Abstract
It has been reported that bacteriophage P1 injects DNA into serovar Choleraesuis without evidence of productive infection. However, we found that P1 generates progeny and is capable of transduction in serovar Choleraesuis. This is not the case with other serovars of Salmonella enterica we tested. Therefore, P1 could play a role in serovar Choleraesuis evolution and contribute to its genetic manipulation and analysis.
Keywords: Salmonella, Choleraesuis, Bacteriophage P1, Genetic transduction
1. Introduction
P1 acts as a generalized transducing phage in strains of Escherichia coli and Shigella dysenteriae (Lennox, 1955); it is also able to infect Myxococcus xanthus (Kaiser and Dworkin, 1975) and is therefore considered to exhibit a wide host range. This notion is reinforced by the observation that P1-sensitive mutants are readily obtained in Klebsiella, Enterobacter, Cit-robacter and Erwinia, bacteria normally resistant to infection by P1 (Goldberg et al., 1974). Due to its capacity to encap-sidate a 93,601 bp genome (Lobocka et al., 2004), P1 is not only able to transduce bacterial DNA fragments of 91–100 kb (Provence and Curtiss, 1994), but also conjugative R plasmids in E. coli (Watanabe et al., 1968). Therefore, P1 and P1-like phages should play an important role in lateral gene transfer among the corresponding infectable bacteria and thus in the evolution of such microorganisms.
Salmonella enterica serovars are not particularly susceptible to P1 and usually galE mutants need to be derived for successful P1 infection (Ornellas and Stocker, 1974). Nevertheless, Kelly et al. (1992) used P1L4 to transduce chromosomal genes into wild-type S. enterica serovar Choleraesuis without further reporting on P1 behavior in this bacterium. This gave us an indication that serovar Choleraesuis could be a host in which P1 could become established, proliferate and eventually out-transduce genetic material. In this study we show that P1 is able to lysogenize wild-type serovar Choler-aesuis, to generate progeny in this bacterium and to mediate transduction of a model plasmid contained therein and also of chromosomal loci.
2. Materials and methods
2.1. Biologicals
The main bacterial strains, plasmid and phage used are listed in Table 1. Additional bacterial strains are indicated in Table 2 or in the text. The primers for the traJ gene of plasmid pLM2 were purchased from Integrate DNA Technologies (Coralville, IA). Bacteriological media and/or components were from Oxoid (Basingstoke, UK). Antibiotics and reagents were from Sigma (St. Louis, Mo).
Table 1.
Main bacterial strains and plasmid used in this study.
| Strains and plasmid | Description | Source or reference |
|---|---|---|
| χ289 E. coli K-12 | F−supE42 λ − T3r | R. Curtiss III |
| χ1849 E. coli K-12 | F−tonA53 dapD8 minA1 purE41 supE42 Δ40[gal-uvrB] λ −minB2 his-53 nalA25 metC65 oms-1 T3r Δ29[dio-asd] ilv-277 cycB2 cycA1 hsdR2; amber suppressor | R. Curtiss III |
| χ1849 E. coli K-12 (pLM2) | F−tonA53 dapD8 minA1 purE41 supE42 Δ40[gal-uvrB] λ −minB2 his-53 nalA25 metC65 oms-1 T3r Δ29[dio-asd]ilv-277 cycB2 cycA1 hsdR2; amber suppressor Kmr, Apr(am), Tetr(am) | This study |
| χ1912 E. coli C Nalr | Spontaneous Nalr mutant, naturally restrictionless | This study |
| χ1912T E. coli C Nalr | Spontaneous Nalr mutant, naturally restrictionless, P1clm,clr100 | This study |
| χ1912C E. coli C Nalr | Spontaneous Nalr mutant, naturally restrictionless, P1 cured | This study |
| χ1932T E. coli K-12 | F− prototroph, T6s, λ −nalA, Strs, T3r, P1clm,clr100 | R. Curtiss III |
| χ1932C E. coli K-12 | F− prototroph, T6s, λ −, nalA, Strs, T3r, P1 cured | This study |
| χ2605 E. coli K-12 | Smr, Lac−, Sup0 | R. Curtiss III |
| χ2605 E. coli K-12 (pLM2) | Smr, Lac−, Sup0, Kmr, Tetr(am), Apr(am) | This study |
| χ3246 S. Choleraesuis | Wild-type, pig isolate, Smr, Eryr, Lac− | R. Curtiss III |
| χ3246T S. Choleraesuis | Cmr, Smr, Lac−, Tets, P1clm,clr100 | This study |
| χ3246 S. Choleraesuis (pLM2) | Smr, Lac−, Kmr, Apr(am), Tetr(am) | This study |
| χ3246T S. Choleraesuis (pLM2) | Cmr, Smr, Lac−, Kmr, Apr(am), Tetr(am), P1clm,clr100 | This study |
| χ3246R S. Choleraesuis | Smr, Rifr, Lac− | This study |
| χ3246R S. Choleraesuis (pLM2) | Smr, Rifr, Lac−, Kmr, Apr(am), Tetr(am) | This study |
| χ4390 S. Choleraesuis | Nals, Tetr, Mot−fli-8007∷Tn10 | R. Curtiss III |
| χ4390T S. Choleraesuis | Tetr, Cmrr, Mot−fli-8007∷Tn10, P1clm,clr100 | This study |
| χ3246R2 S. Choleraesuis | Smr, Nalr, Lac− Mot+ | This study |
| Plasmid | ||
| pLM2 | RP4 derivative, Kmr, Tetr(am), Apr(am); 60 kb. | 11 |
Table 2.
Susceptibility of Salmonella serovars to P1clm,clr100 infection and productive lisogenization.
| Strain | P1clm,clr100 infection | None | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| LB agar | LB agar (Cm) | LB agar | LB agar (Cm) | |||||
|
|
|
|
|
|||||
| 30 °C | 42 °C | 30 °C | 42 °C | 30 °C | 42 °C | 30 °C | 42 °C | |
| χ3201 S. Agonab | + | − | + | − | + | + | − | − |
| χ3215 S. Londona | + | + | + | + | + | + | − | − |
| χ3217 S. Montevideoa | + | + | + | + | + | + | − | − |
| χ3218 S. Nienstedtena | + | + | + | + | + | + | − | − |
| χ3219 S. Othmarschena | + | + | + | + | + | + | − | − |
| χ3225 S. Virchowa | + | + | + | + | + | + | − | − |
| χ3228 S. Bovisa | + | − | + | + | + | + | − | − |
| χ3229 S. Anatuma | + | + | + | + | + | + | − | − |
| χ3246 S. Choleraesuisb | + | − | + | − | + | + | − | − |
| χ3247 S. Choleraesuisb | + | − | + | − | + | + | − | − |
| χ3000 S. Typhimurium | + | + | − | − | + | + | − | − |
| χ289 E. coli K-12 | + | − | + | − | + | + | − | − |
+: growth; −: no growth.
P1 infectable and transposition derivatives.
P1 infectable and lysogenizable.
2.2. Techniques
Molecular techniques were performed as described (Sambrook and Russell, 2001). Genetic manipulations were basically according to Miller (1972) and Provence and Curtiss (1994). The latter are briefly described below.
2.3. Lysogenization
P1cml,clr100-susceptible bacterial strains were lysogenized by infecting log-phase cells grown with aeration at 37 °C in LB broth (Miller, 1972) supplemented with 10 mM CaCl2. P1cml,clr100 was added at a multiplicity of infection (m.o.i.) of 1 and after incubation at 30 °C for 30 min the bacteria-bacteriophage mix was plated onto LB agar (1.5%) supplemented with chloramphenicol (Cm, 12.5 μg ml−1). Cm-resistant lysogenic colonies were recovered after a 48 h incubation at 30 °C.
2.4. Preparation of P1cml,clr100 lysates
Lysogenic bacterial strains were grown with aeration at 30 °C in 10 ml of LB broth supplemented with 10 mM MgSO4 up to a density of 2 × 108 cells ml−1. Then the culture was shifted to 42 °C and incubated further for 35 min; finally, it was kept at 37 °C for an hour until lysis was apparent. Chloroform (100 μl) was added and cell debris removed by centrifugation at 9300 × g in an Eppendorf 5415D centrifuge. Lysates were kept at 4 °C under chloroform until they were used.
2.5. Transduction
Recipient bacterial strains were grown overnight in LB broth with aeration at 37 °C. Cells were pelleted by centrifugation and resuspended in an equivalent volume of MC (100 mM MgSO4, 5 mM CaCl2). Then, 0.1 ml of the cell suspension was mixed with 0.1 ml of the P1cml,clr100 corresponding donor lysate and incubated at 30 °C for 30 min, period after which 0.2 ml of 1 M sodium citrate was added together with 3 ml of LB soft agar (0.65%). The entire mix was plated onto appropriate selective LB agar plates to recover transductant colonies after incubation at 37 °C for 48 h.
2.6. P1cml,clr100 adsorption determinations
To determine adsorption of P1cml,clr100 to serovar Chol-eraesuis cells and to control bacteria, exponentially growing cultures (1 × 108 cells ml−1) were infected with the phage at an m.o.i. of 1. Samples of infected cultures were taken at time 0 and up to 20 min incubation at 37 °C, centrifuged to pellet cells with adsorbed phage and the supernatants transferred to fresh tubes and treated with chloroform. Titration of phage remaining in supernatants was performed using the agar layer technique as described by Goldberg et al. (1974) employing E. coli χ289 as indicator strain.
3. Results and discussion
3.1. Lysogenization of serovar Choleraesuis
To test whether P1 could establish lysogeny in serovar Choleraesuis and other Salmonella serovars, we infected wild-type strains with P1cml,clr100. This phage derivative confers Cm resistance to lysogens and renders them thermosensitive as they are unable to grow at 42 °C. Using the lysogenization procedure described above we had no difficulty in obtaining Cmr serovar Choleraesuis χ3246 colonies. These were tested for thermosensitivity and were shown to be unable to grow at 42 °C as opposed to 30 °C (see Fig. 1A for representative results). The lysogens we have tested are stable for at least 81 generations.
Fig. 1.
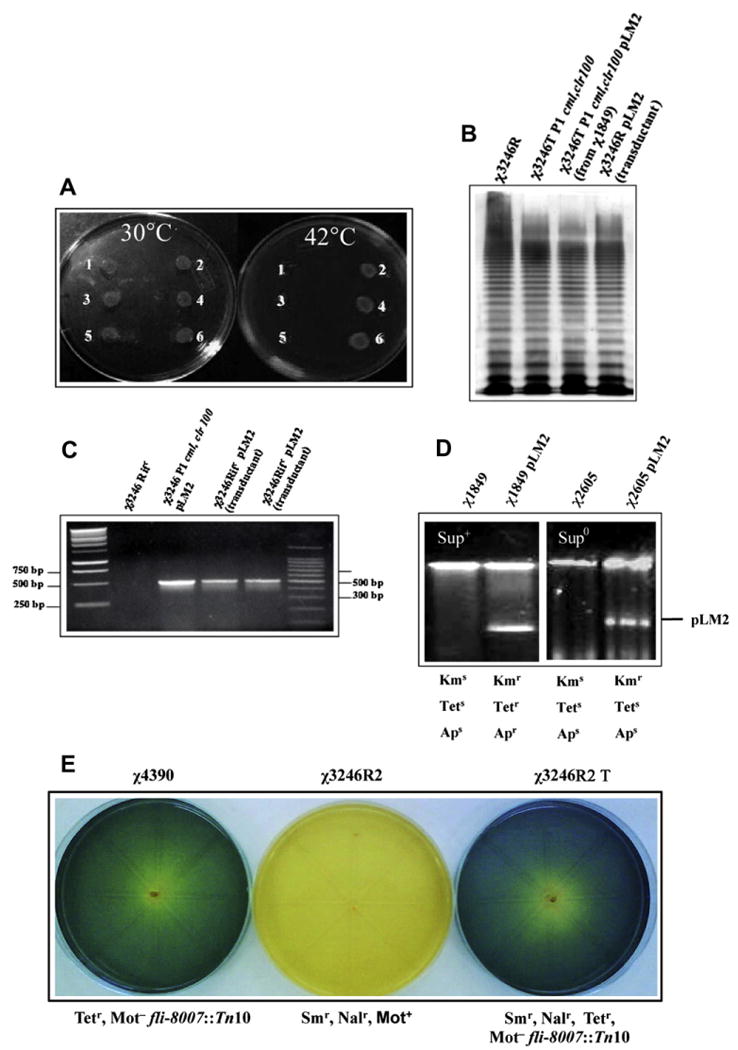
A. Spots tests of P1 lysogens. 1 and 3 correspond to two different Cmr derivates of serovar Choleraesuis χ3246 obtained by infection with P1cml,clr100; 5 corresponds to E. coli χ1912T; 2 and 4 correspond to a duplicate (two independent colonies) of wild-type serovar Choleraesuis χ3246; 6 corresponds to E. coli C χ1912C. B. LPS profile; C. PCR verification of traJ gene (500 bp) present in pLM2, outer lanes are 1 kb and 100 bp DNA ladder respectively; D. Plasmid profile and antibiotic patterns of the bacterial strains indicated.
However, when we attempted lysogenization of other serovars of S. enterica with P1cml,clr100 no positive results were obtained. We were only able to detect Cm-resistant transposition derivatives that grew well both at 30 °C and 42 °C. All these results are shown in Table 2. Lysogens were always obtained with χ289, an E. coli K-12 strain used as positive control.
Lysates induced in χ3246T serovar Choleraesuis strains form plaques on χ1912 E. coli C at 37 and 42 °C. However, plaques were not detected in wild-type serovar Choleraesuis under the same plating conditions. We also determined that P1cml,clr100 established lysogeny at a frequency of 1 × 10−4 lysogens/pfu, a value similar to that reported for mutants of serovar Typhimurium (Goldberg et al., 1974), probably galE, reported to be susceptible to P1 infection (Ornellas and Stocker, 1974). In contrast, we found that serovar Choler-asuis lysogens maintain the galactose-fermenting phenotype on McConkey-galactose plates, and their smooth LPS profile (Fig. 1B), an indication that P1 naturally infects wild-type serovar Choleraesuis. In addition, we noted that lysogenization in serovar Choleraesuis was approximately two orders of magnitude lower than that reported for E. coli strains (Thomas and Kay, 1984), a fact that is probably restriction-related, as P1cml,clr100 was originally propagated in E. coli K-12 χ1932T (with a yield of ∼ 109 pfu/ml). However, when P1cml,clr100 was prepared from a serovar Choleraesuis lysogen (with a low yield of ∼2 × 106 pfu/ml) and used to infect the wild-type strain of Choleraesuis, the frequency of lysogenization was about 4 × 10−3 lysogens/pfu, an observation that suggests additional difficulties for infection over the effect of restriction.
P1 resistance in serovars of Salmonella other than Choleraesuis seems to be due to blockage of P1 adsorption by the O antigen (Ornellas and Stocker, 1974). However, serovar Choleraesuis belonging to group C1, possessing antigen O6,7 (LeMinor, 1991) displays less hindrance to infection by P1, a fact that might reflect increased availability particular to the O6,7 antigen. In fact, we determined that the titer of P1cml,clr100 decreased by 87% in the supernatant of the serovar Choleraesuis strain infected with the phage after 20 min incubation at 37 °C. A similar value (70%) was observed for E. coli χ289. In contrast, only a 17% decrease was observed when using a serovar Typhimurium strain (LT2) and practically no decline in phage titer with a strain of Staphylococcus spp. This points out the particular sensitivity of serovar Choleraesuis to P1cml,clr100 infection. Most importantly, however, Choleraesuis was the only serovar we tested that consistently yielded P1cml,clr100 lysogens that bred true.
3.2. Transduction of plasmid pLM2
We then sought to test the full functionality of P1clm,clr100 contained in serovar Choleraesuis in terms of its ability to transduce genetic material. To this effect, we used plasmid pLM2, a transmissible IncP, RP4-derived plasmid coding Kmr with amber-suppressible Apr and Tetr (Mindich et al., 1976). pLM2 was conjugated at 30 °C as described by Provence and Curtiss (1994) from χ1849 E. coli K-12 harboring pLM2 into serovar Choleraesuis χ3246T Transconjugants were selected at 30 °C on LB agar containing Cm (20 μg/ml) and Km (50 μg/ml). One of these transconjugants was purified and used to prepare P1cml,clr100 lysates by induction at 42 °C Lysates were used to infect (1:1 bacteria/phage) χ3246R serovar Choleraesuis and transductants were selected on LB agar plates containing Rif (100 μg/ml) and Km. We chose to assay transduction into serovar Choleraesuis to minimize restriction-related lowering of transduction frequencies. In six separate experiments, we found a mean transduction frequency of 3.3 × 10−7 trans-ductants/pfu. Presence of pLM2 in the donor strain and in transductants was detected by PCR amplification of the traJ gene with primers 5′-AAGCTCGTCCTGCTTCTCTTCGAT-3′ and 5′-ACTTTCCTTGGTGTATCCAACGGC-3′, followed by agarose (0.8%) gel electrophoresis (Fig. 1C). Plasmid pLM2 contained in serovar Choleraesuis transductants was further checked by conjugating it into the amber suppressor strain E. coli K-12 χ1849. Transconjugants were selected on LB agar plates containing DAP, Nal and Km. From one of the χ1849 transconjugants harboring pLM2 the plasmid was conjugated into the non-suppressor E. coli K-12 χ2605 using Km, Sm and DAP-less selection. These strains showed the expected profile of antibiotic resistance-sensitivity to Km, Ap (25 μg/ml) and Tet (12.5 μg/ml), and the corresponding plasmid content (Fig. 1D). We also verified that P1cml,clr100 lysates prepared from serovar Choleraesuis lysogens containing pLM2 transduced this plasmid into E. coli C χ1912 at a mean frequency of 1 × 10−4 transductants/pfu.
3.3. Transduction of chromosomal markers
In addition, we found that P1cml,clr100 transduces chromosomal genetic material in serovar Choleraesuis. In these experiments, we used strain χ4390, a non-motile mutant due to insertion of transposon Tn10 in the fli gene (Table 1). Therefore, non-motility (Mot−) is associated with Tet resistance. S. Choleraesuis χ4390 was infected with P1cml,clr100 and lysogens were selected on LB Cm Tet agar plates at 30 °C. Derivatives that were Cmr Tetr and grew at 30 °C, but not at 42 °C (χ4390T), were employed to generate P1cml,clr100-transducing lysates to infect motile strain χ3246R2, a nalidixic acid-resistant (Nalr) derivative of a wild-type, motile serovar Choleraesuis strain. Transductants were selected on LB Tet Nal agar plates incubated at 42 °C. The transductants obtained (Trd) consistently were found to be non-motile in OF basal medium (Merck, Darmstadt) supplemented with 1% glucose (Fig. 1E). The mean frequency of transduction obtained from four independent experiments was 8.8 × 10−7 transductants/pfu. Using the same donor strain genetic background, two other chromosomal mutations, metE862∷Tn10 and crp-773∷Tn10, were also transduced at frequencies of 3 × 10−7 and 8.3 × 10−7 respectively. The first mutation confers methionine auxotrophy and the second the inability to ferment maltose in the corresponding transductants. Therefore, it is safe to assume that the phenomenon detected corresponds to P1cml,clr100-mediated generalized transduction.
Taken together, results presented indicate that P1 and P1-related phage might play an important role in lateral gene transfer involving serovar Choleraesuis, other P1-sensitive serovars of Salmonella and P1-susceptible bacteria in the Enterobacteriaceae or other phylogenetic groups. In the case of transduced conjugative plasmids, these could be further dispersed by conjugation into compatible bacterial genetic backgrounds.
In addition, we envisage P1-mediated dispersal of serovar Choleraesuis virulence genes and genes for metabolic functions, scenarios that are known to occur in the complex network of genetic interactions entailed in bacterial evolution (Ochman et al., 2000).
Acknowledgments
We thank the Vice-Rectoría de Investigación y Estudios Avanzados Pontificia Universidad Católica de Valparaíso for financial support. We are particularly grateful to Kenneth Roland (Biodesign Institute at Arizona State University, USA) for his criticism and suggestions and to Natalia Riquelme (Pontificia Universidad Catolica de Valparaiso) for assistance in experiments.
References
- Goldberg RB, Bender RA, Streicher SL. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974;118:810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D, Dworkin M. Gene transfer to a Myxobacterium by Escherichia coli phage P1. Science. 1975;187:653–654. doi: 10.1126/science.803710. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Bosecker BA, Curtiss R., III Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect Immunol. 1992;60:4881–4890. doi: 10.1128/iai.60.11.4881-4890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMinor L. The genus Salmonella. In: Balows AH, Trüper H, Dworkin M, Harder W, Schleifer KH, editors. The Prokaryotes A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. second. III. Springer-Verlag; New York: 1991. [Google Scholar]
- Lennox ES. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:199–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lobocka MB, Rose DJ, Plunkett G, Rusin M, Samojedny A, Lehnherr H, Yarmolinsky MB, Blattner FR. Genome of bacte-riophage P1. J Bacteriol. 2004;186:7032–7068. doi: 10.1128/JB.186.21.7032-7068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory. Cold Spring Harbor; New York: 1972. [Google Scholar]
- Mindich L, Cohen J, Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976;126:177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- Ornellas EP, Stocker BAD. Relation of lipopolysaccharide character to P1 sensitivity in Salmonella typhimurium. J Virol. 1974;60:491–502. doi: 10.1016/0042-6822(74)90343-2. [DOI] [PubMed] [Google Scholar]
- Provence DL, Curtiss R., III . Gene transfer in gram negative bacteria. In: Gerhardt P, Murray RGE, Wood WA, Kreig NR, editors. Methods for General and Molecular Bacteriology. American Society for Microbiology; Washington DC: 1994. [Google Scholar]
- Sambrook J, Russell W. Molecular Cloning; a Laboratory Manual. third. Cold Spring Harbor Press; NY: 2001. [Google Scholar]
- Thomas JM, Kay WW. Effect of bacteriophage P1 lysogeny on lipopolysaccharide composition and the lambda receptor of Escherichia coli. J Bacteriol. 1984;159:1047–1052. doi: 10.1128/jb.159.3.1047-1052.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Furuse C, Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968;96:1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


