Abstract
Serum autoantibodies, directed against oncogenic proteins, have been frequently detected in the sera of breast cancer patients. It is unknown whether serum antibodies that are identified in patients with established disease could also be detected in patients with newly diagnosed disease or even predate the diagnosis of breast cancer. Using sera collected at the time of treatment, at the time of diagnosis, or prior to the time of diagnosis, the current study aimed to address the temporal relationship between breast cancer development and serum antibody response. Starting from serum antibodies to eight known breast cancer antigens, we first identified four serum antibodies, HER-2/neu, p53, CEA, and cyclin B1, which are significantly increased in the sera collected from breast cancer patients at the time of treatment. These antibodies were also elevated in breast cancer sera collected at the time of diagnosis. Lastly, comparison of antibody responses in pre-diagnostic samples from women prior to the development of breast cancer and in controls demonstrated that antibodies to the HER-2/neu and p53 can be detected in sera that were collected on average more than 150 days before a breast cancer diagnosis. These results demonstrated that serum autoantibodies commonly reported in sera from patients with established disease can also be detected in pre-diagnostic sera and may be useful for the early detection of breast cancer.
Keywords: serum antibody, breast cancer, early detection
Introduction
Despite the wide-spread use of mammography for the early detection of breast cancer in the USA, approximately 40% of breast cancers do not have a localized stage at diagnosis (1). The development of new biomarkers that may help in the early detection of breast cancer have the potential to facilitate clinical management of the disease and improve survival rates.
Serum antibodies to oncogenic proteins have been detected in the sera of patients with different types of cancer, including breast cancer (2, 3). For example, in a large study of nearly 10,000 cancer patients with a wide variety of tumors p53 specific antibodies were present in 20–40% of those harboring the p53 missence mutation (4, 5). Our group has demonstrated that HER-2/neu (HER2) antibody immunity can be detected in early stage breast cancer patients and is positively correlated with overexpression of the HER2 protein by the tumor (6, 7). The potential for using serum antibodies as cancer diagnostic biomarkers has also been demonstrated by others (2, 8–10). A tumor antibody signature composed of 22 antigen fragments had 88.2% specificity and 81.6% sensitivity in discriminating between patients with and without prostate cancer (2). Another study of antibodies against defined tumor antigens in serum specimens from 527 patients with cancer and 346 controls found that a panel of seven antigens was useful for the diagnosis of cancer (9). More recently, a study focused on early stage breast cancer showed that a classifier based on 28 serum autoantibodies can discriminate cancer patients from control women with a sensitivity of 80.8% and a specificity of 61.6% (AUC = 0.756) (10). With these encouraging results, we hypothesized that serum antibodies may develop before the clinical diagnosis of disease and may aid in early detection.
The immune system can respond to immunogenic proteins when those proteins are present at low levels not detectable by direct protein screening (11). Theoretically, serum antibodies to oncogenic proteins may develop before the clinical onset of disease. Indeed, it has been shown that serum antibody to p53 can be detected in high risk populations such as patients with chronic obstructive pulmonary disease and Barrett’s esophagus and predate the diagnosis of cancer (4, 12). With respect to breast cancer, there is some evidence that serum antibodies can be detected in early stage cancer patients and in patients with carcinoma in situ (13–15), but it has not been previously explored whether serum antibodies can be detected in asymptomatic populations and predate cancer diagnosis. We assessed whether a panel of candidate tumor antigens that are commonly identified in patients with established disease elicit a similar antibody response in sera from newly diagnosed patients and sera drawn before breast cancer diagnosis.
Materials and Methods
Subjects
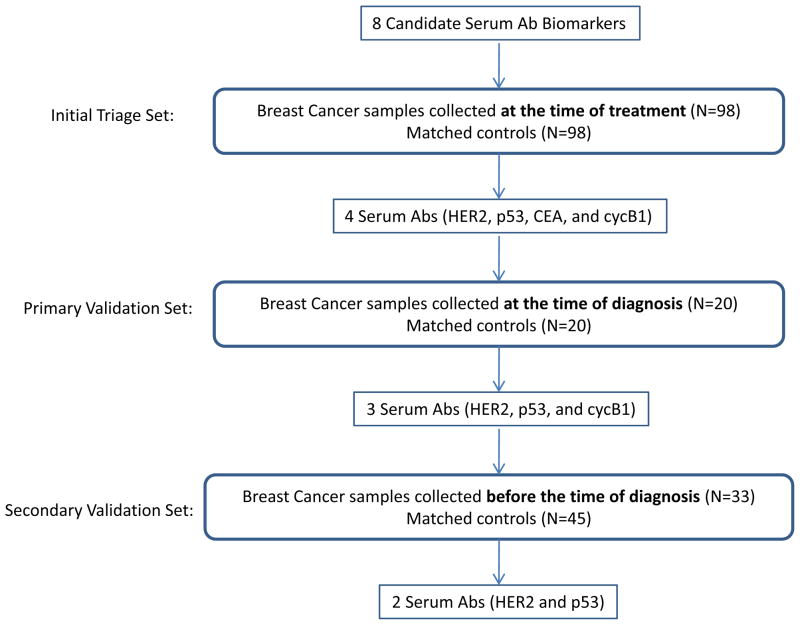
The use of human samples was approved by the University of Washington Institutional Review Board. Three independent sample sets, collected at different times as to breast cancer diagnosis, were used for this biomarker study (Figure 1). The initial triage set consisted of 98 breast cancer samples (age range: 34–76; average: 52) collected at the time when they started treatment (distant from diagnosis) at the Tumor Vaccine Group at University of Washington and 98 age-matched controls (age range: 24–76; average: 52) collected at the Puget Sound Blood Center (Seattle, WA). The stage distribution for the breast cancer patients was 19% stage II, 48% stage III, and 34% stage IV. The primary validation set consisted of sera collected at the time of diagnosis from 20 stage III breast cancer patients and 20 age-matched controls from the MD Anderson Cancer Center (Houston, TX). The secondary validation set consisted of pre-diagnostic samples collected from 78 women that participated in the Women’s Health Initiative (WHI) cohort studies. The WHI is a major epidemiologic and disease prevention project, sponsored by National Institute of Health (NIH) to address the most common causes of death, disability and impaired quality of life in postmenopausal women. It involves 161,808 women aged 50–79, and includes one of the most definitive, far-reaching clinical trials of post-menopausal women’s health ever undertaken. We have analyzed antibody responses in plasma samples collected from 33 cases and 45 controls that were matched for age, date of blood collection and duration of storage (within six months). Breast cancer sera were collected on average more than 150 days (range: 21–262 days) before a breast cancer diagnosis. Controls were selected to be free of breast cancer throughout a follow-up period of 5 years, and cases and controls were selected that were never users of postmenopausal hormone therapy.
Figure 1.
Schematic diagram of study design.
Assessment of autoantibody reactivity using ELISA
The measurement of serum autoantibodies to tumor antigen HER2 was carried out using capture ELISA using SKBR3 cell lysate as the source of HER2 antigen, as previously described (16). In brief, plates were coated with 520C9, a murine monoclonal antibody directed against human HER-2/neu and then incubated with SKBR3 cell lysate. Unbound lysate was washed off before addition of serum samples. After incubation and washing, goat anti-human IgG-HRP conjugate (Zymed) was added and TMB substrate (Kirkegaard and Perry) was used for color development (16). Using Western blot as the “gold standard”, the HER2/neu capture ELISA assay had a sensitivity of 89% and a specificity of 77% (16). Assays for the other tumor antigens were done using recombinant proteins, p53 (Sigma Chemicals, St. Louis, MO), insulin-like growth factor binding protein 2 (IGFBP2, Sigma), carcinoembryonic antigen (CEA, Protein Sciences Corporation, Meriden, CT), MUC-1 (Abnova, Taipei, China), cyclin B1 (cycB1, U.S. Biological, Swampscott, MA), topoisomerase II alpha (TOPO2α, Topogen, Columbus, OH), and cathepsin D (catD, U.S. Biological). The recombinant proteins were coated overnight on 96-well plates in carbonate buffer. After blocking, the sera was added in duplicate titration sets and the plate was developed as described previously (17).
Assays of serum autoantibodies to tumor antigens using protein arrays
Assays of serum autoantibodies to tumor antigens in the WHI samples were done using customized protein microarrays as previously described (18). In brief, recombinant proteins were arrayed in duplicate onto nitrocellulose-coated slides using a contact printer. Serum samples were hybridized with the protein microarray for 3 hours at 4 °C. Slides were then incubated with Cy5-labeled anti-human IgG for 1 hour at 4 °C. Local background-subtracted median spot intensities for downstream statistical analysis were generated using GenePix software.
Statistical analysis
The level of serum antibodies between cancer and control were compared using an unpaired Student t test with Welch’s correction. Difference in the incidence of a serum antibody response between cancer cases and controls was evaluated using a χ2 test. The sensitivity and specificity of the test based on a single or combination of antibodies were evaluated using the receiver-operating-characteristic (ROC) curve analysis, leading to estimates of the area under the curve (AUC), with 95% confidence intervals. Statistical analysis was carried out in SPSS software, version 15.0.
Results
Antibodies to HER2, p53, CEA, and cycB1 are increased in sera collected distant from diagnosis or at the time of diagnosis
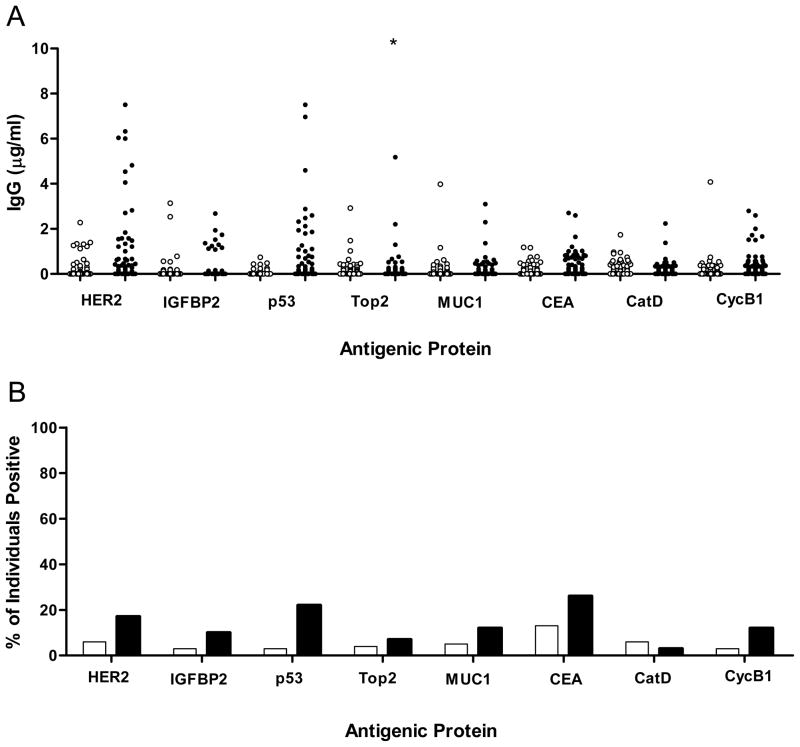
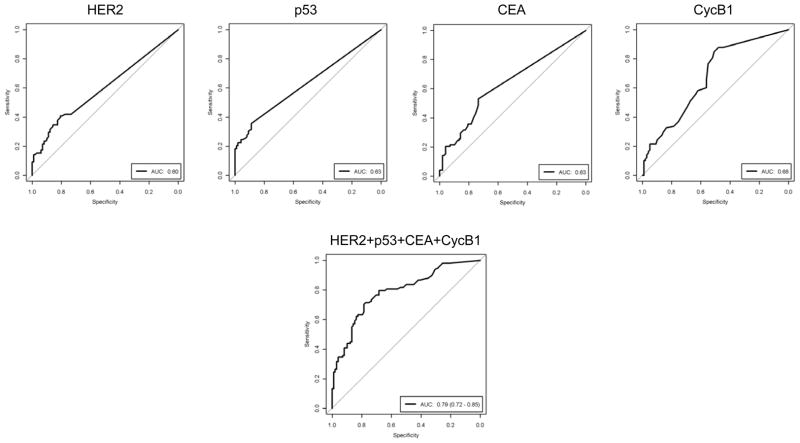
We first evaluated serum antibody levels using sera collected at the time of treatment (distant from diagnosis). Eight candidate tumor antigens selected based on prior studies (HER2, IGFBP2, p53, Topo2α, MUC1, CEA, catD, cycB1) (17) were investigated for autoantibodies in these sera. Four antigens (HER2, p53, CEA, and cycB1) exhibited statistically significantly increased autoantibody responses in cancer patients compared to controls: HER2 (0.67±0.15 μg/ml in case vs. 0.14±0.04 μg/ml in control, p=0.0012), p53 (0.49±0.17 μg/ml in case vs. 0.04±0.01 μg/ml in control, p=0.0005), CEA (0.27±0.05 μg/ml in case vs. 0.10±0.02 μg/ml in control, p=0.0022), and cycB1 (0.34±0.05 μg/ml in case vs. 0.17±0.04 μg/ml in control, p=0.011; p=0.0001 when one outlier value in the control group was removed) (Fig. 2A). A sample was defined as positive if the magnitude of response was greater than mean+2SD of the previously analyzed reference population. The percentage of individuals that are positive for the antibody response, as judged by this criteria, was also significantly higher in cancer patients than controls for the 4 antibodies: HER2 (17% in case vs. 6% in control, p=0.02), p53 (22% vs. 3%, p<0.0001), CEA (26% vs. 13%, p=0.03), and cycB1 (12% vs. 3%, p=0.03) (Fig. 2B). It is noted that none of the single antigen antibody response fractions was higher than 30%. However, sixty-one percent of patients have an antibody response to at least one of the 4 antigens. The sensitivity and specificity of tests based on individual markers or the sum of the 4 serum autoantibody IgG levels are shown in Figure 3. The unweighted sum of the 4 antibody levels results in an AUC of 0.79 (95% CI: 0.73–0.85, p<0.001). At a cutoff value of 0.43 μg/ml total serum antibodies (sum of 4 antibody levels), the assay has a sensitivity of 79% and a specificity of 68% in the samples collected at a time distant from diagnosis.
Figure 2. Breast cancer patients have elevated levels of serum antibodies to HER2, p53, CEA, and cyclin B1.
(A) Shown are the magnitude of antibody responses (μg/ml) in 98 breast cancer patients (filled circles) and 98 controls (empty circles). Each data point represents the value from an individual cancer or control donor. *, indicates two out-of-range values, 11.26 and 11.50 μg/ml. (B) The columns represent the percentage of individuals positive for a single antibody response in breast cancer patients (black columns) and control donors (white columns).
Figure 3. Sensitivity and specificity of the assay based on individual or combination of 4 serum antibodies for differentiating between breast cancer and control in the initial triage set.
ROC curves indicate the level of serum antibody (IgG) levels in sera from cancer patients (N=98), as compared with the Ab levels in sera from control donors (N=98). AUC denotes area under the curve.
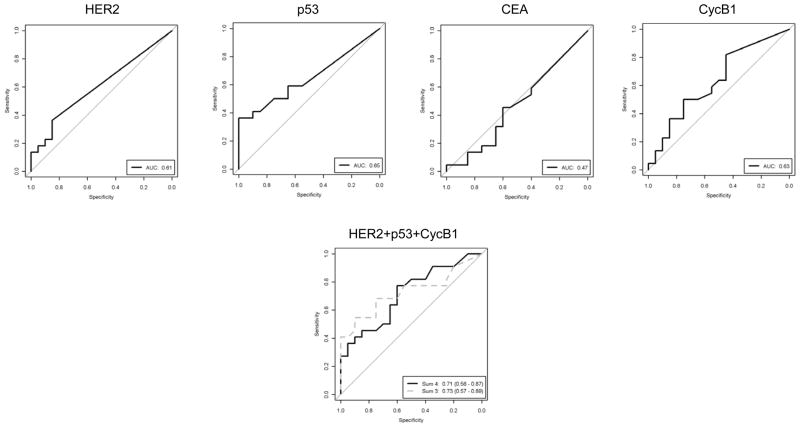
We then determined the performance of these four antibody levels in sera from newly diagnosed breast cancer patients. As shown in Figure 4, three of the four antibodies, HER2, p53, and cycB1, were more frequently detected in sera collected from patients with newly diagnosed breast cancer than controls. An unweighted combination of the three Abs results an AUC of 0.73 (95% CI: 0.56 to 0.87, p=0.018).
Figure 4. Sensitivity and specificity of the assay based on individual or combination of 3 serum antibodies for differentiating between breast cancer and control in the validation set of samples from newly diagnosed patients.
ROC curves indicate the level of antibodies to HER2, p53, CEA, and cycB1, or the combination of them in sera from cancer patients (N=20), as compared with the Ab levels in sera from control donors (N=20). ROC curves based on both sum 3 (HER2+p53+cycB1, dotted grey line) and sum 4 (HER2+p53+CEA+cycB1, solid line) are shown.
Antibodies to HER2 and p53 can be detected in pre-diagnostic sera
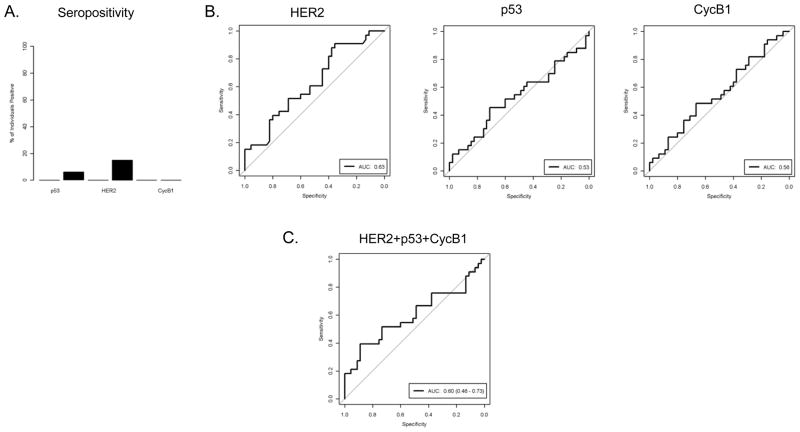
We next questioned whether these serum antibodies can be detected in sera drawn before the time of diagnosis. Using pre-diagnostic sera from the WHI study, we found that 15% of cases were positive for HER2 Abs, and 6% were positive for p53 Abs, while no controls were positive for Abs to either protein (Fig. 5A). No tested samples were positive for cycB1 Abs. This is concordant with the presented findings in late-stage patients, where HER2 and p53 had a higher incidence of Abs than cycB1 (Fig 2B). The performance of each individual serum antibody and combinations are shown in Fig. 5B and C. The pre-diagnostic sera have significantly increased serum antibody responses to HER2 (AUC=0.63, p=0.026) but the responses to p53 and cycB1 were not different from control. Because the pre-diagnostic sera were obtained at a wide range of time (ranging 22 to 262 days before diagnosis), we questioned whether HER2 antibody might develop over time and is more frequently found in samples collected close to the time of diagnosis. As shown in Figure 6, there is no clear association between HER2 antibody level and the time prior to diagnosis, and the 5 positive samples (shown in black filled dots) spread over the range of time prior to diagnosis.
Figure 5. Sensitivity and specificity of the assay based on individual or combination of 3 serum antibodies for differentiating between breast cancer and control pre-diagnostic sera from WHI study.
(A) The seropositivity of antibodies to p53, HER2, and cycB1 in control and cases in WHI samples. The black bars indicate response rates in cases. The response rate in control was zero for all three antibodies. (B) ROC curves indicate the level of serum antibody (IgG) level to individual antigens, HER2, p53, and cycB1, in sera from cancer patients (N=33), as compared with the Ab levels in sera from control donors (N=45). AUC denotes area under the curve. (C) ROC curve indicates the level of serum antibody (IgG) level to the combination of HER2, p53, and cycB1 in sera from cancer patients and control. AUC denotes area under the curve.
Figure 6. Distribution of HER2 antibody responses over time prior to diagnosis.
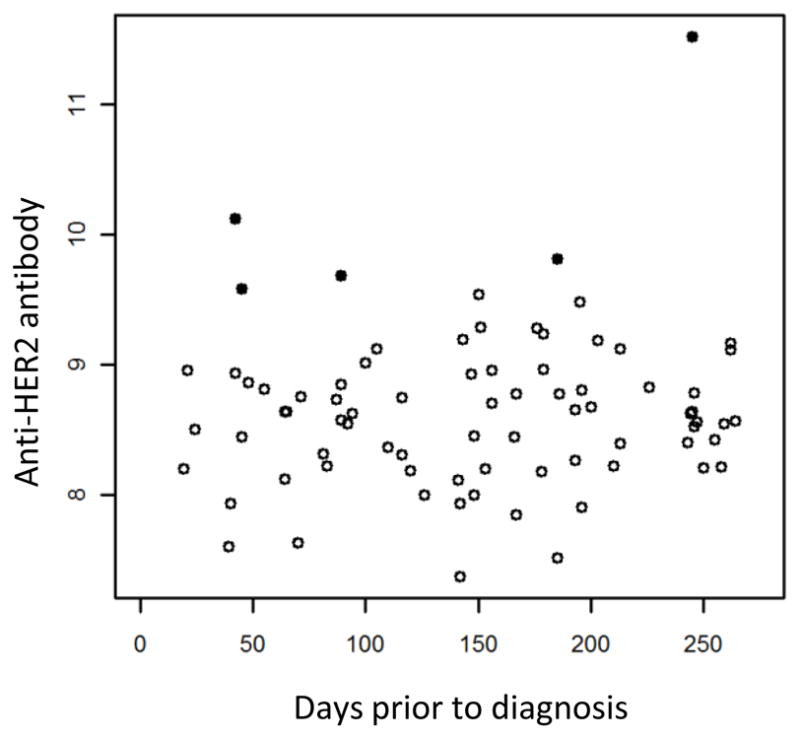
Shown are anti-HER2 IgG levels (log2 transformed fluorescent intensities) in the 78 WHI sample (33 cases and 45 controls). The 5 samples that are positive for HER2 antibody response are shown as filled black dots. The other samples are shown as unfilled circles.
Discussion
Studies described here show that multiple cancer specific autoantibodies are present in the sera of breast cancer patients collected distant from diagnosis or at the time of diagnosis, and the combination of autoantibodies can significantly discriminate cancer patients from controls. We further show that a fraction of these autoantibodies are present in pre-diagnostic sera and is potentially useful for early detection.
Due to the limited availability of pre-diagnostic sera, the standard approach for biomarker discovery is to use samples from symptomatic patients and often from patients with advanced disease (19). Established cancer may behave differently from pre-clinical disease, so it remains unknown whether markers identified from patients with established disease can also apply to samples collected at earlier time points, at the time of diagnosis or even before diagnosis. Investigations in ovarian cancer have shown that none of the biomarker panels that were discovered in diagnostic samples of ovarian cancer could be validated in pre-diagnostic samples (20). Interestingly, our results suggest that autoantibodies discovered in sera from established disease may also be detectable in pre-diagnostic sera. Indeed, out of the 4 serum antibody markers identified from sera collected at time of treatment, three of them (HER2, p53, and cycB1) can be validated in independent serum samples collected at the time of diagnosis. Furthermore, two of them (HER2 and p53) can be detected in WHI samples that were collected on average at 153 days before diagnosis. The biological differences between breast and ovarian tumor development could have contributed to the observed difference on whether serum antibodies from post-diagnostic samples may apply to pre-diagnostic samples. Our results on p53 are also consistent with previous reports on lung cancer that serum antibody to p53 can be detected in high risk populations before clinical diagnosis of cancer (4, 5, 12).
The two antigens that induce serum antibodies in pre-diagnostic sera, p53 and HER2, both play critical roles in tumor development. p53 is a nuclear phosphoprotein that normally acts as a tumor suppressor by inhibiting uncontrolled cell growth. Mutation in p53, which can occur in up to 50% of all cancers, inactivates the normal function of p53 and results in “immortalized cells” (17). Previous studies on lung cancer have shown that the anti-p53 antibody can be detected in high risk populations prior to clinical diagnosis of cancer (4, 12). Using WHI samples, our study demonstrated that p53 antibody can be detected in 6% of women who subsequently developed breast cancer. Similar to p53, HER2 is a receptor tyrosine kinase that plays an important role in tumorigenesis. The amplification and overexpression of HER2 occurs in 10%–30% of human primary breast cancers and is associated with aggressive tumor behavior and poor prognosis (21). We have previously shown that HER2 antibodies at titers of >1:100 were detected in 12 of 107 (11%) early stage breast cancer patients versus 0 of 200 (0%) controls (13). Chapman et al also reported that anti-HER2 antibody can be detected in 13% of patients with DCIS (22). HER2 overexpression has been reported in DCIS and has been shown to be a predictor for transition from in situ to invasive breast cancer (23). Using the WHI sample set, the current study shows HER2 antibody can be detected in 15% pre-diagnostic sera from cancer patients and none of the matched controls, suggesting it may be a useful marker for early detection. Although preliminary, these results indicate the potential of using serum antibodies as a breast screening test in an asymptomatic population.
A major strength of our study is that the samples were collected from sources that avoided systemic biases, which has contributed to the lack of validation of some previously reported biomarkers. Pepe and colleagues have described the PRoBE (prospective-specimen collection, retrospective blinded evaluation) design to eliminate systemic bias (24). The WHI samples were collected before diagnosis and fit the recommended PRoBE design. To our knowledge, our study represents the first report of the occurrence of serum autoantibody responses in pre-diagnostic sera from breast cancer patients. However, it has to be noted that the current study only used a very limited number of WHI samples (33 cases and 45 controls). As a result, there are only 5 cases positive for HER2 and 2 cases positive for p53. Therefore, caution has to be taken as to not over interpret the data. The fact that the 5 HER2 positive samples spread over the range in their time prior to diagnosis suggests that anti-HER2 antibody could develop well ahead of clinical detectable breast cancer. Out of the 2 cases that are positive for p53, one case is also positive for HER2. Again, the small sample size makes it impossible to determine the overall degree of overlap in antibody response in pre-diagnostic samples.
Mammography, the current screening modality, has a specificity over 90%, but the sensitivity ranges between 30% and 90% and is dependent on age and breast density (25–27). The sensitivity is lower in pre-menopausal women with dense breast tissue (26, 27). Furthermore, cancers found in younger women tend to be more aggressive and grow faster (27). Therefore, a blood-based test that may improve the sensitivity of mammography will be very valuable. The low percentages of patient population tested positive for HER2 (15%) and p53 (6%) in pre-diagnostic samples in the current study shows that these two antibodies are not ready to have immediate clinical impact. However, more antibodies may be identified in pre-diagnostic sera and the combination of them might be useful. In the current study, we don’t have enough clinical subtype information to determine the potential association between serum anti-HER2 antibody and tissue positivity for HER2, but we have previously shown that presence of HER2 antibody is significantly associated with HER2 overexpression in tissue (7). We also don’t have sufficient information to address whether the presence of endogenous HER2 antibody is associated with responsiveness to Herceptin treatment. However, a study by Taylor et al has shown that anti-HER2 humoral immunity is induced during trastuzumab therapy (from 29% to 56%) and correlates with favorable clinical response (28).
In summary, results from this study show that autoantibodies to tumor antigens can be detected in serum samples collected at different time points relative to diagnosis, and biomarkers identified using sera from patients having established disease can also be detected in pre-diagnostic sera. The sample sizes used in the current study are relatively small, especially the pre-diagnostic sera from WHI study. Therefore, the results are preliminary and need verification in future studies with larger sample size. The lack of comparison to benign condition is also a limit. However, these novel preliminary findings are encouraging and warrant future large scale studies to further investigate the potential of developing a serum antibody-based assay for screening asymptomatic populations.
Acknowledgments
Dr. Mary Disis has received commercial research grant from GSK and Hemispherix. She is a consultant for Roche, BMS, VentiRx, and Immunovaccine. She owns patents filed by University of Washington that may be related to the reported study.
This work was supported by funding from the Avon Foundation and Susan G. Komen for the Cure Foundation. We thank G. Mills for making samples available for this study.
Abbreviations
- AUC
area under the curve
- CEA
carcinoembryonic antigen
- CI
confidence interval
- cycB1
cyclin B1
- ELISA
enzyme-linked immunosorbant assay
- HER2
Human epidermal growth factor receptor 2
- IGFBP2
insulin-like growth factor binding protein 2
- ROC
receiver operating characteristic curve
- SEREX
serological screening of cDNA expression library
- TAA
tumor associated antigen
- WHI
Women’s Health Initiative
Footnotes
Other authors have no Conflicts of Interest to disclose.
References
- 1.Cancer Facts and Figures. American Cancer Society; 2006. [Google Scholar]
- 2.Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–35. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–33. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Karjalainen A, Koskinen H, Hemminki K, Vainio H, Shnaidman M, et al. p53 autoantibodies predict subsequent development of cancer. Int J Cancer. 2005;114:157–60. doi: 10.1002/ijc.20715. [DOI] [PubMed] [Google Scholar]
- 5.Lubin R, Zalcman G, Bouchet L, Tredanel J, Legros Y, Cazals D, et al. Serum p53 antibodies as early markers of lung cancer. Nat Med. 1995;1:701–2. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]
- 6.Disis ML, Cheever MA. HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv Cancer Res. 1997;71:343–71. doi: 10.1016/s0065-230x(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 7.Goodell V, Waisman J, Salazar LG, de la Rosa C, Link J, Coveler AL, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–54. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 8.Zhong L, Ge K, Zu JC, Zhao LH, Shen WK, Wang JF, et al. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008;10:R40. doi: 10.1186/bcr2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koziol JA, Zhang JY, Casiano CA, Peng XX, Shi FD, Feng AC, et al. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin Cancer Res. 2003;9:5120–6. [PubMed] [Google Scholar]
- 10.Anderson KS, Sibani S, Wallstrom G, Qiu J, Mendoza EA, Raphael J, et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J Proteome Res. 2011;10:85–96. doi: 10.1021/pr100686b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn OJ. Immune response as a biomarker for cancer detection and a lot more. N Engl J Med. 2005;353:1288–90. doi: 10.1056/NEJMe058157. [DOI] [PubMed] [Google Scholar]
- 12.Trivers GE, De Benedetti VM, Cawley HL, Caron G, Harrington AM, Bennett WP, et al. Anti-p53 antibodies in sera from patients with chronic obstructive pulmonary disease can predate a diagnosis of cancer. Clin Cancer Res. 1996;2:1767–75. [PubMed] [Google Scholar]
- 13.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–7. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 14.Desmetz C, Bascoul-Mollevi C, Rochaix P, Lamy PJ, Kramar A, Rouanet P, et al. Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin Cancer Res. 2009;15:4733–41. doi: 10.1158/1078-0432.CCR-08-3307. [DOI] [PubMed] [Google Scholar]
- 15.Regele S, Kohlberger P, Vogl FD, Bohm W, Kreienberg R, Runnebaum IB. Serum p53 autoantibodies in patients with minimal lesions of ductal carcinoma in situ of the breast. Br J Cancer. 1999;81:702–4. doi: 10.1038/sj.bjc.6690751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodell V, Disis ML. Human tumor cell lysates as a protein source for the detection of cancer antigen-specific humoral immunity. J Immunol Methods. 2005;299:129–38. doi: 10.1016/j.jim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–94. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Madoz-Gurpide J, Misek DE, Kuick R, Brenner DE, Michailidis G, et al. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J Proteome Res. 2004;3:261–7. doi: 10.1021/pr049971u. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs I, Menon U. The sine qua non of discovering novel biomarkers for early detection of ovarian cancer: carefully selected preclinical samples. Cancer Prev Res (Phila) 2011;4:299–302. doi: 10.1158/1940-6207.CAPR-11-0048. [DOI] [PubMed] [Google Scholar]
- 20.Zhu CS, Pinsky PF, Cramer DW, Ransohoff DF, Hartge P, Pfeiffer RM, et al. A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer. Cancer Prev Res (Phila) 2011;4:375–83. doi: 10.1158/1940-6207.CAPR-10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 22.Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–73. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 23.Roses RE, Paulson EC, Sharma A, Schueller JE, Nisenbaum H, Weinstein S, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–9. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–8. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinclair N, Littenberg B, Geller B, Muss H. Accuracy of screening mammography in older women. AJR Am J Roentgenol. 2011;197:1268–73. doi: 10.2214/AJR.10.5442. [DOI] [PubMed] [Google Scholar]
- 26.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081–7. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 27.Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40–49 years. J Natl Cancer Inst. 2004;96:1432–40. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- 28.Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–43. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]