Abstract
Life-history strategies describe that ‘slow’- in contrast to ‘fast’-living species allocate resources cautiously towards reproduction to enhance survival. Recent evidence suggests that variation in strategies exists not only among species but also among populations of the same species. Here, we examined the effect of experimentally induced stress on resource allocation of breeding seabirds in two populations with contrasting life-history strategies: slow-living Pacific and fast-living Atlantic black-legged kittiwakes. We tested the hypothesis that reproductive responses in kittiwakes under stress reflect their life-history strategies. We predicted that in response to stress, Pacific kittiwakes reduce investment in reproduction compared with Atlantic kittiwakes. We exposed chick-rearing kittiwakes to a short-term (3-day) period of increased exogenous corticosterone (CORT), a hormone that is released during food shortages. We examined changes in baseline CORT levels, parental care and effects on offspring. We found that kittiwakes from the two populations invested differently in offspring when facing stress. In response to elevated CORT, Pacific kittiwakes reduced nest attendance and deserted offspring more readily than Atlantic kittiwakes. We observed lower chick growth, a higher stress response in offspring and lower reproductive success in response to CORT implantation in Pacific kittiwakes, whereas the opposite occurred in the Atlantic. Our findings support the hypothesis that life-history strategies predict short-term responses of individuals to stress within a species. We conclude that behaviour and physiology under stress are consistent with trade-off priorities as predicted by life-history theory. We encourage future studies to consider the pivotal role of life-history strategies when interpreting inter-population differences of animal responses to stressful environmental events.
Keywords: trade-offs, stress hormones, reproduction, populations, seabirds, life-history theory
1. Introduction
Animals respond to changes in the environment, which allows them to reproduce and survive under stressful conditions [1–3]. However, limited time and energy to fuel these responses impose trade-offs between different interests, for example whether to prioritize reproduction or survival [4]. According to life-history theory, these differences in prioritization are manifested in different life-history strategies [5–7]. The ‘slow-living’ strategy is expressed as improved self-maintenance, and thus survival, but decreased investment in reproduction. By contrast, the opposite pattern of prioritization of fecundity over survival is found in the ‘fast-living’ strategy. These trade-offs are expected to manifest most strongly under stressful conditions, for example when breeding animals face food limitations [4].
Life-history theory predicts that different paces of life are expressed in contrasting behavioural and physiological traits of individuals [4,8–11]: fast-living individuals are expected to be more risk-taking, maintain higher metabolism and invest less in costly defence mechanisms that would promote survival at the expense of fecundity. Recently, life-history theory has been used to explain interspecific responses in allocation of resources to changes in the environment [4]. However, differences in the life-history strategies among populations of the same species have also been documented [10]. Among populations with substantial gene flow, these differences may reflect short-term phenotypic responses to a given set of environmental conditions instead of genetically fixed strategies [12,13]. However, the differences may also be fixed at the population level, for example, among genetically distant populations [14,15]. In the marine environment, such intraspecific differentiation in life-history traits occurs at the ocean scale [16]. A well-studied example is the difference in life-history traits between North Pacific and North Atlantic populations of black-legged kittiwakes (Rissa tridactyla, hereafter ‘kittiwake’), a long-lived seabird species. These populations are distinct in morphological [17], vocal [18] and genetic characteristics [19]. Even though all kittiwakes are relatively slow-living animals (maximum reported lifespan is 28 years [20]) when compared with species with shorter lifespans, consistent differences in life-history traits are found between the populations. Pacific kittiwakes appear slower-living compared with their Atlantic conspecifics: Pacific kittiwakes live longer, whereas their fecundity is lower compared with Atlantic kittiwakes, which produce more offspring but have lower adult survival rates [16,21–24]. However, these differences might also reflect currently diverging large-scale inter-ocean patterns of food supply, which may be less variable in the Atlantic than in the Pacific [12,16]. To test whether such differences in life-history strategies reflect environmental conditions or are genetically fixed, an experimental manipulation is necessary [13,14].
Glucocorticoid hormones (i.e. corticosterone in birds, reptiles and rodents, cortisol in most mammals) have been proposed as physiological mediators between environmental conditions and life-history strategies in animals [9,25–27]. Glucocorticoid levels trace the stability and quality of the environment, and trigger behavioural and metabolic responses to cope with changing conditions [28,29]. They are therefore strong candidates as regulators of fitness trade-offs and may play a pivotal role in the adaptation of strategies that maximize fitness in a given environment. However, the relationship between glucocorticoids and fitness components such as reproduction is far from consistent [29], indicating differences in glucocorticoid responsiveness between species and populations. In kittiwakes, corticosterone (CORT) concentrations correlate strongly with food availability in the environment [30], and affect parental and foraging behaviours [31]. Through manipulations of glucocorticoid levels, it is thus possible to mimic the acute stress induced by environmental challenges in free-living animals and to study their reproductive responses, instead of attempting to alter a complex marine environment.
To test the effects of a transient stressful event on reproductive behaviour of kittiwakes, we experimentally induced acute stress in individuals breeding at two colonies that belong to the Pacific and Atlantic kittiwake populations: Middleton Island in the Gulf of Alaska, North Pacific and Kongsfjorden on Svalbard, North Atlantic. Because within-ocean genetic structuring of kittiwake populations is negligible [32], these two colonies are likely to be representative of other colonies in the respective oceanic regions. At both colonies, individuals received subcutaneous silastic CORT implants for a 3-day period. These implants increase circulating CORT levels within the physiological range over approximately 3 days in kittiwakes, with the peak produced within the first 2 days [31,33,34]. After 3 days, the concentrations are expected to return to pre-implantation levels, owing to increased clearance and/or the negative feedback mechanism that downregulates the endogenous hormone production [33].
By conducting the CORT implant experimental manipulations, we tested the hypothesis that reproductive investment of chick-rearing kittiwakes under stressful conditions reflects their life-history strategies. We adopted two previously advanced hypotheses [24,35] to represent the hypothesized strategies for the two populations and formulate our specific predictions (figure 1): (i) under the fixed investment hypothesis parental effort in raising young is ‘fixed’ and, to avoid effects on their own survival, these parents will allocate less to parental care when facing stressful conditions, leading to negative impacts on offspring; (ii) under the flexible investment hypothesis parents buffer their offspring from negative impacts of detrimental environmental conditions via an increase of parental effort. We predict that under acute stress, behaviour of slow-living Pacific kittiwakes will support hypothesis (i), and they will invest more reluctantly in offspring care than Atlantic kittiwakes. Specifically, we predict that acute stress will reduce nest attendance in CORT-implanted Pacific birds, and their chicks will be affected negatively (i.e. they will exhibit higher mortality, slower growth and/or an increased adrenocortical function than controls). In contrast to slow-living Pacific kittiwakes, the behaviour of fast-living Atlantic kittiwakes will support hypothesis (ii); they will respond to induced stress by increasing the amount of resources allocated to their young to compensate for the (hormonally signalled) reduced resource availability. This will be reflected in increased nest attendance of CORT-implanted Atlantic birds, and—because the physiological signal of food stress in this situation does not reflect actual resource availability—higher chick growth, lower adrenocortical function and higher survival compared with controls (figure 1).
Figure 1.
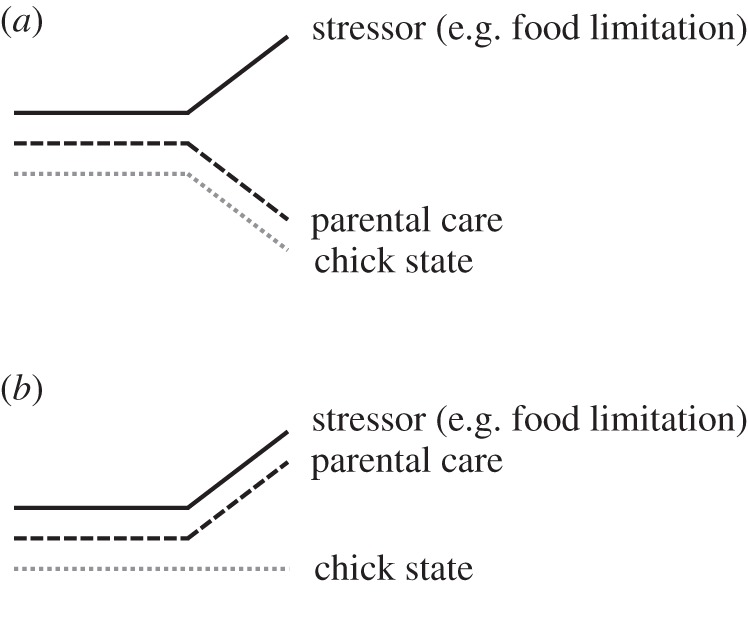
Schematic of study hypotheses. (a) The fixed investment hypothesis states that parental care is not matched to compensate fluctuations in food availability, and chicks bear negative consequences when food conditions deteriorate. This hypothesis is used to predict adult kittiwake responses and effects on offspring for the Pacific colony. (b) The flexible investment hypothesis states that parental care tracks food availability (i.e. care increases when food conditions deteriorate, in order to maintain chick state). This hypothesis is used to predict adult responses and effects on chicks for the Atlantic colony. However, because food stress in this study is induced physiologically and does not reflect actual food availability, offspring at the Atlantic colony are predicted to show an improving state.
To disentangle effects of current foraging conditions versus life-history strategies on reproductive decisions, we experimentally improved the food availability in a subset of CORT-implanted and control kittiwakes in the Pacific colony by providing them with a food supplement. If food availability rather than life-history strategy is important, then a reduction in parental care during stress, as expected for Pacific kittiwakes, will be ameliorated by the food supplement. Similarity of behavioural responses to induced stress between food-supplemented and unfed individuals will further support the role of life-history strategy rather than current foraging conditions as a determinant of reproductive decisions.
2. Material and methods
(a). Study system
Kittiwakes are medium-sized gulls with a circumpolar breeding distribution. They are colonial cliff-nesters with biparental care, and usually raise one or two chicks annually. We focused on two colonies: a North Pacific colony at Middleton Island in the Gulf of Alaska (59°26′ N, 146°21′ W) and a North Atlantic colony at Kongsfjorden in the Svalbard archipelago (78°59′ N, 12°06′ E). At the Pacific colony, kittiwakes breed on artificial ledges built on vertical walls of an abandoned radar tower, whereas at the Atlantic colony they breed on natural cliffs.
(b). Implant treatment
We simulated acute stress by treating birds with CORT implants during early chick-rearing in 2010 (Pacific colony: n = 30) and 2011 (Atlantic colony: n = 19). The implantation procedures were carried out following the established protocols to allow direct comparisons of bird responses between the focal colonies [31,34]. Briefly, kittiwakes were implanted subcutaneously between the shoulders with a silastic tube 25 mm in length (Dow Corning) and filled with crystallized CORT (Sigma Aldrich, ref. C2505). Small incisions were made at both ends of the implant to facilitate the release of CORT, which effectively reproduces the approach in earlier field and laboratory studies in which CORT levels of kittiwakes were elevated by two CORT implants with single cuts [31,36]. The amount of CORT contained in a single implant was not limiting in our study, because more than half of the crystallized CORT was left in all implants at removal (J.S. 2010–2011, personal observation). A control group of kittiwakes received empty implants (Pacific colony: n = 31; Atlantic colony: n = 18). Long-term exposure to exogenous CORT has been shown to increase mortality of adult kittiwakes [31,37]. In this study, we aimed to induce only a short-term exposure to stress, and thus planned to recapture all kittiwakes and remove their implants after a 3-day period.
(c). Hormones
Baseline CORT was measured immediately before and 3 days after implantation. At each occasion, birds were captured on their nests, and CORT was measured in blood samples drawn from the brachial vein within 3 min of capture. This provided us with measurements of baseline CORT unaffected by the capturing and handling procedure (details in [30]). Blood samples were kept on ice until centrifugation within a few hours after collection to separate red blood cells and plasma. Plasma samples were then kept frozen until analyses of CORT in a radioimmunoassay, which has been described in detail earlier [38].
(d). Food treatment in the Pacific colony
Food availability was directly manipulated in a subset of CORT-implanted and control individuals at the Pacific colony. A subset of kittiwakes that nest at the radar tower was supplemented with food (hereafter ‘fed’; nCORT = 15, ncontrol = 15), as opposed to ‘unfed’ kittiwakes without additional food provision (nCORT = 15, ncontrol = 16). Occupants of each fed nest (parents and chicks) were offered ad libitum capelin (Mallotus villosus) through a plastic pipe three times daily (08.00, 13.00 and 18.00 h) throughout the breeding period. Despite this improvement in food availability, food-supplemented birds continue to forage at sea, and the consumption of supplemented fish varies with natural food availability [39,40]. The supplementation therefore improves but does not completely override natural food availability [39,41,42].
(e). Parental care
All birds were individually marked, allowing us to track behaviour of mates within a pair. To quantify parental care, nest attendance was observed every 30 min for 12 h on the day prior to and during the 2 days following implantation. However, because we observed an unexpectedly strong response to the implant treatment in the Pacific colony (see Results), we also quantified the nest deserting behaviour, which was defined as a bird's abandoning the nest site for the duration of the reproductive season. Because kittiwakes usually continue to guard and defend their nest sites even when chicks are lost, and to account for chick mortality, we defined birds that lost chicks but continued attending their nests as not deserting. Deserting birds could not be recaptured for blood sampling, and we were thus not able to measure post-implant baseline CORT levels in these individuals. Individual condition was a factor that we initially considered as a potentially important determinant of parental care in kittiwakes [34]. However, because we currently lack an accepted concise measure for individual condition in general [43] or for kittiwakes in particular [41], and the commonly used indicator of condition (body mass scaled for structural size, where size is measured as the first principal of headbill, wing and tarsus lengths [41]) had no significant effects on behavioural responses in our study (see electronic supplementary material, appendix S1), we did not include this factor in our main analysis.
(f). Chick state
The state of offspring was quantified by three measures. Growth of chicks was measured as the change in body mass (g d−1) between early (at the time of parent implantation) and late chick-rearing (approx. three weeks later). During the later measurement, we also measured the adrenocortical function (assessed as the adrenocortical response to a standardized stressor of capture and restraint, hereafter ‘stress response’ [44]) of chicks by capturing and holding them individually in a breathable cotton bag for approximately 1 h, while obtaining blood samples at 0, 30 and 50 min post-capture [44–46]. In kittiwakes, this measure provides a proxy of the nutritional history of individuals during the previous two to three weeks, thereby gauging how well parents fed offspring and approximating the physiological state of the offspring (a higher response indicates nutritional stress) [44]. These CORT samples were processed and analysed as described for baseline samples above, and stress response defined as the increase in CORT concentrations from baseline to maximum levels within 50 min (Δng ml−1). Chick survival was measured by observing focal nests throughout the chick-rearing period until fledging (approx. 40 day post-hatch) and recording the number of live chicks in each nest.
(g). Statistical analyses
All analyses were performed with R [47]. Means are reported with associated standard errors. We used the Akaike information criterion (AIC) model selection approach to test the effects of implants, ocean origin and food availability on adult (i) changes in baseline CORT and (ii) changes in nest attendance, and offspring (iii) survival, (iv) growth and (v) stress response. The candidate models for the ocean comparison included an implant-effect model, a colony-effect model, a model describing contrasting effects of implants between the colonies (interaction model) and a null model. The candidate set for the food comparison included an implant-effect model, a food-effect model, a model describing contrasting effects of implants between the food treatments (interaction model) and a null model. For continuous response variables with no need for correction of pseudoreplication (i, ii), we fitted linear models; for continuous variables with pseudoreplication owing to chicks sharing nests (iv, v), we fitted linear mixed effects models with ‘nest’ as the random variable; whereas we fitted a generalized linear model with binomial error distribution for the discrete response variable (iii). We selected models based on their AICc values, and excluded all models with AICc > 4, because low AICc values (e.g. 2) are considered too strict a cut-off point [48]. All selected models are presented with their AIC weights and evidence ratios. We considered our hypothesis supported (i.e. that responses of kittiwakes to the CORT treatment are predictable based on their contrasting life-history strategies) whenever the interaction model was selected into the top set of models, and the direction of effects matched the predictions of the fixed versus flexible investment hypothesis for Pacific and Atlantic kittiwakes, respectively.
3. Results
(a). Parental baseline corticosterone levels
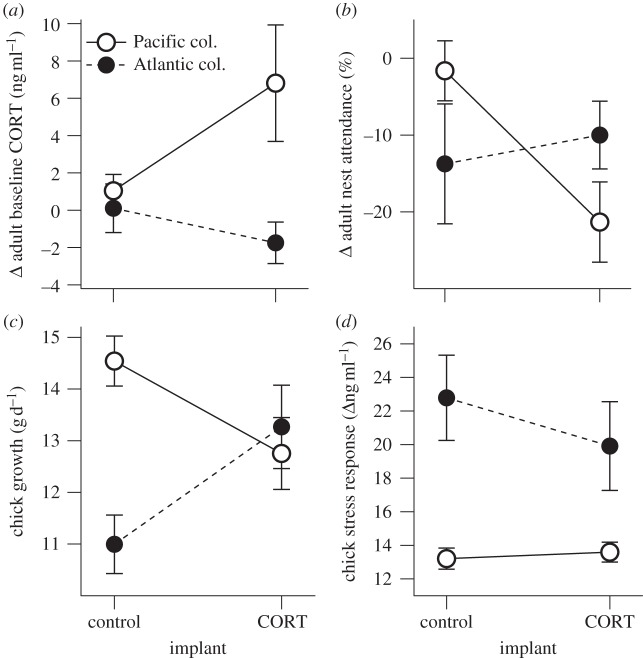
On average, kittiwakes from the Atlantic colony had higher levels of baseline CORT (7.4 ± 0.6 ng ml−1) compared with kittiwakes from the Pacific colony (4.9 ± 0.5 ng ml−1 in unfed individuals; ANOVA: F1,65 = 13.331, p < 0.001), but there was considerable overlap at the individual level (see electronic supplementary material, appendix S2). Food supplementation further decreased baseline CORT levels in kittiwakes from the Pacific colony (to 3.0 ± 0.3 ng ml−1; ANOVA: F1,59 = 10.907, p = 0.002). There was strong evidence from model selection that implants had different effects between colonies on the change of baseline CORT between pre-implantation and day three post-implantation (table 1). The implantation procedure did not affect CORT levels of control birds (figure 2). At the end of the 3-day implantation period, baseline levels of CORT remained increased by about twofold in unfed kittiwakes from the Pacific colony, whereas baseline levels had at this point returned to below pre-implantation concentrations in kittiwakes from the Atlantic colony (figure 2). Changes in baseline CORT in kittiwakes from the Pacific colony were similar between unfed and fed kittiwakes, because none of the selected models included an interaction between food treatment and type of implant (table 1; electronic supplementary material, appendix S3).
Table 1.
Model selection results (displayed are selected models with ΔAIC < 4, and null model) for effect of colony identity (Pacific or Atlantic colony), type of implant (CORT or control) and food treatment (unfed or fed) on baseline CORT and adult nest attendance. Effects of food treatment analysed for the Pacific colony only (food comparison). Indicated in italics is the interaction model that supports the study hypothesis. d.f., degrees of freedom; AICc, AIC corrected for finite sample size; ΔAIC, difference between the AICc of this model and the AICc of the best model; wi, ratio of AICc values for this model relative to the whole set of candidate models (weight); e.r., evidence ratio describing the relative weight of this model against the best model.
| response variable | model | d.f. | AICc | ΔAICc | wi | e.r. |
|---|---|---|---|---|---|---|
| colony comparison | ||||||
| baseline CORT | implant + colony + impl × col | 5 | 536.9 | 0.00 | 1.000 | 1.0 |
| (null model) | 2 | 549.9 | 13.03 | — | — | |
| nest attendance | implant + colony + impl × col | 5 | –8.4 | 0.00 | 0.769 | 1.0 |
| implant | 3 | –6.0 | 2.41 | 0.231 | 3.3 | |
| (null model) | 2 | –1.4 | 6.94 | — | — | |
| food comparison | ||||||
| baseline CORT | implant | 3 | 334.1 | 0.00 | 0.740 | 1.0 |
| implant + food | 4 | 336.2 | 2.09 | 0.260 | 2.8 | |
| (null model) | 2 | 340.9 | 6.88 | — | — | |
| nest attendance | implant | 3 | –15.5 | 0.00 | 0.734 | 1.0 |
| implant + food | 4 | –13.5 | 2.03 | 0.266 | 2.8 | |
| (null model) | 2 | –3.1 | 12.43 | — | — | |
Figure 2.
Effects of CORT implantation on (a,b) parental and (c,d) offspring parameters of unfed kittiwakes at the Atlantic and Pacific colonies. Parental parameters are measured (a) as the change of baseline CORT levels between before and 3 days after implantation, and (b) as the change in adult nest attendance from before to after implantation. Chick parameters are measured (c) as growth of body mass (g d−1) during a three week period after implantation, and (d) as chick stress response (i.e. the increase in CORT from baseline to maximum in response to an acute stressor, at the end of that period).
(b). Parental care
Kittiwakes responded differently to CORT implant treatment at the two colonies, as evidenced by model selection for changes in nest attendance in which the most selected model included an interaction between implant and colony (table 1). There was considerably less evidence from model selection for an effect of implant alone on nest attendance (table 1). CORT-implanted kittiwakes from the Pacific colony decreased their nest attendance compared with controls (difference between means of unfed kittiwakes = −19.7%), whereas we observed the opposite response in kittiwakes from the Atlantic colony, where nest attendance was somewhat higher in CORT-implanted individuals than in controls (difference between means = +3.8%; figure 2; electronic supplementary material, appendix S3). Food supply did not affect the response in the Pacific colony, because nest attendance was similarly reduced in CORT-implanted individuals that were unfed or fed (no implant × food interaction in any of the selected models; table 1; electronic supplementary material, appendix S2).
In CORT-implanted kittiwakes, only kittiwakes from the Pacific colony deserted their offspring (table 2). None of the controls (Atlantic or Pacific) deserted their offspring (table 2). Food supplement did not affect this response in the Pacific colony, because fed kittiwakes that were CORT-implanted deserted their nests at similar rates compared with unfed kittiwakes (table 2), whereas all fed control birds continued attending their breeding sites (table 2).
Table 2.
Effects of colony identity (Pacific or Atlantic colony) and type of implant (CORT or control) on response of unfed adult kittiwakes to desert offspring (number of individuals). Numbers in parentheses indicate the similar response of fed individuals in the Pacific colony.
| desert | stay | ||
|---|---|---|---|
| Pacific colony | control | 0(0) | 16(15) |
| CORT | 5(4) | 10(11) | |
| Atlantic colony | control | 0 | 18 |
| CORT | 0 | 19 |
(c). Effects on offspring
There were multiple indications that offspring in the Pacific colony suffered negative consequences when a parent was experimentally stressed, whereas this was not the case for the Atlantic colony.
(i). Chick survival
Model selection yielded some evidence that chicks of CORT-implanted birds survived differently between the colonies (one of three selected models included an interaction between implant and colony; table 3). While CORT implantation led to improved reproductive success in the Atlantic colony (42.3% chick survival versus 33.3% in controls; table 4), there was an opposite effect in the Pacific (72.7% versus 91.7% chick survival in CORT-implanted and controls, respectively; table 4). Food supplementation in the Pacific colony alleviated this negative effect on offspring, because chick survival in CORT-implanted and control-fed kittiwakes was the same (at 95.8%; table 4), and none of the selected models included an interaction between implant and food (table 3).
Table 3.
Model selection results (displayed are selected models with ΔAIC < 4, and null model) for the effects of colony identity (Pacific or Atlantic colony), type of implant (CORT or control) and food treatment (unfed or fed) on offspring of implanted kittiwakes. Effects on offspring are measured as chick survival, chick growth and chick stress response. Effects of food treatment analysed for the Pacific colony only (food comparison). Indicated in italics is the interaction model that supports the study hypothesis. d.f., degrees of freedom; AICc, AIC corrected for finite sample size; ΔAIC, difference between AICc of this model and the AICc of the best model; wi, ratio of AICc values for this model relative to the whole set of candidate models (weight); e.r., evidence ratio describing the relative weight of this model against the best model.
| response variable | model | d.f. | AICc | ΔAICc | wi | e.r. |
|---|---|---|---|---|---|---|
| colony comparison | ||||||
| chick survival | colony | 2 | 101.8 | 0.00 | 0.611 | 1.0 |
| implant + colony | 3 | 103.8 | 1.99 | 0.226 | 2.7 | |
| implant + colony + impl × col | 4 | 104.4 | 2.64 | 0.163 | 3.8 | |
| (null model) | 1 | 128.6 | 26.77 | — | — | |
| chick growth | implant + colony + impl × col | 6 | 415.1 | 0.00 | 1.000 | 1.0 |
| (null model) | 3 | 431.3 | 16.12 | — | — | |
| chick stress response | colony | 4 | 968.9 | 0.00 | 0.428 | 1.0 |
| implant + colony + impl × col | 6 | 969.3 | 0.38 | 0.355 | 1.2 | |
| implant + colony | 5 | 970.3 | 1.36 | 0.217 | 2.0 | |
| (null model) | 3 | 993.4 | 24.46 | — | — | |
| food comparison | ||||||
| chick survival | food | 2 | 112.9 | 0.00 | 0.722 | 1.0 |
| implant + food | 3 | 114.8 | 1.92 | 0.277 | 2.6 | |
| (null model) | 1 | 130.5 | 17.62 | — | — | |
| chick growth | implant + food | 5 | 323.5 | 0.00 | 0.499 | 1.0 |
| implant | 4 | 324.4 | 0.86 | 0.324 | 1.5 | |
| implant + food + impl × food | 6 | 325.6 | 2.07 | 0.177 | 2.8 | |
| (null model) | 3 | 328.5 | 5.03 | — | — | |
| chick stress response | implant + food + impl × food | 6 | 815.3 | 0.00 | 1.000 | 1.0 |
| (null model) | 2 | 822.3 | 7.05 | — | — | |
Table 4.
Effects of colony identity (Pacific or Atlantic colony) and type of implant (CORT or control) on loss of at least one offspring in unfed adult kittiwakes. Numbers in parentheses indicate the different effect for individuals that were fed in the Pacific colony.
| lost ≥ 1 | survived | ||
|---|---|---|---|
| Pacific colony | control | 2(1) | 14(14) |
| CORT | 5(1) | 10(14) | |
| Atlantic colony | control | 13 | 5 |
| CORT | 12 | 7 |
(ii). Chick growth
Model selection provided strong evidence for different effects of parental implants on chick growth between the colonies (table 3). Chicks of CORT-implanted parents from the Pacific colony gained less mass between the approximate ages of 10 and 25 days than chicks in control nests (difference between means of unfed kittiwakes = −1.8 g d−1; figure 2; electronic supplementary material, appendix S4). By contrast, chicks from the Atlantic colony with CORT-implanted parents gained more mass than controls (difference between means = +2.3 g d−1; figure 2). There was limited evidence that food supplementation altered this effect by decreasing the growth difference between CORT and control nests (one out of three selected models included the interaction between implant and food; table 3; electronic supplementary material, appendix S4).
(iii). Chick stress response
Model selection indicated that implants may have affected chicks differently in the Pacific and the Atlantic colony, but evidence was ambiguous, because the interaction model was indistinguishable from others (table 3). Chicks from the Atlantic colony with a CORT parent tended to have a lower stress response than chicks in control nests (difference between means = −2.9 ng ml−1), whereas the opposite was the case at the Pacific colony (difference between means of unfed kittiwakes = +0.4 ng ml−1; figure 2). Food supplementation did not change the direction but further increased this effect (interaction between implant and food in the selected model; table 3; electronic supplementary material, appendix S4).
4. Discussion
Life-history strategies of animals can explain patterns in short-term allocation of limited resources to reproduction versus other functions or behaviours that promote survival. Previously, this has been demonstrated through interspecific comparisons. We tested, for the first time, whether these predictions hold for different allocation patterns between populations of a long-lived animal in which populations show diverging life-history traits. Overall, our results support the hypothesis that reproductive responses of parental birds to environmental fluctuations and the effects on their chicks can be predicted based on population-specific life-history strategies (table 4).
We found that behavioural responses to acute stress are consistent with the predictions deduced from the life-history strategies of Pacific and Atlantic kittiwakes. When challenged with acute stress, kittiwakes in the Pacific colony decreased nest attendance and often deserted their offspring. This was also true for Pacific kittiwakes that were experimentally supplemented with food, supporting the conclusion that reproductive responses to stress reflected strategies rather than current foraging conditions. The opposite patterns occurred in kittiwakes in the Atlantic colony, which continued breeding when challenged with CORT and had a slightly higher nest attendance compared with controls. These results support predictions of the fixed investment hypothesis for parental behaviour of individuals breeding at the slow-living Pacific kittiwake colony, whereas the results for the fast-living Atlantic colony supported the predictions of the flexible investment hypothesis. Overall, there was evidence that reproductive success (as measured in offspring survival, growth and stress response) of the slow-living, but not the fast-living colony was negatively affected by stress.
Our study represents a novel comparative approach for testing responses to stress between populations of a single wild animal species in the context of life-history theory. Our methodology allows us to make direct comparisons of responses between the two study colonies and to speculate on the mechanisms underlying life-history strategies. However, we can generalize our interpretations by evaluating our findings in the context of earlier studies. The kittiwake has been a focal species in numerous other studies examining responses of breeding individuals to stressors, including studies in both oceans that have manipulated stress hormones. The short-term response in Atlantic kittiwakes to CORT implantation appears consistent throughout years, because previous studies also found that these birds do not desert but usually maintain nest attendance during the first 2–3 days when CORT-implanted [33,34]. In this context, it was also observed that CORT-implanted kittiwakes with a higher body condition index spent more time flying than controls [34]. However, it remains to be tested whether such a change in behaviour improves self-investment only, as suggested by the authors based on the larger body mass gain in CORT birds compared with controls [34], or if it also increases the food supply to offspring. In another study, Atlantic female kittiwakes implanted with CORT before egg-laying had an increased breeding success compared with controls [49]. By contrast, kittiwakes in a Pacific study responded with a decrease in nest attendance to CORT implantation within the first 2–3 days, and their offspring were left unguarded for longer periods of time [31]. Another Pacific study found a similar effect when CORT-implanted kittiwakes reduced provisioning rates to their offspring, and chicks of CORT-implanted parents had a higher stress response than controls [50]. Finally, a Pacific study reported the negative effect of high baseline CORT levels on kittiwake laying success [40], demonstrating that strategic reproductive decisions may already be taken early in the breeding season. The rates of desertion that were observed during our study, however, have not been documented before. Additional evidence that the reduction of parental effort in response to stress observed in the Pacific was strategic derives from our feeding experiment, because optimal feeding conditions (in experimentally fed Pacific birds) did not affect changes in nest attendance or desertion rates. This further supports the study hypothesis that life-history strategies can predict population-specific behavioural responses to physiological stress. Thus, although our experimental study is limited to the two colonies in which we challenged kittiwakes with stress hormones, the results obtained are in line with a number of other experimental studies. Our study allowed a direct inter-colony comparison of responses to stress, because timing and methodology were identical at both colonies, and suggests that responses are likely to represent general inter-ocean patterns. Specifically, results of this and other studies indicate that at least some differences between populations in reproductive responses to environmental stress reflect different life-history strategies.
Results of this study may also explain how differences in life-history strategy are mediated physiologically. Although CORT levels in kittiwakes fluctuate substantially across regions and years, which is likely to be a reflection of variable environmental conditions [29,30,41], our results are consistent with earlier studies in that we found moderately elevated baseline CORT levels in Atlantic adult kittiwakes compared with many colonies in the Pacific [30,34,40,51–53]. In addition, Atlantic chicks circulated higher glucocorticoid levels compared with their Pacific counterparts (see electronic supplementary material, appendix S4). Because moderate increases in glucocorticoids often enhance rather than interfere with reproductive effort in animals [28], a higher hypothalamic–pituitary–adrenal (HPA) axis activity at the Atlantic colony may be an adaptive mechanism to the fast-living strategy that helps to prioritize reproduction by increasing the work effort of parents [54–56]. Such an enhancement of parental effort and reproductive performance is, in our study, not a result of a suppression of the HPA axis in response to exogenous CORT, as previously suggested [49]. Specifically, similar adrenocortical responses to acute stress of capture and handling between CORT-implanted and control kittiwakes indicated that Atlantic parents had not experienced a suppression of their HPA axis in response to a 3-day exposure to exogenous CORT (see electronic supplementary material, appendix S5). Similarly, our observation that during the experimental exposure to stress hormones some Pacific but not Atlantic kittiwakes abandoned reproduction indicates that threshold levels at which an abandonment of reproduction is triggered may be set differently in the two populations. However, the exact physiological adjustments that would allow this adaptation (for example, changes in the hormone receptors and binding proteins [27,57] or interactions with other hormones [58]) remain to be explored. Alternatively, the higher observed CORT levels at the Atlantic colony could indicate that kittiwakes that are more sensitive to elevated CORT levels (i.e. slow-living phenotypes) could have deserted breeding before the commencement of our study. However, no chicks were observed left alone during our presence in the colony, indicating that kittiwakes did not desert their chicks before the experiment. Instead, failed breeding was most often associated with predation by avian predators (glaucous gulls, Larus hyperboreus). Potentially more important for variation in CORT signatures than a ‘pre-selection’ of attending breeders may have been the seasonal food conditions. Baseline CORT in kittiwakes strongly correlates with food availability [30], and the higher levels at the Atlantic colony may simply reflect worse food conditions. While we cannot exclude the possibility that food instead of life-history strategies may have determined inter-colony differences in CORT levels (see above), assuming an environmental cause makes the observed differences in behavioural responses even more notable. Even when under higher food stress, Atlantic kittiwakes did not desert nests, and CORT-implanted Atlantic individuals maintained nest attendance similar to that of Pacific and higher than Atlantic controls. In conclusion, it may be speculated whether CORT has been a mediator in an adaptation of an ocean-specific strategy in kittiwakes to differing environmental conditions, which supports suggestions that glucocorticoids may play an important role in the evolution of life-history strategies in animals [9,25–27].
The fine-tuning of the HPA feedback system is a suggested control point for reproductive responses of animals, and may therefore be important in the context of life-history strategies [9,56]. How kittiwakes deal with increased exogenous CORT in terms of hormone metabolism and negative feedback in our study may explain why they can differ in behavioural responses. Atlantic kittiwakes quickly attenuated high levels of CORT induced by implants, thereby possibly avoiding behaviour that directs them away from reproduction. Whether this is a fast and strong suppression of endogenous CORT production or a physiological mechanism that quenches circulating CORT is unclear. That attenuation of high CORT levels may indeed be related to life-history strategies is supported by comparisons with other implant studies. Here and in another study of Pacific kittiwakes, CORT levels were still elevated by more than twofold 3 days after CORT implantation [31]. In the Atlantic, however, CORT levels have at this time usually returned back to or fallen below pre-implantation levels [34,37,49]. This underlines the pivotal mechanistic role that the regulation of and sensitivity to glucocorticoids may play in shaping individuals' and populations’ responses to stressful conditions.
The observed responses in adult birds to induced stress led to an increase in chick growth of Atlantic CORT-implanted parents, whereas in Pacific kittiwakes, CORT implants resulted in lower chick growth. Additionally, some of our evidence suggested that reproductive success was negatively affected and that stress incurred by offspring was higher in the Pacific. Overall reproductive success was lower in the Atlantic than in the Pacific colony, which contradicts the general patterns in life-history traits. However, personal observations in the field indicate that this was caused by a much higher predation rate by large gulls (L. hyperboreus in the Atlantic; glaucous-winged gulls, Larus glaucescens, in the Pacific) in the Atlantic colony. We observed several incidents of such nest predation during the time we spent in the Atlantic colony, whereas no single incident was observed in the Pacific colony, despite the presence of a large number of predatory gulls on the island. As described earlier, the colony in the Pacific is exceptional in that the birds nest on artificial ledges around a tower, which are well protected against predation [39,59]. Individuals breeding on natural cliffs on the same island have very low fecundity and frequently suffer complete breeding failure [22,59]. We therefore suspect that chick loss would have been higher overall and particularly in CORT nests if the experimental nests were situated in the natural breeding habitat.
In summary, our study demonstrates that differences in life-history strategies within a species can shape individual responses to environmental stress. This seems particularly important in the light of ongoing and predicted changes in marine ecosystems, which are expected to change prey abundance, distribution and seasonality, and thus availability, to marine top predators [60,61]. However, this study can be viewed only as an initial step in exploring population responses to environmental stress as determined by the life-history strategies. We lack the consistent long-term and widespread sampling that is required to reliably make broader generalizations. While we have focused on inter-population differences, future studies will have to increase efforts to explore also the impact of individual differences in life histories within colonies and regions. It has recently become clear that maternal effects or conditions that individuals experience during early life can set them up to pursue distinct strategies throughout the rest of their lives [62–64]. Thus, intraspecific phenotypic flexibility seems high to start with, but genetic isolation such as in kittiwakes may potentially increase differences. Ignoring differences associated with life-history strategies may be problematic, because they could lead to equivocal predictions on the effect of environmental change on individuals and populations.
Acknowledgements
We are very grateful to all those who contributed to the collection of our field data: Ine Dorresteijn, Rebecca C. Young, Vegard S. Bråthen, Charlotte Lassen, Elin Noreen, Tim van Nus and the USGS Middleton volunteers. We thank Zhenya Kitaiskaia for her work on the corticosterone assays. Finally, we thank Wojtek Moskal and staff of the Sverdrup Research Station for logistic support. Earlier versions of the manuscript were improved by comments from Z.M. Benowitz-Fredericks, R.C. Young, J. Welcker, S. Vincenzi and three referees. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US Government.
Permissions to perform the experiments were given by the Norwegian Animal Research Authority and the Governor of Svalbard (Norway), and the US Fish and Wildlife Service, the Alaska Department for Fish and Game and the Institutional Animal Care and Use Committee/University of Alaska Fairbanks (USA).
Funding statement
This work was supported by the Research Council of Norway (SPORE project, grant no. 196181; MariClim project, grant no. 165112). J.S. was supported by a Graduate Student Research Award from the North Pacific Research Board. J.S. and C.B. received support by Arctic Field Grants from the Svalbard Science Forum.
References
- 1.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 2.Morris WF, et al. 2008. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89, 19–25 (doi:10.1890/07-0774.1) [DOI] [PubMed] [Google Scholar]
- 3.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 4.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126 (doi:10.1146/annurev.ecolsys.32.081501.114006) [Google Scholar]
- 5.Gaillard JM, Pontier D, Allaine D, Lebreton JD, Trouvilliez J, Clobert J. 1989. An analysis of demographic tactics in birds and mammals. Oikos 56, 59–76 (doi:10.2307/3566088) [Google Scholar]
- 6.Stearns SC. 1977. The evolution of life history traits: a critique of the theory and a review of the data. Annu. Rev. Ecol. Syst. 8, 145–171 (doi:10.1146/annurev.es.08.110177.001045) [Google Scholar]
- 7.Williams JB, Miller RA, Harper JM, Wiersma P. 2010. Functional linkages for the pace of life, life-history, and environment in birds. Integr. Comp. Biol. 50, 855–868 (doi:10.1093/Icb/Icq024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara JM, Houston AI. 1996. State-dependent life histories. Nature 380, 215–221 (doi:10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 9.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- 10.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Careau V, Garland T. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiol. Biochem. Zool. 85, 543–571 (doi:10.1086/666970) [DOI] [PubMed] [Google Scholar]
- 12.Lahann P, Dausmann KH. 2011. Live fast, die young: flexibility of life history traits in the fat-tailed dwarf lemur (Cheirogaleus medius). Behav. Ecol. Sociobiol. 65, 381–390 (doi:10.1007/S00265-010-1055-4) [Google Scholar]
- 13.Palacios MG, Sparkman AM, Bronikowski AM. 2012. Corticosterone and pace of life in two life-history ecotypes of the garter snake Thamnophis elegans. Gen. Comp. Endocrinol. 175, 443–448 (doi:10.1016/J.Ygcen.2011.11.042) [DOI] [PubMed] [Google Scholar]
- 14.Lancaster LT, Hazard LC, Clobert J, Sinervo BR. 2008. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 21, 556–565 (doi:10.1111/J.1420-9101.2007.01478.X) [DOI] [PubMed] [Google Scholar]
- 15.Cheviron ZA, Brumfield RT. 2009. Migration–selection balance and local adaptation of mitochondrial haplotypes in rufous-collared sparrows (Zonotrichia capensis) along an elevational gradient. Evolution 63, 1593–1605 (doi:10.1111/J.1558-5646.2009.00644.X) [DOI] [PubMed] [Google Scholar]
- 16.Suryan RM, Saba VS, Wallace BP, Hatch SA, Frederiksen M, Wanless S. 2009. Environmental forcing on life history strategies: evidence for multi-trophic level responses at ocean basin scales. Prog. Oceanogr. 81, 214–222 (doi:10.1016/j.pocean.2009.04.012) [Google Scholar]
- 17.Chardine JW. 2002. Geographic variation in the wingtip patterns of black-legged kittiwakes. Condor 104, 687–693 (doi:10.1650/0010-5422(2002)104[0687:GVITWP]2.0.CO;2) [Google Scholar]
- 18.Mulard H, Aubin T, White JF, Wagner RH, Danchin E. 2009. Voice variance may signify ongoing divergence among black-legged kittiwake populations. Biol. J. Linn. Soc. 97, 289–297 (doi:10.1111/j.1095-8312.2009.01198.x) [Google Scholar]
- 19.McCoy KD, Boulinier T, Tirard C. 2005. Comparative host-parasite population structures: disentangling prospecting and dispersal in the black-legged kittiwake Rissa tridactyla. Mol. Ecol. 14, 2825–2838 (doi:10.1111/j.1365-294X.2005.02631.x) [DOI] [PubMed] [Google Scholar]
- 20.Robinson RA, Clark JA.2012. The online ringing report: bird ringing in Britain & Ireland in 2011. Thetford, UK: BTO. http://www.bto.org/ringing-report .
- 21.Coulson JC. 2002. Why do adult kittiwakes survive so long but breed so poorly in the Pacific? J. Avian Biol. 33, 111–112 (doi:10.1034/j.1600-048X.2002.t01-1-330201.x) [Google Scholar]
- 22.Hatch SA, Roberts BD, Fadely BS. 1993. Adult survival of black-legged kittiwakes Rissa tridactyla in a Pacific colony. Ibis 135, 247–254 (doi:10.1111/j.1474-919X.1993.tb02841.x) [Google Scholar]
- 23.Frederiksen M, Harris MP, Wanless S. 2005. Inter-population variation in demographic parameters: a neglected subject? Oikos 111, 209–214 (doi:10.1111/j.0030-1299.2005.13746.x) [Google Scholar]
- 24.Golet GH, Irons DB, Estes JA. 1998. Survival costs of chick rearing in black-legged kittiwakes. J. Anim. Ecol. 67, 827–841 (doi:10.1046/j.1365-2656.1998.00233.x) [Google Scholar]
- 25.Finch C, Rose M. 1995. Hormones and the physiological architecture of life history evolution. Q. Rev. Biol. 70, 1–52 (doi:10.1086/418864) [DOI] [PubMed] [Google Scholar]
- 26.Miles DB, Sinervo B, Hazard LC, Svensson EI, Costa D. 2007. Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Funct. Ecol. 21, 653–665 (doi:10.1111/j.1365-2435.2007.01304.x) [Google Scholar]
- 27.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212 (doi:10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 29.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 30.Kitaysky AS, Piatt JF, Hatch SA, Kitaiskaia EV, Benowitz-Fredericks ZM, Shultz MT, Wingfield JC. 2010. Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct. Ecol. 24, 625–637 (doi:10.1111/j.1365-2435.2009.01679.x) [Google Scholar]
- 31.Kitaysky AS, Wingfield JC, Piatt JF. 2001. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 12, 619–625 (doi:10.1093/beheco/12.5.619) [Google Scholar]
- 32.Friesen VL, Burg TM, McCoy KD. 2007. Mechanisms of population differentiation in seabirds. Mol. Ecol. 16, 1765–1785 (doi:10.1111/j.1365-294X.2006.03197.x) [DOI] [PubMed] [Google Scholar]
- 33.Angelier F, Clement-Chastel C, Welcker J, Gabrielsen GW, Chastel O. 2009. How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct. Ecol. 23, 784–793 (doi:10.1111/j.1365-2435.2009.01545.x) [Google Scholar]
- 34.Angelier F, Clement-Chastel C, Gabrielsen GW, Chastel O. 2007. Corticosterone and time-activity budget: an experiment with black-legged kittiwakes. Horm. Behav. 52, 482–491 (doi:10.1016/j.yhbeh.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 35.Erikstad KE, Sandvik H, Fauchald P, Tveraa T. 2009. Short- and long-term consequences of reproductive decisions: an experimental study in the puffin. Ecology 90, 3197–3208 (doi:10.1890/08-1778.1) [DOI] [PubMed] [Google Scholar]
- 36.Kitaysky AS, Kitaiskaia E, Piatt J, Wingfield JC. 2003. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 43, 140–149 (doi:10.1016/S0018-506x(02)00030-2) [DOI] [PubMed] [Google Scholar]
- 37.Goutte A, Angelier F, Welcker J, Moe B, Clement-Chastel C, Gabrielsen GW, Bech C, Chastel O. 2010. Long-term survival effect of corticosterone manipulation in black-legged kittiwakes. Gen. Comp. Endocrinol. 167, 246–251 (doi:10.1016/j.ygcen.2010.03.018) [DOI] [PubMed] [Google Scholar]
- 38.Kitaysky AS, Piatt JF, Wingfield JC. 2007. Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352, 245–258 (doi:10.3354/meps07074) [Google Scholar]
- 39.Gill VA, Hatch SA. 2002. Components of productivity in black-legged kittiwakes Rissa tridactyla: response to supplemental feeding. J. Avian Biol. 33, 113–126 (doi:10.1034/j.1600-048X.2002.330201.x) [Google Scholar]
- 40.Lanctot RB, Hatch SA, Gill VA, Eens M. 2003. Are corticosterone levels a good indicator of food availability and reproductive performance in a kittiwake colony? Horm. Behav. 43, 489–502 (doi:10.1016/S0018-506X(03)00030-8) [DOI] [PubMed] [Google Scholar]
- 41.Schultner J, Kitaysky AS, Welcker J, Hatch SA. 2013. Fat or lean: adjustment of endogenous energy stores to predictable and unpredictable changes in allostatic load. Funct. Ecol. 27, 45–55 (doi:10.1111/j.1365-2435.2012.02058.x) [Google Scholar]
- 42.Gill VA, Hatch SA, Lanctot RB. 2002. Sensitivity of breeding parameters to food supply in black-legged kittiwakes Rissa tridactyla. Ibis 144, 268–283 (doi:10.1046/j.1474-919X.2002.00043.x) [Google Scholar]
- 43.Green AJ. 2001. Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82, 1473–1483 (doi:10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2) [Google Scholar]
- 44.Kitaysky AS, Piatt JF, Wingfield JC, Romano M. 1999. The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B 169, 303–310 (doi:10.1007/s003600050225) [Google Scholar]
- 45.Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. 2001. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B 171, 701–709 (doi:10.1007/s003600100230) [DOI] [PubMed] [Google Scholar]
- 46.Brewer JH, O'Reilly KM, Buck CL. 2008. Effect of investigator disturbance on corticosterone concentrations of black-legged kittiwake chicks. J. Field Ornithol. 79, 391–398 (doi:10.1111/J.1557-9263.2008.00187.X) [Google Scholar]
- 47.R Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 48.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 49.Goutte A, Clement-Chastel C, Moe B, Bech C, Gabrielsen GW, Chastel O. 2011. Experimentally reduced corticosterone release promotes early breeding in black-legged kittiwakes. J. Exp. Biol. 214, 2005–2013 (doi:10.1242/Jeb.051979) [DOI] [PubMed] [Google Scholar]
- 50.Shultz MT. 2007. Stress physiology in breeding seabirds: coping with a variable food supply. MSc thesis, University of Alaska, Fairbanks, AK [Google Scholar]
- 51.Chastel O, Lacroix A, Weimerskirch H, Gabrielsen GW. 2005. Modulation of prolactin but not corticosterone responses to stress in relation to parental effort in a long-lived bird. Horm. Behav. 47, 459–466 (doi:10.1016/j.yhbeh.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 52.Goutte A, Angelier F, Chastel CC, Trouve C, Moe B, Bech C, Gabrielsen GW, Chastel O. 2010. Stress and the timing of breeding: glucocorticoid-luteinizing hormones relationships in an arctic seabird. Gen. Comp. Endocrinol. 169, 108–116 (doi:10.1016/j.ygcen.2010.07.016) [DOI] [PubMed] [Google Scholar]
- 53.Buck CL, O'Reilly KM, Kildaw SD. 2007. Interannual variability of black-legged kittiwake productivity is reflected in baseline plasma corticosterone. Gen. Comp. Endocrinol. 150, 430–436 (doi:10.1016/j.ygcen.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 54.Bokony V, Lendvai AZ, Liker A, Angelier F, Wingfield JC, Chastel O. 2009. Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598 (doi:10.1086/597610) [DOI] [PubMed] [Google Scholar]
- 55.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 56.Boonstra R, McColl CJ. 2000. Contrasting stress response of male arctic ground squirrels and red squirrels. J. Exp. Zool. 286, 390–404 (doi:10.1002/(SICI)1097-010X(20000301)286:4<390::AID-JEZ7>3.0.CO;2-O) [PubMed] [Google Scholar]
- 57.Breuner CW, Orchinik M. 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175, 99–112 (doi:10.1677/joe.0.1750099) [DOI] [PubMed] [Google Scholar]
- 58.Spee M, Marchal L, Lazin D, Le Maho Y, Chastel O, Beaulieu M, Raclot T. 2011. Exogenous corticosterone and nest abandonment: a study in a long-lived bird, the Adelie penguin. Horm. Behav. 60, 362–370 (doi:10.1016/J.Yhbeh.2011.07.003) [DOI] [PubMed] [Google Scholar]
- 59.Hatch SA. 2013. Kittiwake diets and chick production signal a 2008 regime shift in the Northeast Pacific. Mar. Ecol. Prog. Ser. 477, 271–284 (doi:10.3354/meps10161) [Google Scholar]
- 60.Frederiksen M, Edwards M, Richardson AJ, Halliday NC, Wanless S. 2006. From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 75, 1259–1268 (doi:10.1111/j.1365-2656.2006.01148.x) [DOI] [PubMed] [Google Scholar]
- 61.Harley CDG, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (doi:10.1111/j.1461-0248.2005.00871.x) [DOI] [PubMed] [Google Scholar]
- 62.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 63.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 64.Vincenzi S, Hatch S, Mangel M, Kitaysky A. 2013. Food availability affects onset of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20130554. (doi:10.1098/rspb.2013.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]