Key Points
Maturation, homeostasis, and function of peripheral B lymphoid cells require Rictor, an essential mTOR complex 2 component.
Rictor regulates survival of B cells and their balance of proapoptotic vs antiapoptotic gene expression.
Abstract
The mammalian target of rapamycin (mTOR), an essential serine/threonine kinase, functions in biochemically distinct multiprotein complexes, but little is known about roles of the complexes in B cells. The acutely rapamycin-sensitive mTOR complex 1 (mTORC1) is defined by a core subunit Raptor, whereas mTORC2 lacks Raptor and, instead, has Rictor and SIN1 as distinct essential components. We now show that homeostasis and function of B cells require Rictor. Conditional deletion of Rictor before lymphoid specification impaired generation of mature follicular, marginal zone, and B1a B lymphocytes. Induced inactivation in adult mice caused cell-autonomous defects in B lymphoid homeostasis and antibody responses in vivo, along with affecting plasma cells in bone marrow. Survival of B lymphocytes depended on Rictor, which was vital for normal induction of prosurvival genes, suppression of proapoptotic genes, nuclear factor κB induction after B-cell receptor stimulation, and B-cell activating factor–induced nuclear factor κB2/p52 generation. Collectively, the findings provide evidence that mTOR signaling affects survival and proliferation of mature B lymphocytes, and establish Rictor as an important signal relay in B-cell homeostasis, fate, and functions.
Introduction
Humoral immunity relies on suitable pools of mature B-cell subsets, and their capacity for clonal expansion and differentiation into antibody-secreting cells.1 After successful immunoglobulin gene rearrangements in B lineage–committed bone marrow (BM) cells, immature B cells emigrate from the BM and undergo peripheral maturation1,2; the lack of successful Ig heavy-chain gene rearrangement entails insufficient survival signaling.3 At multiple stages, B lymphocytes undergo selection to delete or render hyporeactive those cells whose antigen receptor (B-cell receptor; BCR) is autoreactive.4,5 This vetting leads to peripheral repertoires of functional mature B cells that can be clonally activated, proliferate, and differentiate into plasma cells, germinal center B cells, or assume other B lineage fates if their BCR appropriately binds antigen and other stimuli are present.6
Antigen encounters typically occur long after B-cell maturation, so mechanisms maintaining these populations are vital for immune fitness. Maintenance depends on signaling initiated by the BCR3 and receptors for B-cell activating factor (BAFF),7,8 and long life spans of memory B cells and antibody-secreting plasma cells are critical for humoral defenses against recurrent infections by a particular pathogen.9 The BCR also initiates signaling essential for antigen-specific clonal expansion, which determines the number of cells available for differentiation into plasma cells and the levels of antibody achieved after immune challenge.6,9 These same processes are important in B lymphoid cancers and diseases driven by sustained breaches in peripheral B-cell tolerance. Thus, elucidation of key signal relays connecting the BCR to survival or proliferation is a priority in developing new strategies for manipulation of antibody responses, autoimmunity, or cancers.
Induced loss of BCR expression by mature B lymphocytes caused progressive depletion of these cells, indicating that B cells require tonic BCR signaling to persist.3 Importantly, a constitutively active mutated catalytic subunit for the lipid kinase phosphatidylinositol 3-kinase (PI3K) prevented this loss of B lymphocytes after BCR deletion,10 indicating that PI3K activates pathways central to survival signaling. In addition, loss-of-function analyses affecting catalytic or regulatory subunits of PI3K observed impairment of early B lineage development.11,12 These findings suggest that a qualitative feature or the magnitude of PI3K-initiated signaling is vital for the BCR to effect development and cell maintenance. This underscores the importance of dissecting separable functions of BCR activation of PI3K pathways in development, maintenance, and proliferation.
PI3K functions by generating phosphatidylinositol (3, 4, 5) triphosphate (PIP3). This lipid signal affects numerous signaling pathways as it recruits PH domain-containing proteins to membrane locales, thereby approximating multiple kinases, adapters, and substrates to be phosphorylated.13 Components of the network downstream from PI3K include diverse serine-threonine kinases.13,14 Transfer experiments repopulating recipient mice with Akt1/2-deficient fetal liver cells provided evidence supporting Akt as a major effector downstream from PI3K in B lineage selection into marginal zone (MZ) and B1 B-cell subpopulations as well as in B-cell survival.15 However, B lineage precursors and immature B cells in BM were unaffected by Akt1/2 deficiency. The O-class forkhead box transcription factors are direct targets of Akt, which inactivates FoxO isoforms by phosphorylation and consequent nuclear export.16 This might suggest that decreased FoxO protein levels in the B lineage would enhance B-cell development. However, multistage analyses of B lineage development in a loss-of-function model for FoxO1 showed that complete lack of this transcription factor caused blocks at early or late stages of B lineage development.17 Adding further complexity along this branch of PI3K signaling, the PI3K-initiated signal that could restore homeostasis and survival after BCR elimination could be replaced by decreasing FoxO1 levels.10 Taken together, these findings suggest a complex activity-response curve with respect to Akt and the modulation of nuclear FoxO in the B lineage.
Akt is activated by binding of its PH domain to PIP3 on membranes and proximity to the similarly recruited phosphoinositide-dependent protein kinase 1 (PDK1), which phosphorylates a threonine residue conserved in the activation loop of all 3 Akt proteins.14 Activity and conformation of each Akt isoform can then be modulated by serine phosphorylation in a hydrophobic motif (HM) shared with protein kinases C (PKC) and other enzymes in a family termed “AGC kinases.”18-20 PKC, DNA-PKs, and TANK121-23 can phosphorylate the Akt HM, but mammalian target of rapamycin (mTOR) is the main kinase for this site.24-26 mTOR forms 2 complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 is rapidly but incompletely inhibited by the immune suppressant rapamycin and phosphorylates ribosomal S6 kinase (S6K) and eukaryotic translation initiation factor 4E binding protein, thereby regulating cell size and proliferation in response to nutrients and mitogenic signals.27 mTORC2 excludes an essential structural component of mTORC1, Raptor, and instead contains Rictor and SIN1.28 mTORC2 modifies the HMs of Akt, PKC isoforms, and serum- and glucocorticoid-induced protein kinase 1 (SGK1) as well as Tsc2, whose phosphorylation and inactivation can enhance mTORC1 activity. In T lymphoid cells and fibroblasts, mTORC2 has no apparent impact on cell survival and only a modest effect on proliferation.25,26,29
To investigate survival and differentiation signals initiated by the BCR and PI3K, we analyzed a loss-of-function model for mTORC2. Specifically, we used a conditional Rictor allele that can be disrupted after stage-specific expression or chemical activation of Cre recombinase. Rictor deletion early in B lymphoid ontogeny had at most a modest effect on pro- and pre-B–cell progression in the BM. However, development, survival, and function of mature B lineage cells in the periphery manifested striking abnormalities, with antibody production severely impaired when mature B cells lost Rictor expression after completing their development. In contrast to T cells,26 Rictor was vital for B-cell survival, suggesting that it participates in relaying BCR survival signals initiated via PI3K.
Methods
Detailed Methods are outlined in the supplemental Materials.
Mice, BM chimeras, and adoptive transfers
C57BL/6 (B6) (Vav-Cre) or ROSA26-Cre-ERT2) traits underwent introgression into a B6 Rictorfl/fl line.29 Mice were used in experimental protocols approved by the Institutional Animal Care and Use Committee and monitored by the Office of Animal Welfare Assurance. Mixed competitive chimeras, in which BM or lymphoid organ cells were obtained from B6 (CD45.2) WT, B6 Rictorfl/fl, and B6-CD45.1 mice and then transferred into recipients, were generated as described and were analyzed after treatment of the transfer recipients with tamoxifen (3 mg × 3 doses).29
Immunization, ELISA
Serum antibody was analyzed by enzyme-linked immunosorbent assay (ELISA) using nitrophenyl (NP)-bovine serum albumin–coated plates after mice were immunized with NP-keyhole limpet hemocyanin or NP-ovalbumin in alum as described.26 Antibody-secreting cells were determined by ELISpot assays using 96-well filter plates coated with NP-bovine serum albumin; anti-NP IgM and IgG1 were detected using isotype-specific secondary antibodies (Southern Biotech).
Immunohistochemistry, B-cell isolation, and flow cytometry
Immunohistochemistry was performed on frozen sections of spleens as described.30 Immunofluorescent staining of cell suspensions was conducted as described.26 Mature B cells were purified by depleting AA4.1+ and Thy1.2+ cells using biotinylated antibodies and IMag streptavidin particles (BD). B lineage cells were isolated from suspensions using anti-B220 microbeads and magnetic cell sorting (Miltenyi).
Cell culture; BrdU incorporation assay
Splenocytes or B cells were cultured as described26 after stimulation with anti-IgM, BAFF, or lipopolysaccharide (LPS); addition of 5-bromo-2-deoxyuridine (BrdU) to the medium; and flow cytometric analyses of anti-BrdU stains as described.26
Western blotting; measurements of RNA
Cell lysates were analyzed by immunoblotting.26 RNA levels were measured in triplicate by quantitative reverse-transcription polymerase chain reaction (qRT2-PCR) as described,29 using primer pairs tabulated in supplemental Table 1.
Electrophoretic mobility shift assay
B-cell nuclear extracts were assayed using a nuclear factor κB (NF-κB) oligonucleotide as described.29
Results
Rictor and the ontogeny of B-cell subsets in the periphery
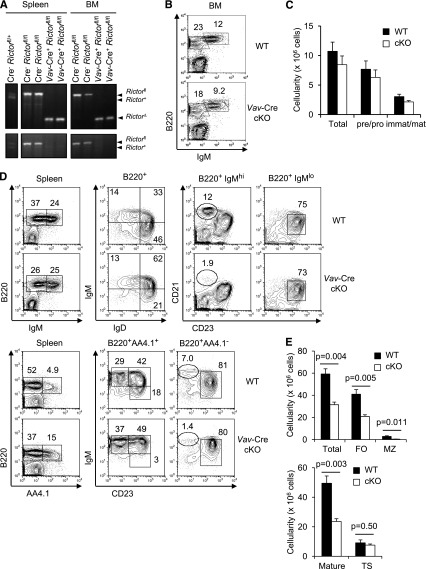
B-cell development requires functional PI3K p110α and p110δ catalytic subunits,31 which increase activity of both mTOR complexes. When Rictor, an essential subunit of mTOR complex 2, was deleted using the Vav-Cre transgene, which generally excises target genes before lymphopoiesis,29 the conditional alleles were completely excised in B lineage cells purified from the BM and spleen of Vav-Cre Rictorfl/fl mice (Figure 1A). Despite efficient deletion, little impact on early B lineage stages was apparent in the BM (Figure 1B-C). Of note, IgDhi IgM+ mature B cells (likely the recirculating population) were decreased in BM of Rictor-deficient mice (supplemental Figure 1A), and B-cell subsets were significantly perturbed in the secondary lymphoid organs of Vav-Cre Rictorfl/fl mice. The frequencies and numbers of mature B lymphocytes, including follicular (FO – IgMlo CD21lo CD23hi or AA4.1- IgMlo CD23hi) and MZ (MZ – IgMhi CD21hi CD23lo; alternatively, AA4.1- IgMhi CD23lo) B cells, were reduced, whereas the prevalence of transitional B cells (B220+ AA4.1+) and the IgMhi population increased (Figure 1D-E). Immunohistochemistry of cKO spleens revealed less B cells outside of the Moma1+ rim and, intriguingly, disrupted B-cell follicles (supplemental Figure 1B). Because cellularity was reduced, transitional B-cell numbers were similar to those of WT controls despite their increased prevalence (Figure 1D-E). Moreover, we observed increased ratios of T1 (IgMhi CD23−) to T2 (IgMhi CD23+) transitional B-cell subsets but depletion of the T3 transitional subset (IgMlo CD23+), suggesting involvement of Rictor in a signaling pathway crucial for survival, proliferation, or differentiation of transitional B cells. Of note, we observed modest decreases in B cells of lymph nodes and several nonlymphoid organs (supplemental Figure 1C-D), so the observations were not due to abnormal localization. Deficiency of PTEN, a lipid phosphatase that dephosphorylates PIP3, leads to a preferential increase in MZ and peritoneal B1 B cells.32 Conversely, the lack of Rictor affected MZ and peritoneal B1a B cells more severely than other populations such as follicular B cells (supplemental Figure 1E). Taken together, these results indicated that Rictor promotes the production of fully mature B cells.
Figure 1.
Ablation of Rictor impairs developmental progression at the late stage of B lineage. (A) Excision of Rictor in B lymphoid cells. B lineage cells were purified from the spleen and BM of Vav-Cre Rictorfl/fl and control mice, and PCR was performed using 2 different sets of primers detecting Rictor of WT (Rictor+), conditional (Rictorfl), and deleted (RictorΔ) alleles (upper panels), and for Rictor+ and Rictorfl alleles, respectively (bottom panels). (B-E) BM and spleen cells of 10- to 12-week-old Vav-Cre Rictorfl/fl (cKO) or control mice (WT) were analyzed using flow cytometry (n = 8 WT vs 8 cKO). (B) Shown are the B220 vs IgM profiles of BM cells in the viable lymphoid gate with frequencies (%) of the indicated subsets. (C) B lineage cell numbers (mean ± standard error of the mean [SEM]) in the BM were calculated from flow analyses after counting cells in each marrow: “total,” B220+; “pre/pro,” B220+ IgM-; “immat/mat,” immature/mature, ie, B220+ IgM+. (D) Flow cytometry of spleen cells in the viable lymphoid gate or further gates as indicated above panels. (E) Shown are mean (± SEM) cell numbers of the indicated subsets in spleen: FO, follicular B cells (B220+ AA4.1- IgMmed CD23hi); MZ, MZ B cells (B220+ AA4.1- IgMhi CD23lo); mature B cells (B220+ AA4.1-); TS, transitional B cells (B220+ AA4.1+).
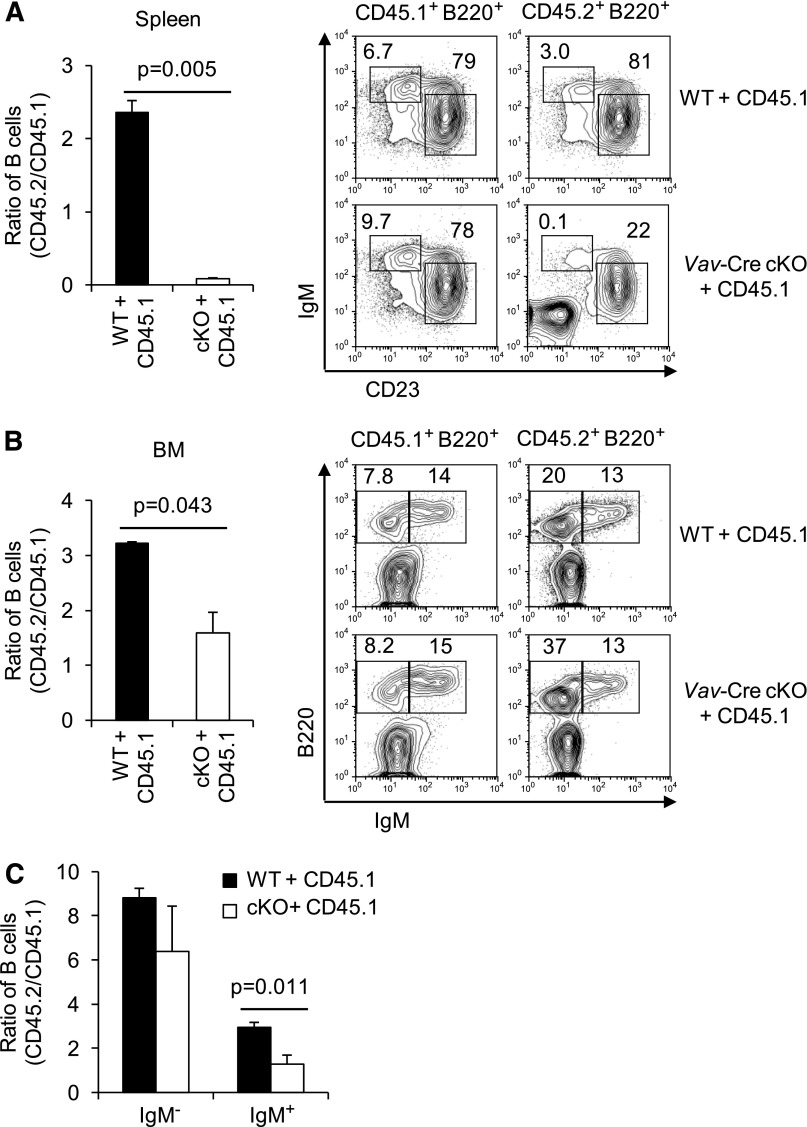
Vav-Cre also deletes the conditional Rictor allele outside of the B lineage.29 Moreover, the developmental defects might be less extensive when mTORC2-deficient B lineage cells lack competition from more fit WT cells. Therefore, we generated BM chimeras in which RAG2° recipients received a mixture of donor marrows from B6-CD45.1 mice and from B6 (CD45.2)-WT or -Vav-Cre Rictorfl/fl animals. Marrow programmed to delete Rictor exhibited a competitive disadvantage in generation of mature splenic B cells compared with WT controls (Figure 2A). Despite a hematopoietic compartment generating normal lineages (WT CD45.1 donor marrow), decreased CD45.2 to CD45.1 competitor ratios and reduced FO and MZ-phenotype B cells from Vav-Cre Rictor cKO (CD45.2) marrow were observed. Also, Vav-Cre Rictorfl/fl cells yielded lower B lineage cellularity in marrow, and the efficiency of their transition from IgM− to IgM+ status was reduced (Figure 2B-C). However, these decreases were modest compared with the impact on mature B cells. Collectively, these findings demonstrate a cell-autonomous role for Rictor in the establishment of mature B-cell populations.
Figure 2.
Defects in repopulation of Rictor-deficient B cells in a competitive fitness model. BM cells of WT or Vav-Cre Rictorfl/fl mice (CD45.2) were mixed with constant fractions of allotypically disparate WT CD45.1 marrow cells, and transferred into lethally irradiated CD45.1 recipients. Spleen (A) and BM (B-C) were analyzed 12 weeks after transplantation by counting cells of each organ and flow cytometry (n = 6 WT vs 6 cKO). Ratio of CD45.2+ B cells to CD45.1+ competitors in each recipient was calculated using flow analyses in the viable B220+ gates (left). Shown are representative fluorescence-activated cell sorter (FACS) profiles in the gates as indicated in the above panels (right). (C) Ratios of CD45.2+ IgM- B cells and CD45.2+ IgM+ B cells to each CD45.1+ competitor in BM were calculated using flow cytometry as in (B).
Rictor regulates survival, proliferation, and homeostasis of B cells
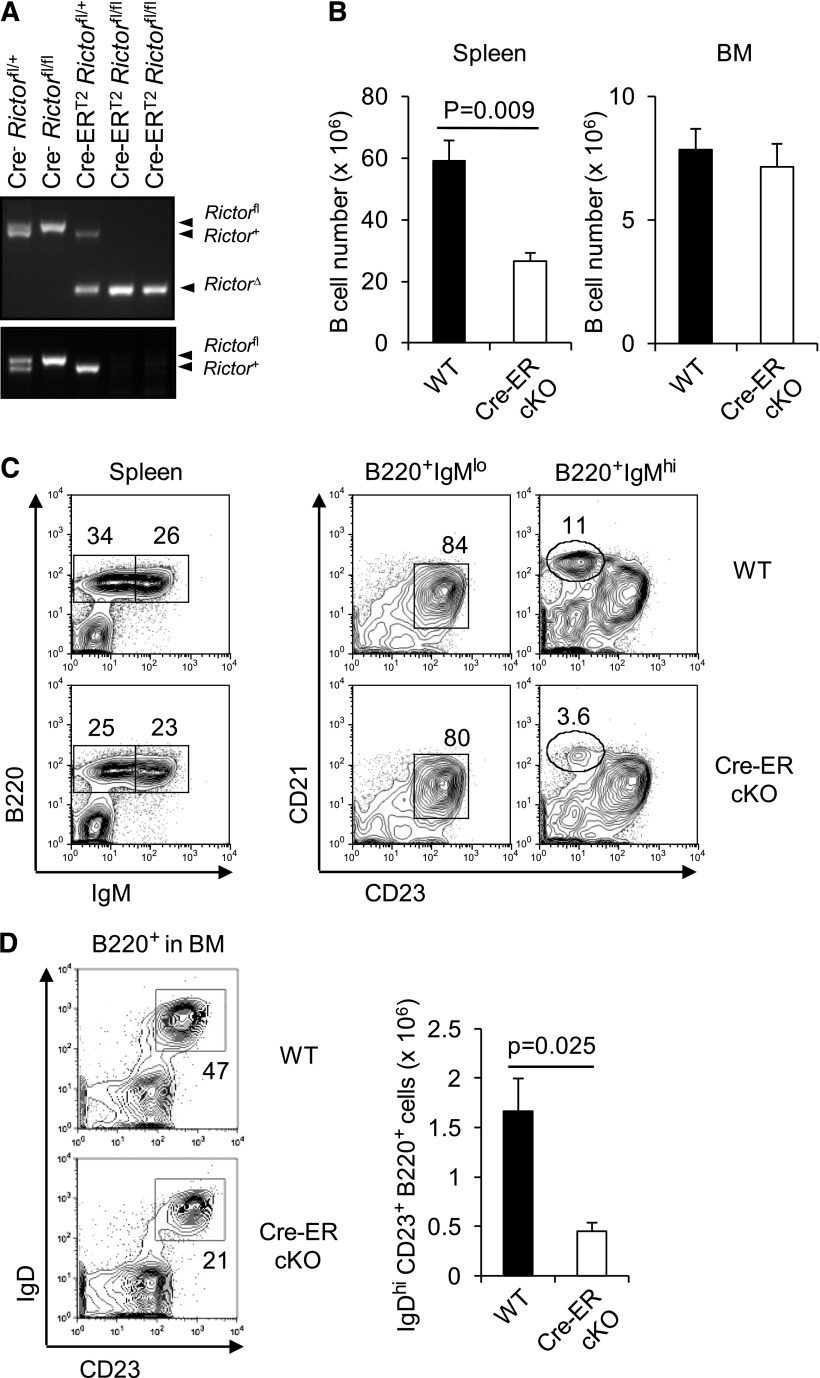
To investigate if Rictor or mTORC2 integrity participates in B-cell maintenance, we used a Cre-ERT2 transgene to effect inducible deletion.33 Short-term tamoxifen treatment of adult Cre-ERT2 Rictorfl/fl mice sufficed to excise both Rictor alleles efficiently (Figure 3A). No defect was apparent 2 weeks after the first tamoxifen injection (data not shown). After 4 weeks, however, the B lineage acquired abnormalities similar to those observed in the Vav-Cre Rictorfl/fl mice when tamoxifen-driven locus inactivation was effected in adults. Thus, treated mice exhibited reduced peripheral B-cell numbers and decreased MZ B cells (Figure 3B-C). Although Rictor deletion impairs thymic production of new T cells,29 B-cell maintenance is T cell independent. B lineage numbers in the BM were normal (Figure 3B), but IgDhi CD23hi (recirculating) mature B cells in marrow were sensitive to acute Rictor deletion (Figure 3D). Together, these results indicate that Rictor mediates maintenance of mature B cells.
Figure 3.
Effect of acute Rictor deletion on B lymphoid cells. ROSA26-Cre-ERT2 Rictorfl/fl mice were treated with tamoxifen as described in “Methods.” (A) PCR was performed to determine Rictor excision using peripheral B cells of mice of the indicated genotypes 2 weeks after starting tamoxifen injection as in Figure 1A. (B) Shown are mean (± SEM) numbers of B lineage cells in the spleen and BM of Cre-ERT2 Rictorfl/fl (cKO) or control (WT) mice 4 weeks after starting tamoxifen treatment (6 WT vs 6 cKO; 2 separate replicate experiments, each of 3 vs 3 mice). (C) Representative FACS profiles with frequencies of the indicated subsets in the viable lymphoid gates of the spleen or further gates as indicated in the above panels. (D) Decreased recirculating mature B cells in the BM of Rictor-deficient mice. Shown are representative FACS profiles with frequencies of IgDhi CD23hi cells in the B220+ gates (left) and mean (± SEM) numbers of the population in the BM of Cre-ERT2 Rictorfl/fl (cKO) or control (WT) mice 4 weeks after tamoxifen treatment (right): n = 6 vs 6.
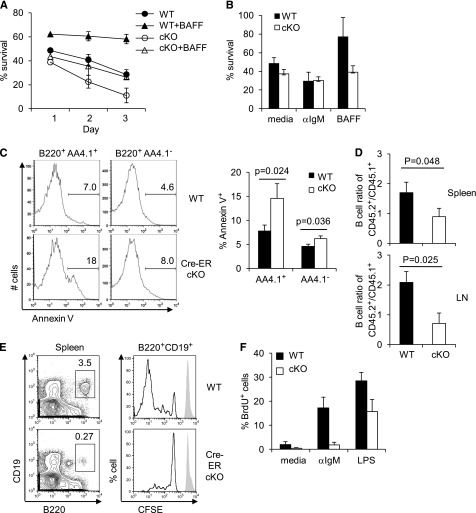
Survival of mature B cells is promoted through the BCR and the cytokine BAFF.3,7 When peripheral B cells were cultured in vitro, Rictor-deficient B cells died faster than WT controls (Figure 4A). BAFF mitigated this death of WT B cells but was less effective with Rictor-deficient samples (Figure 4A-B). Both mature and transitional B cells lacking Rictor exhibited increased annexin-V+ frequencies, and PARP cleavage and Caspase-3 activation were also greater for the Rictor-deficient B cells (Figure 4C; supplemental Figure 2). Thus, this protein assists survival signaling in B cells. To assess the impact on homeostasis of mature B cells in vivo, LN cells of WT and Cre-ERT2 Rictorfl/fl mice were mixed with CD45.1 lymphocytes and transferred into RAG2° mice, after which mice were treated with tamoxifen. Four weeks later, Rictor-depleted (Cre-ERT2 Rictorfl/fl) B cells had dropped substantially in relationship to the cotransferred CD45.1 cells, whereas WT controls remained at normal ratios (Figure 4D). Lymphopenic environments induce homeostatic proliferation of B lymphoid cells,34 but divisions of Rictor-depleted B cells were substantially reduced after their transfer into RAG2° recipients (Figure 4E). In vitro proliferation of Rictor-deficient B cells in response to BCR stimulation and, to a lesser extent, after LPS treatment, was also impaired (Figure 4F). Combined with other results, we conclude that Rictor is crucial for maintenance and homeostasis of the B lineage, in part by promoting mature B lymphocytes’ survival.
Figure 4.
Impaired survival, proliferation, and persistence of Rictor-deficient B cells. (A-B) Spleen cells were isolated from Cre-ERT2 Rictorfl/fl (cKO) or control (WT) mice 2 weeks after starting tamoxifen treatment, and were cultured for indicated times (A) or for 1 day (B) in the presence or absence of BAFF. Viable cells were counted and analyzed by flow cytometry, and B-cell survival was determined by calculating the percentages of live B-cell (7-AAD- B220+) numbers relative to input. Shown are mean (± SEM) for the 3 mice of each genotype within 1 representative analysis of 3 independent replicate experiments yielding the same results. (C) Increased apoptosis of Rictor-deficient B cells. Rictor was acutely depleted in the Cre-ERT2 Rictorfl/fl mice as in Figure 3, and annexin-V staining was performed using splenic B cells. Shown are representative histograms in the transitional (B220+ AA4.1+) and mature (B220+ AA4.1-) B-cell gates (left), and mean (± SEM) frequencies of annexin-V+ B cells (right): n = 5 vs 5. (D) Competitive homeostasis of mature B cells. LN cells of Cre-ERT2 Rictorfl/fl mice (CD45.2) or WT CD45.2 controls were mixed with equal numbers of WT CD45.1 LN cells, and transferred into Rag2 −/− mice. Mice were then treated with tamoxifen and were analyzed by flow cytometry 4 weeks after the adoptive transfer. Shown are mean (± SEM) ratios of CD45.2 B cells to CD45.1 competitors in the spleen and LN (n = 6 WT vs 6 cKO). (E) Homeostatic proliferation of Rictor-deficient B cells. LN cells of donors [Cre-ERT2 Rictorfl/fl (cKO) mice or controls] were labeled with CFSE and then were transferred into Rag2 −/− mice, after which mice were treated with tamoxifen. Shown are representative histograms of CSFE fluorescence measured in the CD19+ B220+ viable lymphoid gate 4 weeks after transfer and initiation of tamoxifen (n = 4 WT vs 4 cKO). (F) Spleen cells were harvested from Cre-ERT2 Rictorfl/fl (cKO) or control (WT) mice 2 weeks after starting tamoxifen treatment and then were cultured (2 days) in the presence or absence of anti-IgM or LPS, followed by addition of BrdU and further culture (18 hours). Percentages of BrdU+ B cells were measured by flow analyses in the viable cell gate. Shown is 1 result representative of those in 3 independent experiments, each consisting of 4 mice of each genotype.
Rictor-dependence of NF-κB and balanced regulation of survival genes
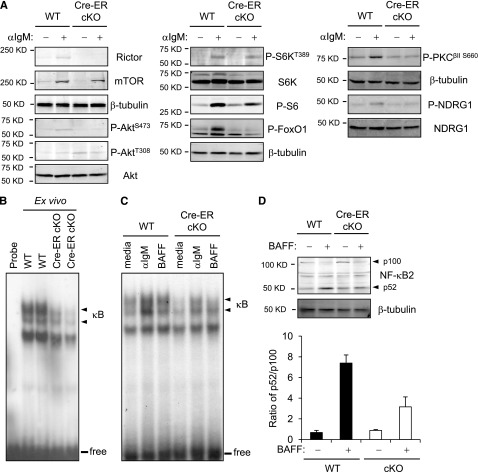
Both BCR and BAFF receptor engagement increase Akt(S473) phosphorylation in WT B cells.35,36 This induction was impaired in Rictor-deficient B cells (Figure 5A and data not shown); intriguingly, Akt(T308) phosphorylation was reproducibly enhanced. Consistent with impaired Akt function at a nuclear target after Rictor depletion, much less phosphorylation of FoxO1 was observed (Figure 5A). Rictor depletion also impaired HM phosphorylation of PKC and activity of SGK1 as determined by decreased P-NDRG1 downstream from SGK1 (Figure 5A).20 Although phosphorylation of the ribosomal protein S6 and total S6K decreased modestly, there was no substantial effect on phosphorylation of the mTORC1 target in S6K. BCR signaling activates the canonical NF-κB pathway, whereas BAFF stimulates proteolytic processing of p100 NF-κB2 into the p52 subunit.37,38 To ensure that proportions of the B lineage subsets in cKO samples were similar to controls, we used mature B cells purified from WT and ROSA26-Cre-ERT2 Rictorfl/fl mice 10 to 14 days after tamoxifen injection. Initial and BCR-induced nuclear NF-κB in Rictor-deficient B cells were diminished, and BAFF-induced conversion of p100 into p52 was less efficient (Figure 5B-D; supplemental Figure 3A). Thus, the absence of Rictor impaired stimulation of the classical and noncanonical NF-κB pathways by receptors essential for B-cell maintenance (supplemental Figure 3B).
Figure 5.
Attenuated nuclear induction of NF-κB and NF-κB2 processing of Rictor-deficient B cells. (A) Aliquots of B cells or mature B cells purified after tamoxifen pretreatment of Cre-ERT2 Rictorfl/fl or control mice (as in Figure 3A) were extracted before or after culture with anti-IgM (15 minutes) and then were analyzed by immunoblotting. Shown are signal images for the indicated antibody in 1 of 3 independent experiments with comparable results. (B) Nuclear extracts were prepared from mature B cells of Cre-ERT2 Rictorfl/fl or control mice pretreated with tamoxifen, and analyzed by EMSA using a consensus NF-κB probe. Shown is the autoradiograph of results from 1 replicate representative of 3 independent experiments: arrowhead indicates complexes formed with NF-κB proteins, and line indicates free probe bands. (C) B cells of Cre-ERT2 Rictorfl/fl or control mice pretreated with tamoxifen were treated with anti-IgM (1 hour), BAFF (4 hour), or medium, after which nuclear induction of NF-κB was analyzed as in (B). (D) Processing of NF-κB2 from the p100 precursor to p52 form. Western blotting was performed on extracts of B cells of Cre-ERT2 Rictorfl/fl or control mice pretreated with tamoxifen (as in panel A) after culture (24 hours) with or without BAFF. Shown is a representative result from among 3 independent experiments, along with the mean (± SEM) ratio of p52/p100 calculated after quantitation of p100 and p52 band intensities.
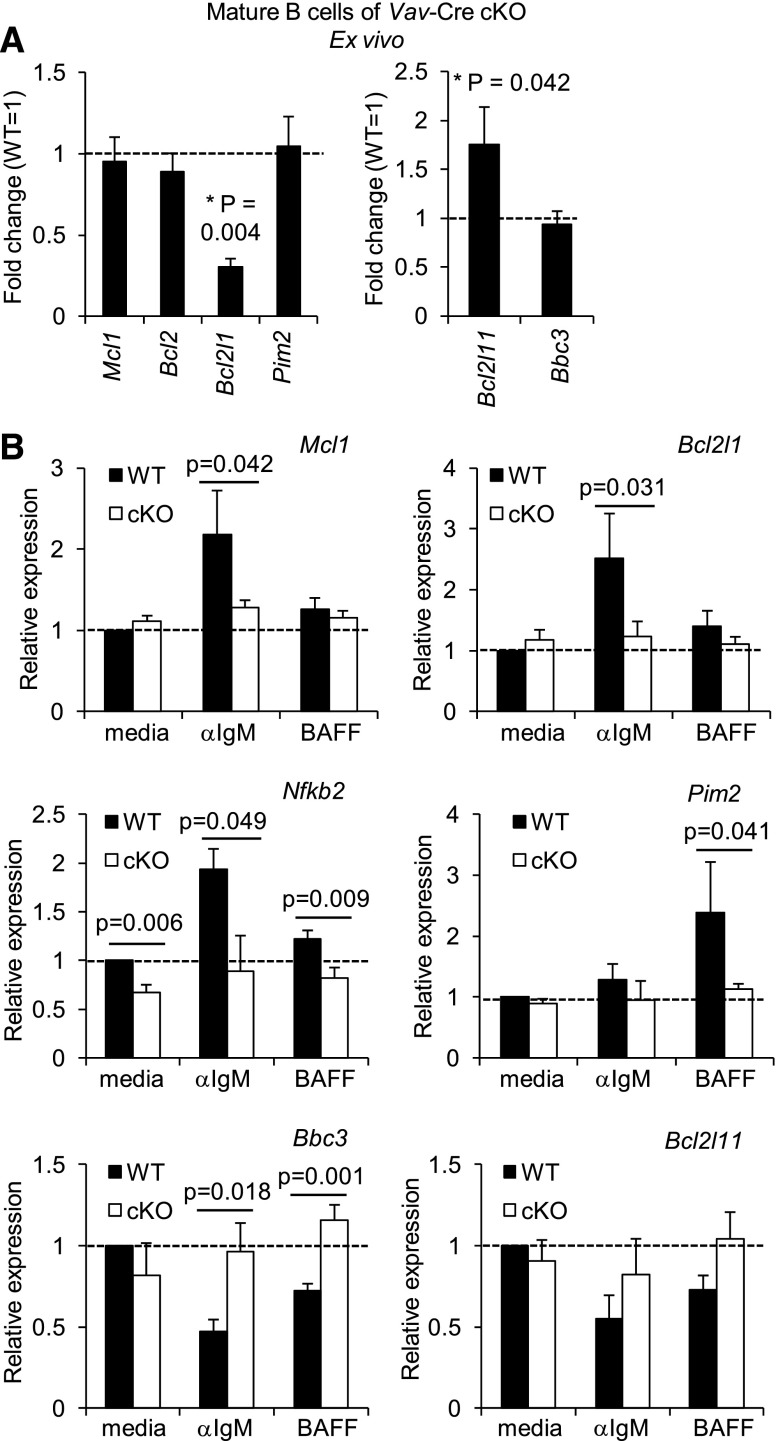
To explore the signaling defects in relation to B-cell survival, we analyzed expression of proapoptotic and antiapoptotic genes in B cells lacking Rictor. RNA encoding the prosurvival protein Bcl-XL (Bcl2l1) decreased and FoxO-regulated proapoptotic Bim (Bcl2l11) messenger RNA (mRNA) increased in freshly isolated mature B cells of Vav-Cre Rictorfl/fl mice (Figure 6A). Changes in survival gene regulation were also observed when purified B cells rendered acutely Rictor-deficient (tamoxifen-treated WT and ROSA26-Cre-ERT2 Rictorfl/fl mice before any perturbation in numbers or subsets) were stimulated through the BCR or with BAFF. In contrast to controls, BCR stimulation of acutely Rictor-deficient B cells induced neither the NF-κB target Nfkb2 nor the prosurvival genes Mcl1 and Bcl2l1 (Figure 6B). This impaired induction was confirmed for Mcl-1 and Bcl-XL proteins (supplemental Figure 3C-D). Conversely, BCR-driven suppression of proapoptotic genes Bbc3 (encoding Puma) and Bcl2l11 (Bim) was attenuated in Rictor-deficient B cells (Figure 6B). PIM kinases enhance B-cell survival, and NF-κB2 mediates BAFF induction of PIM2.39 BAFF failed to induce PIM2 mRNA in Rictor-deficient B cells, consistent with their attenuated NF-κB2 generation (Figure 6B). These data indicate that Rictor assists in setting an appropriate balance of prosurvival and proapoptotic gene expression in B cells.
Figure 6.
Rictor regulates induction of prosurvival genes and suppression of proapoptotic genes. (A) Relative expression of Bcl2 family genes ex vivo. Mature (AA4.1−) B cells were purified from Vav-Cre Rictorfl/fl (cKO) or control mice and were analyzed by qRT2-PCR. Shown are the mean (± SEM) concentrations of mRNA in cKO relative to WT, averaging 5 mice. (B) qRT2-PCR was performed on RNA from purified B cells of Cre-ERT2 Rictorfl/fl or control mice, pretreated with tamoxifen (2 weeks; before observed distortion in B-cell populations) as in Figure 3A, after culture (24 hours) with anti-IgM or BAFF. Shown are the means (±SEM) of relative expression for the indicated mRNA averaging results from 3 (BAFF) or 4 (anti-IgM) independent experiments.
B-cell–intrinsic Rictor contribution to antibody responses
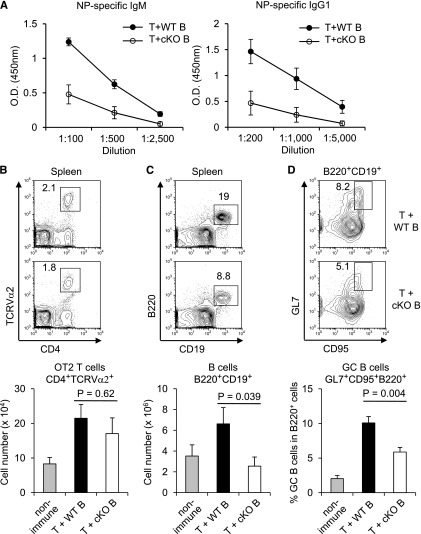
Antibody responses depend on the clonal expansion of antigen-reactive B cells, germinal center formation, and the differentiation and maintenance of plasma cells.6 Both T-independent and antigen-specific, T-dependent antibody responses were profoundly reduced when Rictor was deleted in hematopoietic compartments using Vav-Cre (supplemental Figure 4A-B). This impact was most striking for high-affinity antibodies of class-switched isotypes. Moreover, germinal centers in the spleens of immunized Rictor-deficient mice were sparse and smaller (supplemental Figure 4C). Comparison of these defects to our previous findings with depletion of Rictor in mature T cells26 and the numbers of FO B-phenotype cells suggested that Rictor also acts within B cells in antibody responses. Because Vav-Cre affects other lineages, RAG2° mice were immunized after receiving normal CD4 T cells mixed with purified B cells of Cre-ERT2 Rictorfl/fl mice or WT controls 2 weeks after tamoxifen injections. Mice with WT B cells produced robust hapten-specific IgM and IgG1 after immunization (Figure 7A). However, antibody responses were decreased (∼16-fold lower titer) when acutely Rictor-depleted B cells were used along with the same normal T-cell help, and less antibody-secreting cells were generated (supplemental Figure 5). Rictor-depleted donor B-cell populations expanded less than controls, whereas the WT CD4 T-cell populations were comparable (Figure 7B-C). High-affinity and class-switched antibodies are promoted by the germinal center reaction. Despite suitable T-cell help, frequencies of germinal center-phenotype B cells were reduced when Rictor-depleted B cells were used (Figure 7D). Thus, we conclude that Rictor within B lineage cells promoted population expansion and the antibody response. Overall, we propose that Rictor promotes the development of the preimmune B-cell populations followed by survival during and after clonal expansion.
Figure 7.
B-cell–intrinsic role of Rictor in antibody responses. B cells purified from Cre-ERT2 Rictorfl/fl mice pretreated with tamoxifen, or controls, were transferred together with equal mixed fractions of normal CD4+ T cells from WT and OT-II TCR transgenic mice into Rag2 −/− recipients. Sera of these recipient mice were analyzed 3 weeks after immunization with NP-conjugated ovalbumin. (A) Mean (± SEM) results from ELISA detecting NP-specific IgM and IgG1 (n = 6 vs 6). (B-D) Spleen cells were analyzed by flow cytometry, and donor-derived cell numbers (mean ± SEM; n = 6 vs 6) were calculated.
Discussion
B lymphocyte survival, expansion, and differentiation require PI3K activation by BCR and BAFF receptors.1,13 PI3K is essential for multiple aspects of B lineage biology and cancers,13 but much needs to be elucidated about the contributions of distinct signaling branches downstream from it. Our data show that development, homeostasis, and immune function of mature B cells depend on Rictor, a protein central to mTORC2. Conditional deletion of Rictor provides evidence that it is critical for relaying signals from the BCR to NF-κB, which can be mediated by Akt, PKC isoforms, and SGK1 (supplemental Figure 3B).40-43 BAFF-induced NF-κB2 processing also was attenuated by Rictor depletion, suggesting a role in transducing BAFF-R signals that promote B-cell maturation and subsequent survival.7,8 Indeed, deletion of Rictor from adult mice attenuated B-cell persistence in vivo, associated with impaired survival signaling and proapoptotic and antiapoptotic gene regulation, and crippled antibody responses. These defects, together with lower cell-cycling efficiency, suggest that B-cell–intrinsic contributions of mTORC2 to survival signaling and clonal expansion are major elements in the defective antibody responses. Intriguingly, TLR4-driven B-cell proliferation and in vitro antibody production exhibited less impact of Rictor deficiency (Figure 4F; supplemental Figure 6), suggesting that antigen-driven, BCR-initiated signals may depend more stringently on Rictor than TLR-initiated processes.
It is revealing to integrate these findings with other aspects of PI3K signaling. TORC2 directly modifies 3 types of enzymes: Akt, PKC isoforms, and SGK1.20,24,44 Loss-of-function experiments with Akt yielded results paralleling those presented here.15 Lymphoid progenitors unable to express either Akt1 or 2 yielded increased pre-B cells in the BM but half as many FO B cells, almost no MZ B cells, and a reduced B1 lineage. Strikingly, the impact of Rictor on maturation appeared nearly as substantial as that of Akt(1, 2). Akt is activated by PIP3-mediated recruitment to the proximity of an essential serine-threonine kinase PDK1, which phosphorylates the activation loop (“T308”) of Akt isoforms. Akt is catalytically active after activation loop phosphorylation by PDK1, but its activity and the persistence of T308-phosphorylation are increased by phosphorylation of the conserved HM (S473) of Akt.18,19 Several possibilities might explain why a modification fine-tuning catalytic activity yields as strong a phenotype as the absence of Akt(1, 2). The Akt3 isoform may be sufficiently functional in B lineage cells that the Akt phenotype was only partially penetrant, or the biochemical dose-response curve for Akt may require a level of catalytic activity not achieved without HM phosphorylation. Moreover, Rictor-dependent, Akt-independent pathways may contribute to the defects reported here inasmuch as mTORC2 phosphorylates the HM of PKC isoforms and of SGK1, probably affecting B-cell fitness.36
An intriguing possibility is that phosphorylation and structural rearrangement of the Akt HM promote functional targeting of biologically important substrates.19 In B-cell precursors lacking SIN1, a loss of Akt(S473) phosphorylation was accompanied by an increase in P-Akt(T308),45 which we also observed in Rictor-deficient B cells. Thus, the impact of mTORC2 on T-loop phosphorylation differs in thymocytes, T cells, and others.25,26,29,46 SGK1, another downstream target of mTORC2, regulates FoxO1,20 and SIN1 and Rictor loss each dramatically impaired FoxO1 phosphorylation. Together, these findings suggest that mTORC2 may regulate downstream effectors such as Akt and SGK1 in the nuclei of B cells35 and their modulation of FoxO1.45
The relationship of Rictor to mature B-cell homeostasis matches observations made when a constitutively active PI3K p110α substituted for the BCR to promote persistence.10 In that setting, either a strongly active Akt or decreased FoxO1 could mitigate the impact of BCR deletion in lieu of active PI3K. However, Rictor loss also decreased the efficiency of BAFF-induced noncanonical NF-κB activity, which is linked to survival signaling.38,47 The developmental effects of FoxO1 perturbation at different stages of B lineage ontogeny are more diverse, suggesting complex dose-response curves. FoxO1 deletion early in B lineage development caused blocks at pro- and pre-B–cell stages attributed to a failure to express RAG and the IL-7 receptor.17 Akt(S473) phosphorylation impedes FoxO1 function, so absence of FoxO1 might be expected to phenocopy increased rather than decreased Akt activity. However, fetal liver progenitors lacking SIN1 yielded cells with decreased P-FoxO1, Il7r, Rag, and formation of IgM+ pre-B cells,45 analogous to the marrow of Mb1-Cre, Foxo1fl/fl mice.17 SIN1 is essential early in fetal development, so the Sin1−/− system analyzes the progeny of fetal liver stem cells, whereas the conditional Rictor and FoxO1 experiments scored progeny of adult marrow stem cells. Recent findings with conditional Pten deletion show that the PI3K pathway affects the fetal blood progenitors very differently from stem cells of the adult marrow.48 Because of inherent differences between conditional and complete deletion, trace function may have persisted that supported progression to early transitional B-cell stages in cKO mice. Notwithstanding these issues, the striking similarities between Akt1/2 deficiency and Rictor loss suggest that our findings are mTORC2 dependent.
Another striking phenotype observed in Rictor-deficient mice was a defect of MZ B cells, which were much more sensitive to acute deletion of Rictor than FO B cells. MZ B cells show higher activation of PI3K signaling pathways than FO B cells.49 Consistent with roles of PIP3 and Akt in preferentially promoting the MZ fate, decreasing the PIP3 phosphatase PTEN increased MZ B-cell numbers, whereas Akt was vital for generating MZ B cells.15,32 These results suggest that PIP3 levels guide fate decisions of mature B cells in part through mTORC2 regulation of Akt. Although PI3K is activated in a myriad of ways, it is intriguing that Notch 2 signaling both activates this lipid kinase and promotes MZ B-cell development,50 because mTORC2 relays Notch signals in pre-T–cell differentiation and expansion.29
Our data indicated that Rictor was vital for both NF-κB pathways. Rictor deletion impaired induction of NF-κB–responsive Bcl2 family genes and the NF-κB2–dependent survival gene Pim2, and regulation of the FoxO-dependent proapoptotic genes encoding Bim and Puma was disrupted. Peripheral B-cell maturation requires classical NF-κB activation, IKK1, and generation of p50 NF-κB1 or NF-κB2.30,38,47 IKK2 and NEMO, which effect the canonical NF-κB pathway, were crucial for generation of MZ B cells but not for FO B cells, followed by a requirement for IKK2 in survival and expansion of the mature B cells.47 Conversely, IKK1 or combined absence of p50 and p52 ablated the follicular B-cell population; however, because B lineage ontogeny was normal in the absence of p52 alone, IKK1 probably exerts additional influences on B-cell development.30,38 Overall, then, our findings provide mechanistic insights into Rictor mediation of the survival and maturation of B cells though regulation of Akt, FoxO1, and the NF-κB signaling pathways.
Supplementary Material
Acknowledgments
We thank Vanderbilt cores and institutional Cancer Center Support Grant and Diabetes Center grants.
This study was supported by National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL106812 to M.B.), and National Institute of Allergy and Infectious Diseases (grant AI041649 to R.R.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.L. designed and conducted all experiments, and cowrote the manuscript; L.H., S.H.C., and A.R. codesigned some analyses and experiments; K.T.N. assisted in analysis and interpretation; J.W.T. assisted in the design and interpretation of the experiments; J.J. and R.C.R. designed, performed, interpreted the immunohistochemical analyses; and M.B. guided the experimental design, interpretations, and manuscript preparation, organized conduct of the research, and cowrote the manuscript.
The current address for K.T.N. is Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul 120-750, Korea.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark Boothby, HPI Division, PMI Dept, Vanderbilt University School of Medicine, 1161 21st Ave South/AA-4214 Medical Center North, Nashville, TN 37232-2363; e-mail: mark.boothby@vanderbilt.edu.
References
- 1.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 2.Schlissel MS. Regulating antigen-receptor gene assembly. Nat Rev Immunol. 2003;3(11):890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 3.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 4.Nemazee DA, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 5.Nossal GJ, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci USA. 1980;77(3):1602–1606. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 7.Batten M, Groom J, Cachero TG, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192(10):1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson JS, Schneider P, Kalled SL, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192(1):129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 12.Fruman DA, Snapper SB, Yballe CM, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283(5400):393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 13.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228(1):253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- 15.Calamito M, Juntilla MM, Thomas M, et al. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood. 2010;115(20):4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Dengler HS, Baracho GV, Omori SA, et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9(12):1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan TO, Tsichlis PN. PDK2: a complex tail in one Akt. Sci STKE. 2001;(66):pe1. doi: 10.1126/stke.2001.66.pe1. [DOI] [PubMed] [Google Scholar]
- 19.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22(17):6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416(3):375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami Y, Nishimoto H, Kitaura J, et al. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279(46):47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279(39):41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 23.Xie X, Zhang D, Zhao B, et al. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci USA. 2011;108(16):6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 25.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Lee K, Gudapati P, Dragovic S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Nam KT, Cho SH, et al. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209(4):713–728. doi: 10.1084/jem.20111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci USA. 2007;104(15):6359–6364. doi: 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramadani F, Bolland DJ, Garcon F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3(134):ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4(3):287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202(11):1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabatingan MS, Schmidt MR, Sen R, Woodland RT. Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J Immunol. 2002;169(12):6795–6805. doi: 10.4049/jimmunol.169.12.6795. [DOI] [PubMed] [Google Scholar]
- 35.Astoul E, Watton S, Cantrell D. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145(7):1511–1520. doi: 10.1083/jcb.145.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patke A, Mecklenbräuker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203(11):2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5(6):435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 38.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3(10):958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 39.Enzler T, Bonizzi G, Silverman GJ, et al. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25(3):403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Krappmann D, Patke A, Heissmeyer V, Scheidereit C. B-cell receptor- and phorbol ester-induced NF-kappaB and c-Jun N-terminal kinase activation in B cells requires novel protein kinase C’s. Mol Cell Biol. 2001;21(19):6640–6650. doi: 10.1128/MCB.21.19.6640-6650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su TT, Guo B, Kawakami Y, et al. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol. 2002;3(8):780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K, Babic I, Nathanson D, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-kappaB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22(11):1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27(14):1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazorchak AS, Liu D, Facchinetti V, et al. Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol Cell. 2010;39(3):433–443. doi: 10.1016/j.molcel.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki Y, Derudder E, Hobeika E, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24(6):729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11(3):415–428. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37(10):2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 50.Hampel F, Ehrenberg S, Hojer C, et al. CD19-independent instruction of murine marginal zone B-cell development by constitutive Notch2 signaling. Blood. 2011;118(24):6321–6331. doi: 10.1182/blood-2010-12-325944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.