Abstract
Context:
Questionnaire studies linked symptoms of obstructive sleep apnea (OSA) to the risk of gestational diabetes mellitus (GDM). Whether this association is present when OSA is assessed objectively by polysomnography is not known.
Objective:
The objective of the study was to assess the relationship between pregnancy, OSA, and GDM.
Design, Setting, and Participants:
We conducted observational case-control studies using polysomnography in 15 nonpregnant, nondiabetic women (NP-NGT), 15 pregnant women with normal glucose tolerance (P-NGT), and 15 pregnant women with GDM (P-GDM). The groups were frequency matched for age and race/ethnicity. Pregnant women were studied during the late second to early third trimester.
Main Outcome Measures:
Comparisons of OSA diagnosis and sleep parameters between NP-NGT and P-NGT to assess the impact of pregnancy and between P-NGT and P-GDM to explore the association between GDM and OSA were measured.
Results:
Compared with NP-NGT, P-NGT women had a higher apnea hypopnea index (AHI) (median 2.0 vs 0.5, P = .03) and more disrupted sleep as reflected by a higher wake time after sleep onset (median 66 vs 21 min, P < .01) and a higher microarousal index (median 16.4 vs 10.6, P = .01). Among the pregnant women, P-GDM had markedly lower total sleep time (median 397 vs 464 min, P = .02) and a higher AHI (median 8.2 vs 2.0, P = .05) than P-NGT women. OSA was more prevalent in P-GDM than in P-NGT women (73% vs 27%, P = .01). After adjustment for prepregnancy body mass index, the diagnosis of GDM was associated with a diagnosis of OSA [odds ratio 6.60 (95% confidence interval 1.15–37.96)]. In pregnancy, after adjusting for prepregnancy body mass index, higher microarousal index significantly associated with higher hemoglobin A1c and fasting glucose levels. Higher oxygen desaturation index was associated with higher fasting glucose levels.
Conclusion:
Pregnancy is associated with sleep disturbances. Sleep is more disturbed in GDM than in P-NGT women. There is a strong association between GDM and OSA.
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of upper airway closures or partial collapse during sleep, resulting in intermittent hypoxia. The gold standard for diagnosis is polysomnography (PSG), which quantifies the number of apneas and hypopneas per hour of sleep, yielding an apnea-hypopnea index (AHI). A diagnosis of OSA is made when the AHI is 5 or greater. In 1993 the prevalence of OSA in the general US population was estimated at 24% in men and 9% in women (1). Current estimates of OSA, especially among obese individuals, are much higher: between 33% and 77% in men and 11%–46% in women (2). This increased prevalence has been attributed, at least in part, to the epidemic of obesity that has developed over the last few decades. OSA is a major risk factor for insulin resistance, independent of body mass index (BMI), and is present in up to 86% of obese patients with type 2 diabetes (3, 4). Increasing severity of OSA is associated with poorer glycemic control (3).
During pregnancy, poor sleep quality and decreased sleep duration are common, even in the absence of OSA (5). Prospective studies show that symptoms of OSA increase during pregnancy, especially in women whose BMI exceeds 25 kg/m2 (6). OSA in pregnancy is associated with preeclampsia, intrauterine growth retardation and preterm delivery (7–11). Changes in hormonal and physical factors during pregnancy can promote or protect against OSA. Protection from OSA is imparted by a preference for lateral sleeping position and the decrease in rapid eye movement (REM) sleep that is characteristic of later stages of pregnancy (7). Conversely, OSA risk may be increased in pregnant women because of higher rates of CO2 production, decreased functional residual volume from upward displacement of the diaphragm, and mucosal edema of the airways.
Using survey tools, we (12) as well as others (9, 13, 14) have reported associations between sleep disturbances (short sleep duration, frequent snoring by self-report, and elevated risk of OSA) with glucose intolerance and gestational diabetes mellitus (GDM) independent of BMI. Our survey of 169 women in the second trimester of pregnancy found that women with increased OSA risk based on a questionnaire assessment had a 3-fold increase in risk of being diagnosed with GDM (12). In addition, women with self-reported sleep duration less than7 hours per night had a 2.4-fold increase, and those with both increased OSA risk and short sleep had a 3.4-fold increase in the risk of being diagnosed with GDM, compared with women without these sleep disturbances.
There are very few studies of sleep and risk of GDM that are based on objective measures of sleep quality using PSG recording. A recent retrospective analysis of a hospital database revealed that increasing severity of OSA as quantified by PSG was associated with an increased risk of composite adverse pregnancy outcomes (pregnancy related hypertension, GDM, or preterm delivery) (15). However, only 6 of 143 women had GDM; interestingly, all 6 women had OSA. In another study, OSA diagnosed within 1 year prior to the index delivery was associated with an increased risk of GDM with an odds ratio of 1.63, after adjusting for obesity (16). However, BMI values were not reported and the nonpregnant control subjects did not have a PSG.
Although these previous studies suggest an association between objectively assessed OSA and GDM, there has not been a single study that performed and compared PSG in pregnant women with and without GDM while controlling for potential confounders. Because age, race, and BMI can influence the risk of OSA as well as of GDM, these factors should be taken into account when comparing groups. Because symptoms of OSA can increase during pregnancy, controlling for the timing of PSG relative to the stage of pregnancy is also important.
The aims of this study were as follows: 1) to evaluate the impact of pregnancy on objective sleep quality (including the presence and severity of OSA and other sleep variables such as sleep fragmentation), 2) to explore the association between OSA and GDM using PSG, the gold standard test for assessing OSA, and 3) to explore the relationship between sleep parameters and glucose control in pregnancy. We hypothesized that pregnancy is associated with sleep disturbances and that there is a significant association between GDM and OSA, after adjusting for confounders.
Research Design and Methods
To examine the impact of pregnancy on sleep quality and the association between OSA and GDM, we conducted observational case control studies and compared metabolic and PSG measures in three groups of subjects: nonpregnant, nondiabetic women (NP-NGT; n = 15), pregnant women with normal glucose tolerance (P-NGT; n = 15), and pregnant women with GDM (P-GDM; n = 15). The three groups were matched for race and age at the time of PSG using a frequency-matching approach. In addition, we frequency matched the prepregnancy BMI of P-NGT to the BMI at the time of the PSG for NP-NGT and performed PSG during the late second to early third trimester in all pregnant participants.
Pregnant adult women with a singleton pregnancy who were in their late second to early third trimester who had either normal glucose tolerance (P-NGT) or GDM (P-GDM) were invited to participate. The women were recruited from the Obstetrics Clinic at the University of Chicago as well as from advertisement fliers posted within the University of Chicago Medical Center. Written informed consent was obtained from each woman. The study was approved by the University of Chicago Institutional Review Board.
Normal glucose tolerance (NGT) during pregnancy was defined by a glucose value at 1 hour after a 50-g oral glucose administration that did not exceed 140 mg/dL. GDM was defined as two abnormal values from a confirmatory 100-g oral glucose tolerance test (OGTT) (Carpenter-Coustan criteria) (17) or if a 1-hour glucose value after 50 g OGTT is 200 mg/dL or greater. Exclusion criteria included multiple pregnancies; known diabetes prior to pregnancy; preexisting sleep disorders; severe pulmonary, cardiac, or renal disease; steroid use; substance abuse; current neurological or psychiatric disorders; use of prescription or over-the-counter medications known to affect sleep or glucose metabolism; cigarette smoking; significant alcohol (≥7 drinks/wk) or caffeine consumption (≥400 mg/d); recent travel across time zones; and shift work.
Nonpregnant women with normal glucose tolerance (NP-NGT) were included as controls for P-NGT subjects. The NP-NGT participants were healthy overweight or obese women who had a physical examination, routine laboratory tests, and overnight PSG followed by a 75-g OGTT as part of screening procedures for participation in research studies at the University of Chicago. NGT in nonpregnant women was defined as fasting plasma glucose less than 100 mg/dL and 2-hour value after a 75-g oral glucose administration of less than 140 mg/dL.
Baseline data collected in all participants included race, age at the time of the PSG, height, weight, and a detailed medical and family history. Prepregnancy weight (by recall) was collected in pregnant women. Subjects were scheduled for full overnight PSG. For 3 days and nights prior to the overnight PSG, the subjects wore a wrist activity monitor (Actiwatch, Mini-Mitter Co Inc) to determine habitual bedtimes. For each participant, data derived from these ambulatory recordings were used to set sleep and wake times for the laboratory PSG (Neorofaz EEG 1100; Nihon Kohden). PSG involved the recording of electroencephalograms (frontal, central, and occipital), electroculograms, electromyelogram, and two-lead electrocardiogram. Oronasal airflow was monitored via thermistor and nasal pressure transducers, chest and abdominal effort via respiratory inductance plethysmography, snoring via microphone, and pulse oximetry. Recordings were visually scored in 30-second epochs. Obstructive events (ie, apneas and hypopneas) and microarousals were scored according to established criteria (18). Hypopneas were scored according to rule 4B in the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events (18). OSA was deemed to be present if the AHI was 5 or greater. The severity of OSA was graded as mild (AHI ≥ 5 and < 15), moderate (AHI ≥ 15 and < 30), or severe (AHI ≥ 30). Wake time after sleep onset (WASO) was the total time (in minutes) that participants were awake between sleep onset and end of recording. T90 is the percentage of total sleep time in which the oxygen saturation remains below 90%. Oxygen desaturation index (ODI) is the average number of times per hour of sleep time that the oxygen saturation drops by 3% or more.
After an overnight fast, on the morning after the PSG, blood samples were collected for glucose, insulin, and hemoglobin A1c (HbA1c). P-NGT women underwent a 100-g OGTT to ensure normal glucose tolerance [defined as having all glucose values under the diagnosis criteria for GDM (17) as well as a fasting glucose value less than 92 mg/dL (19)]. Homeostasis model assessment insulin resistance index (HOMA-IR) values were calculated using the following formula: HOMA-IR = glucose (milligrams per deciliter) × insulin (microunits per milliliter)/405.
Statistical analysis
The analytical plans for this study were designed to assess the following: 1) the impact of pregnancy on sleep and metabolic measures in women with normal glucose tolerance; 2) an association between a diagnosis of GDM and a diagnosis of OSA during pregnancy, adjusting for potential confounders; and 3) the relationship between sleep and metabolic parameters during pregnancy. Two-sample t tests and Wilcoxon rank-sum tests were used to analyze differences in continuous variables between study groups. The nonparametric Wilcoxon rank-sum test was used when variables were not normally distributed, based on results from the Shapiro-Wilk test. χ2 and Fisher's exact tests were used to analyze differences in categorical variables between study groups. Logistic regression analysis was performed to explore the association between GDM and OSA, adjusting for prepregnancy BMI, which was considered a potential confounder. The rule of thumb that one independent variable can be included in the logistic regression model for every 10 subjects in the smallest outcome group was followed. Spearman rank correlation was used to analyze the relationship between sleep parameters (AHI, microarousal index, T90, lowest oxygen saturation, and ODI) and metabolic parameters (fasting glucose and HbA1c levels) in pregnant women. Partial correlation based on ranks was used to further adjust these correlations for prepregnancy BMI. The analyses were performed using Stata Version 12.1 (StataCorp). Data are presented as mean ± SD in which the data were normally distributed or median (interquartile range) in which the data were not normally distributed.
Results
Thirty-six pregnant women and 15 nonpregnant women consented for PSG. Three pregnant women with NGT failed to come to their scheduled PSG. Of the 33 pregnant women who underwent PSG, two women with an abnormal 1-hour glucose value after a 50-g OGTT did not have GDM after a confirmatory 100-g OGTT, and one woman had an abnormal fasting glucose level after a normal screening 50-g OGTT. Their data were excluded from the analyses. This resulted in 15 P-NGT, 15 P-GDM, and 15 NP-NGT who were included in the final analyses.
Comparison between NP-NGT and P-NGT women
Table 1 summarizes the demographic, metabolic, and PSG characteristics of 15 nonpregnant (NP-NGT) and 15 pregnant (P-NGT) participants with normal glucose tolerance of similar age and race. Fasting glucose levels in NP-NGT women were higher than in P-NGT women but all were within the normal range. There were no significant differences in fasting insulin, HbA1c levels, or fasting HOMA-IR between the two groups.
Table 1.
Demographic, Metabolic, and Polysomnography Data in NP-NGT, P-NGT, and P-GDM Subjects
| NP-NGT | P-NGT | P-GDM | P Value (NP-NGT vs P-NGT)a | P Value (P-NGT vs P-GDM)a | |
|---|---|---|---|---|---|
| Age, y | 29.2 ± 5.2 | 28.5 ± 5.9 | 29.2 ± 5.6 | .71 | .73 |
| BMI on PSG day, kg/m2 | 31.0 ± 4.3 | 34.3 ± 7.2 | 39.6 ± 9.7 | .58b | .10 |
| Prepregnancy BMI, kg/m2 | N/A | 29.7 ± 7.5 | 37.4 ± 9.2 | N/A | .02 |
| Race/ethnicity, n, % | 1.00c | 1.00c | |||
| Caucasian | 2 (13) | 1 (7) | 1 (7) | ||
| African American | 12 (80) | 12 (80) | 12 (80) | ||
| Hispanic | 1 (7) | 1 (7) | 1 (7) | ||
| Asian | 0 (0) | 1 (7) | 1 (7) | ||
| Fasting glucose, mg/dL | 89.4 ± 5.0 | 82.4 ± 6.2 | 94.9 ± 14.8 | <.01 | <.01 |
| Fasting insulin, pmol/L | 51 (36–82) | 54 (34–88) | 81 (69–126) | .68d | .02d |
| Fasting HOMA-IR | 1.6 (1.0–2.5) | 1.5 (0.9–2.5) | 3.1 (1.8–4.2) | .55d | .01d |
| HbA1c, % | 5.3 ± 0.3 | 5.2 ± 0.3 | 5.8 ± 0.5 | .64 | <.01 |
| Total sleep time, min | 454 (442–487) | 464 (438–478) | 397 (356–440) | 1.00d | .02d |
| REM sleep, % | 23.0 ± 4.2 | 18.8 ± 4.2 | 19.5 ± 4.2 | .01 | .65 |
| Stage 1, % | 6.0 (3.5–6.7) | 11.3 (8.0–14.7) | 9.7 (9.0–12.6) | <.01d | .66d |
| Stage 2, % | 59.9 ± 7.6 | 61.1 ± 6.0 | 60.7 ± 7.8 | .63 | .86 |
| Slow-wave sleep, % | 10.3 (6.7–15.5) | 8.9 (2.1–11.1) | 8.4 (0.5–13.0) | .15d | .69d |
| Microarousal index | 10.6 (6.4–14.9) | 16.4 (11.3–19.5) | 20.3 (14.4–29.9) | .01d | .08d |
| WASO, min | 21 (13–42) | 66 (39–89) | 58 (50–108) | <.01d | .56d |
| AHI | 0.5 (0.1–1.2) | 2.0 (1.0–5.0) | 8.2 (1.1–17.4) | .03d | .05d |
| T90 | 0 (0–0.07) | 0 (0–0.04) | 0.08 (0–0.75) | .57d | .03d |
| Lowest O2 saturation | 90 (86–92) | 92 (89–93) | 87 (85–90) | .18d | .02d |
| ODI | 0.13 (0.01–0.43) | 0.18 (0–0.98) | 1.0 (0.3–3.0) | .52d | .11d |
Abbreviation: N/A, not available. Data are expressed as mean ± SD or median (interquartile range) unless otherwise noted.
P values are from t tests unless otherwise noted.
Compared with prepregnancy BMI of P-NGT subjects.
Fisher's exact test.
Wilcoxon rank-sum test.
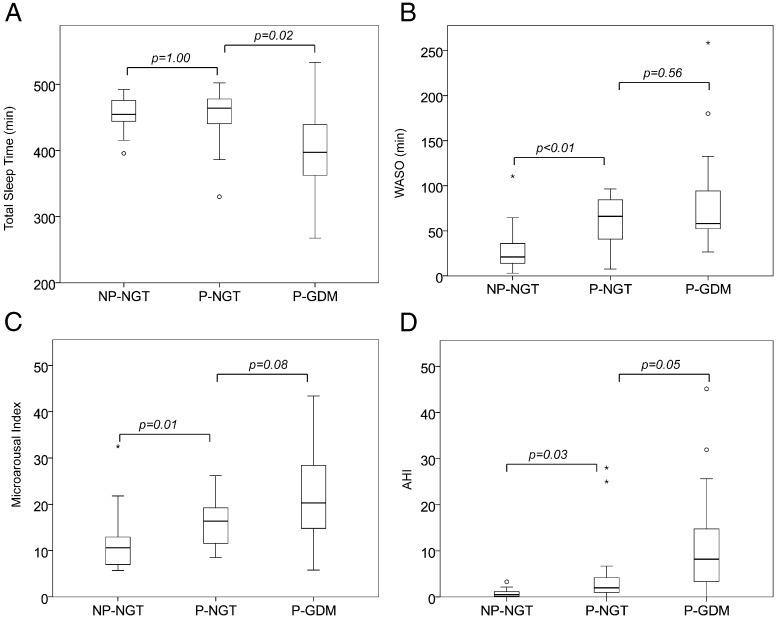
The mean BMI value of NP-NGT women was similar to the prepregnancy BMI of the P-NGT women, allowing us to explore the relationship between pregnancy and sleep variables. Total sleep time during the PSG was not affected by pregnancy. However, P-NGT women had significantly more WASO (P < .01), with a significantly higher microarousal index (P = .01), thus reflecting disrupted sleep. In addition, the median AHI in P-NGT women was significantly higher than that of NP-NGT women (2.0 vs 0.5, respectively; P = .03). Four P-NGT women (27%) had OSA (two with moderate and two with mild OSA), whereas none of the NP-NGT participants had OSA. This elevated prevalence of OSA in P-NGT women relative to NP-NGT participants failed to reach statistical significance (P = .10). The P-NGT group spent less time in REM sleep (P = .01) and more time in stage 1 sleep (P < .01), consistent with previously documented changes in sleep architecture during late pregnancy (7).
Comparison between P-NGT and P-GDM women
Fifteen P-GDM women and 15 age- and race-matched P-NGT women underwent PSG at an average gestational age of 28.2 ± 3.7 and 30.9 ± 2.0 weeks, respectively. The demographic, metabolic, and PSG data of both groups of pregnant participants are shown in Table 1. P-GDM women had a significantly higher prepregnancy BMI than did the P-NGT group, and their BMI at the time of the PSG also tended to be higher. A majority of these women were overweight (BMI 25.0–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) based on the prepregnancy BMI (93% of P-GDM women and 67% of P-NGT women). P-GDM women gained less weight from prepregnancy period until the time of PSG than P-NGT women (BMI increased by 2.2 ± 2.0 vs 4.6 ± 1.9 kg/m2, P = .003). As expected, fasting glucose, insulin, HbA1c levels, and HOMA-IR in the P-GDM women were significantly higher than those in the P-NGT women.
Total sleep time in P-GDM participants was more than 1 hour shorter than in P-NGT women, and P-GDM women tended to have more fragmented sleep as indicated by a higher microarousal index. Time spent in each stage of sleep (REM, stage 1, stage 2, and slow wave sleep) was similar between the two groups. The median AHI was more than 4-fold higher in P-GDM women, and their lowest oxygen saturation levels were significantly lower, whereas T90 values were significantly higher compared with the P-NGT women. Eleven GDM women (73%) met diagnostic criteria for OSA (2 with severe, 2 with moderate, and 7 with mild), compared with only 4 P-NGT women (27%) (P = .01).
Because BMI is considered a potential confounder in the relationship between GDM and OSA, a logistic regression analysis adjusting for prepregnancy BMI was conducted to explore the association of GDM with OSA. In this model, prepregnancy BMI did not emerge as a significant predictor of OSA (P = .71). The diagnosis of GDM, however, was found to be associated with an increased likelihood of having OSA, with an adjusted odds ratio of 6.60 (95% confidence interval 1.15–37.96, P = .03).
Figure 1 illustrates the salient PSG findings of the study.
Figure 1.
PSG findings in NP-NGT, P-NGT, and P-GDM women. A, Total sleep time. B, WASO. C, Microarousal index. D, AHI.
The relationship between sleep parameters and glycemia during pregnancy
When data from all pregnant women were analyzed together, AHI significantly correlated with fasting glucose concentrations [correlation coefficient (r) between fasting glucose and AHI was 0.36, P = .05] as well as HbA1c (r = 0.42, P = .02) (Table 2). In addition, the microarousal index and duration of exposure to significant oxygen desaturation (T90) correlated positively with fasting glucose and HbA1c levels. More frequent desaturation events (ODI) correlated with higher fasting glucose levels.
Table 2.
Correlations Between Sleep and Glycemic Parameters in Pregnant Women (r)
| Bivariate analysis (r) |
Prepregnancy BMIadjusted (r) |
|||
|---|---|---|---|---|
| HbA1c | Fasting Glucose | HbA1c | Fasting Glucose | |
| AHI | 0.42a | 0.36b | 0.35 | 0.33 |
| Microarousal index | 0.40a | 0.41a | 0.37b | 0.40a |
| T90 | 0.40a | 0.38a | 0.31 | 0.36 |
| Lowest O2 saturation | −0.30 | −0.19 | −0.17 | −0.11 |
| ODI | 0.29 | 0.39a | 0.18 | 0.37b |
P < .05.
P = .05.
After adjustment for prepregnancy BMI, more sleep fragmentation as indicated by higher microarousal index remained significantly associated with higher HbA1c and fasting glucose levels, and higher ODI remained significantly associated with higher fasting glucose levels.
Discussion
Our study is the first to use PSG to evaluate sleep quality, including OSA, in GDM women compared with NGT women of similar age and race/ethnicity during the similar stage of pregnancy, with adjustment for prepregnancy BMI. An important aspect of our study design is that lights off and lights on for the PSG were timed to the usual sleep habits of the participants to increase relevance to real-life conditions and avoid potential confounding effects of abnormal circadian timing. We found that nearly 75% of women with GDM had OSA. This high prevalence is remarkably similar to the prevalence reported in nonpregnant women and men with type 2 diabetes (3). GDM women had less total sleep time as well as a trend toward poorer sleep quality, which are PSG findings typically found among nonpregnant women and men with OSA. The diagnosis of GDM was associated with an almost 7 times greater odds of having OSA, adjusted for prepregnancy BMI. Our finding that the diagnosis of GDM is associated with OSA in pregnant women is consistent with evidence from our previous study that relied upon questionnaire data (12). Although a few previous studies have reported an association between GDM and a diagnosis of OSA based on PSG (8, 15, 16), our study makes an important contribution because for the first time GDM women were compared with carefully matched non-GDM women, and all were studied during a very similar stage of pregnancy.
In women with normal glucose tolerance, we found that pregnancy was associated with increased OSA risk and more disturbed sleep. P-NGT women with a prepregnancy BMI similar to the BMI of NP-NGT participants had a significantly higher AHI and more wake time after sleep onset. These changes may put pregnant women, especially those who are overweight, at risk of developing OSA. These findings are congruent with prospective data in pregnant women, especially those with higher BMI (6, 20).
In addition, we found significant correlations between sleep and metabolic parameters in pregnant women. After adjustment for prepregnancy BMI, higher microarousal index and more frequent oxygen desaturation events were associated with higher fasting glucose levels. These associations between sleep disturbances and reduced glucose tolerance are in agreement with those in nonpregnant populations (3, 22, 23).
The reason that we found a strong association between GDM and OSA, as well as correlations between sleep and metabolic parameters in pregnant women, is likely due to the fact that OSA is a risk factor for abnormal glucose metabolism. OSA is a complex sleep disorder involving intermittent hypoxia and transient arousals, resulting in fragmented sleep, shallow sleep with low amounts of slow wave sleep, and generally reduced total sleep time. There is strong evidence that each of the components of OSA is linked to an increased risk of diabetes. In a nonpregnant population, 1–2 weeks of sleep restriction results in a marked decrease in glucose tolerance in response to an iv glucose bolus, with a reduction in insulin sensitivity that is not compensated for by increased insulin release (24). A recent study showed that 4 days of experimental sleep restriction in healthy lean participants resulted in impaired intracellular insulin signaling and an insulin-resistant state in human adipocytes (25). Experimental sleep fragmentation and suppression of slow-wave sleep have been shown to cause reduced insulin sensitivity as well as increased sympathetic nervous system activity (26, 27). In addition, acute exposure to intermittent hypoxia in healthy volunteers was associated with decreased insulin sensitivity and impaired glucose tolerance (28). It is likely that all of the components of OSA contribute to our findings of the association between GDM and OSA. Because P-GDM women gained less weight than P-NGT women, the strong association between GDM and OSA in the current study cannot be explained by gestational weight gain.
The prevalence of OSA in pregnancy is currently unknown because there is no large population-based study. Because the upper airways are significantly narrowed later in pregnancy, symptoms or severity of OSA may improve after delivery (29). Nevertheless, the presence of OSA and sleep disturbances in pregnancy have been shown to be associated with worsened pregnancy outcomes such as preterm delivery, small-for-gestational-age newborns, and pregnancy-associated hypertension (7–12). Previous studies have shown that poor maternal sleep and intermittent hypoxia can lead to increased oxidative stress and proinflammatory cytokine release (TNF-α, and IL-6) as well as increased sympathetic activation, peripheral vasoconstriction, and endothelial dysfunction (11, 30). These are likely contributors to the development of pregnancy related adverse outcomes. Early continuous positive airway pressure (CPAP) use in women with hypertension and chronic snoring was associated with better blood pressure control and pregnancy outcomes (31). CPAP treatment in nonpregnant type 2 diabetes patients with OSA has been shown to improve glucose control in some (32) but not in others (21).There are currently no data on the effects of OSA treatment in GDM women.
Our study is limited by its relatively small sample size (n = 45), although the findings were not equivocal and were based on rigorous quantitative assessments and careful matching of participants with and without GDM. Nonetheless, the high prevalence of OSA in GDM needs to be documented in a larger population. In addition, the cross-sectional design does not provide information regarding the direction of causality between OSA and GDM. Future research is needed to explore the possible relationship between severity of OSA and glycemic control. Because most of our GDM participants with OSA had only mild OSA, it remains to be proven whether CPAP treatment will affect glucose metabolism and pregnancy outcomes. The possibility of having of OSA should be considered in pregnant women who are diagnosed with GDM, especially if other risk factors for OSA are present including obesity and hypertension. Conversely, for women with an established diagnosis of OSA who become pregnant, consideration should be given to early screening for GDM.
In summary, we found that pregnancy is associated with significant disturbances in sleep quality in general and an increased risk for OSA, particularly in the presence of GDM.
Acknowledgments
We thank Harry Whitmore, Renate Schneider, and Abeer Rue (Section of Endocrinology, University of Chicago) and staff of the Clinical Resource Center (University of Chicago) for their assistance in the study.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The ResMed Foundation had no role in the study design, data collection, analysis, interpretation, or writing of the report.
This study was supported by a grant from the Diabetes Research Training Center at the University of Chicago (P60 DK20595), a specialized Center of Research on Women's Health (Grant 5P50HD057796), a Program Project Grant (PO1AG11412) from the National Institute on Aging, the Blum-Kovler Family Foundation, and Grant UL1-RR024999 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The Clinical Resource Center is supported by Clinical Translational Science Award UL1-RR024999. This study was also supported by the ResMed Foundation.
Address for S.R. after September 15, 2013: Division of Endocrinology, Department of Medicine, Ramathibodi Hospital, Mahidol University, Rama VI Road, Rachathewi, Bangkok 10400, Thailand. E-mail: sreutrak10800@gmail.com.
Disclosure Summary: S.R., N.Z., K.W., H.H.K., and M.I. had nothing to declare. E.V.C. receives grant support from Philips/Respironics, the ResMed Foundation, and Amylin/Lilly; is a consultant for Pfizer Inc and Viropharma; an associate editor for the journal Sleep and for a volume entitled Sleep Loss and Obesity: Intersecting Epidemics published by Springer Science and Business, LLC.; and serves as an expert witness for Lamson, Dugan, and Murray, LLP (Omaha, Nebraska). D.A.E. received research support from the ResMed Foundation as well as the Kovler Family Foundation.
Footnotes
- AHI
- apnea-hypopnea index
- BMI
- body mass index
- CPAP
- continuous positive airway pressure
- GDM
- gestational diabetes mellitus
- HbA1c
- hemoglobin A1c
- HOMA-IR
- homeostasis model assessment insulin resistance index
- NGT
- normal glucose tolerance
- NP-NGT
- nonpregnant, nondiabetic women
- ODI
- oxygen desaturation index
- OGTT
- oral glucose tolerance test
- P-GDM
- pregnant women with GDM
- P-NGT
- pregnant women with NGT
- OSA
- obstructive sleep apnea
- PSG
- polysomnography
- r
- correlation coefficient
- REM
- rapid eye movement
- T90
- percentage of total sleep time in which the oxygen saturation remains below 90%
- WASO
- wake time after sleep onset.
References
- 1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235 [DOI] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599 [DOI] [PubMed] [Google Scholar]
- 3. Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Facco FL, Kramer J, Ho KH, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83 [DOI] [PubMed] [Google Scholar]
- 6. Maasilta P, Bachour A, Teramo K, Polo O, Laitinen LA. Sleep-related disordered breathing during pregnancy in obese women. Chest. 2001;120:1448–1454 [DOI] [PubMed] [Google Scholar]
- 7. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–1417 [DOI] [PubMed] [Google Scholar]
- 8. Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261–265 [DOI] [PubMed] [Google Scholar]
- 9. Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36:849–855 [DOI] [PubMed] [Google Scholar]
- 10. Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet. 2008;100:141–146 [DOI] [PubMed] [Google Scholar]
- 11. Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16:574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reutrakul S, Zaidi N, Wroblewski K, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34:2454–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Facco FL, Liu CS, Cabello AA, Kick A, Gribman WA, Zee PC. Sleep-disordered breathing: a risk factor for adverse pregnancy outcomes? Am J Perinatol. 2012;29:277–282 [DOI] [PubMed] [Google Scholar]
- 16. Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136.e1–5 [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association Standard of medical care in diabetes. Diabetes Care. 2010;33:S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iber C, Ancoli-Israel S, Chesson C, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed American Academy of Sleep Medicine; 2007 [Google Scholar]
- 19. American Diabetes Association Standards of Medical Care in Diabetes. Diabetes Care. 2012;35:S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–1305 [DOI] [PubMed] [Google Scholar]
- 21. West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep. 2008;31:1018–1024 [PMC free article] [PubMed] [Google Scholar]
- 23. Lesser DJ, Bhatia R, Tran WH, et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr Res. 2012;72:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 25. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards N, Blyton DM, Hennessy A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep. 2005;28:737–741 [DOI] [PubMed] [Google Scholar]
- 30. Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21 [DOI] [PubMed] [Google Scholar]
- 32. Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452 [DOI] [PubMed] [Google Scholar]