Abstract
Context:
Isolated prolactin (PRL) deficiency is a rare entity of unknown etiology manifesting as failure of puerperal lactogenesis.
Objective:
The aim of the study was to determine the cause of isolated PRL deficiency in an affected woman.
Design and Setting:
We examined genetic and autoimmune causes of isolated PRL deficiency at academic medical centers.
Patient:
The patient was a 39-year-old woman with puerperal alactogenesis after two deliveries and undetectable PRL. The other pituitary axes, serum calcium levels, and cranial magnetic resonance imaging were normal.
Intervention:
Recombinant human PRL (r-hPRL) was administered to the patient.
Main Outcome Measures:
We measured the sequencing of candidate genes and immunofluorescence analysis of autoantibodies directed against pituitary endocrine cells.
Results:
There were no rare sequence variants in the genes encoding for PRL, putative PRL-releasing peptide, putative PRL-releasing peptide receptor, or in other genes important for lactotroph lineage development (POU1F1, PROP1, LHX3, LHX4, HESX1, OTX2, and LSD1). The patient serum, on the contrary, contained autoantibodies that specifically recognized a subset of PRL-secreting cells but not PRL itself or any other pituitary cells or hormones. The mother was able to lactate fully after 17 days of treatment with r-hPRL 60 μg/kg every 12 hours, but alactogenesis resumed after treatment was completed.
Conclusions:
These studies report a new autoimmune etiology for women with isolated PRL deficiency and puerperal alactogenesis.
Prolactin (PRL) deficiency can be defined as the loss of function of anterior pituitary cells secreting PRL, with resulting decreased or absent serum levels of PRL. PRL deficiency can occur in association with other anterior pituitary hormone defects or in isolation.
Combined PRL deficiency represents the overwhelming majority of cases and recognizes both acquired and genetic causes (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). It can be found in all states of anterior pituitary impairment, including vascular lesions (such as the ischemic necrosis of Sheehan syndrome), pituitary or parasellar tumors (mainly nonsecreting adenoma and craniopharyngioma), autoinflammatory conditions (hypophysitis and sarcoidosis), and infections (such as tuberculosis). The incidence of acquired severe PRL deficiency increases with the number of other anterior pituitary hormone defects and should be considered a marker of extensive pituitary damage (1). Combined PRL deficiency is also reported in genetic defects of G proteins underlying the resistance to PTH (pseudohypoparathyroidism) (2) and in those of POU1F1, PROP1, LHX3, LHX4, HESX1, and OTX2 transcription factors, which direct the lineage differentiation of lactotrophs (3–6).
Isolated PRL deficiency is extremely rare and manifests clinically only in women after delivery with the lack of puerperal lactogenesis (7). Although PRL is important for ovulation in rodents (8), a role in humans is unclear because manifestations such as decreased fertility have not been associated with PRL deficiency. There have also been no reports of abnormalities in men. The diagnosis is established by demonstrating borderline low or undetectable levels of PRL, which do not increase upon stimulation with TRH or antidopaminergic agents. There have been only six cases of isolated PRL deficiency published in the English literature (summarized in Table 1) (9–13). The first two patients, reported by Turkington (12) in 1972, were women in their late twenties who complained of easy fatigability and inability to lactate after each of two successive pregnancies. With one exception (10), all women were able to conceive spontaneously, despite some having irregular menstrual cycles. All puerperae had a normal physical examination, sellar radiographic appearance, and hormonal tests, except for undetectable levels of PRL. One report suggested a genetic basis for the PRL deficiency because it was observed in both the index case and her mother (13). No study, however, investigated the etiology of PRL deficiency in these women.
Table 1.
Key Characteristics of Women With Isolated PRL Deficiency and Puerperal Alactogenesis
| ID | Age, y | Parity | Maternal History of Puerperal Alactogenesis | Past Medical History | Gestational Breast Enlargement | Menstrual Cycle | Conception | Chief Clinical Complaint | Sella Turcica Imaging | Basal PRL Levels, ng/mL | PRL Levels After Stimulation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 2 | Not mentioned (likely no) | None | Yes | Not mentioned | Not mentioned (likely spontaneous) | Fatigability, alactogenesis after both deliveries | Normal | <2 | No response after phenothiazine | 12 |
| 2 | 29 | 2 | Not mentioned (likely no) | None | Yes | Not mentioned | Not mentioned (likely spontaneous) | Fatigability, alactogenesis after both deliveries | Normal | <2 | No response after phenothiazine | 12 |
| 3 | 30 | 2 | No | None | Yes | Regular | Spontaneous | Alactogenesis after both deliveries | Normal | <3.5 in 27 of 28 measurements | Blunt response to TRH and metoclopramide | 11 |
| 4 | 36 | 2 | No | None | Yes | Irregular | Induced by clomiphene citrate | Alactogenesis after both deliveries | Not done | <0.1 | No response to TRH | 10 |
| 5a | 25 | 6 | Yes | None | Not mentioned | Regular | Spontaneous | Alactogenesis after both deliveries | Normal | <1.9 | Undetectable after chlorpromazine | 13 |
| 6 | 26 | 1 | No | None | No | Irregular | Spontaneous | Alactogenesis | Not done | <0.1 | Undetectable after TRH stimulation | 9 |
| 7 | 36 | 2 | No | Hypothyroid Hashimoto thyroiditis | Yes | Regular | Spontaneous | Alactogenesis after both deliveries | Normal | Undetectable | Not responsive to domperidone | Present case |
The mother of this patient also had an inability to lactate after her six pregnancies, with serum PRL levels undetectable and not responsive to chlorpromazine, suggesting the possibility of a genetic basis.
We report a new case of isolated PRL deficiency where genetic and autoimmune etiologies were examined.
Case Report
The patient presented at age 39 years with lactation insufficiency after each of two pregnancies. She underwent normal thelarche and pubarche and had regular menstrual cycles. At age 36, she became pregnant spontaneously with her first child, had normal pregnancy-associated breast enlargement, delivered uneventfully with no excessive blood loss, and was able to express colostrum postpartum but never produced milk. Her PRL was 6.5 ng/mL 10 days postpartum despite a combination of breast stimulation with the use of a Lact-Aid device, breast pumping eight times per day, and domperidone treatment. PRL declined to undetectable 10 days later despite these continued measures. Other pituitary axes were normal, including a morning cortisol of 31.1 μg/dL (normal, >18), TSH of 1.14 μU/L (normal, 0.2–6) with a free T4 index of 13.9 ng/dL (normal, 8–22), and IGF-I of 277 ng/mL (normal, 114–492). Her reproductive axis was normal, as evidenced by regular menstrual cycles, spontaneous pregnancies, and normal hormones (LH, 0.5 IU/L; FSH, 2.0 IU/L; estradiol, 111 pg/mL; and progesterone, 20.4 ng/mL, indicating a luteal phase sample). Serum calcium was normal at 9.6 mg/dL, and a cranial magnetic resonance imaging showed no abnormalities. She conceived her second pregnancy after trying for 8 months. The pregnancy and postpartum were again associated with breast fullness, colostrum production postpartum, but no increase in milk production. Past medical history was notable for treated Hashimoto hypothyroidism, diagnosed at age 33 years with an elevated TSH and positive thyroperoxidase antibodies. Her mother and sister had no difficulty breast-feeding. Her physical examination at 8 weeks postpartum demonstrated height of 150 cm, body mass index of 24 kg/m2, Tanner stage V breast development, but no glandular fullness and no milk expression. Repeat cortisol, free T4, and IGF-I were normal at the time of the study, and PRL was undetectable despite the use of a Lact-Aid device.
After providing written informed consent, the patient participated in a study of recombinant human PRL (r-hPRL) treatment at 8 weeks postpartum as part of a Massachusetts General Hospital study of mothers with lactation insufficiency (NCT00181623) (14, 15). Briefly, both breasts were pumped simultaneously, and milk volume was measured using a syringe. The patient continued to pump once in the morning throughout the study but fed her baby at the breast at least eight times per day using a Lact-Aid supplemental device as needed. Blood samples were obtained immediately before the onset of pumping, then every 30 minutes for a total of 3 hours to measure PRL. Three hours after the initiation of pumping, r-hPRL (60 μg/kg) was administered sc, and blood samples were taken every 2 hours for the next 6 hours (Figure 1). The patient self-administered r-hPRL every 12 hours for the subsequent 28 days, returning for blood sampling on days 7 and 28. Blood was sampled 1 week later as on day 1 to document changes in endogenous PRL levels.
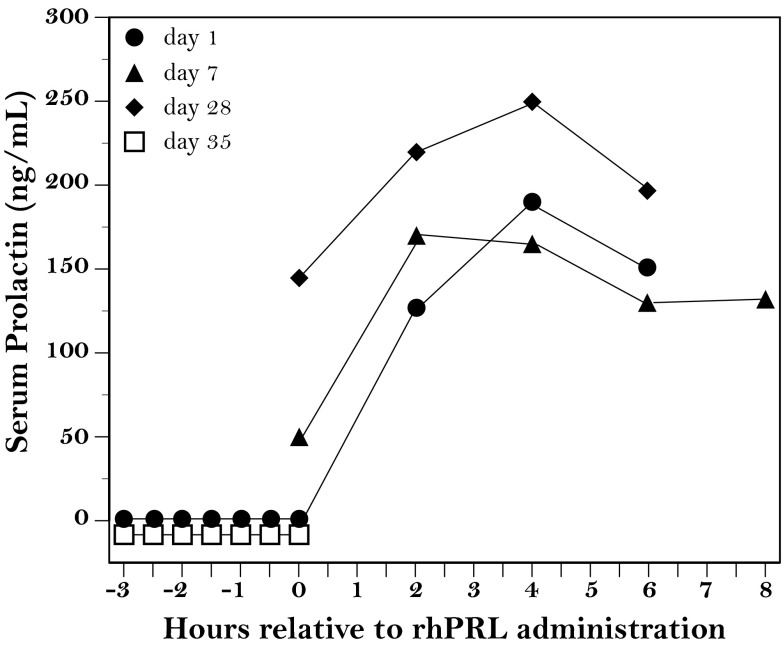
Figure 1.
Serum PRL levels in a woman with isolated PRL deficiency. Serum PRL was measured during breast pumping (indicated by the time from −3 to 0 h) and up to 8 hours after administration of r-hPRL (60 μg/kg every 12 h). Measurements were performed on injection days 1 (closed circles), 7 (closed triangles), and 28 (closed diamonds), as well as 1 week after the last r-hPRL injection (d 35, open squares).
Serum PRL, undetectable at baseline, increased to a peak of 192 ng/mL 4 hours after the first injection of r-hPRL (Figure 1, closed circles). Similar increases were noted after the injections on day 7 (Figure 1, closed triangles) and day 28 (Figure 1, closed diamonds). Breast milk first appeared as drops on day 3 with manual expression and increased to 129 mL after one pumping session on day 28. Supplemental formula was no longer needed after day 17. After stopping the injections of r-hPRL on day 28, breast milk volume returned to zero, and serum PRL was again undetectable (Figure 1, open squares).
Overall, the clinical, endocrine, and therapeutic findings established a diagnosis of isolated PRL deficiency, unresponsive to antidopaminergics, and suggested an abnormality in PRL or the lactotrophs. Genetic and immunological studies were then performed to assess the cause of this deficiency.
Genetic Studies
Peripheral blood DNA was amplified by PCR and then sequenced to identify the exons and intron/exon borders of PRL, putative PRL-releasing peptide, and putative PRL-releasing peptide receptor (16), as well as those of seven other genes involved in the lactotroph lineage determination (PIT1, PROP1, LHX3, LHX4, HESX1, OTX2, and LSD1; Supplemental Table 2) (3–6). We found no mutations, most likely excluding a genetic basis for the patient's isolated PRL deficiency.
Immunological Studies
We next analyzed the patient's serum for the presence of autoantibodies directed against PRL and lactotrophs using indirect double immunofluorescence. As the substrate for immunofluorescence, we used a human pituitary gland collected at autopsy, snap-frozen in liquid nitrogen, and embedded in OCT compound (Sakura Finetek). The gland was cut at the cryostat (Leica CM-1950) into 5-μm sections, which were then fixed in ice-cold acetone, air dried, and blocked with protein block, a commercial serum-free reagent (DAKO, catalog no. X0909).
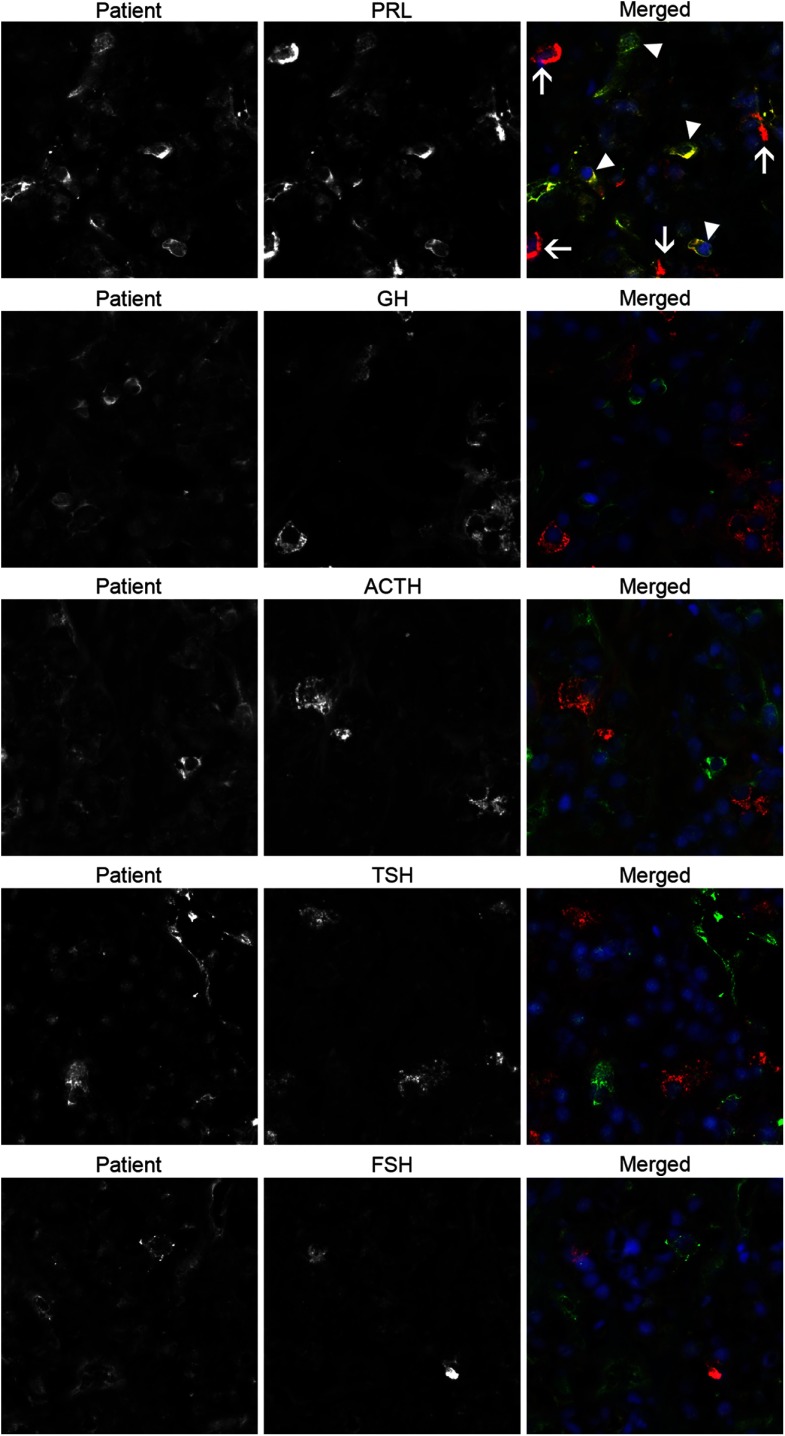
Sections were then incubated overnight at 4°C with the patient serum (diluted 1:10 in PBS) and one of following antibodies: anti-PRL, anti-GH, anti-FSH subunit-β, anticorticotropin, or anti-TSH subunit-β. All antibodies were purchased from the National Hormone & Peptide Program, except for the anti-PRL (Santa Cruz Biotechnology). After the overnight incubation, sections were washed in PBS supplemented with 0.2% Tween, and then incubated for 30 minutes at room temperature with a matching anti-Ig secondary antibody conjugated to a green (FITC) or red (Dylight 649) fluorochrome. After a final wash, sections were counterstained with DAPI (Roche) to identify the nuclei and with Sudan black to decrease the background autofluorescence (17). Sections were then mounted in 80% glycerol and analyzed using an AxioImager A2 fluorescence microscope (Carl Zeiss) equipped with a digital camera. The patient's serum recognized specifically cytosolic antigens in PRL-secreting cells and in particular only in a subset of PRL-secreting cells (Figure 2, top right panel, arrowheads). There were, in fact, other PRL-secreting cells that were not recognized by the patient's autoantibodies (Figure 2, top right panel, arrows). No recognition was found for cells secreting GH, ACTH, TSH, or FSH (Figure 2, second to fifth row).
Figure 2.
The patient autoantibodies recognize a specific subset of PRL-secreting cells in double immunofluorescence experiments using human pituitary as the substrate. Each horizontal series of 3 panels represents the staining obtained using only the patient serum (left panels), only a commercial anti-hormone antibody (center panels), or the two combined (right panels). The top 3 panels represent the experiment with the anti-PRL antibody. It shows that the patient antibodies recognize some PRL-secreting cells that are also recognized by the commercial anti-PRL antibody, and thus appear yellows when the two images are merged (top right panel, arrow heads). It also shows that there are other PRL-secreting cells that are not recognized by the patient serum (top right panel, arrows). Performing the same experiments with commercial antibodies to GH (second row), ACTH (third row), TSH (fourth row), or FSH (fifth row) showed no colocalization of the staining.
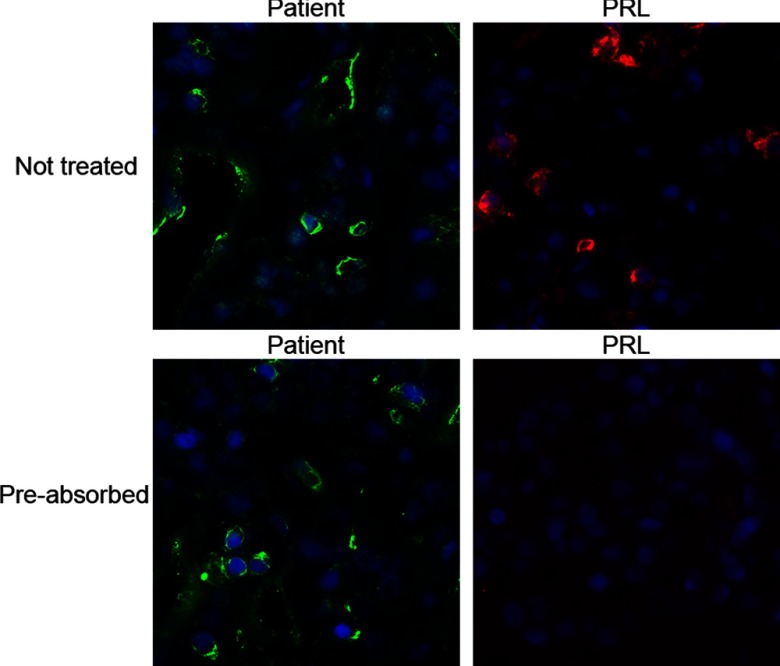
To determine whether the patient's autoantibodies recognized PRL itself or some other antigen expressed by PRL-secreting cells, we performed a preabsorption experiment. Before using the patient's serum or the commercial anti-PRL antibody in the immunofluorescence assay described above, the two reagents were incubated overnight at 4°C without (Figure 3, top panels) or with (Figure 3, lower panels) 100 μg/mL of full-length r-hPRL (ab51703; Abcam). Results showed that the patient recognition was not abolished by preincubation with the PRL antigen (Figure 3, lower left panel), indicating that the patient's autoantibodies recognized some other antigen expressed by the PRL-secreting cells but not PRL itself. On the contrary, recognition of the commercial anti-PRL antibody was abolished by the preabsorption with PRL (Figure 3, lower right panel).
Figure 3.
Preabsorption of the patient serum and the commercial anti-PRL antibody using r-hPRL. This preabsorption abolished the signal from anti-PRL antibody (lower right panel) but did not affect the recognition by the patient serum (lower left panel), indicating that the autoantigen(s) recognized by the patient was not PRL itself.
Discussion
The study reports the seventh patient with isolated PRL deficiency and the first where detailed investigations determining the etiology of the condition were performed. The patient presented in the postpartum with inability to lactate and showed no endocrine abnormality except for undetectable PRL levels. Breast milk production did not respond to stimulation with the antidopaminergic drug domperidone, but was instead restored by the treatment with r-hPRL (14, 15). Because domperidone acts by inhibiting the main inhibitor of PRL secretion, dopamine, it requires functionally intact PRL-secreting cells in order to increase PRL secretion. The lack of response to domperidone, as well as the restoration of milk production upon r-hPRL administration, indicated the existence of a specific defect in PRL-secreting cells, involving either PRL itself or other factors that collectively contribute to the synthesis and release of PRL. We reasoned that the two most likely mechanisms underlying this defect would be genetic or autoimmune.
Although isolated PRL deficiency has its own entry in the OMIM database (264110), where it is considered to be an autosomal recessive trait, no genetic abnormalities have been identified thus far. In one of the six previously published patients (patient 5 in Table 1), however, a genetic basis is highly possible because the mother of the index case also had puerperal alactogenesis (13). In our patient, we could not demonstrate a mutation in the coding region of the PRL gene, its putative releasing hormone or receptor (16), or in other genes that have been reported to be abnormal when PRL deficiency is associated with the deficiency of other anterior pituitary hormones or are responsible for lactotrophs lineage development, overall making a genetic basis unlikely. We unraveled, instead, an autoimmune basis for the deficiency, supported by the coexistence in the same patient of another disease that has a clear autoimmune pathogenesis (Hashimoto thyroiditis). Although Hashimoto thyroiditis, by virtue of being one of the most prevalent autoimmune diseases, could have been found in this patient just by chance, it is now recognized that different autoimmune diseases tend to occur together in the same patient (comorbidity) or family (familial aggregation), supporting shared pathogenic mechanisms.
Autoimmune responses directed against PRL-secreting cells appear only rarely in the literature (18–20). Bottazzo et al (18) first reported that 19 of 287 patients (7%) with one or more autoimmune endocrinopathies had serum autoantibodies recognizing cytosolic antigens in anterior pituitary cells. Using four of the strongest sera, the authors showed that the antibodies specifically recognized antigens contained in the cytosolic granules of PRL-secreting cells, but not PRL itself (18). Horvath et al (20) reported the case of a 35-year-old woman with histologically proven autoimmune hypophysitis and barely detectable (0.64 ng/mL) PRL. She had a normal pregnancy and lactation 12 years previously and presented for symptoms of mass effect (headache and visual disturbances) and gonadotropin dysfunction (oligomenorrhea). Histology of the resected pituitary specimen showed a marked destruction of the acinar architecture, with lymphocyte and plasma cell infiltration in some areas, often organizing into germinal centers, and fibrosis in other areas. Immunohistochemistry for anterior pituitary hormones revealed a marked reduction of the PRL cell population, whereas the other cell types were conserved. There were no serum studies in this patient, but the morphological studies clearly indicated a long-standing autoimmune reaction specifically targeting PRL-secreting cells. Finally, De Bellis et al (19) recently reported the case of a 34-year-old woman who presented with puerperal alactogenesis after the delivery of her first child. The patient, who also had autoimmune hypothyroidism, was found to have a combined deficiency of PRL and GH associated with the presence of serum antibodies recognizing PRL- and GH-secreting cells. Our findings of antibodies to PRL cells in a patient with isolated PRL deficiency lend support to the notion that pituitary antibodies can be clinically significant, although the role and function of PRL antibodies remain to be established.
In summary, we report the first case of isolated PRL deficiency in which etiopathogenic investigations were performed. The work demonstrates the presence of circulating autoantibodies recognizing some antigens in PRL-secreting cells but not the hormone itself or any other pituitary cells or hormones. The work should also alert physicians to the possibility of PRL deficiency from causes other than Sheehan's syndrome in mothers who fail to lactate.
Supplementary Material
Acknowledgments
The study was supported by National Institutes of Health Grant DK080351 (to P.C.), and by March of Dimes Birth Defects Foundation Research Grant 6-FY04-76 and National Center for Research Resources General Clinical Research Centers Program Grant M01-RR-01066 (to C.K.W.). S.I. was supported in part by a fellowship from the Manpei Suzuki Diabetes Foundation. The patient was part of clinical trial no. NCT00181623.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- PRL
- prolactin
- r-hPRL
- recombinant human PRL.
References
- 1. Toledano Y, Lubetsky A, Shimon I. Acquired prolactin deficiency in patients with disorders of the hypothalamic-pituitary axis. J Endocrinol Invest. 2007;30:268–273 [DOI] [PubMed] [Google Scholar]
- 2. Carlson HE, Brickman AS, Bottazzo GF. Prolactin deficiency in pseudohypoparathyroidism. N Engl J Med. 1977;296:140–144 [DOI] [PubMed] [Google Scholar]
- 3. Mullen RD, Colvin SC, Hunter CS, et al. Roles of the LHX3 and LHX4 LIM-homeodomain factors in pituitary development. Mol Cell Endocrinol. 2007;265–266:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol. 2011;7:727–737 [DOI] [PubMed] [Google Scholar]
- 5. Romero CJ, Pine-Twaddell E, Radovick S. Novel mutations associated with combined pituitary hormone deficiency. J Mol Endocrinol. 2011;46:R93–R102 [DOI] [PubMed] [Google Scholar]
- 6. Zhu X, Wang J, Ju BG, Rosenfeld MG. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kauppila A. Isolated prolactin deficiency. Curr Ther Endocrinol Metab. 1997;6:31–33 [PubMed] [Google Scholar]
- 8. Goffin V, Bouchard B, Ormandy CJ, et al. Prolactin: a hormone at the crossroads of neuroimmunoendocrinology. Ann N Y Acad Sci. 1998;840:498–509 [DOI] [PubMed] [Google Scholar]
- 9. Douchi T, Nakae M, Yamamoto S, Iwamoto I, Oki T, Nagata Y. A woman with isolated prolactin deficiency. Acta Obstet Gynecol Scand. 2001;80:368–370 [PubMed] [Google Scholar]
- 10. Falk RJ. Isolated prolactin deficiency: a case report. Fertil Steril. 1992;58:1060–1062 [DOI] [PubMed] [Google Scholar]
- 11. Kauppila A, Chatelain P, Kirkinen P, Kivinen S, Ruokonen A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64:309–312 [DOI] [PubMed] [Google Scholar]
- 12. Turkington RW. Phenothiazine stimulation test for prolactin reserve: the syndrome of isolated prolactin deficiency. J Clin Endocrinol Metab. 1972;34:246–249 [PubMed] [Google Scholar]
- 13. Zargar AH, Masoodi SR, Laway BA, Shah NA, Salahudin M. Familial puerperal alactogenesis: possibility of a genetically transmitted isolated prolactin deficiency. Br J Obstet Gynaecol. 1997;104:629–631 [DOI] [PubMed] [Google Scholar]
- 14. Powe CE, Allen M, Puopolo KM, et al. Recombinant human prolactin for the treatment of lactation insufficiency. Clin Endocrinol (Oxf). 2010;73:645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powe CE, Puopolo KM, Newburg DS, et al. Effects of recombinant human prolactin on breast milk composition. Pediatrics. 2011;127:e359–e366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinuma S, Habata Y, Fujii R, et al. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276 [DOI] [PubMed] [Google Scholar]
- 17. Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW. Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem. 1999;47:229–236 [DOI] [PubMed] [Google Scholar]
- 18. Bottazzo GF, Pouplard A, Florin-Christensen A, Doniach D. Autoantibodies to prolactin-secreting cells of human pituitary. Lancet. 1975;2:97–101 [DOI] [PubMed] [Google Scholar]
- 19. De Bellis A, Colella C, Bellastella G, et al. Late primary autoimmune hypothyroidism in a patient with post-delivery autoimmune hypopituitarism associated with antibodies to growth hormone- and prolactin-secreting cells [published online ahead of print January 3, 2013]. Thyroid. doi:10.1089/thy.2012.0482 [DOI] [PubMed] [Google Scholar]
- 20. Horvath E, Vidal S, Syro LV, Kovacs K, Smyth HS, Uribe H. Severe lymphocytic adenohypophysitis with selective disappearance of prolactin cells: a histologic, ultrastructural and immunoelectron microscopic study. Acta Neuropathol. 2001;101:631–637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.