, 649–656 (doi:10.1111/j.1365-2656.2011.01939.x) [DOI] [PubMed] [Google Scholar]
- 45.Liebig J, Peeters C, Hölldobler B. 1999. Worker policing limits the number of reproductives in a ponerine ant. Proc. R. Soc. Lond. B 266, 1865–1870 (doi:10.1098/rspb.1999.0858) [Google Scholar]
- 46.Duchateau MJ, Velthuis HHW. 1989. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 51, 199–213 (doi:10.1111/j.1570-7458.1989.tb01231.x) [Google Scholar]
- 47.Ratnieks FLW, Helanterä H. 2009. The evolution of extreme altruism and inequality in insect societies. Phil. Trans. R. Soc. B 364, 3169–3179 (doi:10.1098/rstb.2009.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratnieks FLW, Wenseleers T. 2008. Altruism in insect societies and beyond: voluntary or enforced? Trends Ecol. Evol. 23, 45–52 (doi:10.1016/j.tree.2007.09.013) [DOI] [PubMed] [Google Scholar]



