Abstract
Male-derived sex-peptide (SP) induces profound changes in the behaviour of Drosophila females, resulting in decreased receptivity to further mating and increased egg laying. SP can mediate the switch in female reproductive behaviours via a G protein-coupled receptor, SPR, in neurons expressing fruitless, doublesex and pickpocket. Whether SPR is the sole receptor and whether SP induces the postmating switch in a single pathway has not, to our knowledge been tested. Here we report that the SP response can be induced in the absence of SPR when SP is ectopically expressed in neurons or when SP, transferred by mating, can access neurons through a leaky blood brain barrier. Membrane-tethered SP can induce oviposition via doublesex, but not fruitless and pickpocket neurons in SPR mutant females. Although pickpocket and doublesex neurons rely on G(o) signalling to reduce receptivity and induce oviposition, G(o) signalling in fruitless neurons is required only to induce oviposition, but not to reduce receptivity. Our results show that SP's action in reducing receptivity and inducing oviposition can be separated in fruitless and doublesex neurons. Hence, the SP-induced postmating switch incorporates shared, but also distinct circuitry of fruitless, doublesex and pickpocket neurons and additional receptors.
Keywords: sex-peptide, sexual behaviour, sex-peptide targets, blood brain barrier, neurohormonal signalling
1. Introduction
Mating profoundly alters the physiology and behaviour of many species. To guarantee reproductive success, control of pregnancy-associated alterations in behaviours is believed to be mostly hard-wired into the brain. In many species including most insects, male-derived substances transferred during mating impact on female physiology and postmating behaviours probably by interfering with female control of reproduction [1–3]. How mating-induced alterations of reproductive physiology and behaviour are implemented into the female brain, however, is not well understood, but probably includes multi-level control involving stimulatory and inhibitory neuronal circuits adapting the postmating response to the physiological status of the female and to environmental conditions.
In Drosophila, the 36-amino acid sex-peptide (SP) is the main regulator of the postmating response in females and is transferred by males together with sperm and accessory gland fluid during mating [4–7]. SP leads to rejection of courting males and increases egg production and oviposition [4–8], but also leads to other behavioural and physiological changes. These include an increase in feeding, a change in food choice and sleep, and stimulation of the immune system [9–13]. In addition, SP is required for release of stored sperm and imposes costs of mating [14,15].
A high-affinity receptor for SP, SPR, has been identified and its presence is required for the postmating switch resulting in reduced receptivity (readiness to mate) and increased oviposition [16]. SPR encodes a G protein-coupled receptor and can activate trimeric G proteins with either Gαi or Gαo subunits when heterologously expressed in cell culture. Despite the highly specific behavioural response, however, SPR is broadly expressed in the nervous system and the oviduct. SPR also binds myoinhibitory peptides (MIPs) with high affinity, which do not induce the SP-mediated postmating switch [17–19]. It therefore appears that SPR has additional functions.
Insights into the circuitry mediating the SP-induced postmating switch has come from the analysis of SP-insensitive egghead (egh) mutants. These studies revealed the requirement for a subset of afferent ventral nerve cord interneurons that express apterous (ap) and project to central parts of the brain [20]. Other attempts to map the neuronal circuitry mediating the SP response used membrane-bound SP (mSP) or RNAi knock-down of SPR in restricted patterns [21–24]. These studies identified three restricted neuronal expression patterns, where SPR expression in SPR mutants can restore, or expression of mSP can induce the SP response. These neurons are defined by the expression of the sex-determination genes doublesex (dsx) and fruitless (fru), and of pickpocket (ppk), a DEG/ENaC channel involved in nociception [21,22,24,25]. In both the central nervous system and PNS, dsx and fru are expressed in approximately 700 and approximately 2000 neurons, respectively, in characteristic patterns, while ppk is expressed exclusively in a subset of peripheral nervous system (PNS) neurons [21,24,26–28]. Overlapping expression of these three genes has been identified in a subset of genital tract neurons that project to central areas of the brain suggesting that these neurons signal the presence of SP to higher order processing centres in the brain [21,22,24]. Dsx-expressing neurons in the abdominal ganglion (Abg) that project to the genital tract and the central brain are also involved in the SP response [22].
Binding sites of SP have been identified in many parts of the nervous system including, most prominently, the ventral nerve cord, all afferent nerves, the neck connective and distinct areas in the brain [29,30]. Consistent with the complexity of the SP-induced postmating switch, SPR is broadly expressed in a similar pattern [16]. As we consistently observed an SP-induced increase in oviposition in SPR mutant females, we were wondering whether SPR is the sole receptor for SP and whether there were multiple pathways by which SP can induce the postmating switch. Here, we show that SP can induce the postmating behavioural switch in the absence of SPR and that SP binding sites are present on afferent nerves and the genital tract in SPR mutant females. Our results indicate that the SP response in the absence of SPR is dependent on passage of SP through the blood brain barrier (BBB). Intriguingly, analysis of the SP response in females with a leaky BBB also demonstrates a role for SPR in inhibiting egg laying independent of the regulation of receptivity. Separation of the control of egg laying from receptivity is further revealed by mSP expression in dsx neurons in SPR mutant females. Regulation of receptivity is also separable from ovipositon in fru, but not dsx and ppk neurons through inhibition of G(o) signalling. Together, these results suggest that multiple pathways regulate the SP-induced postmating switch.
2. Results
To examine whether SP can induce postmating behaviour in the absence of SPR, we delivered SP directly to neurons by a transgene, which expresses the SP gene under the control of a neuronal elav promoter [31]. This construct contains the signal peptide resulting in secretion of SP. In wild-type females, delivery of SP to neurons reduces receptivity and induces oviposition indistinguishably from delivery to the genital tract by mating or to the haemolymph by secretion of an ectopically expressed SP gene in the fat body (figure 1a,b) [5]. In SPR mutant females, neuronal expression of SP can induce the full response in reducing receptivity and inducing oviposition (figure 1a,b). By contrast, delivery of SP to the genital tract by mating or to the haemolymph by fat body expression in SPR mutant females does not reduce receptivity, but results in a small but significant increase in oviposition (figure 1a,b). Hence, SP can trigger the postmating switch in SPR mutant females, but only when delivered directly to neurons.
Figure 1.
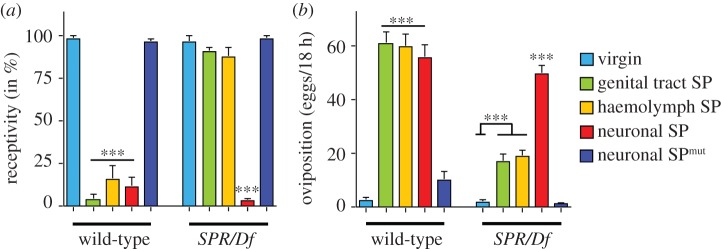
Neuronally expressed SP reduces receptivity and increases oviposition in SPR mutant females. (a) Receptivity of wild-type and SPR/Df virgin and mated females and females expressing the SP gene under the yp1 promoter secreting SP to the haemolymph (YPhsSPg, haemolymph SP) or under the neuron-specific elav promoter secreting SP from neurons (elavSP, neuronal SP), or the non-functional SP-SA mutant under the elav promoter (elavSPmut) in wild-type and SPR/Df background was measured seven hours after mating or in virgins of YPhsSPg, elavSP and elavSPmut by counting mating females in a 1 h time period. Means with the standard error for three experiments with 18–21 females each are shown. Statistically significant differences are indicated by asterisks (***p < 0.0001). (b) Oviposition of wild-type and SPR/Df virgin and mated females and females expressing the SP gene under the yp1 promoter secreting SP to the haemolymph (YPhsSPg, haemolymph SP) or under the neuron-specific elav promoter secreting SP from neurons (elavSP, neuronal SP), or the non-functional SP-SA mutant under the elav promoter (elavSPmut) in wild-type and SPR/Df background is shown as means of eggs laid in 18 h for 20 females each with the standard error. Statistically significant differences are indicated by asterisks (***p ≤ 0.0001).
To validate the specificity of transgenically supplied SP to neurons, we examined the effect of a non-functional SP. Reduction of receptivity, induction of oviposition and binding to SP targets in the brain have been mapped to C-terminal amino acids (aa) 17–36 of SP and mutating the last cysteine abolishes both behavioural responses as well as binding in the nervous system [29,30]. In agreement with this, ectopic neuronal expression of SP with the last two aa changed to SA (neuronal SPmut) does not result in reduced receptivity nor in an increase in oviposition (figure 1a,b). Therefore, the postmating switch of reproductive behaviours in SPR mutant females is also mediated by the C-terminal part of SP.
In SPR mutant females, SP present in the haemolymph (see the electronic supplementary material, figure S1) seems not to reach neuronal target(s), which are separated from the haemolymph by a sheath of glial cells forming the BBB [32,33]. We therefore wanted to know whether a leaky BBB would allow SP to reach its neuronal target(s) and restore the SP response in SPR mutant females in the physiological context of mating. The moody G protein-coupled receptor is expressed in the BBB and its knock-down results in a leaky BBB [32,33]. Wild-type females that express moody -RNAi in glial cells using repoGAL4 are viable and behave as wild-type females with regard to mating (figure 2a,b). SPR mutant females with a leaky BBB respond to SP delivered by mating comparable with wild-type females, e.g. receptivity is reduced and oviposition is increased (figure 2a,b). In these females, SP is the mediator of the behavioural switch because females mated with males lacking SP (SP0) behave very much like virgins (figure 2a,b). These results suggest that SP enters the haemolymph and needs to pass through the BBB to reach the relevant neurons to induce the SP response in SPR mutant females.
Figure 2.
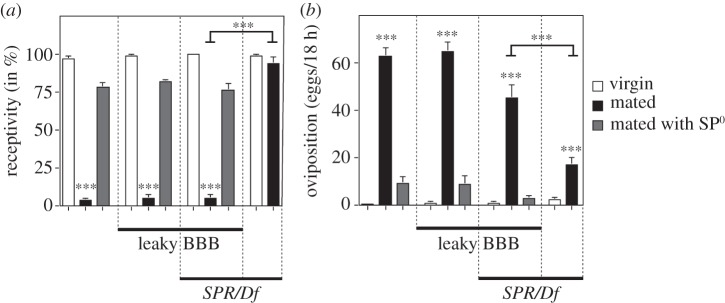
A leaky BBB rescues receptivity and oviposition in SPR mutant females after mating and SP injection. (a) Receptivity of wild-type and SPR/Df virgin and mated females with and without a leaky BBB (repoGAL4 UAS moody RNAi) was measured 7 h after mating with wild-type or SP-deficient males (SP0) by counting mating females in a 1 h time period. Means with the standard error for three experiments with 18–21 females each are shown. Statistically significant differences are indicated by asterisks (***p < 0.0001). (b) Oviposition of wild-type and SPR/Df virgin and mated females with and without a leaky BBB (repoGAL4 UAS moody RNAi) is shown as means of eggs laid in 18 h for 10 females each with the standard error, except for SPR/Df, which were 20 females. Statistically significant differences are indicated by asterisks (***p < 0.0001).
To elucidate the characteristics of the SP response in the presence of a leaky BBB and in the absence of SPR, we injected SP into the haemolymph to analyse the dose response and response time to reduce receptivity and induce oviposition. In females with a leaky BBB, the critical concentration to reduce receptivity was lowered about fivefold (from 600 to 90 fmole) compared with control females, while in SPR mutant females with a leaky BBB about a 2.5-fold higher dose was required to reduce receptivity (from 0.6 to 1.5 pmole, see the electronic supplementary material, figure S2a). For oviposition, females with a leaky BBB laid the maximum amount of eggs with 0.25 pmole of injected SP, while higher concentrations inhibited egg laying (see the electronic supplementary material, figure S2b). In SPR mutant females with a leaky BBB, 15 pmole of SP was required for laying a similar amount of eggs as control females (see the electronic supplementary material, figure S2b).
Next, we analysed the time required to induce the SP response after injection of SP (see the electronic supplementary material, figure S2c and 2d). For measuring receptivity, anaesthesia of wild-type females by cold and injection does not impact on their ability to mate immediately after recovery and all females mated within a period of an hour (see the electronic supplementary material, figure S2c). We observed that receptivity of control females as well as of SPR mutant females with a leaky BBB was 50% reduced after about an hour following injection, while injected SP reduced receptivity in females with a leaky BBB instantly (see the electronic supplementary material, figure S2c). To analyse the onset of egg laying, we measured the time it takes to induce ovulation, that is the presence of an egg in the uterus (see the electronic supplementary material, figure S2d). In this assay, females with a leaky BBB initiated ovulation with a delay of about an hour which is similar to control females (50 min), while SPR mutant females with a leaky BBB initiate ovulation with a delay of 75 min and these females also ovulate at a lower frequency (see the electronic supplementary material, figure S2d). These results reveal that egg laying is more sensitive to injected SP in the presence of SPR. Injection of SP into the haemolymph induces ovulation faster (50 min) than mating (210 min), but this delay is not owing to the presence of sperm (see the electronic supplementary material, figure S3).
In wild-type females, neuronal expression of mSP or SPR can mediate the postmating switch in ppk, fru and dsx neurons [16,21,22,24]. Now we wanted to assess whether SP switches female postmating behaviour also in SPR mutant females via these previously identified neurons. In SPR mutant females, expression of mSP in dsx neurons resulted in increased oviposition but did not affect receptivity. SPR mutant females expressing mSP in fru and ppk patterns behaved like virgin females and did not reduce receptivity and increase oviposition (figure 3a,b). Consistent with previously published data, expression of mSP in wild-type females with ppkGal4, fruGal4 or dsxGal4 reduced receptivity and increased oviposition similar to mating (figure 3a,b) [21,22,24]. These results indicate that SP can induce egg laying independent of reducing receptivity and that this response is mediated by dsx neurons.
Figure 3.
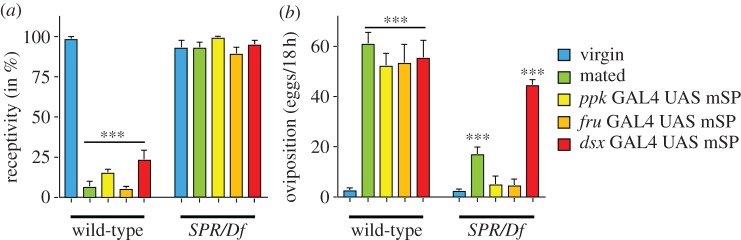
SP induces oviposition via dsx neurons in SPR mutant females. (a) Receptivity of virgin or mated wild-type females, and of virgin females expressing mSP in ppk, fru and dsx patterns in wild-type or SPR/Df background was measured 7 h after mating by counting mating females in a 1 h time period. Means with the standard error for three experiments with 15–21 females each are shown. Statistically significant differences are indicated by asterisks (***p < 0.0001). (b) Oviposition of virgin or mated wild-type females, and of virgin females expressing mSP in ppk, fru and dsx patterns in wild-type or SPR/Df background is shown as means of eggs laid in 18 h for 10 females each with the standard error, except for virgin and mated SPR/Df, which were 20 females. Statistically significant differences are indicated by asterisks (***p < 0.0001).
The SPR G protein-coupled receptor can activate trimeric G proteins containing either Gαi or Gαo in cell culture [22]. Although SPR signals through G(o) in ppk neurons to mediate the postmating switch [21], it has not been tested whether fru and dsx neurons also signal through G(o) or whether these neuronal populations can be further divided by the signalling characteristics of SPR. Pertussis toxin (PTX) selectively and irreversibly inactivates G(o) by ADP-ribosylation of the alpha subunit, and is specific for G(o) signalling as Drosophila does not have a transducing homologue, and Gαi in Drosophila does not have the PTX recognition site [34–36]. To assess whether SPR signalling via Gα0 also mediates the switch in postmating behaviours in fru and dsx neurons, we used conditional expression of tet0-PTX by the UAS-rtTA doxycyclin-inducible transactivator [37] and tested mature 3–5 day-old virgin females for their response to injected SP in receptivity and oviposition. Conditional PTX expression in mature virgin females in ppk, fru and dsx patterns did not alter their postmating behaviour, e.g. receptivity was high and egg laying was low (figure 4a,b). Likewise, conditional expression of PTX in the eye using GMR Gal4 does not alter the response to SP (figure 4a,b). By contrast, conditional PTX expression in ppk and dsx neurons of mature virgin females inhibited their response to injected SP, resulting in mating and egg laying similar to virgin females (figure 4a,b). Intriguingly, conditional PTX expression in fru neurons of mature virgin females only inhibited SP-induced egg laying, but these females still responded to SP in reducing receptivity comparable with SP-injected control females expressing PTX in the eye (figure 4a,b). The same result was obtained in response to mating (data not shown). These results indicate that SPR can regulate receptivity in fru neurons by a G(o)-independent pathway, and therefore further suggest that receptivity and oviposition can be regulated by distinct neuronal pathways.
Figure 4.
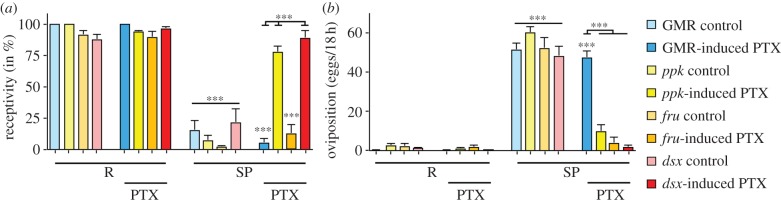
fru neurons are distinct from ppk and dsx neurons in regulating receptivity. (a) Receptivity of Ringer's and SP (3 pmol) injected mature virgin females (3–5 days) conditionally expressing PTX (induced by doxycycline for 1 day) in control (GMR) or in ppk, fru and dsx patterns was measured 5 h after injection by counting mating females in a 1 h time period. Means with the standard error for three experiments with 18–21 females each are shown. Statistically significant differences are indicated by asterisks (***p < 0.0001). (b) Oviposition of Ringer's and SP (3 pmol) injected mature virgin females (3–5 days) conditionally expressing PTX (induced by doxycycline for 1 day) in control (GMR) or in ppk, fru and dsx patterns is shown as means of eggs laid in 18 h for 10 females each with the standard error. Statistically significant differences are indicated by asterisks (***p < 0.0001).
3. Discussion
After mating, Drosophila females exert complex behavioural changes most prominently indicated by refractoriness to further mating and by increased egg laying. Based on the complexity of the SP-induced postmating response and the numerous SP binding sites in the nervous system of females, multi-level control of female postmating behaviours by SP has been suggested [38]. Indeed, our results argue that the switch in postmating behaviours can be induced in several ways by distinct neuronal circuitry and receptors.
(a). The sex-peptide response is mediated by shared and distinct circuitry of ppk, fru and dsx neurons
In principle, SP could act in higher order processing centres in the brain or through neurons that project to these areas to induce the postmating switch centrally. Indeed, a set of neurons have been identified in the genital tract, which project to the central brain [21,22,24]. These neurons express ppk, fru and dsx. Expression of SPR in these patterns restores the SP response in SPR mutant females. In addition, a central role for projections from ap interneurons in the ventral nerve cord to the brain has also been demonstrated by the analysis of egh mutants which are insensitive to SP owing to defects in these projections [20].
In the genital tract, ppk, fru and dsx neurons express in overlapping as well as in unique neuronal populations at anatomical distinct locations, suggesting that these neuronal populations are functionally different [21,22,24]. Indeed, our results show that the two main postmating responses, receptivity and egg laying, are mediated by distinct populations of fru neurons. One population regulates receptivity, is PTX insensitive and independent of G(o) signalling, while another population regulates oviposition and relies on G(o) signalling instead. These receptivity regulating fru neurons are also different from ppk and dsx neurons regulating receptivity, because they are PTX sensitive and rely on G(o) signalling. Hence, these experiments suggest distinct neuronal populations through which SP regulates the postmating switch (see the electronic supplementary material, figure S5).
Functional differences between neuronal populations expressing ppk, fru, dsx and ap have also become evident when neuronal activity was compromised in these neurons. Inhibiting neuronal transmission in dsx or ap neurons results in insensitivity to SP, e.g. these females do not lay eggs and remate despite having received SP [20,22,27]. dsx and ap neurons regulating PMRs are stimulatory, because inhibiting neuronal transmission in virgin females does not result in increased oviposition and reduced receptivity [20,22,27]. By contrast, silencing fru or ppk neurons results in the opposite effect, which is reduced receptivity and increased egg laying in virgin females [21,24,39]. Based on these results, the SP-induced postmating switch can be separated into two pathways: in one pathway, SP stimulates neuronal activity in dsx and indirectly in ap neurons to reduce receptivity and induce oviposition. In the other pathway, SP inhibits neuronal activity in fru and ppk neurons to reduce receptivity and induce oviposition. In virgin females, neuronal activity of fru and ppk neurons is required for high receptivity and low oviposition (see the electronic supplementary material, figure S5).
Combining the results from these two experimental approaches therefore argues for multiple pathways through which SP can induce the postmating switch in reproductive behaviours. High-resolution mapping of neuronal circuits and analysis of their activity changes upon SP encounter in the future will provide the topological features of the circuitry governing the SP-induced postmating switch of reproductive behaviours.
(b). Multiple receptors mediate the sex-peptide response
SPR mutant females consistently showed a significant increase in oviposition after mating or injection of SP, indicating that SPR might not be the sole receptor for mediating the postmating response. Indeed, neuronal expression of SP in SPR mutant females or delivery of SP by mating in SPR mutant females with a leaky BBB completely restores the SP response in receptivity and oviposition. Upon injection of SP into the haemolymph of SPR mutants with a leaky BBB, higher amounts of SP are required and it also takes longer to induce the postmating switch compared with wild-type females with a leaky BBB. These results indicate that SPR potentiates the response to SP by increasing the sensitivity. Whether this occurs at the level of signalling, however, is not clear as the delay in the response could require higher amounts of SP to compensate for its degradation in the haemolymph.
A puzzling discovery has been that SPR can bind MIPs in Drosophila and activate the receptor in cell culture, but injection of MIPs neither induces the SP response nor do MIPs act antagonistically to inhibit the SP response [19,40]. SPR is evolutionarily well conserved and functions in other insects also with ligands seemingly different from SP [19,40]. Therefore, SPR signalling might have a role in regulating access of peptides to neurons, which is consistent with its broad expression in Drosophila. A role for SPR in regulating access of peptides to their target could explain, why we did not detect a reduction of receptivity in SPR mutants after mating and suggests that SP can regulate receptivity and oviposition through distinct neuronal populations. As broad expression of SPR in all neurons or glia does not rescue the SP response in SPR mutant females (data not shown), ligand-activated signalling of SPR at the BBB needs to be localized to allow SP and other peptides to reach their neuronal target(s).
(c). Routes of sex-peptide to its targets
Injection of SP into the haemolymph triggers the postmating switch indistinguishable from mating [30,41]. It has therefore been argued that SP enters the circulatory system to reach its target, which is supported here by detecting SP in the haemolymph at concentrations sufficient to induce the postmating switch. Intriguingly, facilitated access of injected SP to neurons through a leaky BBB leads to an instant response in reducing receptivity, while in wild-type females this response is delayed by 50 min. This delay suggests that SP needs to pass through the BBB once in the haemolymph. The start of egg laying after mating is delayed for about 210 min, but is shortened to 50 min if SP is injected [29]. This seems to be the time it takes to induce egg laying because it is not further reduced by a leaky BBB. Hence, the delay in inducing oviposition between mating and injection of SP indicates that SP passes through the genital tract and enters the haemolymph to trigger oviposition. It is thus very unlikely that the receptor for SP is localized on a sensory structure projecting to the lumen of the uterus; at least for inducing oviposition. The time response for receptivity after mating cannot be tested, because male substances other than SP transferred during mating reduce short-term remating [4].
If SP is injected into the haemolymph of SPR mutant females with a leaky BBB, higher concentrations are required to reduce receptivity and also more time is needed to induce oviposition. These altered features of the response could reflect suboptimal delivery to neuronal targets, resulting in a longer exposure to degradation in the haemolymph rather than reduced affinity to these additional receptor(s) because the binding constant is in the same range as for binding to SPR [16].
An intriguing role for the BBB is further revealed in allowing access of SP to those target neurons, which mediate the postmating switch. If SP is injected into females with a leaky BBB, SP acts at lower concentrations. At higher concentrations SP completely inhibits oviposition, but its effect in reducing receptivity is only marginally compromised (see the electronic supplementary material, figures S2b and S5). Thus, once in the haemolymph, SP seems to be delivered to a dedicated set of neurons.
(d). Multi-level control of the postmating switch by sex-peptide
Ours and previous SP injection experiments have shown that both receptivity and oviposition are altered at the same critical concentration [42]. An interpretation of these results would be the presence of an SP receptor on a single type of neuron, which then induces the postmating switch by signalling to higher order processing centres. However, our results suggest presence of SP receptor(s), which bind SP with similar affinity, on different types of neurons, responsible either for reducing receptivity or inducing oviposition (see the electronic supplementary material, figure S5). This is supported by our findings that SP-mediated regulation of receptivity can be separated from oviposition in fru and dsx neurons. First, fru neurons can be split into a PTX-insensitive population, which regulates receptivity and does not express ppk, and a PTX-sensitive population, which regulates receptivity and oviposition, and expresses ppk. PTX-sensitive fru neurons express ppk because ppkGAL80 can inhibit the SP response when mSP is expressed via fruGAL4 and seem epistatic to PTX-insensitive fru neurons [21]. Second, a subset of dsx neurons contains an additional SP receptor(s), which can induce oviposition, but have no effect on receptivity. Potentially, these dsx neurons are part of a motor output programme and could induce oviposition by dsx neurons projecting from the Abg to the genital tract [22].
Diversification in the control of the postmating switch through SP is indicated by the characteristics of ppk, fru and dsx neurons, which can be inhibitory, stimulatory and/or PTX sensitive (see the electronic supplementary material, figure S5). Although ppk, fru and dsx neurons overlap in a small population of neurons in the genital tract, the diverse properties of ppk, fru and dsx neurons makes it unlikely that the SP response is entirely regulated by these neurons co-expressing ppk, fru and dsx. The presence of additional neurons, which can mediate the SP response, has also been indicated by ppkGAL80 inhibition of dsxGAL4 UAS mSP expression. In this experiment, the SP response is only partially suppressed [22]. It is conceivable that the SP response in ppk, fru and dsx neurons is mediated by an interconnected hierarchical arrangement of inhibitory and stimulatory circuits. Fine mapping of the neuronal circuitry involved in the SP response will be required to determine whether stimulatory dsx+/SPR+ and SPR− and inhibitory fru+/ppk+/SPR+ neurons comprise separate populations for receptivity and oviposition (see the electronic supplementary material, figure S5).
In conclusion, separation of SP's action in reducing receptivity from inducing oviposition in some neurons, but not in others argues for multiple pathways through which SP can induce the postmating behavioural switch.
4. Material and methods
Flies were kept on standard cornmeal-agar food (1% industrial-grade agar, 2.1% dried yeast, 8.6% dextrose, 9.7% cornmeal and 0.25% Nipagin, all in (w/v)) in a 12 L : 12 D cycle. Injections and behavioural assays were performed on sexually mature 3–5 day-old virgin females as described previously [8,20]. For injections, female flies were cooled to 4°C and 3 pmol SP, or as otherwise stated, in 50 nl Ringer's solution was injected. Spermless males were generated by crossing tud1/Df(2R)Exel6072 females to wild-type males. ANOVA followed by planned pairwise comparisons with Fisher's protected least significant difference was done for statistical analysis using StatView. Generation of the SP construct for neuronal expression and transgenic flies by phiC31-mediated transformation has been described previously [31] using landing sites at 28E (PBac{y+-attP-3B}VK00002) and 76A (PBac{y+-attP-3B}VK00002). SPR/Df females were generated by crossing females for the larger deficiency Df(1)JC70/FM7 with SPR mutant males (Df(1)Exel6234). moody RNAi lines P{GD709}v1800 and P{100674}v109601 were used and yielded indistinguishable results. repoGAL4 is an enhancer-trap insert in the repo gene. For transgenic delivery of SP to the haemolymph via secretion from fat body expression under the yp1 promoter, a YPhsSPg insert on the second chromosome was used [5] and mSP was expressed from an insert on the third chromosome [23]. fruGal4 and dsxGal4 are Gal4 insertions into the endogenous locus, and for expression in the ppk and GMR, patterns inserts on the second chromosome were used [21,27,28]. For conditional expression of PTX with doxycycline, the UAS-rtTA tet0-PTX20f line with both inserts on the second chromosome was used [37]. Doxycyline was applied (200 µl 10 mg ml−1) to the surface of standard food and females were exposed for 24 h.
Acknowledgements
We thank T. Aigaki, B. Dickson, S. Goodwin, Y. N. Jan, E. Kubli, G. Roman, and the Bloomington and VDRC Stock Centers for fly stocks; E. Kubli for antibodies; R. Cook and M. Li for help with cloning; M. Li for generating transgenes; M. Wheatley for discussions; and G. Roman, J. Levine and J. C. Billeter for comments on the manuscript.
Funding statement
This work was supported by the University of Birmingham and NSF grant IOS-0919697 to B.D.
References
- 1.Gillott C. 2003. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48, 163–184 (doi:10.1146/annurev.ento.48.091801.112657) [DOI] [PubMed] [Google Scholar]
- 2.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40 (doi:10.1146/annurev-ento-120709-144823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith LC. 2008. Neuroscience: love hangover. Nature 451, 24–25 (doi:10.1038/451024a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Kubli E. 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 9929–9933 (doi:10.1073/pnas.1631700100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aigaki T, Fleischmann I, Chen PS, Kubli E. 1991. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron 7, 557–563 (doi:10.1016/0896-6273(91)90368-A) [DOI] [PubMed] [Google Scholar]
- 6.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. 2003. From the cover: the sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA 100, 9923–9928 (doi:10.1073/pnas.1631635100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. 1988. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 54, 291–298 (doi:10.1016/0092-8674(88)90192-4) [DOI] [PubMed] [Google Scholar]
- 8.Soller M, Bownes M, Kubli E. 1999. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208, 337–351 (doi:10.1006/dbio.1999.9210) [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro C, Dickson BJ. 2010. Sex peptide receptor and neuronal TOR/S6 K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 20, 1000–1005 (doi:10.1016/j.cub.2010.03.061) [DOI] [PubMed] [Google Scholar]
- 10.Isaac RE, Li C, Leedale AE, Shirras AD. 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. R. Soc. B 277, 65–70 (doi:10.1098/rspb.2009.1236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. 2006. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol. 16, 692–696 (doi:10.1016/j.cub.2006.02.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domanitskaya EV, Liu H, Chen S, Kubli E. 2007. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS J. 274, 5659–5668 (doi:10.1111/j.1742-4658.2007.06088.x) [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Zipperlen P, Kubli E. 2005. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 15, 1690–1694 (doi:10.1016/j.cub.2005.08.048) [DOI] [PubMed] [Google Scholar]
- 14.Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186, 595–600 (doi:10.1534/genetics.110.119735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wigby S, Chapman T. 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321 (doi:10.1016/j.cub.2005.01.051) [DOI] [PubMed] [Google Scholar]
- 16.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 (doi:10.1038/nature06483) [DOI] [PubMed] [Google Scholar]
- 17.Poels J, Van Loy T, Vandersmissen HP, Van Hiel B, Van Soest S, Nachman RJ, Vanden Broeck J. 2010. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell Mol. Life Sci. 67, 3511–3522 (doi:10.1007/s00018-010-0393-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, et al. 2010. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl Acad. Sci. USA 107, 6520–6525 (doi:10.1073/pnas.0914764107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanaka N, Hua YJ, Roller L, Spalovska-Valachova I, Mizoguchi A, Kataoka H, Tanaka Y. 2010. Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proc. Natl Acad. Sci. USA 107, 2060–2065 (doi:10.1073/pnas.0907471107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soller M, Haussmann IU, Hollmann M, Choffat Y, White K, Kubli E, Schafer MA. 2006. Sex-peptide-regulated female sexual behavior requires a subset of ascending ventral nerve cord neurons. Curr. Biol. 16, 1771–1782 (doi:10.1016/j.cub.2006.07.055) [DOI] [PubMed] [Google Scholar]
- 21.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (doi:10.1016/j.neuron.2008.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezaval C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22, 1155–1165 (doi:10.1016/j.cub.2012.04.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama S, Kaiser K, Aigaki T. 1997. Ectopic expression of sex-peptide in a variety of tissues in Drosophila females using the P[GAL4] enhancer-trap system. Mol. Gen. Genet. 254, 449–455 (doi:10.1007/s004380050438) [DOI] [PubMed] [Google Scholar]
- 24.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518 (doi:10.1016/j.neuron.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 25.Zhong L, Hwang RY, Tracey WD. 2010. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 20, 429–434 (doi:10.1016/j.cub.2009.12.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. 2005. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 [DOI] [PubMed] [Google Scholar]
- 27.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. 2010. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 (doi:10.1038/nn.2515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. 2005. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (doi:10.1016/j.cell.2005.04.026) [DOI] [PubMed] [Google Scholar]
- 29.Ding Z, Haussmann I, Ottiger M, Kubli E. 2003. Sex-peptides bind to two molecularly different targets in Drosophila melanogaster females. J. Neurobiol. 55, 372–384 (doi:10.1002/neu.10218) [DOI] [PubMed] [Google Scholar]
- 30.Ottiger M, Soller M, Stocker RF, Kubli E. 2000. Binding sites of Drosophila melanogaster sex peptide pheromones. J. Neurobiol. 44, 57–71 (doi:10.1002/1097-4695(200007)44:1<57::AID-NEU6>3.0.CO;2-Q) [PubMed] [Google Scholar]
- 31.Haussmann IU, Li M, Soller M. 2011. ELAV-mediated 3′-end processing of ewg transcripts is evolutionarily conserved despite sequence degeneration of the ELAV-binding site. Genetics 189, 97–107 (doi:10.1534/genetics.111.131383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. 2008. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 28, 587–597 (doi:10.1523/JNEUROSCI.4367-07.2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. 2005. Moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 123, 145–156 (doi:10.1016/j.cell.2005.07.029) [DOI] [PubMed] [Google Scholar]
- 34.Thambi NC, Quan F, Wolfgang WJ, Spiegel A, Forte M. 1989. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J. Biol. Chem. 264, 18 552–18 560 [PubMed] [Google Scholar]
- 35.Ferris J, Ge H, Liu L, Roman G. 2006. G(o) signaling is required for Drosophila associative learning. Nat. Neurosci. 9, 1036–1040 (doi:10.1038/nn1738) [DOI] [PubMed] [Google Scholar]
- 36.Fremion F, Astier M, Zaffran S, Guillen A, Homburger V, Semeriva M. 1999. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J. Cell Biol. 145, 1063–1076 (doi:10.1083/jcb.145.5.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madalan A, Yang X, Ferris J, Zhang S, Roman G. 2012. G(o) activation is required for both appetitive and aversive memory acquisition in Drosophila. Learn. Mem. 19, 26–34 (doi:10.1101/lm.024802.111) [DOI] [PubMed] [Google Scholar]
- 38.Kubli E, Bopp D. 2012. Sexual behavior: how sex peptide flips the postmating switch of female flies. Curr. Biol. 22, R520–R522 (doi:10.1016/j.cub.2012.04.058) [DOI] [PubMed] [Google Scholar]
- 39.Kvitsiani D, Dickson BJ. 2006. Shared neural circuitry for female and male sexual behaviours in Drosophila. Curr. Biol. 16, R355–R356 (doi:10.1016/j.cub.2006.04.025) [DOI] [PubMed] [Google Scholar]
- 40.Hanin O, Azrielli A, Applebaum SW, Rafaeli A. 2011. Functional impact of silencing the Helicoverpa armigera sex-peptide receptor on female reproductive behaviour. Insect Mol. Biol. 27, 161–167 (doi:10.1111/j.1365-2583.2011.01122.x). [DOI] [PubMed] [Google Scholar]
- 41.Soller M, Bownes M, Kubli E. 1997. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur. J. Biochem. 243, 732–738 (doi:10.1111/j.1432-1033.1997.00732.x) [DOI] [PubMed] [Google Scholar]
- 42.Schmidt T, Choffat Y, Klauser S, Kubli E. 1993. The Drosophila melanogaster SP: a molecular analysis of structure-function relationships. J. Insect Physiol. 39, 361–368 (doi:10.1016/0022-1910(93)90023-K) [Google Scholar]


