Key Points
Ovariectomy expands short-term hemopoietic stem and progenitor cells and improves engraftment and host survival after bone marrow transplantation.
T cells are required for ovariectomy to expand hemopoietic stem and progenitor cells.
Abstract
Estrogen deficiency expands hemopoietic stem and progenitor cells (HSPCs) and mature blood lineages, but the involved mechanism and the affected HSPC populations are mostly unknown. Here we show that ovariectomy (ovx) expands short-term HSPCs (ST-HSPCs) and improves blood cell engraftment and host survival after bone marrow (BM) transplantation through a dual role of the T-cell costimulatory molecule CD40 ligand (CD40L). This surface receptor is required for ovx to stimulate T-cell production of Wnt10b, a Wnt ligand that activates Wnt signaling in HSPCs and stromal cells (SCs). Moreover, CD40L is required for ovx to increase SC production of the hemopoietic cytokines interleukin (IL)-6, IL-7, and granulocyte macrophage–colony-stimulating factor. Attesting to the relevance of CD40L and Wnt10b, ovx fails to expand ST-HSPCs in CD40L-null mice and in animals lacking global or T-cell expression of Wnt10b. In summary, T cells expressed CD40L, and the resulting increased production of Wnt10b and hemopoietic cytokines by T cells and SCs, respectively, plays a pivotal role in the mechanism by which ovx regulates hemopoiesis. The data suggest that antiestrogens may represent pharmacological targets to improve ST-HSPC function through activation of the microenvironment.
Introduction
Estrogen (E) is known to decrease early hemopoietic precursors via an E receptor α–dependent mechanism1 and the number of hemopoietic stem and progenitor cells (HSPCs) that migrate to the thymus.2 Conversely, menopause causes an expansion of hemopoietic precursors.1,3,4 The acute effects of menopause are modeled by ovariectomy (ovx) that, like natural menopause, causes bone loss5 and promotes hemopoiesis.3,4 Effects of ovx that are relevant for hemopoiesis are an expansion of the stromal cell (SC) pool6 and increased SC production of hemopoietic cytokines such as interleukin (IL)-6, IL-7, and macrophage–colony-stimulating factor (M-CSF).3,7,8
The mechanism by which ovx regulates hemopoietic cells remains largely undetermined. In addition, it is presently unknown whether ovx regulates HSPC function. Because osteoclasts derived by hemopoietic cells and osteoblasts are part of the HSPC niche, E may regulate bone turnover and hemopoiesis through the same mechanisms. E prevents bone loss by enhancing osteoclast apoptosis,9 blocking reactive oxygen species production in the bone marrow (BM),10 and blunting osteoblast production of osteoclastogenic cytokines.11 In addition, T lymphocytes play a pivotal role in the mechanism of action of E in bone, as T cell–deficient mice are protected against ovx-induced bone loss.12 One mechanism involved is an ovx-induced expression of the costimulatory molecule CD40 ligand (CD40L) by T cells. This surface receptor is required for ovx to expand SCs and osteoblasts, regulate SC production of the osteoclastogenic factors M-CSF, receptor activator of NF-κB ligand, and osteoprotegerin, and upregulate osteoclast formation.13 CD40L exerts its effects by binding to CD4014 and several integrins.15,16 CD40 is expressed on antigen-presenting cells,17 hemopoietic progenitors,18 and osteoblasts.19 Because of its regulatory effects on osteoblasts11,13 and hemopoietic progenitors,18 T cell–expressed CD40L may play a role in the stimulatory effects of ovx on hemopoiesis.
Another intracellular system that regulates HSPC expansion is Wnt signaling.20,21 Its activation in SC and HSPCs by Wnt ligands produced in the BM microenvironment is required for HSPC expansion and survival. E blunts the expression of Wnt7a in the uterus,22 suggesting that E may regulate hemopoiesis by repressing Wnt activation. Because T cells secrete Wnt10b and T cell–produced Wnt10b plays a pivotal role in the stimulatory effects of parathyroid hormone (PTH) on HSPCs,23 an additional mechanism by which E regulate hemopoiesis might involve suppression of Wnt10b production by T cells.
To investigate the hypothesis that ovx initiates changes in HSPC function through T cell–mediated mechanisms, we evaluated the role of T cells and T cell–expressed CD40L and Wnt10b on the expansion of hemopoietic cells induced by ovx. We show that T cell–expressed CD40L induces T-cell production of Wnt10b. The resulting activation of Wnt signaling in SCs and HSPCs, along with a CD40L-driven increase in the production of hemopoietic cytokines by SCs, plays a pivotal role in the mechanism by which ovx regulates hemopoiesis and promotes survival after BM transplantation.
Methods
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. Additional information is provided as supplemental Methods on the Blood Web site. Information on T-cell transfers, cell purifications, in vitro SCs and T-cell cocultures, flow cytometry, cell sorting, transplantation experiments, real-time reverse transcription-polymerase chain reaction, cytokine assays, and statistical analysis is provided in the supplemental Methods. Procedures and assays were carried out as previously described.23
Results
Ovx expands short-term HSPC/multipotent progenitors and mature hemopoietic lineages through T cells
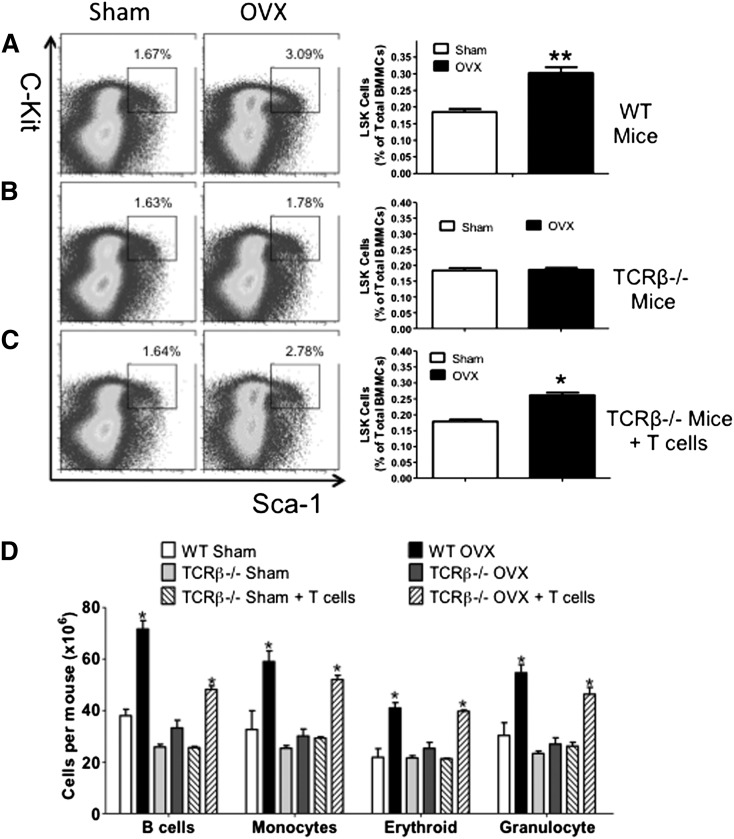
Time course studies revealed that ovx increased the fraction of BM HSPCs, defined as Lin−Kit+Sca-1+ (LSK) cells by ∼1.5-fold at 2, 4, and 8 weeks (supplemental Figure 1A). LSKs include long-term HSPCs (LT-HSPCs), short-term HSPCs (ST-HSPCs), and multipotent precursors (MPPs). LT-HSPCs are CD150+CD48−, whereas ST-HSPCs/MPPs are CD150−CD48−.24,25 We found that ovx increased the number of ST-HSPC/MPPs (supplemental Figure 1B), B-lineage cells (B220+), monocytes (CD11b+), erythroid cells (TER 119+), and granulocytes (Ly6G/Ly6C+) (supplemental Figure 1C) by two- to threefold at weeks 2, 4, and 8. By contrast, ovx did not increase the frequency of LT-HSPCs at any time.
To investigate the role of T cells in the effects of ovx on HSPCs, wild-type (WT) mice and congenic TCRβ−/− mice, a strain devoid of αβ T cells, were euthanized 2 weeks after ovx or sham operation. Controls included TCRβ−/− mice subjected to adoptive transfer of WT T cells 3 weeks before surgery, a procedure that is followed by the expansion of the donor T cells.13,26,27 The expansion of adoptively transferred T cells was verified by flow cytometry (supplemental Figure 2). Ovx increased the fraction of BM LSK cells by ∼1.5-fold in WT mice and TCRβ−/− mice reconstituted with T cells, whereas it had no effect on LSK population in T cell–deficient TCRβ−/− mice (Figure 1A-C). Moreover, ovx increased the number of B-lineage cells, monocytes, erythroid cells, and granulocytes by two- to threefold in the BM of WT mice and TCRβ−/− mice reconstituted with T cells (Figure 1D). Ovx had no effects in any of these lineages in BM from congenic TCRβ−/− mice.
Figure 1.
Effect of ovx on HSPC expansion in T cell–replete and T cell–deficient mice. (A-C) Effects of ovx on the relative frequency of LSK cells in WT mice, TCRβKO mice, and TCRβKO mice previously subjected to adoptive transfer of T cells. Lin− cells were gated and analyzed for Sca-1 and c-Kit expression using isotype control settings. (Left) Representative flow cytometric dot plots from 1 mouse per group. The black box delineates c-Kit+ Sca-1+ cells. Parent population is Lin−. Data are expressed as percentage of total Lin− cells. (Right) Mean ± standard error of the mean (SEM) for each group. Data are expressed as percentage of total BM mononucleated cells (BMMCs). (D) Effect of ovx on the number of BM B cells, monocytes, erythroid cells, and granulocytes. n = 10 mice per group. *P < .05 and **P < .01 compared with the corresponding sham-operated group.
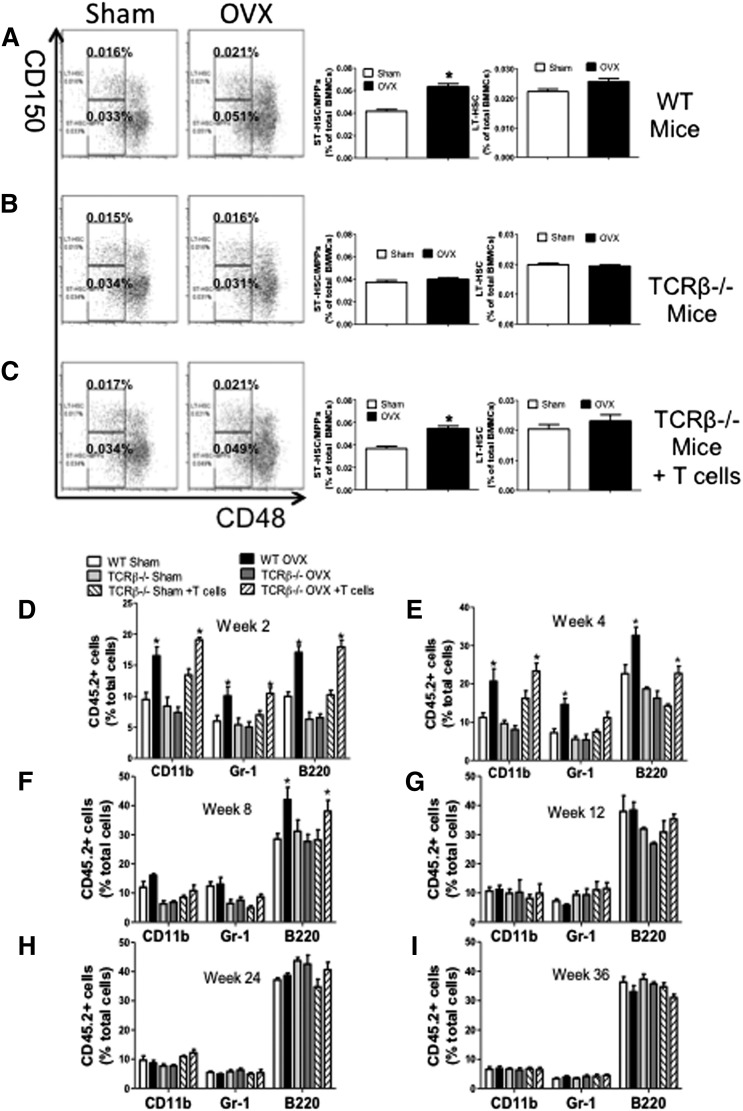
We also found that ovx increased the frequency of ST-HSPC/MPPs in BM samples from WT mice and TCRβ−/− mice subjected to adoptive transfer of WT T cells (Figure 2A,C), whereas it had no effects in BM samples from TCRβ−/− mice (Figure 2B). Ovx did not increase the frequency of LT-HSPCs in any group of mice. Thus, ovx expands HSPCs with limited self-renewal through a mechanism involving T cells.
Figure 2.
Effect (mean + SEM) of ovx on the expansion of ST-HSPCs and LT-HSPCs in T cell–replete and T cell–deficient mice. (A-C) Effects of ovx on the relative frequency of CD150−CD48− LSK cells (ST-HSPCs/MPPs) and CD150+CD48− LSK cells (LT-HSPCs) in WT, TCRβKO, and TCRβKO mice previously reconstituted with T cells. (Left) Representative flow cytometric dot plots from 1 mouse per group using the signalling lymphocyte activation molecule receptors CD150 and CD48. Parent population is Lin−Sca1+c-Kit+. The upper boxes delineate LT-HSPCs. The lower boxes delineate ST-HSPCs + MPPS. (Right) Mean + SEM for each group. Data are expressed as percentage of BMMCs. n = 10 mice per group. (D-I) Effect of ovx on peripheral blood cell expansion after primary competitive repopulation. The panels show the percentage of CD45.2+ myeloid cells (CD11b+), granulocytic cells (GR-1+), and B lineage cells (B220+) in the peripheral blood of lethally irradiated WT recipient mice that received CD45.2+ BM donor cells mixed in a 1:2 ratio with CD45.1+ competitor BM cells. CD45.2+ BM cells were obtained from WT, TCRβKO, and reconstituted TCRβKO mice subjected to sham operation or ovx 2 weeks earlier. CD45.1+ BM cells were obtained from intact WT mice. *P < .05 compared with the corresponding sham-operated group.
Ovx increases short-term but not long-term hemopoietic engraftment through T cells
Whether T cells are required for ovx to expand ST-HSPCs/MPPs was further investigated using competitive repopulation assays.28 CD45.2+ WT mice, TCRβ−/− mice, and TCRβ−/− previously reconstituted with WT CD45.2+ T cells were sham operated or ovx. BM was harvested 2 weeks later, mixed with BM from intact CD45.1+ WT mice at a ratio of 1:2 (donor:competitor), and injected into lethally irradiated intact CD45.1+ recipient mice. The peripheral blood of recipient mice was then analyzed by flow cytometry every 2 to 4 weeks up to 36 weeks. Recipient mice transplanted with BM from ovx WT or reconstituted mice had greater reconstitution in the myeloid (CD11b+), granulocytic (Gr-1+), and B-cell (B220+) compartments at 2 and 4 weeks after transplantation compared with recipients transplanted with BM from sham-operated donors (Figure 2D-E). However, the engraftment advantage conferred by ovx ceased at week 8 for the myeloid and granulocytic lineages (Figure 2F) and at 12 weeks for the B-cell lineage (Figure 2G). Recipients of BM from ovx TCRβ−/− mice did not demonstrate greater reconstitution in any lineage at all time points (Figure 2D-I). These findings demonstrate that T cells are required for ovx to expand ST-HSPCs.
Expansion of the ST-HSPC pool may be associated with depletion of the LT-HSPC pool.29 To investigate this matter, secondary transplantation was performed 36 weeks after the primary transplantation. At this time point, the frequency of donor-derived LSK cells (CD45.2+ LSKs) in the BM of all primary recipients was the same in all groups (supplemental Figure 3).
Engraftment in the secondary recipients was measured at 10 weeks, a time point when ovx no longer induced engraftment superiority in the primary recipients. These studies demonstrated equal reconstitution in all compartments in all groups (supplemental Figure 4), indicating that the accelerated short-term engraftment induced by ovx through T cells did not hamper long-term repopulation.
The engraftment superiority induced by ovx may be due to an increase in the number of ST-HSPCs or qualitative changes such as increasing in homing or engraftment efficiency. To investigate this issue, we transplanted 500 or 750 ST-HSPCs sorted from intact mice. We found superior engraftment with 750 ST-HSPCs, demonstrating the relevance of the quantitative changes induced by ovx (supplemental Figure 5A-C). Next we assessed the engraftment achieved with 500 and 750 ST-HSPCs from ovx donors in comparison with 500 ST-HSPCs from sham donors. When equal numbers of ST-HSPCs were transplanted, we found an engraftment advantage with ST-HSPCs from ovx donors (supplemental Figure 5D-F), demonstrating that ovx alters the quality of ST-HSPCs. Comparisons of 750 ST-HSPCs from ovx mice and 500 ST-HSPCs from sham donors revealed an even greater engraftment with 750 ovx ST-HSPCs, confirming that both quantitative and qualitative changes in ST-HSCs account for the overall effect of ovx.
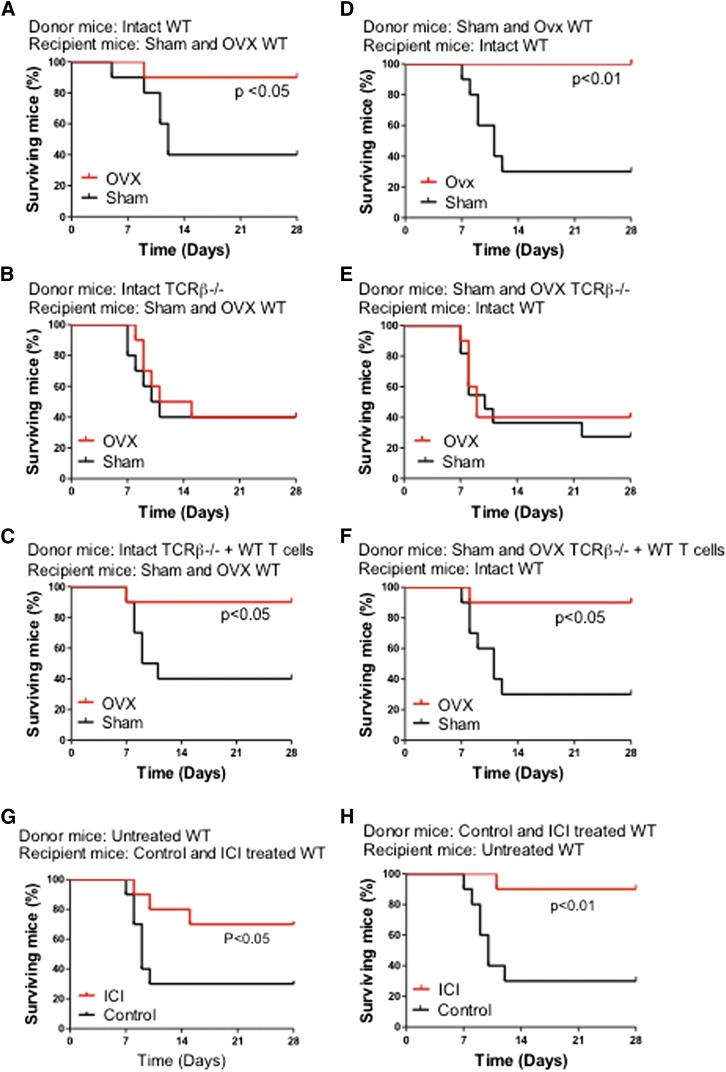
Ovx improves survival after BM transplantation through T cells
To determine the relevance of the effects of ovx on HSPCs, we assessed the survival of mice undergoing myeloablative BM transplantation using a limiting number of donor cells derived from T cell–replete and T cell–null mice. Thus, 8- to 10-week-old WT mice were lethally irradiated and transplanted with BM cells harvested from WT mice, TCRβ−/− mice, and TCRβ−/− mice subjected to adoptive transfer of WT T cells 3 weeks earlier. In a first experiment, donor mice were left intact, whereas recipient mice were either ovx or sham operated 2 weeks before the day of the BM transplantation (Figure 3A-C). In a second experiment, donor mice were either ovx or sham operated 2 weeks before the BM harvest, whereas recipient mice were left intact (Figure 3D-F). In both experiments, survival at 4 weeks of sham-operated mice was 30% to 40% regardless of whether the donor mice were T cell replete or T cell deficient. Ovx of either donor mice or recipient mice increased survival by two- to threefold at 4 weeks of recipients transplanted with T cell–replete BM. Ovx had no effect on the survival of mice transplanted with BM from T cell–null mice. Thus, in a setting of therapeutic need, ovx affects HSPCs expansion only in the presence of T cells. The increase in survival induced by ovx was mirrored by an increase in the relative frequency of LSKs in the BM of recipient mice (supplemental Figure 6).
Figure 3.
Effects of ovx and of the antiestrogen ICI 182780 on the survival of lethally irradiated WT mice transplanted with a limiting number of BM cells derived from WT mice, TCRβ−/− mice, and TCRβ−/− mice previously subjected to adoptive transfer of T cells. (A-C) Donor mice were intact. Recipient mice were sham operated or ovx 2 weeks before BM transplantation. (D-F) Donor mice were sham operated or ovx 2 weeks before BM transplantation. Recipient mice were intact. (G) Donor mice were untreated. Recipient mice were treated with vehicle or ICI182780 (100 μg/mouse subcutaneously, twice a week for 4 weeks) starting at the time of transplantation. (H) Donor mice were treated with vehicle or ICI182780. Recipient mice were untreated. n = 10 per group.
To further investigate whether E deprivation improves survival after BM transplantation, mice were treated with the E antagonist ICI 182780 (100 μg/mouse subcutaneous, twice a week for 4 weeks). BM was then harvested and transplanted into lethally irradiated untreated recipients. In a second experiment, BM was harvested from untreated donors and transplanted into recipient mice treated with ICI 182780 for 4 weeks prior to lethal irradiation and BM transplantation. In both experiments, survival at 4 weeks of control mice was ∼30%. Treatment with ICI 182780 of either donor mice or recipient mice increased survival by approximately threefold of recipient mice at 4 weeks (Figure 3G-H). The increase in survival induced by ICI 182780 was mirrored by an increase in the relative frequency of LSKs in the BM of recipient mice (supplemental Figure 7).
To investigate whether the increase in survival induced by ovx results from quantitative and/or qualitative changes in ST-HSPCs, we transplanted 300 or 450 ST-HSPCs from intact mice and found higher survival in mice transplanted with 450 ST-HSPCs (supplemental Figure 8A). Next we transplanted 300 ST-HSPCs from sham-operated or ovx donors and found a higher survival rate in recipients of ST-HSPCs from ovx mice (supplemental Figure 8B). However, an even greater survival advantage was conferred by the transplantation of 450 ST-HSPCs from ovx mice as opposed to 300 ST-HSPCs from sham-operated mice (supplemental Figure 8C). These data indicate that ovx improves survival through quantitative and qualitative changes in ST-HSPCs.
T cells regulate hemopoietic cells and hemopoietic cytokines through CD40L
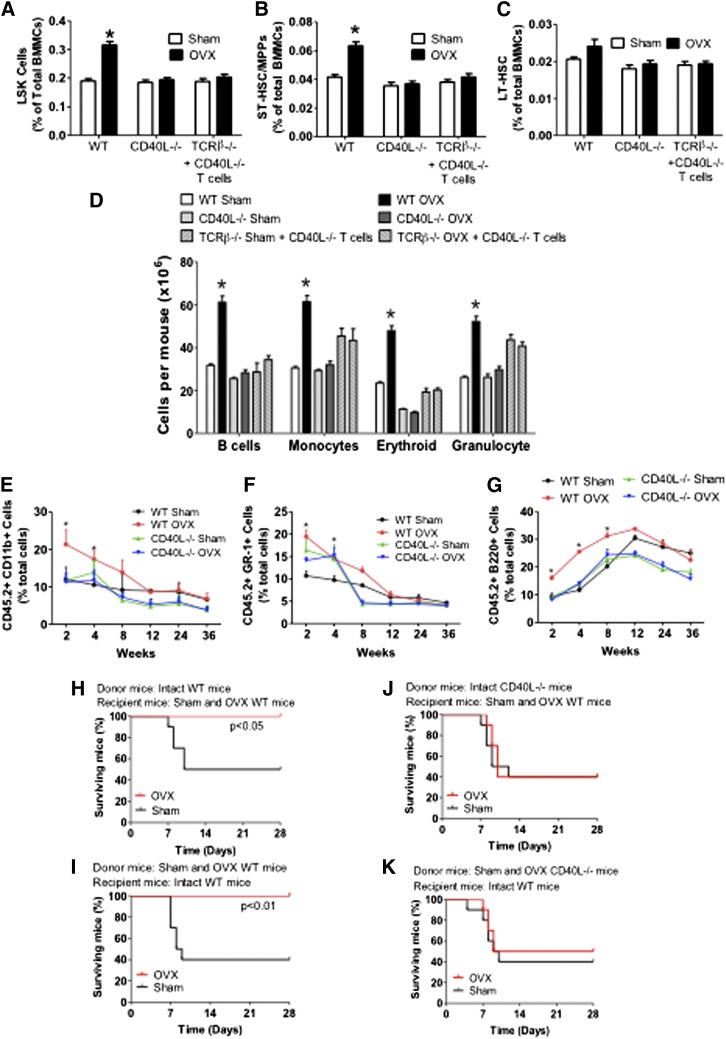
Investigations of the effects of ovx on the expansion of HSCPs in CD40L−/− mice and TCRβ−/− mice previously reconstituted with CD40L−/− T cells revealed that ovx increased the fraction of LSKs by ∼1.5-fold in WT mice, whereas it had no effect on LSKs in CD40L−/− mice and TCRβ−/− mice previously reconstituted with CD40L−/− T cells (Figure 4A).
Figure 4.
Analysis of the effects (mean + SEM) of ovx in WT and CD40L−/− mice and TCRβ−/− mice previously reconstituted with CD40L−/− T cells. (A) Effects of ovx on the relative frequency of BM LSK cells. (B-C) Effect of ovx on the relative frequency of ST-HSPCs/MPP and LT-HSPCs. (D) Effect of ovx on the number of BM B cells, monocytes, erythroid cells, and granulocytes. (E-G) Effect of ovx on peripheral blood cell expansion after primary competitive repopulation. In these experiments, CD45.2 CD40L−/− and control WT mice were killed after 2 weeks of ovx or sham operation. BM was then mixed with BM from intact CD45.1+ WT mice at a ratio of 1:2 (donor/ competitor) and injected into lethally irradiated CD45.1+ recipient mice. The percentage of CD11b+, GR-1+, and B220+ cells in the peripheral blood of lethally irradiated WT recipient mice are shown. Recipient mice received CD45.2+ BM donor cells from WT and CD40L−/− mice previously subjected to sham operation or ovx mixed in a 1:2 ratio with CD45.1+ competitor BM cells from intact WT mice. (H-K) Survival analysis of WT mice transplanted with limiting number of BM cells derived from WT and CD40L−/− mice. (H-J) Donor mice were intact WT or CD40L−/− mice. Recipient mice were WT mice subjected to sham operation or ovx 2 weeks before the BM transplantation. (I-K) Donor mice were sham operated or ovx 2 weeks before the BM transplantation. Recipient mice were intact WT mice. n = 10 per group. *P < .05 compared with the corresponding sham-operated group.
Analysis of the signalling lymphocyte activation molecule markers CD48 and CD150 revealed that ovx expanded ST-HSPCs/MPPs in WT mice but not in CD40L−/− mice and TCRβ−/− mice previously reconstituted with CD40L−/− T cells (Figure 4B). Ovx did not increase the frequency of LT-HSPC in all mice (Figure 4C). Moreover, ovx increased the number of B-lineage cells (B220+), monocytes (CD11b+), erythroid cells (TER 119+), and granulocytes (Ly6G/Ly6C+) by ∼1.5- to 2.0-fold in control mice but not in CD40L−/− mice and TCRβ−/− mice previously reconstituted with CD40L−/− T cells (Figure 4D).
To confirm that CD40L is required for ovx to improve the short-term repopulation, we conducted additional competitive repopulation experiments using CD40L−/− mice. The peripheral blood of recipient mice that were transplanted with BM from ovx control WT mice demonstrated greater reconstitution in the myeloid (CD11b+) and granulocytic (Gr-1+) compartments at 2 and 4 weeks after transplantation compared with recipients transplanted with BM from sham-operated donors (Figure 4E-F). However, the engraftment advantage conferred by ovx ceased at week 8 after transplantation. Transplantation of BM from ovx control WT mice increased the engraftment of B220+ cells for 8 weeks (Figure 4G). The engraftment superiority conferred by ovx was no longer significant starting at 12 weeks after transplantation. By contrast, ovx did not confer engraftment superiority at any time point in mice transplanted with BM from CD40L−/− mice, thus demonstrating a requirement for CD40L expressed on T cells.
Next we assessed the effect of ovx on the survival of mice transplanted with BM from WT and CD40L−/− mice. In 1 experiment, donor mice were left intact while recipient mice were ovx or sham-operated 2 weeks before the day of BM transplantation (Figure 4H,J). In another, donor mice were ovx or sham-operated 2 weeks before the BM harvest while recipient mice were left intact (Figure 4I,K). Ovx of either donor or recipient mice increased the survival at 4 weeks of recipients transplanted with control BM by approximately twofold. However, ovx had no effect on the survival of mice transplanted with BM from CD40L−/− mice.
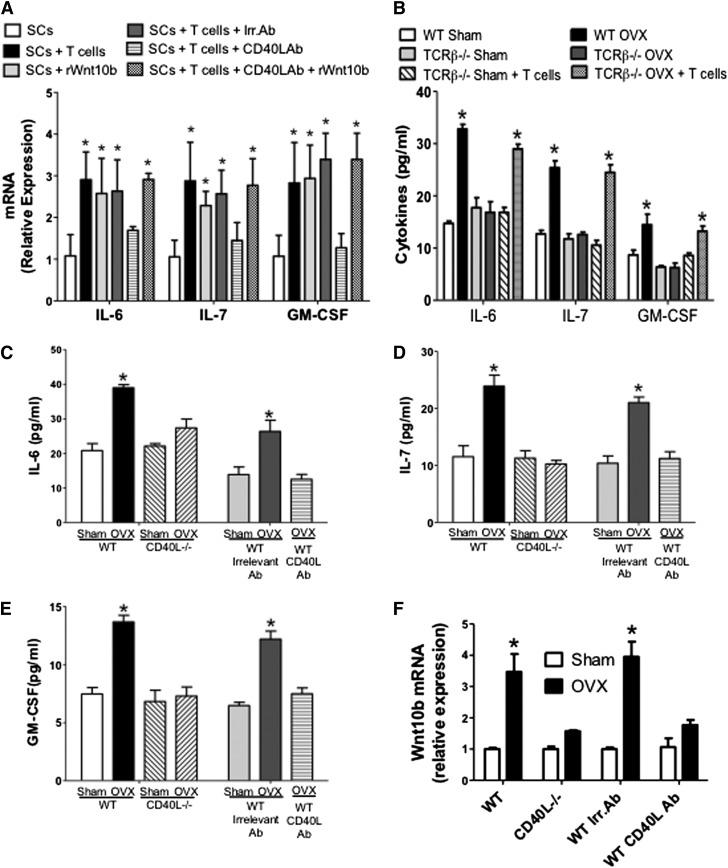
Next we investigated whether ovx regulates SCs and their production of hemopoietic factors through CD40L expressed on T cells. First, SCs from intact mice were cocultured for 7 days with T cells or Wnt10b. We found that incubation with T cells or rWnt10b increased the SC mRNA levels of IL-6, IL-7, and granulocyte-M-CSF (GM-CSF) by two- to threefold (Figure 5A). Addition of anti-CD40L monoclonal antibody (mAb), but not irrelevant mAb, blocked the stimulatory effects of T cells, whereas it did not blunt the effect of rWnt10b. These findings suggest that T cells upregulate the SC production of hemopoietic factors through CD40L and that CD40L is upstream of Wnt10b. Second, we found that ovx increased the SC production of IL-6, IL-7, and GM-CSF by approximately twofold in WT mice and T cell–deficient mice previously reconstituted with WT T cells (Figure 5B), but not in T cell–deficient mice, thus demonstrating that ovx regulates SC cytokine production through T cells.
Figure 5.
Role of CD40L on the production of hemopoietic cytokines by SCs. (A) The SC expression of IL-6, IL-7, and GM-CSF mRNAs was measured in SCs cultured alone or in the presence of T cells, anti-CD40L Ab, or rWnt10b. (B) Levels of IL-6, IL-7, and GM-SCF in the culture media of SCs from sham-operated and ovx WT mice, TCRβKO mice, and TCRβKO mice previously subjected to adoptive transfer of WT T cells. (C-E) Levels of IL-6, IL-7, and GM-SCF as measured by enzyme-linked immunosorbent assay in the 48-hour culture media of SCs purified from sham operated and ovx WT and CD40L−/− mice. The first 4 bars to the left show data from WT and CD40L−/− mice. The last 3 bars to the right show data from WT mice treated with irrelevant Ab or MR-1 Ab. In these experiments, BM was cultured for 1 week, SCs were then purified, and cytokine was measured by enzyme-linked immunosorbent assay in the 48-hour culture media. (F) Effect of ovx on the mRNA expression of Wnt10b in BM T cells. The first 4 bars to the left show WT and CD40L−/− mice. The last 4 bars to the right show WT mice treated with irrelevant Ab or MR-1 Ab. *P < .05 compared with the corresponding sham-operated group.
Finally, we analyzed the effects of ovx on the production of IL-6, IL-7, and GM-CSF by SCs in CD40L−/− mice and WT mice treated with MR-1, an Ab that neutralizes CD40L in vivo.30 These experiments revealed that ovx increased the levels of IL-6 (Figure 5C), IL-7 (Figure 5D), and GM-CSF (Figure 5E) approximately twofold in the media of SCs from control mice but not in that from CD40L−/− mice and MR-1–treated WT mice.
T cells regulate HSPCs through Wnt10b
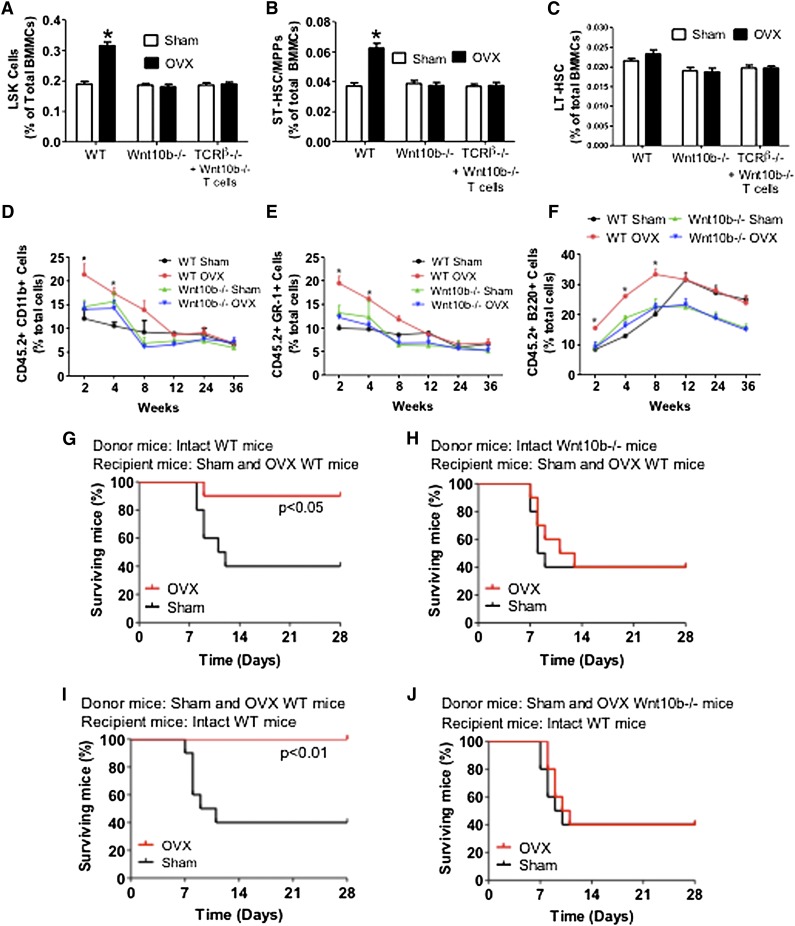
Because ovx increases T-cell activation13 and T-cell activation stimulates T cells to produce Wnt10b,31 we investigated whether ovx increases Wnt10b production by T cells and whether such an effect is mediated by CD40L. Thus, untreated WT mice, CD40L−/− mice, and WT mice treated with irrelevant Ab or the anti-CD40L Ab were sham operated or ovx and euthanized 2 weeks later. Analysis of T-cell mRNA expression revealed (Figure 5F) that Wnt10b mRNA level was increased by ovx in T cells from WT mice and WT mice treated with irrelevant mAb but not in T cells from CD40L−/− mice and WT mice treated with MR-1. Moreover, ovx increased the frequency of LSKs in the BM of WT mice by approximately twofold, whereas it had no effect in Wnt10b−/− mice and TCRβKO mice reconstituted with Wnt10b−/− T cells (Figure 6A). Ovx also expanded ST-HSPCs/MPPs in WT mice but not in Wnt10b−/− mice and TCRβKO mice reconstituted with Wnt10b−/− T cells (Figure 6B), thus demonstrating that ovx expands STHSPCs/MPPs through T cell–produced Wnt10b. Ovx did not cause the expansion of LT-HSPCs in all mice (Figure 6C). Moreover, ovx failed to expand B cells, monocytes, erythroid cells, and granulocytes in Wnt10−/− mice (supplemental Figure 9). Competitive repopulation assays revealed an increase in the engraftment of cells from ovx WT mice but not Wnt10b−/− mice at weeks 2, 4, and 8 compared with cells from sham-operated WT mice (Figure 6D-F), thus demonstrating that Wnt10b is required for ovx to expand ST-HSPCs.
Figure 6.
Analysis of the effects (mean + SEM) of ovx in WT and Wnt10b−/− mice. (A) Effects of ovx on the relative frequency of BM LSK cells. (B-C) Effect of ovx on the relative frequency of ST-HSPCs/MPP and LT-HSPCs. (D-F) Effect of ovx on peripheral blood cell expansion after primary competitive repopulation. The percentage of CD11b+, GR-1+, and B220+ cells in the peripheral blood of lethally irradiated WT recipient mice are shown. CD45.2+ WT mice and Wnt10b−/− mice were killed 2 weeks after ovx or sham operation. Their BM was then mixed with BM from intact CD45.1+ WT mice at a ratio of 1:2 and injected into lethally irradiated CD45.1+ host mice. (G-J) Survival analysis of WT mice transplanted with limiting number of BM cells derived from WT and Wnt10b−/− mice. (G-H) Donor mice were intact WT or Wnt10b−/− mice. Recipients were WT mice subjected to sham operation or ovx 2 weeks before transplantation. (I-J) Donor mice were WT mice subjected to sham operation or ovx 2 weeks before transplantation. Recipient mice were intact WT mice. n = 10 in each group. *P < .05 compared with the corresponding sham-operated group.
Next we assessed the effect of ovx on the survival after transplantation of BM derived from WT and Wnt10b−/− mice. First, donor mice were left intact, whereas recipient mice were either ovx or sham operated 2 weeks before starting the day of BM transplantation (Figure 6G-H). Second, donor mice were ovx or sham operated 2 weeks before the BM harvest, whereas recipient mice were left intact (Figure 6I-J). Ovx of either donor or recipient mice increased the survival at 4 weeks of recipients transplanted with WT BM by ∼2.5-fold, whereas ovx had no effect on the survival of mice transplanted with BM from Wnt10−/− mice. Thus, T cells and Wnt10b are required for ovx to activate Wnt signaling in HSPCs and increase survival after BM transplantation.
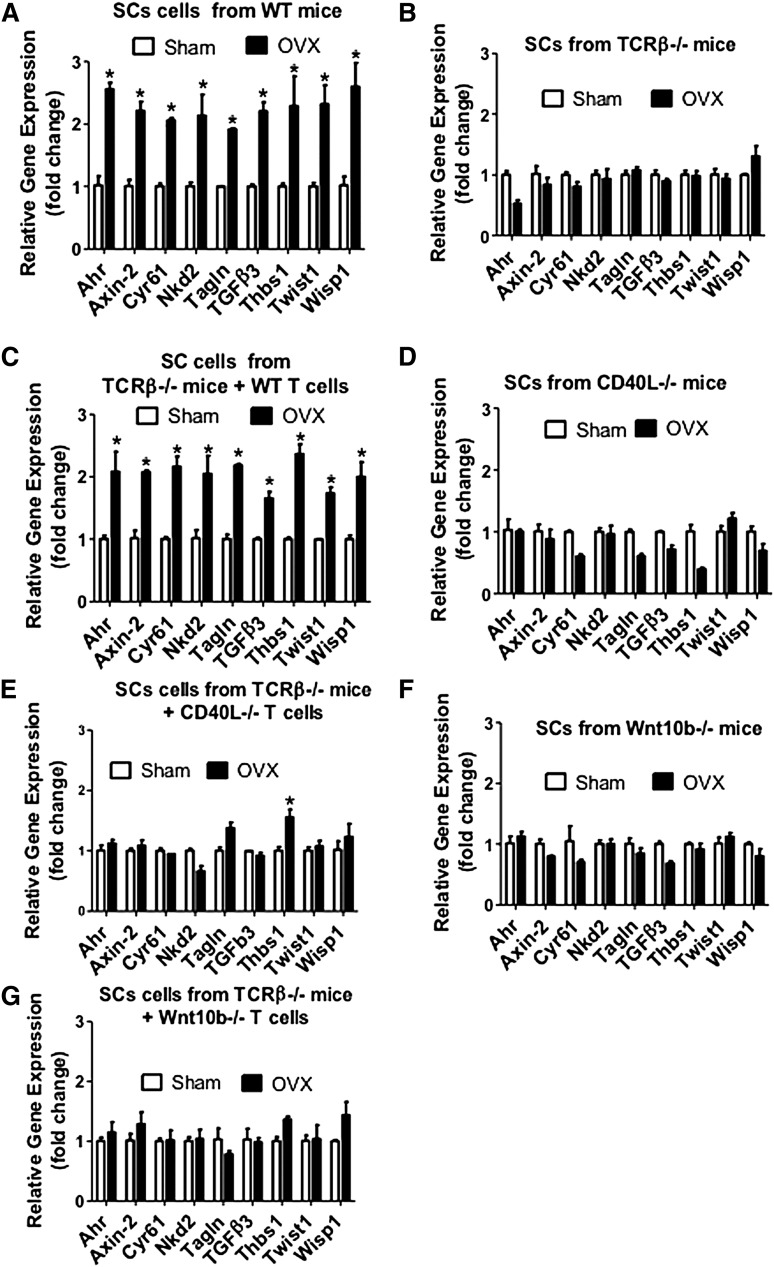
Activation of Wnt signaling in both SCs and HSPCs promotes HSPC expansion and self-renewal.20,21,32 Therefore, we sought to investigate whether ovx activates Wnt signaling in SCs and HSPCs through T cell–produced Wnt10b. Thus, we harvested SCs from ovx or sham-operated mice and analyzed mRNA expression of genes specifically upregulated by Wnt signaling.33 The analyzed genes were aryl-hydrocarbon receptor, axin2, cysteine-rich protein 61, naked cuticle 2 homolog, transgelin, transforming growth factor β3, thrombospondin 1, Twist gene homolog 1, and Wnt1 inducible signaling pathway protein 1. The levels of mRNA for these genes were all increased by ovx in SCs from T cell–replete mice and T cell–deficient mice previously subjected to transfer of WT T cells (Figure 7A,C). By contrast, ovx had no effect on SCs from T cell–deficient mice (Figure 7B), CD40L−/− mice (Figure 7D), TCRβ−/− mice previously reconstituted with CD40L−/− T cells (Figure 7E), Wnt10b-null mice (Figure 7F), and T cell–deficient mice reconstituted with Wnt10b−/− T cells (Figure 7G).
Figure 7.
Effect (mean + SEM) of ovx on the SC expression of mRNA of genes known to be up-regulated by Wnt signaling. BM harvested at death was cultured for 1 week. SCs were purified, and mRNA levels were determined by real-time reverse transcription-polymerase chain reaction. SCs were obtained from (A) WT mice, (B) TCRβKO mice, (C) TCRβKO mice previously subjected to adoptive transfer of WT T cells, (D) CD40L−/− mice, (E) TCRβ−/− mice previously reconstituted with CD40L−/− T cells, (F) Wnt10b−/− mice, and (G) TCRβKO mice previously subjected to adoptive transfer of Wnt10b−/− T cells. The Wnt-dependent genes analyzed were aryl-hydrocarbon receptor (Ahr), axin2, cystein-rich protein 61 (Cyr61), naked cuticle 2 homolog (Nkd2), transgelin (tagln), transforming growth factor β 3 (TGFβ3), thrombospondin 1 (Thbs1), Twist gene homolog 1 (Twst1), and Wnt1 inducible signaling pathway protein 1 (Wisp1). n = 5 mice per group. *P < .05 compared with the corresponding sham-operated group.
Analysis of HSPCs revealed that the levels of mRNA for the 9 tested genes were all increased by ovx in HSPCs from WT mice and T cell–deficient mice reconstituted with WT T cells (supplemental Figure 10). By contrast, ovx did not increase the expression of Wnt-dependent genes in HSPCs from TCRβ−/− mice, CD40L−/− mice, TCRβ−/− mice previously reconstituted with CD40L−/− T cells, Wnt10b-null mice, or T cell–deficient mice reconstituted with T cells from Wnt10b−/− mice. These data indicate that ovx up-regulates Wnt signaling in HSPCs through T cell–produced Wnt10b. We also found that ovx induces Wnt-dependent genes in ST-HSC/MPP but not in LT-HSCs (supplemental Figure 11A-B). We also found that ovx upregulates Wnt genes in B cells but not in other lineages (supplemental Figure 11C-F).
Discussion
We report that ovx increases ST-HSPC function and improves blood cell engraftment and survival after BM transplantation. The regulatory effect of ovx on ST-HSPCs requires T cells and expression of CD40L (supplemental Figure 12). CD40L upregulates T-cell production of Wnt10b that expands HSPCs by activating Wnt signaling in SCs and ST-HSPCs. We also found that ovx upregulates the SC production of hemopoietic cytokines through CD40L and Wnt10b.
We report that ovx induces both quantitative and qualitative changes in ST-HSPCs that lead to enhanced engraftment and increase survival after BM transplantation. Although the duration of superior engraftment induced by ovx varied somewhat among lineages, long-term reconstitution was not increased in any experiment. These findings are similar to those of an earlier report that T cells are required for PTH to expand ST-HSPCs.23 Because T cells mediate the effects of ovx and PTH on HSPC expansion, it could be argued that ovx regulates HSPCs by increasing PTH signaling in T cells. This hypothesis was excluded by the finding that ovx equally expands HSPCs in control and PTH/PTH-related protein receptorT cells −/− mice, a strain with a silent PTH/PTH-related protein receptor in all T cells34,35 (supplemental Figure 13). Together, these results demonstrate that T cells are a targetable component of the HSPC niche, a concept supported by anatomical and functional evidence. For example, regulatory T cells are now known to reside in the proximity of HSPCs and to be required for allo-HSPC persistence.36
Secondary transplantation experiments revealed that ovx has no negative effects on long-term hemopoietic expansion. Thus, ovx does not expand ST-HSPCs at the expense of LT-HSPC self-renewal. This finding may reflect a complex effect of ovx on the niche, leading the niche to restrain the HSPC pool even when it is stimulated to expand. The data highlight the potential benefit of therapeutic strategies designed to stimulate HSPCs expansion indirectly through targeting of the niche. Attesting to the feasibility of this approach, we found that treatment of donor mice with ICI 182780, an antiestrogen approved for the treatment of breast cancer, improves survival after BM transplantation.
Our data point to a pivotal mechanistic role of T cell–produced Wnt10b and CD40L as mediators of the effects of ovx in HSPCs. CD40L is a T-cell surface receptor required for ovx to sustain T-cell activation.13 We found CD40L to act upstream of Wnt10b, as ovx increased T-cell production of Wnt10b and activated Wnt signaling in SCs and LSKs in WT but not in CD40L−/− mice. This finding is consistent with the notion that Wnt10b is primarily produced by activated T cells.31 To what extent T cells affect HSPC expansion by regulating the osteoblastic vs the hemopoietic components of the niche has not been addressed in the current investigation and remains to be determined.
We also found CD40L to be implicated in the expansion of more mature hemopoietic lineages. In fact, ovx failed to stimulate B cells, monocytes, and granulocytes in the BM of mice lacking global or T cell–specific CD40L expression. The expansion of these lineages is dependent on SC cytokine production.37 Both E1,38 and CD40L38,39 regulate SC production of soluble and membrane bound factors, which are critical regulators of hemopoiesis. Among them are IL-6, IL-7, and GM-CSF. Because ovx failed to regulate the production of these cytokines in SCs from T cell– and CD40L-null mice, ovx likely up-regulates the expansion of committed hemopoietic lineages by increasing the SC production of cytokines through T cell–expressed CD40L. IL-6 limits HSPC apoptosis in concert with fms-like tyrosine kinase 3 ligand.40 Therefore, IL-6 is likely to contribute to the HSPC expansion induced by ovx. Whether other cytokines produced by SCs play a contributory role in HSPCs expansion remains to be determined.
We previously found Wnt10b to play an essential role in the expansion of HSPCs induced by PTH.23 In this study, we found Wnt10b to be equally pivotal for the expansion of HSPCs and more mature hemopoietic cells induced by ovx. Therefore, Wnt10b is a central common mediator of the effects of calciotrophic hormones on HSPCs.
Our results align with those of earlier studies on the role of Wnt signaling in HSPC biology. Studies in models of hemopoietic regeneration have revealed not only that the production of Wnt10b by hemopoietic cells and SCs is strongly upregulated in conditions of hematopoietic recovery,41 but also that Wnt10b directly induces HSPCs expansion.41 Moreover, it is well established that canonical Wnt signaling regulates HSPCs expansion.20,21,32 However, until recently, there has been significant controversy as gain- and loss-of-function studies led to the conclusion that activation of Wnt signaling enhances or depletes the HSPC pool according to the experimental strategy.20,32,42-45 A recent report has, however, disclosed that canonical Wnt signaling regulates hematopoiesis in a dose-dependent fashion.46 Accordingly, a mild increase in Wnt signaling activation in HSPCs results in HSPC expansion, whereas a marked increase in Wnt signaling impairs HSPC self-renewal.46 Hence, we found that the increased production of Wnt10b by T cells resulted in a moderate (approximately two- to threefold) stimulation of Wnt signaling in LSKs and ST-HSPCs. However, our study did not formally demonstrate a causal relationship between Wnt activation and ST-HSPC expansion.
In summary, our findings provide a new insight into the signaling integration between cells of the immune system, bone cells, and HSPCs, and the impact of such cellular interaction on hematopoiesis. Understanding the means by which E regulates T cells may therefore yield novel therapeutic strategies for hematologic disorders and BM transplantation.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Diabetes, Digestive and Kidney Disease (DK091780), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR49659 and AR061453). M.N.W. gratefully acknowledges financial support by Biomedical Laboratory Research and Development Service of the VA Office of Research and Development grant 5I01BX000105, National Institute of Arthritis and Musculoskeletal and Skin Diseases grants AR059364, AR056090, and AR053607, National Institute of Aging grant AG040013, and the Georgia Research Alliance.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.-Y.L. conducted the research and analyzed data; J.A. conducted the research; L.M.C. wrote the manuscript; T.F.L. provided mice and wrote the manuscript; M.N.W. designed the research and wrote the manuscript; and R.P. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roberto Pacifici, Division of Endocrinology, Metabolism and Lipids, Emory University School of Medicine, 101 Woodruff Circle, Room 1309, Atlanta, GA 30322; e-mail: roberto.pacifici@emory.edu.
References
- 1.Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA. Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology. 2000;141(7):2309–2318. doi: 10.1210/endo.141.7.7560. [DOI] [PubMed] [Google Scholar]
- 2.Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 2006;176(12):7371–7378. doi: 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
- 3.Jilka RL, Passeri G, Girasole G, et al. Estrogen loss upregulates hematopoiesis in the mouse: a mediating role of IL-6. Exp Hematol. 1995;23(6):500–506. [PubMed] [Google Scholar]
- 4.Erben RG, Raith S, Eberle J, Stangassinger M. Ovariectomy augments B lymphopoiesis and generation of monocyte-macrophage precursors in rat bone marrow. Am J Physiol. 1998;274(3 Pt 1):E476–E483. doi: 10.1152/ajpendo.1998.274.3.e476. [DOI] [PubMed] [Google Scholar]
- 5.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 6.Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101(9):1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110(11):1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J Clin Invest. 2000;105(9):1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328(3):688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 12.Pacifici R. Role of T cells in ovariectomy induced bone loss—revisited. J Bone Miner Res. 2012;27(2):231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- 13.Li JY, Tawfeek H, Bedi B, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci USA. 2011;108(2):768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 15.André P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin—dependent mechanism. Nat Med. 2002;8(3):247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 16.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA. 2003;100(21):12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grammer AC, Lipsky PE. CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signaling mechanisms. Adv Immunol. 2000;76:61–178. doi: 10.1016/s0065-2776(01)76019-1. [DOI] [PubMed] [Google Scholar]
- 18.Saeland S, Duvert V, Caux C, et al. Distribution of surface-membrane molecules on bone marrow and cord blood CD34+ hematopoietic cells. Exp Hematol. 1992;20(1):24–33. [PubMed] [Google Scholar]
- 19.Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144(5):1761–1769. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- 20.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26(3):414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi K, Erikson DW, Tilford SA, et al. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80(5):989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JY, Adams J, Calvi LM, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012;120(22):4352–4362. doi: 10.1182/blood-2012-06-438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegué E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126(2):415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Qian WP, Dark K, et al. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci USA. 2004;101(47):16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Grassi F, Ryan MR, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55(1):77–81. [PubMed] [Google Scholar]
- 29.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 30.Fernández FG, McKane B, Marshbank S, Patterson GA, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. J Heart Lung Transplant. 2005;24(7 Suppl):S232–S238. doi: 10.1016/j.healun.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Hardiman G, Albright S, Tsunoda J, McClanahan T, Lee F. The mouse Wnt-10B gene isolated from helper T cells is widely expressed and a possible oncogene in BR6 mouse mammary tumorigenesis. Gene. 1996;172(2):199–205. doi: 10.1016/0378-1119(96)00109-6. [DOI] [PubMed] [Google Scholar]
- 32.Scheller M, Huelsken J, Rosenbauer F, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7(10):1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 33.Jackson A, Vayssière B, Garcia T, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36(4):585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Tawfeek H, Bedi B, Li JY, et al. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS ONE. 2010;5(8):e12290. doi: 10.1371/journal.pone.0012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedi B, Li JY, Tawfeek H, et al. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci USA. 2012;109(12):E725–E733. doi: 10.1073/pnas.1120735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gsalpha-dependent signaling pathways. Proc Natl Acad Sci USA. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Gregorio GB, Yamamoto M, Ali AA, et al. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J Clin Invest. 2001;107(7):803–812. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Wu X, Terauchi M, et al. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8(2):132–145. doi: 10.1016/j.cmet.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pirih FQ, Michalski MN, Cho SW, et al. Parathyroid hormone mediates hematopoietic cell expansion through interleukin-6. PLoS ONE. 2010;5(10):e13657. doi: 10.1371/journal.pone.0013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Congdon KL, Voermans C, Ferguson EC, et al. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells. 2008;26(5):1202–1210. doi: 10.1634/stemcells.2007-0768. [DOI] [PubMed] [Google Scholar]
- 42.Luis TC, Weerkamp F, Naber BA, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113(3):546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111(1):160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 45.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 46.Luis TC, Naber BA, Roozen PP, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9(4):345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.