Abstract
Environmental and genetic stress have well-known detrimental effects on ejaculate quality, but their concomitant effect on male fitness remains poorly understood. We used competitive fertilization assays to expose the effects of stress on offensive sperm competitive ability in the beetle Callosobruchus maculatus, a species where ejaculates make up more than 5% of male body mass. To examine the effects of environmental and genetic stress, males derived from outcrosses or sib matings were heat shocked at 50°C for 50 min during the pupal stage, while their siblings were maintained at a standard rearing temperature of 28°C. Heat-shocked males achieved only half the offensive paternity success of their siblings. While this population exhibited inbreeding depression in body size, sperm competitiveness was unaffected by inbreeding, nor did the effect of heat shock stress on sperm competitiveness depend on inbreeding status. In contrast, pupal emergence success was increased by 34% among heat-stressed individuals, regardless of their inbreeding status. Heat-shocked males' ejaculate size was 19% reduced, but they exhibited 25% increased mating duration in single mating trials. Our results highlight both the importance of stress in postcopulatory sexual selection, and the variability among stressors in affecting male fitness.
Keywords: Ejaculate size, heat shock, Hsp, inbreeding, paternity, sperm competition, stress
Introduction
As the vehicle for male gametes, the ejaculate plays a central role in determining male reproductive fitness. The importance of both the sperm and nonsperm components (seminal fluid proteins) of the ejaculate in postcopulatory natural and sexual selection are now well recognized (Simmons and Fitzpatrick 2012). For example, sperm numbers, motility, and morphology all have effects on male fitness (Froman et al. 2002; Gage and Morrow 2003). As do the quantities of seminal fluid and accessory gland products that can have profound effects on sperm competition by modifying female reproductive behavior (Chapman 2001; Simmons 2001; Gillott 2003). Although many components of ejaculates have well-established genetic bases (Hales et al. 1989; Ducrocq and Humblot 1995; Swanson et al. 2001; Birkhead et al. 2005; Dowling et al. 2007; Dobler and Hosken 2009), estimates for heritability and additive genetic variance in sperm-competitive performance are generally modest (reviewed in: Simmons and Moore 2009). The influence of environmental effects on sperm competitive success is increasingly recognized (e.g., oviposition site availability: Eady et al. 2004; larval density, nutrition: Amitin and Pitnick 2007; adult density: Crean and Marshall 2008; sperm competition risk: DelBarco-Trillo 2011; immune insult: McNamara et al. 2013).
Through its well-established effects on ejaculate quality, stress is often presumed to be an important environmental source of variance in male fitness (Campbell et al. 1992; Pérez-Crespo et al. 2008; Hansen 2009). While the cause of stress can vary considerably (e.g., heat, excess reactive oxygen species, or physical handling), cellular responses to stress-related damage in affected tissues are remarkably consistent. Stress induces the expression of heat shock proteins (Hsp), molecular chaperones that repair cellular damage associated with stress (reviewed in Sørensen et al. 2003). Partly due to their resource requirements and demands on the transcriptional machinery, the expression of these gene products imposes its own costs (Feder et al. 1992; Krebs and Feder 1998), and the survival benefits of expression typically trade-off against other fitness components (Hoffmann 1995). While the adverse effects of heat stress on sperm production and function are well studied (Hansen 2009), knowledge about its consequences on other seminal components remains limited.
Hsp expression also appears to mitigate the consequences of genetic stress. A recent study in Caenorhabditis elegans showed that induced Hsp expression reduced the penetrance of a late-onset detrimental mutation (Casanueva et al. 2012). In a similar fashion, Hsp expression might be expected to counter the effects of the expression of detrimental alleles in homozygotes after inbreeding. Kristensen et al. (2002) found raised levels of Hsp70, a widely expressed and inducible chaperone, in unstressed inbred Drosophila larvae. While environmental stress usually worsens inbreeding depression (Armbruster and Reed 2005), inbred genotypes in Drosophila buzzatii show less of a decline in hatching success following heat shock than do outbred genotypes (Dahlgaard and Loeschcke 1997). However, attenuating effects of exposure to one stressor on susceptibility to another are not always supported. For example, in Drosophila melanogaster, the degree of inbreeding has no significant effect on heat stress survival (Dahlgaard et al. 1995). Although Hsp genes are highly conserved, their expression exhibits genetic variation in many species (Sørensen et al. 2003), and may thus show inbreeding depression. How interactions between intrinsic (genetic) and extrinsic stress affect trade-offs with other fitness components remains poorly understood.
In this study, we used the seed beetle Callosobruchus maculatus as a model to study how interactions between acute thermal and genetic stressors affect male fitness. Males transfer costly ejaculates that comprise 5–8% of their body mass (Savalli and Fox 1999). For females, these ejaculates are a source of water and accessory secretions that have complex effects on female fitness (Eady et al. 2007; Edvardsson 2007). As a pest of stored legumes, inbreeding is likely to occur “naturally” after colonization of new food sources. Inbreeding affects the number of sperm transferred per ejaculate (Fox et al. 2012). In densely infested legumes, metabolic processes may strongly elevate temperatures (Utida 1972), which larvae, developing within the beans, cannot escape. Although adult heat shock exposure is generally thought to induce temporary increases in Hsp expression, in zebrafish thermal stress during development produced a permanent increase in thermal tolerance at the expense of body size (Schaefer and Ryan 2006). Because males in C. maculatus typically attain high last-male sperm precedence (see Material and Methods), we examined the effects of thermal and genetic stress on this offensive sperm competitive performance. We manipulated stress by heat shocking one of two male siblings that were derived from inbred or outbred crosses, during the pupal stage. This developmental stage is the most resistant to heat stress (Johnson et al. 2010), and represents a period of major spermatogenesis and proliferation of secretory epithelia (Dumser 1980; Happ 1992). We also examined whether any reductions in sperm competitive ability result from changes in ejaculate size.
Material and Methods
Stock culture
Experimental animals were sourced from a large outbred population that originated from a stock culture held by the Stored Grain Research Laboratory of CSIRO (Canberra, Australia). Beetles were maintained at 28°C in an incubator (Binder KB 240, Germany) on black-eyed beans (Vigna unguiculata). In this population, a single generation of inbreeding causes genetic stress. This is shown by the observation that the offspring from brother–sister matings are significantly lighter at eclosion than outbred offspring (inbred males 2.6% lighter, F1,1604 = 9.8, P < 0.002 and inbred females 5.6% lighter, F1,1777 = 48.2, P < 0.001).
Protocol
We used a split-family design to examine the effects of genetic stress (inbreeding) and environmental stress (heat shock during pupation) on sperm competitiveness of related males (Fig. 1). Parental virgins were derived from the population by haphazardly isolating infested beans in microtubes. Sixty pairs of virgins on the second day after emergence were formed and mated in microtubes. Females were then moved to 55 mL plastic vials (Techno Plas, Australia) with 40 black-eyed beans and allowed to oviposit until death. Infested beans were once again isolated in microtubes to assure virginity among F1 animals. Within each F1 family, a “brother–sister” cross was conducted and an outbred cross was set up using a donor female from the next family within the block (Fig. 1). In outbred populations, inbreeding coefficients increase most strongly in the first generation of inbreeding, which generally predicts the degree of inbreeding depression (Roff 1997). Animals used for these crosses were between 2 and 4 days old. Following mating in microtubes, females were allowed to oviposit until death on 40 black-eyed beans, which were then isolated to capture virgin F2 animals.
Figure 1.
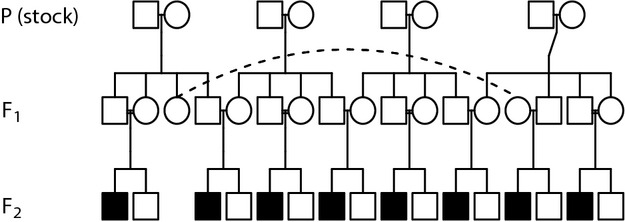
One of 15 replicate blocks within our experimental design. Stock virgins were single mated to produce four F1 families. Within each of these, two F1 siblings were mated to generate inbred F2 offspring, and two siblings were mated to beetles from other families to produce outcrossed F2 offspring. Shortly before emergence (day 26), F2 families were split: part of the pupae received a heat shock treatment (50 min on 50°C) while the remaining pupae were maintained at standard culturing conditions. Resulting F2 males were mated to females previously mated to irradiated males (not shown) to assess the effects of heat shock and inbreeding treatments on sperm-competitive ability. Dashed line indicates mating between nonadjacently depicted families. ○: females; □ males; ▪ heat-shocked males.
On the 26th day after the mating, clutches of inbred and outbred unemerged F2 individuals were randomly split between control and heat shock treatments. Preliminary trials indicated that this day precedes a 5-day period in which two thirds of emergences occur. This timing thus ensures that the majority of animals received heat shock treatment late in the pupal stage. Spermiogenesis in insects generally takes place during the final larval stages (i.e., pupal stage) (Dumser 1980), and spermatophore production in C. maculatus may be incomplete before 24 h postemergence (Eady and Brown 2000). Heat-shock events during this phase therefore are likely to affect sperm production and subsequent ejaculate competitiveness. We exposed pupae inside their beans, inside microtubes, to 50°C for 50 min in the aforementioned incubators. This treatment minimizes any water loss and is expected to induce strong cell-physiological responses, but exert minimal selection through mortality (Johnson et al. 2010). The effect of this stress was assessed by recording F2 eclosion success per randomly selected five beans for all experimental crosses within five blocks (20 families).
From each F1 family, four outbred non heat-shocked female F2 virgin offspring were collected for sperm competition trials. Females were always mated first to an irradiated virgin male (see below) and remated to an experimental male the next day. Female C. maculatus are generally polyandrous, but exhibit a refractory period after mating (Eady 1995). Females were given the opportunity to remate with the same male for 3 days in 15-min mating trials and were discarded if unsuccessful. Due to practical constraints, the ages at second mating of females, irradiated males, and experimental males were variable (median [iqr]: 5 [4–6]; 3.5 [3–5]; 5 [4–6] days, respectively), but controlled statistically. Following successful remating, females were placed in 55 mL plastic vials with 40 black-eyed beans and allowed to oviposit until death. For each female, the number of eggs and F3 emergences were counted.
Irradiation
Irradiated males were produced by isolating infested beans from the stock population. Emerged males were kept in groups of 20 in ventilated vials (d × h = 24 × 64 mm) for 2 days before exposure to 60 Gy gamma radiation from a cobalt-60 source, over a period of 14 min and under nitrogen anesthesia (5 L min−1). Irradiated males' sperm remain functionally competent, but have DNA mutations that result in early embryonic mortality, so F3 could be attributed to experimental males (Parker 1970). Supplementary trials were conducted to confirm the efficacy of irradiation treatment on reducing fertilization ability. Fifty-eight 2-day-old females were successfully mated to either 2-day-old irradiated (27) or nonirradiated virgin males (31) and allowed to oviposit on 40 beans in 55 mL vials. Fecundity (egg number) and emergences were counted. Irradiation of males induced significant embryonic mortality in the offspring they sired (proportion emergence, median [iqr], control: 0.76 [0.61–0.82]; irradiated: 0.06 [0.03–0.11]; Wilcoxon rank sum test, W = 85.5, P < 0.001).
Statistics
The consequences of stress on F2 eclosion success were examined by modeling the effects of experimental treatments (inbreeding, heat shock, and their interaction) with family as a random factor. To assess the effect of our treatments on the sperm competitiveness of F2 males, we analyzed F3 emergence data using generalized mixed-effects modeling with F2 fecundity as binomial totals. To address overdispersion, an observation-level random factor was included in the model. Graphical inspection of the data indicated between-family variation in the effect of heat shock treatment on F3 emergence. The fit of the full model was improved by including a random term that allowed the effects of heat shock treatment to vary by family (likelihood test, 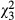 = 12.33, P = 0.006). The full model was weighted by fecundity and included fixed effects for heat shock treatment and inbreeding status, and five covariates to control for any effects of factors not fixed in the experimental design (female age at mating, duration of refractory period after the female's first mating, female body mass at emergence, and the ages of the first and second males at mating). Nonsignificant covariates were eliminated from the model by stepwise backward deletion. The full model also included interactions between heat shock treatment and inbreeding status, and between the ages of the first and second male at mating, but these were dropped due to nonsignificance. All analyses were conducted using R version 2.14.1 (R Development Core Team 2012), with the package “lme4” for mixed modeling (Bates et al. 2011).
= 12.33, P = 0.006). The full model was weighted by fecundity and included fixed effects for heat shock treatment and inbreeding status, and five covariates to control for any effects of factors not fixed in the experimental design (female age at mating, duration of refractory period after the female's first mating, female body mass at emergence, and the ages of the first and second males at mating). Nonsignificant covariates were eliminated from the model by stepwise backward deletion. The full model also included interactions between heat shock treatment and inbreeding status, and between the ages of the first and second male at mating, but these were dropped due to nonsignificance. All analyses were conducted using R version 2.14.1 (R Development Core Team 2012), with the package “lme4” for mixed modeling (Bates et al. 2011).
Results
Heat-shock treatment induced significant viability benefits: stressed pupae showed a 34% increase in eclosion success compared to controls (7.1 vs. 5.3 emergences per five beans; type III ANOVA (analysis of variance) 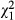 = 22.05, P < 0.001; Fig. 2); neither inbreeding nor its interaction with heat shock affected eclosion (both
= 22.05, P < 0.001; Fig. 2); neither inbreeding nor its interaction with heat shock affected eclosion (both 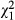 < 1.72, P > 0.19).
< 1.72, P > 0.19).
Figure 2.
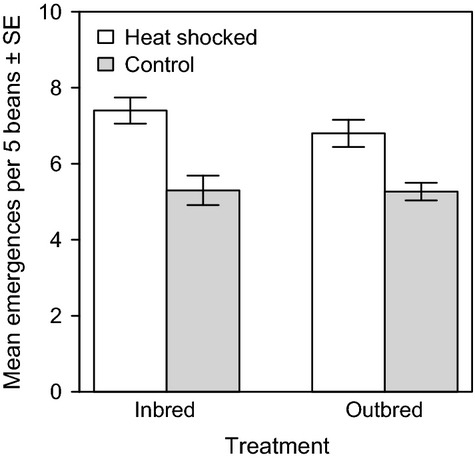
Effects of genetic (inbred vs. outcrossed) and environmental stress (heat shock vs. control) on F2 pupal eclosion success (number of emergences per five beans).
Forty-nine of 240 planned double mating trials were excluded due to failures to mate (n = 22), deaths before remating (n = 1), and other issues that prevented successful crosses. This reduction was not associated with any of the experimental treatments (χ2 test, 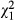 = 0.17, P = 0.68).
= 0.17, P = 0.68).
The reduced model revealed that only male heat shock treatment and female age at the first mating affected focal male paternity (P2) (Table 1). Heat-shocked males sired only half the proportion of offspring that non heat-shocked males gained (0.37 vs. 0.76, resp.; Fig. 3). Whether focal males were derived from in- or outbred crosses did not affect their sperm competitive ability (Fig. 3).
Table 1.
Analysis of deviance table for a generalized linear mixed-effects model of the proportion paternity of the second male to mate (P2)
| β ± SE | 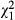 |
P | |
|---|---|---|---|
| Male heat shock | −2.77 ± 0.38 | 54.22 | 0.000 |
| Male inbreeding | −0.01 ± 0.28 | 0.00 | 0.974 |
| Female age at first mating | 0.20 ± 0.09 | 5.58 | 0.018 |
Random terms are not shown.
Figure 3.
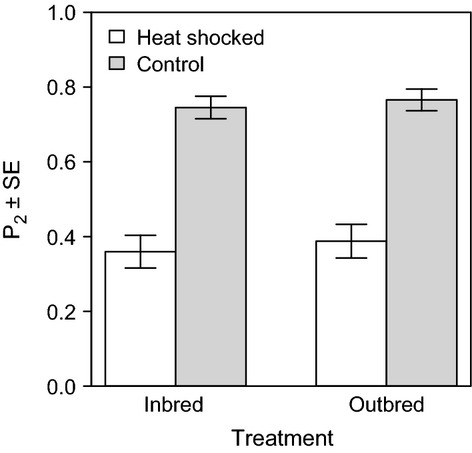
Effects of genetic (inbred vs. outcrossed) and environmental stress (heat shock vs. control) on the paternity over F3 offspring by experimental males (P2).
To examine the mechanism behind the decline in sperm competitive ability after heat shock, we determined ejaculate sizes of 23 heat-shocked and 23 control males between 1 and 3 days after eclosion. Prior to and after a timed mating with a 1-day-old virgin stock female, the male and female were weighed to the nearest 1 μg, and mass changes averaged. Heat-shocked males exhibited a 19% decrease in ejaculate mass (1.361 vs. 1.678 mg) despite 25% longer matings (592 vs. 474 sec) compared to control males (Table 2).
Table 2.
Type II ANCOVA (analysis of covariance) results for the effects of heat shock treatment on transferred ejaculate mass and the duration of mating between virgin beetles
| Term | Ejaculate mass | Mating duration | ||||
|---|---|---|---|---|---|---|
| β | F1,42 | P | β | F1,42 | P | |
| Heat shock | −495.34 | 11.40 | 0.002 | 135.51 | 8.30 | 0.006 |
| Male body mass | 0.06 | 12.15 | 0.001 | −0.01 | 2.06 | 0.159 |
| Female body mass | −0.01 | 2.74 | 0.105 | −0.01 | 5.32 | 0.026 |
Discussion
Thermal stress has well-known detrimental effects on male fertility. Our results indicate that the observed decline in offensive sperm competitiveness in stressed males was associated with reduced ejaculate size. Ejaculate size may affect sperm competitive success in several ways. The benefits of larger ejaculates in non heat-shocked males might simply derive from greater sperm numbers. Indeed, the pattern of sperm precedence in C. maculatus is consistent with sperm displacement from the spermatheca (Eady 1994). Sperm displacement might also be affected via seminal fluid components of the ejaculate, as in Drosophila (Wolfner 1997). However, the effect of quantitative changes in ejaculate components appears to be limited. Among virgin males, variation in ejaculate size has no effect on sperm precedence or female fecundity (Edvardsson and Tregenza 2005). Although mating causes significant ejaculate depletion, with roughly equivalent effects on ejaculate size and sperm numbers, the reduction in last-male sperm precedence due to depletion is far smaller than the reduction observed in this study (Eady 1995; Savalli and Fox 1999). Spermatozoa, spermatocytes, and spermatids do exhibit increased sensitivity to heat stress (Pérez-Crespo et al. 2008). We suggest therefore, that heat stress may have detrimental effects on gamete performance.
We further show that heat-stressed pupae exhibit a marked increase in eclosion success, indicative of up-regulated Hsp expression. Mild heat stress is often found to produce somatic benefits, including delayed senescence and increased longevity (Sørensen et al. 2003). Conversely, previous work indicates that reproductive effort reduces resistance to oxidative stress (Alonso-Alvarez et al. 2004), which is partly mediated by Hsps (Hartwig et al. 2009). The underperformance of control-treated individuals points to a common source of developmental mortality that is mitigated by increased Hsp expression. The pupal stage in holometabolous insects is accompanied by profound changes in transcriptional activity (Arbeitman et al. 2002). Mortality among larvae and unemerged adults in C. maculatus is substantial and heritable (Tran and Credland 1995). The pupal viability benefits associated with heat stress in this study are likely attributable to the roles Hsps play in stabilizing developmental processes (Takahashi et al. 2010) or counteracting the products of detrimental genes (Casanueva et al. 2012).
The evidence for increased investment in Hsps may provide a potential mechanism for the decrease in sperm competitive performance. While gametogenic and secretory tissues, and gametes may suffer direct stress-induced damage, sperm competitive performance may also be affected indirectly by a systemic or tissue-specific developmental trade-off between investment in reproduction and stress resistance. The costs of Hsp up-regulation on cellular functions are often more severe in reproductive cell types. In mice, Hsp expression is triggered at lower temperatures in spermatocytes than somatic reproductive cell types (Sarge 1995). Both germline and somatic reproductive tissues can further have Hsp expression profiles not found in nonreproductive tissues. In Drosophila, Hsp23 and Hsp27 are expressed in the secretory cells of the seminal vesicle and accessory glands, mainly during the pupal stage (Michaud et al. 1997). Expression of Hsp23 is heat-stress inducible and associated with increased stress survival (Arrigo 1987). Up-regulation of similar Hsps might underlie the observed reduction in ejaculate size we observed in heat-shocked C. maculatus.
While body size is susceptible to genetic stress in our population, inbreeding had no direct effect on sperm competitive performance. Single generations of full-sib mating often reduce sperm competitive success (e.g., Simmons 2011), which is sometimes attributed to the relative complexity of spermatogenesis. Inbreeding depression in sperm competition traits has been found in other populations of C. maculatus, although these studies did not directly test offensive performance (Bilde et al. 2009; Fox et al. 2012). Fox et al. (2012) found that inbred males' ejaculates were similarly sized, but contain 17–33% fewer sperm. Given the relatively limited effects of severe sperm depletion on last-male sperm precedence (Eady 1995), any inbreeding depression in sperm numbers appears to have had no detectable effect on offensive sperm competitive success in our population.
Despite the absence of inbreeding depression in sperm competitive success, the effects of environmental stress on competitiveness could still depend on inbreeding status. First, Hsp genes, and the genes involved in their expression, may themselves be subject to inbreeding. Homozygosity at Hsp loci has been linked to reduced sperm numbers (Huang et al. 2002). Furthermore, synergistic effects of stressors are thought to result from competition between different sources of damaged proteins over Hsps (Rutherford 2003). Inbreeding chronically raises Hsp expression (Kristensen et al. 2002). We hence expected the genome-wide stress of inbreeding to interact with the effects of heat shock, but found no such interactions. This result cannot be due to an overall lack of genetic variation in our population, because it exhibits inbreeding depression in other traits (this study; Tomkins et al. 2010). However, previous contradictory findings within populations of C. maculatus show that the effects of environmental stress on inbreeding depression in life-history traits strongly depend on the exact environmental conditions (Fox and Reed 2011; Fox et al. 2011). Despite the generality of the cellular heat shock response, different environmental stressors may exhibit varying interactions with genetic stress. Dahlgaard and Hoffmann (2000) found that inbreeding in D. melanogaster reduced resistance to several stressors, but not to heat knockdown. There are also indications that constitutively raised levels of Hsp due to inbreeding can reduce the costs of environmental stress (Dahlgaard and Loeschcke 1997), potentially masking the effects of other stressors such as heat shock.
In conclusion, we offer, to our knowledge, the first report of viability benefits concomitant with reductions in offensive sperm competitive performance induced by heat stress. This result appears consistent only with developmental up-regulation of Hsps. In C. maculatus, a key invertebrate model system for sexual selection and conflict, ejaculate components have important consequences for female fecundity, longevity, and remating propensity (Edvardsson 2007), highlighting the importance of environmental effects on variance in male postcopulatory success.
Acknowledgments
We thank Kathryn McNamara and Carly Wilson for their technical assistance. We also thank Ernie Steiner and the West Australian Department of Agriculture for access to irradiation facilities. This research was supported by the Australian Research Council.
Conflict of Interest
None declared.
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 2004;7:363–368. doi: 10.1111/j.1461-0248.2004.00594.x. [Google Scholar]
- Amitin EG, Pitnick S. Influence of developmental environment on male- and female-mediated sperm precedence in Drosophila melanogaster. J. Evol. Biol. 2007;20:381–391. doi: 10.1111/j.1420-9101.2006.01184.x. doi: 10.1111/j.1420-9101.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- Arbeitman MN, Furlong EEM, Imam F, Johnson E, Null BH, Baker BS, et al. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–2275. doi: 10.1126/science.1072152. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Arrigo A-P. Cellular localization of HSP23 during Drosophila development and following subsequent heat shock. Dev. Biol. 1987;122:39–48. doi: 10.1016/0012-1606(87)90330-7. doi: 10.1016/0012-1606(87)90330-7. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. Available at http://CRAN.R-project.org/package=lme4.
- Bilde T, Maklakov A, Meisner K, Friberg L, la Guardia U. Sex differences in the genetic architecture of lifespan in a seed beetle: extreme inbreeding extends male lifespan. BMC Evol. Biol. 2009;9:33. doi: 10.1186/1471-2148-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead TR, Pellatt EJ, Brekke P, Yeates R, Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. doi: 10.1038/nature03374. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Pottinger TG, Sumpter JP. Stress reduces the quality of gametes produced by rainbow trout. Biol. Reprod. 1992;47:1140–1150. doi: 10.1095/biolreprod47.6.1140. doi: 10.1095/biolreprod47.6.1140. [DOI] [PubMed] [Google Scholar]
- Casanueva MO, Burga A, Lehner B. Fitness trade-offs and environmentally induced mutation buffering in isogenic C. elegans. Science. 2012;335:82–85. doi: 10.1126/science.1213491. doi: 10.1126/science.1213491. [DOI] [PubMed] [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Crean AJ, Marshall DJ. Gamete plasticity in a broadcast spawning marine invertebrate. Proc. Natl Acad. Sci. USA. 2008;105:13508–13513. doi: 10.1073/pnas.0806590105. doi: 10.1073/pnas.0806590105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgaard J, Hoffmann AA. Stress resistance and environmental dependency of inbreeding depression in Drosophila melanogaster. Conserv. Biol. 2000;14:1187–1192. doi: 10.1046/j.1523-1739.2000.99206.x. [Google Scholar]
- Dahlgaard J, Loeschcke V. Effects of inbreeding in three life stages of Drosophila buzzatii after embryos were exposed to a high temperature stress. Heredity. 1997;78:410–416. doi: 10.1038/hdy.1997.64. doi: 10.1038/hdy.1997.64. [DOI] [PubMed] [Google Scholar]
- Dahlgaard J, Krebs RA, Loeschcke V. Heat-shock tolerance and inbreeding in Drosophila buzzatii. Heredity. 1995;74:157–163. doi: 10.1038/hdy.1995.23. doi: 10.1038/hdy.1995.23. [DOI] [PubMed] [Google Scholar]
- DelBarco-Trillo J. Adjustment of sperm allocation under high risk of sperm competition across taxa: a meta-analysis. J. Evol. Biol. 2011;24:1706–1714. doi: 10.1111/j.1420-9101.2011.02293.x. doi: 10.1111/j.1420-9101.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Dobler R, Hosken DJ. Response to selection and realized heritability of sperm length in the yellow dung fly (Scathophaga stercoraria) Heredity. 2009;104:61–66. doi: 10.1038/hdy.2009.93. doi: 10.1038/hdy.2009.93. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Nowostawski AL, Arnqvist G. Effects of cytoplasmic genes on sperm viability and sperm morphology in a seed beetle: implications for sperm competition theory? J. Evol. Biol. 2007;20:358–368. doi: 10.1111/j.1420-9101.2006.01189.x. doi: 10.1111/j.1420-9101.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- Ducrocq V, Humblot P. Genetic characteristics and evolution of semen production of young Normande bulls. Livest. Prod. Sci. 1995;41:1–10. doi: 10.1016/0301-6226(94)00029-7. [Google Scholar]
- Dumser JB. The regulation of spermatogenesis in insects. Annu. Rev. Entomol. 1980;25:341–369. doi: 10.1146/annurev.en.25.010180.002013. [Google Scholar]
- Eady P. Sperm transfer and storage in relation to sperm competition in Callosobruchus maculatus. Behav. Ecol. Sociobiol. 1994;35:123–129. doi: 10.1007/bf00171502. [Google Scholar]
- Eady PE. Why do male Callosobruchus maculatus beetles inseminate so many sperm? Behav. Ecol. Sociobiol. 1995;36:25–32. doi: 10.1007/BF00175725. [Google Scholar]
- Eady PE, Brown DV. Spermatophore size and mate fecundity in the bruchid beetle Callosobruchus maculatus. Ethol. Ecol. Evol. 2000;12:203–207. [Google Scholar]
- Eady PE, Rugman-Jones P, Brown DV. Prior oviposition, female receptivity and last-male sperm precedence in the cosmopolitan pest Callosobruchus maculatus (Coleoptera: Bruchidae) Anim. Behav. 2004;67:559–565. doi: 10.1016/j.anbehav.2003.07.003. [Google Scholar]
- Eady PE, Hamilton L, Lyons RE. Copulation, genital damage and early death in Callosobruchus maculatus. Proc. Biol. Sci. 2007;274:247–252. doi: 10.1098/rspb.2006.3710. doi: 10.1098/rspb.2006.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson M. Female Callosobruchus maculatus mate when they are thirsty: resource-rich ejaculates as mating effort in a beetle. Anim. Behav. 2007;74:183–188. doi: 10.1016/j.anbehav.2006.07.018. [Google Scholar]
- Edvardsson M, Tregenza T. Why do male Callosobruchus maculatus harm their mates? Behav. Ecol. 2005;16:788–793. doi: 10.1093/beheco/ari055. [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Fox CW, Reed DH. Inbreeding depression increases with environmental stress: an experimental study and meta analysis. Evolution. 2011;65:246–258. doi: 10.1111/j.1558-5646.2010.01108.x. doi: 10.1111/j.1558-5646.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Fox C, Stillwell RC, Wallin W, Curtis C, Reed D. Inbreeding-environment interactions for fitness: complex relationships between inbreeding depression and temperature stress in a seed-feeding beetle. Evol. Ecol. 2011;25:25–43. doi: 10.1007/s10682-010-9376-3. [Google Scholar]
- Fox CW, Xu J, Wallin WG, Curtis CL. Male inbreeding status affects female fitness in a seed-feeding beetle. J. Evol. Biol. 2012;25:29–37. doi: 10.1111/j.1420-9101.2011.02400.x. doi: 10.1111/j.1420-9101.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Froman DP, Pizzari T, Feltmann AJ, Castillo-Juarez H, Birkhead TR. Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc. Biol. Sci. 2002;269:607–612. doi: 10.1098/rspb.2001.1925. doi: 10.1098/rspb.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage MJG, Morrow EH. Experimental evidence for the evolution of numerous, tiny sperm via sperm competition. Curr. Biol. 2003;13:754–757. doi: 10.1016/s0960-9822(03)00282-3. doi: 10.1016/S0960-9822(03)00282-3. [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Hales LA, Savage TF, Harper JA. Heritability estimates of semen ejaculate volume in medium white turkeys. Poult. Sci. 1989;68:460–463. doi: 10.3382/ps.0680460. [Google Scholar]
- Hansen PJ. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:3341–3350. doi: 10.1098/rstb.2009.0131. doi: 10.1098/rstb.2009.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ GM. Maturation of the male reproductive system and its endocrine regulation. Annu. Rev. Entomol. 1992;37:303–320. doi: 10.1146/annurev.en.37.010192.001511. doi: 10.1146/annurev.en.37.010192.001511. [DOI] [PubMed] [Google Scholar]
- Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr. 2009;4:59–67. doi: 10.1007/s12263-009-0113-x. doi: 10.1007/s12263-009-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA. Acclimation: increasing survival at a cost. Trends Ecol. Evol. 1995;10:1–2. [Google Scholar]
- Huang SY, Chen MY, Lin EC, Tsou HL, Kuo YH, Ju CC, et al. Effects of single nucleotide polymorphisms in the 5′-flanking region of heat shock protein 70.2 gene on semen quality in boars. Anim. Reprod. Sci. 2002;70:99–109. doi: 10.1016/s0378-4320(01)00202-0. doi: 10.1016/s0378-4320(01)00202-0. [DOI] [PubMed] [Google Scholar]
- Johnson J, Wang S, Tang J. Estoril, Portugal: 2010. Radio frequency treatments for insect disinfestation of dried legumes. Proceedings of 10th International Working Conference on Stored Product Protection (IWCSPP), June 27–July 2, 2010. [Google Scholar]
- Krebs RA, Feder ME. Experimental manipulation of the cost of thermal acclimation in Drosophila melanogaster. Biol. J. Linn. Soc. 1998;63:593–601. doi: 10.1111/j.1095-8312.1998.tb00331.x. [Google Scholar]
- Kristensen TN, Dahlgaard J, Loeschcke V. Inbreeding affects Hsp70 expression in two species of Drosophila even at benign temperatures. Evol. Ecol. Res. 2002;4:1209–1216. [Google Scholar]
- McNamara KB, Jones E, van Lieshout TH, Simmons LW. Age-dependent trade-offs between immunity and male, but not female, reproduction. J. Anim. Ecol. 2013;82:235–44. doi: 10.1111/j.1365-2656.2012.02018.x. doi: 10.1111/j.1365-2656.2012.02018.x. [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Westwood JT, Tanguay RM. Cell-specific expression and heat-shock induction of Hsps during spermatogenesis in Drosophila melanogaster. J. Cell Sci. 1997;110:1989–1997. doi: 10.1242/jcs.110.17.1989. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Pérez-Crespo M, Pintado B, Gutiérrez-Adán A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 2008;75:40–47. doi: 10.1002/mrd.20759. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Roff DA. Evolutionary quantitative genetics. New York: Chapman & Hall; 1997. [Google Scholar]
- Rutherford SL. Between genotype and phenotype: protein chaperones and evolvability. Nat. Rev. Genet. 2003;4:263–274. doi: 10.1038/nrg1041. [DOI] [PubMed] [Google Scholar]
- Sarge KD. Male germ cell-specific alteration in temperature set point of the cellular stress response. J. Biol. Chem. 1995;270:18745–18748. doi: 10.1074/jbc.270.32.18745. doi: 10.1074/jbc.270.32.18745. [DOI] [PubMed] [Google Scholar]
- Savalli UM, Fox CW. The effect of male mating history on paternal investment, fecundity and female remating in the seed beetle Callosobruchus maculatus. Funct. Ecol. 1999;13:167–177. [Google Scholar]
- Schaefer J, Ryan A. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 2006;69:722–734. doi: 10.1111/j.1095-8649.2006.01145.x. [Google Scholar]
- Simmons LW. Sperm competition and its evolutionary consequences in the insects. Princeton, New Jersey: Princeton University Press; 2001. [Google Scholar]
- Simmons LW. Inbreeding depression in the competitive fertilization success of male crickets. J. Evol. Biol. 2011;24:415–421. doi: 10.1111/j.1420-9101.2010.02179.x. doi: 10.1111/j.1420-9101.2010.02179.x. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Fitzpatrick JL. Sperm wars and the evolution of male fertility. Reproduction. 2012;144:519–534. doi: 10.1530/REP-12-0285. doi: 10.1530/rep-12-0285. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Moore AJ. Evolutionary quantitative genetics of sperm. In: Birkhead TR, Pitnick S, Hosken DJ, editors. Sperm evolution. London: Elsevier; 2009. pp. 405–434. [Google Scholar]
- Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [Google Scholar]
- Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rako L, Takano-Shimizu T, Hoffmann A, Lee S. Effects of small Hsp genes on developmental stability and microenvironmental canalization. BMC Evol. Biol. 2010;10:284. doi: 10.1186/1471-2148-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins JL, Penrose MA, Greeff J, LeBas NR. Additive genetic breeding values correlate with the load of partially deleterious mutations. Science. 2010;328:892–894. doi: 10.1126/science.1188013. doi: 10.1126/science.1188013. [DOI] [PubMed] [Google Scholar]
- Tran BMD, Credland PF. Consequences of inbreeding for the cowpea seed beetle, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) Biol. J. Linn. Soc. 1995;56:483–503. doi: 10.1111/j.1095-8312.1995.tb01106.x. [Google Scholar]
- Utida S. Density dependent polymorphism in the adult of Callosobruchus maculatus (Coleoptera, Bruchidae) J. Stored Prod. Res. 1972;8:111–125. [Google Scholar]
- Wolfner MF. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]


