Abstract
Context:
β-Cell function (BCF) declines over the course of type 2 diabetes, but little is known about BCF changes across glucose tolerance status (GTS) categories, and comparisons of direct vs surrogate measures.
Objective:
To assess longitudinal changes in BCF across GTS.
Design:
The Insulin Resistance Atherosclerosis Study is a multicenter, observational, epidemiologic study.
Setting:
Four clinical centers in the US that could identify subjects likely to have impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).
Patients:
We compared longitudinal changes in BCF in 1052 subjects over 5 years. Subjects were categorized according to baseline GTS: normal glucose tolerance (NGT: n = 547), impaired fasting glucose or impaired glucose tolerance (IFG/IGT: n = 341), and newly diagnosed type 2 diabetes (n = 164).
Interventions:
None.
Main Outcome Measures:
BCF was assessed from a frequently sampled iv glucose tolerance test (AIR, acute insulin response), and the homeostasis model assessment of BCF (HOMA B).
Results:
NGT and IFG/IGT subjects increased their insulin secretion over time, whereas those with type 2 diabetes experienced either decline or little change in BCF. After adjustment for demographic variables and change in insulin resistance, change in HOMA B underestimated the magnitude of changes in BCF, as assessed by change in AIR. Relative to NGT, the 5-year change in insulin secretion in IFG/IGT and type 2 diabetes was 31% and 70% lower (by HOMA B) and 50% and 80% lower (by AIR).
Conclusions:
The decline in BCF over time in IFG/IGT and type 2 diabetes may be more pronounced than previously estimated; HOMA B may underestimate this decline significantly.
Pancreatic β-cell dysfunction is a core disorder in the etiology of type 2 diabetes mellitus (1). Groups known to be at high risk for type 2 diabetes, including those with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), gestational diabetes mellitus, or a positive family history of type 2 diabetes mellitus, have consistently been documented to have β-cell dysfunction (1). In addition, β-cell dysfunction is significantly associated with risk of incident type 2 diabetes mellitus, independently of other known risk factors, including insulin resistance (2). Furthermore, data from the United Kingdom Prospective Diabetes Study suggest a 50% loss of β-cell function (BCF) at the time of type 2 diabetes mellitus diagnosis (3), an observation that further supports the concept of the occurrence of β-cell dysfunction early in the pathogenesis of type 2 diabetes mellitus.
Despite this spectrum of evidence documenting the importance of β-cell dysfunction in diabetes etiology, only limited data are available regarding longitudinal changes in BCF, both overall and across glucose tolerance status (GTS) categories (4–6). Most of these studies have used indirect or surrogate measures of BCF (such as homeostasis model assessment [HOMA] or modeling from an oral glucose tolerance test [OGTT]), with no studies comparing direct vs surrogate measures in their ability to document longitudinal changes, especially taking into account concomitant changes in insulin resistance.
Our objective, therefore, was to assess longitudinal changes in BCF across categories of glucose tolerance, using frequently sampled iv glucose tolerance tests, a direct measure of both insulin secretion and insulin resistance. This enabled us to compare a direct measure of insulin secretion (acute insulin response, or AIR) to a fasting surrogate measure, specifically, the HOMA of BCF (HOMA B), correcting for underlying insulin resistance (SI and fasting insulin). We also assessed whether longitudinal changes in BCF were similar across subgroups of ethnicity and body mass.
Materials and Methods
Study Subjects
The Insulin Resistance Atherosclerosis Study (IRAS) is a multicenter, observational, epidemiologic study of the relationships between insulin resistance, atherosclerosis, and its known risk factors in different ethnic groups and varying states of glucose tolerance. The design and methods of this study have been described in detail (7). A total of 1625 individuals participated in the baseline IRAS examination (56% women), which occurred between October 1992 and April 1994. After an average of 5.2 years (range: 4.5–6.6 years), follow-up examinations of this cohort were conducted using the baseline protocol. The response rate was 81% and those who attended the follow-up examination were similar to those who did not attend in terms of ethnicity, sex, and baseline GTS (normal glucose tolerance [NGT] versus IGT), and body mass index (BMI); (all comparisons, P > .32). The present report includes information on 1052 individuals for whom longitudinal information was available on key variables used in the present analysis (Table 1); individuals who had self-reported diabetes at baseline were excluded. Of the total IRAS population (n = 1625), we excluded 324 who did not return for follow-up, as well as those with diabetes at baseline (n = 249), leading to a total study population of 1052. The IRAS protocol was approved by local institutional review committees, and all participants provided written informed consent.
Table 1.
Anthropometric and Metabolic Characteristics of Subjects at Baseline and 5-year Follow-up Examinations, According to GTS at Baseline, the IRAS
| Variable | GTS (baseline)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NGT (n = 547) |
IFG/IGT (n = 341) |
Diabetes Mellitus (n = 164) |
|||||||
| Baseline | Follow-up | Changeb | Baseline | Follow-up | Changeb | Baseline | Follow-up | Changeb | |
| Age, y | 53.5 (8.6) | — | — | 56.4 (7.9) | — | — | 56.7 (8.1) | — | — |
| Gender, female, % | 56.1 | — | — | 58.5 | — | — | 57.9 | — | — |
| Ethnicity, % | |||||||||
| W | 40.0 | — | — | 39.7 | — | — | 35.4 | — | — |
| AA | 25.4 | — | — | 27.4 | — | — | 39.6 | — | — |
| H | 34.6 | — | — | 32.9 | — | — | 25.0 | — | — |
| BMI, kg/m2 | 27.2 (4.6) | 28.1 (5.0) | 0.82 (2.3)c | 30.2 (6.4) | 30.7 (6.9) | 0.55 (2.3)c | 32.2 (5.7) | 32.0 (5.6) | −0.22 (3.0) |
| Waist, cm | 87.4 (11.5) | 90.4 (12.1) | 3.1 (5.0)c | 94.6 (13.2) | 96.6 (13.6) | 2.1 (5.6)c | 99.3 (12.0) | 100.3 (12.8) | 0.81 (6.1)e |
| Fasting glucose, mg/dL | 93.4 (7.5) | 96.7 (12.9) | 3.4 (11.6)c | 105.4 (10.1) | 114.4 (32.3) | 9.0 (30.0)c | 144.7 (43.2) | 163.5 (65.5) | 18.8 (56.6)c |
| 2-h glucose, mg/dL | 104.6 (20.9) | 124.9 (44.6) | 20.6 (42.4)c | 155.9 (24.7) | 180.4 (70.7) | 24.5 (15.7)cc | 264.2 (68.7) | 286.4 (89.2) | 22.2 (78.6)d |
| Fasting insulin, μU/mL | 13.4 (9.4) | 16.9 (12.3) | 3.5 (11.5)c | 19.2 (20.4) | 22.3 (13.8) | 3.2 (22.1)c | 25.3 (19.4) | 23.6 (3.4) | −1.8 (19.3) |
| SI, ×10−4 min−1 μU−1 mL−1 | 2.7 (2.3) | 1.6 (1.3) | −1.1 (1.8)c | 1.4 (1.3) | 1.0 (1.6) | −0.45 (1.7)c | 0.60 (0.84) | 0.40 (0.59) | −0.24 (0.79)d |
Abbreviations: AA, African Americans; H, Hispanics; W, non-Hispanic whites. Dashes indicate not applicable.
Sample sizes vary slightly due to occasional missing values; data are means (sd), or proportions for gender and ethnicity variables.
Significance of changes between baseline and follow-up within each glucose tolerance group was assessed using the Wilcoxon signed rank test.
P < .0001;
P < .001;
P < .05.
Clinical measurements and procedures
The IRAS protocol required two visits, 1 week apart, of approximately 4 hours each. Subjects were asked that, before each visit they fast for 12 hours, abstain from heavy exercise and alcohol for 24 hours, and refrain from smoking the morning of the examination. During the first visit, a 75 g OGTT was administered, with GTS determined using World Health Organization criteria (8). During the second visit, insulin sensitivity and insulin secretion were determined using frequently sampled iv glucose tolerance tests, with 2 modifications to the original protocol (9). First, an injection of regular insulin, rather than tolbutamide, was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance (10). Second, a reduced sampling protocol (with 12 rather than 30 samples) was employed for efficiency given the large number of participants (11). Insulin sensitivity, expressed as the insulin sensitivity index (SI), was calculated using mathematical modeling methods (MINMOD version 3.0, 1994) (12). Acute insulin response was calculated as the mean plasma insulin concentration at 2 and 4 minutes after the administration of glucose. The repeatability of SI and AIR have been demonstrated in a subsample of the IRAS cohort (13), and the estimate of SI from this modified protocol has been validated against gold standard measures of insulin resistance from the hyperinsulinemic euglycemic clamp technique (r = 0.95) (14). Acute insulin response has been validated by others using gold standard measures of insulin secretion from the hyperglycemic clamp technique (15).
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight/height2 (kg/m2) and was used as an estimate of overall adiposity. Waist circumference was measured to the nearest 0.5 cm using a steel tape (7). Duplicate measures of anthropometry were made following a standardized protocol, and averages were used in the analysis. Ethnicity was assessed by self-report (7).
Laboratory procedures
Glucose concentration was determined using the standard methods described previously (7). Insulin levels were measured using the dextran-charcoal RIA (16), which has a 19% external coefficient of variation. This assay displays a high degree of cross-reactivity with proinsulin. The HOMA B was calculated from fasting glucose and insulin concentrations according to Matthews et al (17). GTS (NGT, IFG, IGT, and diabetes) was defined using World Health Organization criteria (8).
Statistical analyses
Means (±SD) or proportions for subject characteristics at baseline and follow-up were calculated according to baseline GTS (NGT, IFG/IGT, and diabetes). Subjects with IFG or IGT were combined into a single group due to sample size constraints. The distributions of continuous variables were evaluated, and transformations were used in the analysis as required. Given that some subjects had SI = 0, we used the natural log transformation of (SI + 1). Change variables were calculated as follow-up value minus baseline value, with the signed square-root transformation of these change variables being used to normalize distributions. Unadjusted changes in BCF measures (HOMA B and AIR) were evaluated, as were changes adjusted for age, sex, ethnicity, and change in fasting insulin (as a surrogate for insulin resistance for HOMA B models) and age, sex, ethnicity, and change in SI (for AIR models), using analysis of covariance. Adjustments were made using a measure of insulin resistance/insulin sensitivity that, in each case, would have been available from similar data that were used for the parallel measure of secretion. Because 37% of individuals reported using oral glucose-lowering agents at follow-up, we also adjusted for this potential confounder. Changes in subjects with baseline IFG/IGT and type 2 diabetes were assessed relative to changes in subjects with baseline NGT. For these comparisons, analysis of covariance results were indexed by multiplying the means by 100 divided by the mean for NGT subjects. Similar analyses were conducted by subgroups of overweight (BMI < 27 kg/m2 vs ≥ 27) and ethnicity (non-Hispanic white, African American, or Hispanic).
Results
Table 1 presents participant characteristics at both baseline and 5-year follow-up examinations, according to baseline GTS. Subjects with NGT at baseline were younger than subjects with IFG/IGT (P < .0001) and diabetes (P < .0001), respectively. In subjects without diabetes at baseline, BMI and waist circumference increased over 5 years, although these increases were relatively modest (<1 U of BMI and 2–3 cm of waist circumference). In contrast, BMI remained stable in subjects with newly diagnosed diabetes at baseline, and the increase in waist circumference was less pronounced in this group compared to those without diabetes at baseline. Fasting and 2-hour glucose increased over 5 years in all groups, with more marked increases in fasting glucose among those with IFG/IGT or newly diagnosed diabetes at baseline. Fasting insulin concentration increased modestly in those without newly diagnosed diabetes at baseline, in contrast to a slight decrease in those with diabetes. Conversely, SI decreased over time in all glucose tolerance groups (Table 1).
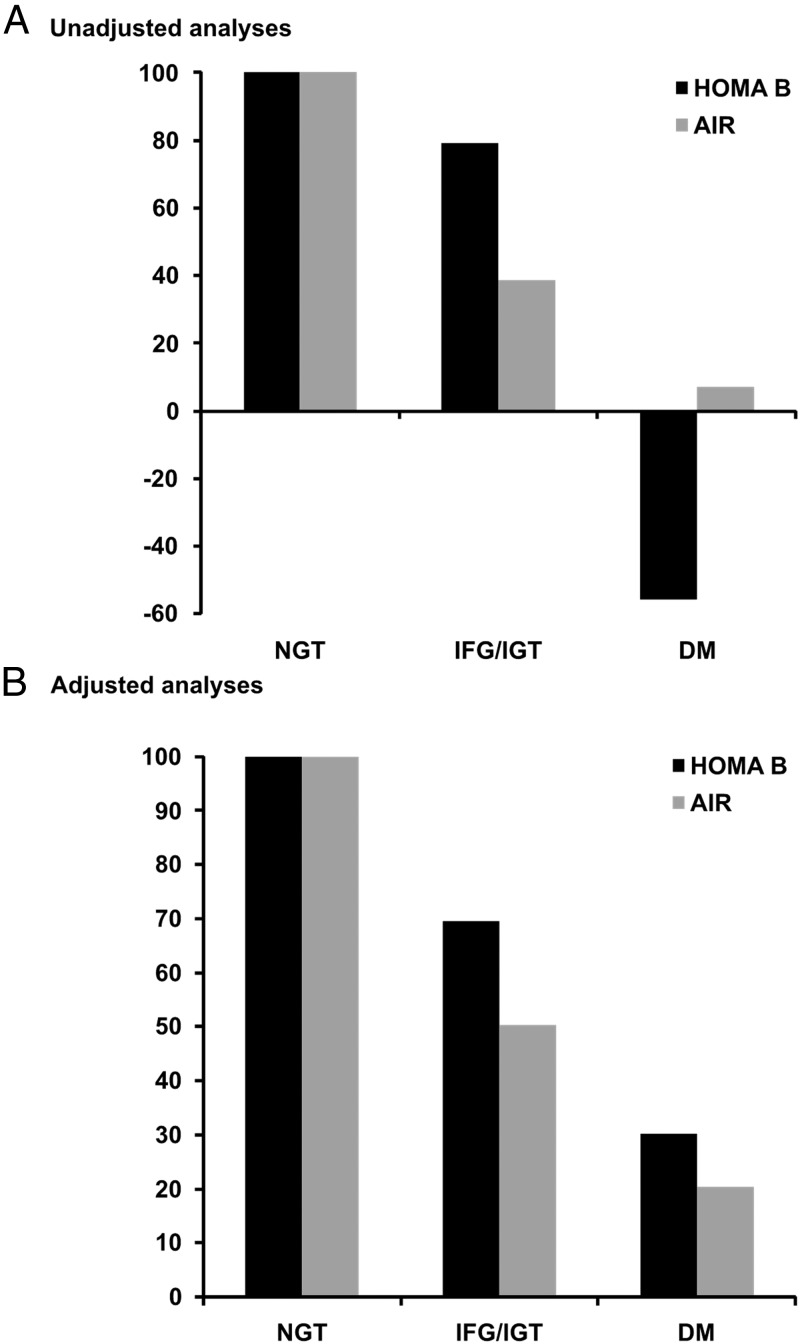
Change in HOMA B was modestly correlated with change in AIR (r = 0.15, P < .001). Longitudinal changes in measures of BCF, according to baseline GTS, are presented in Table 2. HOMA B increased over 5 years in those with NGT or IFG/IGT at baseline, but declined in those with baseline diabetes. Similarly, AIR increased in those with baseline NGT or IFG/IGT, with only a slight increase in those with baseline diabetes. Relative to those with NGT at baseline, HOMA B declined 21% and 156% in those with baseline IFG/IGT and diabetes, respectively, in unadjusted models. Corresponding changes in AIR were 61% and 93%. After adjustment for age, sex, ethnicity, and change in fasting insulin, declines in HOMA B of 31% and 70%, respectively, were noted for subjects with baseline IFG/IGT and diabetes, with corresponding declines in AIR of 50% and 80% after adjustment for age, sex, ethnicity, and change in SI (Table 2, Figure 1). After further adjusting for oral glucose-lowering medication, we found this difference in functional decline in subjects with diabetes at baseline, as assessed using HOMA B or AIR, to be even more pronounced (42% decrease for HOMA B and 91% decrease for AIR). Further adjustment for antihypertensive or lipid-lowering medication did not alter the results (data not shown).
Table 2.
Longitudinal Changes in BCF Measures (follow-up minus baseline) According to GTS at Baseline
| Variable | Baseline GTSa |
||
|---|---|---|---|
| NGT (n = 547) | IFG/IGT (n = 341)b | DM (n = 164)b | |
| Baseline HOMA B | 161.09 (107.51) | 166.21 (165.46) | 128.05 (87.09) |
| Follow-up HOMA B | 183.99 (100.26) | 183.21 (156.48) | 112.48 (80.89) |
| Change in HOMA B (unadjusted) | |||
| Absolute values | 22.84 (109.84) | 16.81 (209.34) | −15.71 (91.90) |
| Transformed valuesc | 2.71 (0.36) | 2.15 (0.45) | −1.51 (0.66)f |
| Indexed values (% drop) | 100 | 79.3 (20.7) | −55.7 (155.7) |
| Change in HOMA B (adjusted)d | |||
| Absolute values | 16.70 (4.89) | 9.65 (6.17) | 19.88 (9.0) |
| Transformed valuesc | 2.36 (0.25) | 1.64 (0.31) | 0.71 (0.45)h |
| Indexed values (% drop) | 100 | 69.5 (30.5) | 30.1 (69.9) |
| Baseline AIR, μU/mL | 73.30 (60.74) | 54.26 (45.29) | 32.13 (30.19) |
| Follow-up AIR, μU/mL | 96.65 (80.87) | 62.48 (59.86) | 34.20 (25.06) |
| Change in AIR (unadjusted) | |||
| Absolute values | 22.55 (59.54) | 8.65 (47.80) | 0.52 (23.74) |
| Transformed valuesc | 2.64 (0.25) | 1.02 (0.32)f | 0.19 (0.48)f |
| Indexed values (% drop) | 100 | 38.6 (61.47) | 7.2 (92.8) |
| Change in AIR (adjusted)e | |||
| Absolute values | 21.41 (2.44) | 10.54 (3.15) | 3.15 (4.92) |
| Transformed valuesc | 2.51 (0.25) | 1.26 (0.32)g | 0.51 (0.51)g |
| Indexed values (% drop) | 100 | 50.26 (49.8) | 20.3 (79.7) |
Abbreviations: DM, diabetes mellitus. Data are least square means and standard errors, except rows indicated by “Indexed values,” which are indexed values and % drop.
Results are absolute values for each group in addition to values indexed to those in the NGT group.
Significance of the differences in changes in β-cell function measures between those with IFG/IGT or DM vs. those with NGT were assessed using analysis of covariance. Transformed outcome values were used for statistical testing.
Signed square-root transformation used for analysis and indexing of changes in HOMA B and AIR; data are LS mean ± SE from analysis of covariance.
Adjusted for age, sex, ethnicity, change in fasting insulin (signed square-root transformation).
Adjusted for age, sex, ethnicity, change in SI (signed square-root transformation).
P < .0001;
P < .001;
P < .01.
Figure 1.
Longitudinal changes in BCF, in individuals with IFG/IGT and diabetes mellitus (DM), indexed to individuals with NGT. Comparison between HOMA B and AIR. A, unadjusted analyses; B, adjusted analyses (for age, sex, and ethnicity and for HOMA B change in fasting and for AIR change in SI, respectively).
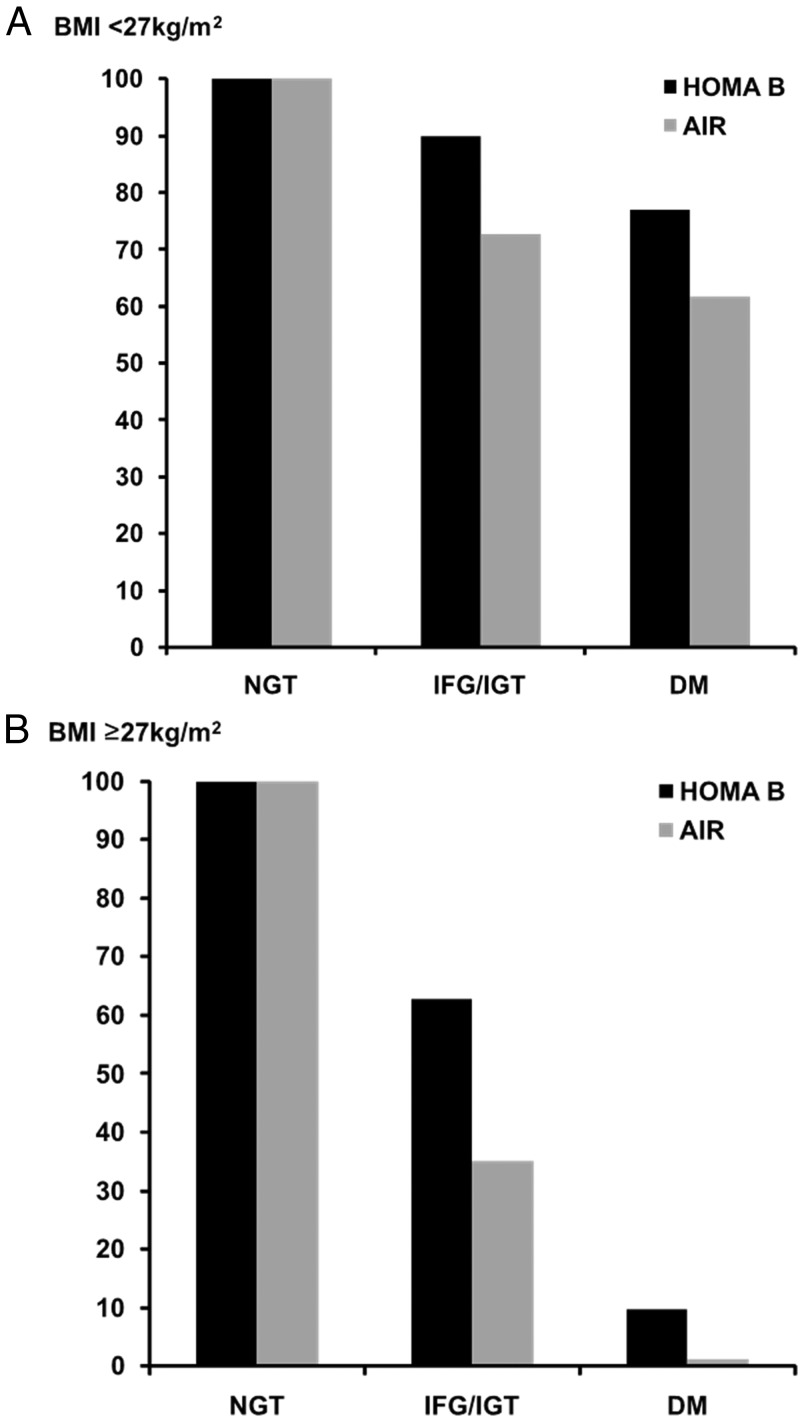
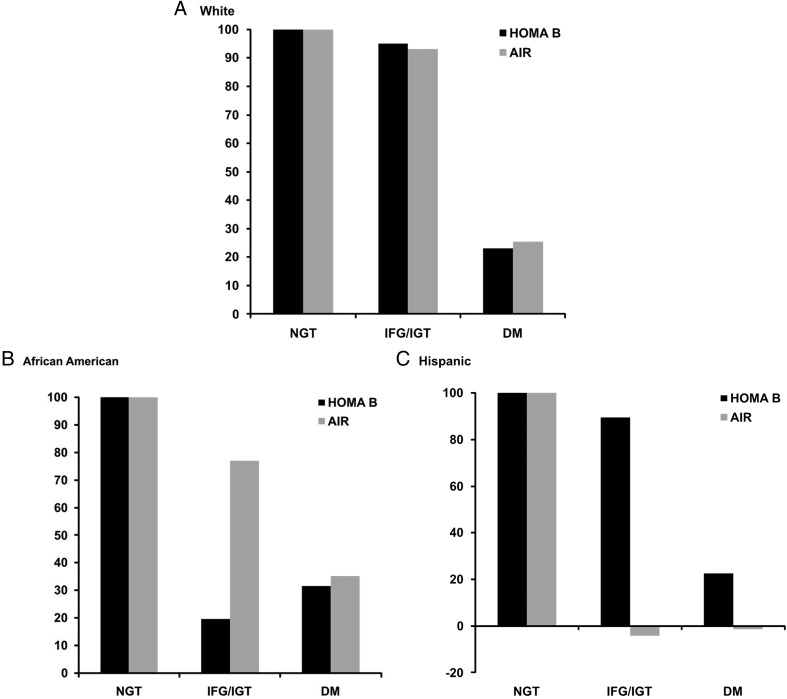
Longitudinal changes in measures of BCF, according to baseline glucose tolerance and by subgroups of BMI, are presented in Table 3 and Figure 2. In general, overweight subjects without diabetes had greater insulin secretion at baseline but more modest increases over 5 years, especially in those with IFG/IGT. Among overweight subjects with diabetes, BCF declined over time, in contrast to a slight increase in subjects with diabetes and BMI < 27 kg/m2. The pattern of relative declines in BCF between HOMA B and AIR were, in general, similar to the full group (ie, HOMA B underestimated β-cell decline relative to AIR, especially among those with IFG/IGT in adjusted models). Furthermore, although this pattern was consistent within the subgroup of Hispanic subjects, it did not necessarily hold for the other ethnic groups (Figure 3). Specifically, among non-Hispanic whites, relative declines in β-cell dysfunction were similar for HOMA B and AIR, whereas HOMA B appeared to overestimate β-cell decline in African Americans, especially those with IFG/IGT.
Table 3.
Longitudinal Changes in BCF Measures (follow-up minus baseline) According to GTS at Baseline and Stratified by Obesity Status, Missing BMI Values in n = 3 Individuals
| Variable | Obesity Statusa |
|||||
|---|---|---|---|---|---|---|
| BMI < 27 kg/m2 |
BMI ≥ 27 kg/m2 |
|||||
| NGT (n = 294) | IFG/IGT (n = 120)b | DM (n = 27)b | NGT (n = 251) | IFG/IGT (n = 220)b | DM (n = 137)b | |
| Baseline HOMA B | 137.07 | 118.80 | 106.18 | 189.88 | 192.07 | 132.39 |
| Follow-up HOMA B | 159.97 | 147.69 | 113.71 | 212.89 | 202.85 | 112.23 |
| Change in HOMA B (unadjusted) | ||||||
| Absolute values | 22.67 | 28.89 | 7.53 | 22.83 | 10.13 | −20.39 |
| Transformed valuesc | 2.94 | 2.95 | 1.43 | 2.46 | 1.70 | −2.10f |
| Indexed values (% drop) | 100 | 100.4 (+0.4) | 48.8 (51.2) | 100 | 70.8 (29.2) | −87.2 (187.2) |
| Change in HOMA B (adjusted)d | ||||||
| Absolute values | 22.94 | 25.82 | 18.32 | 9.47 | 0.80 | 19.36 |
| Transformed valuesc | 2.97 | 2.69 | 2.29 | 1.72 | 1.08 | 0.17i |
| Indexed values (% drop) | 100 | 89.9 (10.1) | 77.1 (22.9) | 100 | 62.7 (37.3) | 9.7 (90.3) |
| Baseline AIR | 61.46 | 39.66 | 30.06 | 87.66 | 62.01 | 32.52 |
| Follow-up AIR | 85.03 | 51.82 | 32.72 | 110.02 | 68.16 | 33.07 |
| Change in AIR (unadjusted) | ||||||
| Absolute values | 23.43 | 12.08 | 8.23 | 21.53 | 6.86 | −1.08 |
| Transformed valuesc | 3.06 | 1.99 | 1.21 | 2.16 | 0.52h | −0.02h |
| Indexed values (% drop) | 100 | 64.8 (35.2) | 39.6 (60.4) | 100 | 23.9 (76.1) | −0.9 (100.9) |
| Change in AIR (adjusted)e | 23.80 | 14.28 | 12.66 | 19.45 | 8.35 | −0.13 |
| Transformed valuesc | 3.11 | 2.26 | 1.92 | 1.95 | 0.68i | 0.02h |
| Indexed values (% drop) | 100 | 72.7 (27.3) | 61.7 (38.3) | 100 | 35.0 (65.0) | 1.2 (98.8) |
Abbreviations: DM, diabetes mellitus. Data are least square means and standard errors, except rows indicated by “Indexed values”, which are indexed values and % drop.
Results are absolute values for each group in addition to values indexed to those in the NGT group.
Significance of the differences in changes in BCF measures between those with IFG/IGT or DM vs those with NGT was assessed using analysis of covariance. Transformed outcome values were used for statistical testing.
Signed square-root transformation used for analysis and indexing of changes in HOMA B and AIR; data are LS mean ± SE from analysis of covariance.
Adjusted for age, sex, ethnicity, and change in fasting insulin (signed square-root transformation).
Adjusted for age, sex, ethnicity, and change in SI (signed square-root transformation).
P < .0001;
P < .001;
P < .01;
P < .05.
Figure 2.
Longitudinal changes in BCF, in individuals with IFG/IGT and diabetes mellitus, indexed to individuals with NGT. Comparison between HOMA B and AIR. Analyses adjusted for age, sex, and for HOMA B change in fasting and for AIR change in SI, respectively, and stratified by ethnicity. A, Non-Hispanic whites; B, African Americans; C, Hispanics.
Figure 3.
Longitudinal changes in BCF, in individuals with IFG/IGT and diabetes mellitus, indexed to individuals with NGT. Comparison between HOMA B and AIR. Analyses adjusted for age, sex, and ethnicity and for HOMA B change in fasting and for AIR change in SI, respectively, and stratified by BMI. A, BMI < 27 kg/m2; B, BMI ≥ 27 kg/m2.
Discussion
Using 5-year longitudinal data from the IRAS study, we report here that BCF increased over time in those with NGT and IFG/IGT, whereas BCF remained stable or declined slightly in those with newly diagnosed diabetes. However, BCF declined markedly in those with IFG/IGT and diabetes after adjustment for covariates, including change in insulin resistance, and relative to changes that occurred among those with NGT. This decline was markedly underestimated using HOMA B compared to AIR. The differences between these two measures are likely due to the fact that they represent different aspects of insulin secretion, one being a dynamic measure in response to a glucose challenge (AIR) and the other being reflective of the fasting steady-state condition (HOMA B).
The time course of β-cell dysfunction in type 2 diabetes, particularly in its early stages, is of practical interest and relevance considering that interventions to prevent, delay, or treat the disease have been demonstrated in some cases to improve BCF (18). Results from cross-sectional studies provide robust evidence that BCF declines progressively with deteriorating glucose tolerance (19), but much less is known about longitudinal changes in BCF. Based on extrapolation of data from the United Kingdom Prospective Diabetes Study, it has been hypothesized that deterioration of BCF (as assessed by HOMA) may commence 10 to 12 years before diagnosis (20). In Pima Indians, a decline in BCF, as assessed using clamp studies, significantly contributed to progression from NGT to IGT, and from IGT to diabetes, respectively (4). In nondiabetic individuals, impaired pancreatic β-cell glucose sensitivity and whole-body insulin sensitivity (based on modeling from OGTT) predicted deteriorating glucose tolerance over 5 years (5). In the IRAS, we have previously shown a decline in insulin sensitivity over time (5.2 years), irrespective of GTS, and we have shown that the change in first phase insulin secretion (AIR) determined GTS at follow-up (6). These results are in line with results from the Botnia Study, which identified the failure of β-cell compensation relative to prevailing insulin resistance as the key defect leading to type 2 diabetes over 6 years (21). The clinical importance of dynamics of BCF over time was confirmed in the Diabetes Prevention Program, where improvements in BCF in response to (any) treatment was associated with lower diabetes risk (22).
In the present report we describe the time course of BCF over 5.2 years, across different glucose tolerance categories and in three ethnic groups with different risks of developing type 2 diabetes. Considering the complexities of the relationship between insulin resistance and insulin secretion, we employed a statistical approach that enabled us to compare longitudinal changes in relation to a normoglycemic population, considering not only baseline data but also changes over time, which would occur as part of the physiological ageing process.
In NGT, insulin secretion increased over time (by as much as 30%), and this increase was not fully explained by change in SI (which decreased during the observation period). The increase in insulin secretion was less pronounced in individuals with IFG/IGT as compared to NGT. Taking changes in insulin sensitivity into account, the difference vs NGT was as much as 31% (using HOMA) and 50% (using AIR) for IFG/IGT, and 70% (HOMA) and 80% (AIR), respectively, for diabetes.
Combining cross-sectional and longitudinal findings, the pattern emerging shows that with deteriorating glucose tolerance (NGT vs IFG/IGT vs diabetes), insulin secretion is decreased, and that over time the capacity to increase insulin secretion (partly in response to declining SI) becomes progressively compromised (from NGT to IFG/IGT to diabetes). HOMA was less sensitive in detecting this pattern than AIR, especially in early stages of glucose intolerance, namely considering differences between NGT and IFG/IGT.
We found that the decline in BCF was consistently seen in all three ethnic groups with diabetes at baseline. In individuals with IFG/IGT at baseline, the decline in BCF and the pattern of how HOMA B and AIR captured the decline differed by ethnicity. In subjects with IFG/IGT at baseline, BCF remained virtually unchanged in whites, declined in African Americans as assessed by HOMA B (and less so as assessed by AIR), and declined in Hispanics, as assessed by AIR (and less so as assessed by HOMA B). These differences may be explained by differences in conversion rates (from IFG/IGT to diabetes) between African Americans and Hispanics or by differences in changes in fasting serum insulin levels, which would affect HOMA B measures. However, we would like to interpret these findings with caution due to small sample sizes in these subgroups.
Previous studies that have evaluated surrogate measures of BCF have focused largely on cross-sectional comparisons of measures against gold standards (23, 24). These articles have reported that measures such as HOMA B and fasting proinsulin and indices such as the insulinogenic index show modest correlation against more detailed measures such as AIR and the hyperglycemic clamp (21, 23). Although both surrogate and direct measures of BCF are known to predict type 2 diabetes (1, 2, 4), few studies have directly compared the two in terms of documenting longitudinal change over time.
The strengths of the present study include the longitudinal design, OGTT-based GTS information, and the availability of both a direct and a surrogate measure of BCF. In addition, multiple ethnic groups were studied and characterized by extensive phenotyping—in particular, insulin sensitivity. The study was limited, however, by a relatively brief period of time between examinations (5.2 y), the limited number of time points for measurements (n = 2), and a lack of additional surrogate measures of BCF. Information on proinsulin concentrations and glucose and insulin data from other time points during the OGTT (eg, 30 min) would have been especially informative, as indices using postchallenge time points, in particular, may capture β-cell dysfunction more accurately. In the clinical decision-making process, nonglycemic effects are considered important, including changes in insulin resistance and insulin secretory capacity (25); our report is supportive of such a therapeutic approach.
In conclusion, our results support the hypothesis that BCF deteriorates progressively over time in subjects with IFG/IGT and diabetes, relative to subjects with NGT. In addition, our results suggest that surrogate measures of BCF markedly underestimate β-cell functional changes. The decline in insulin secretory capacity over time in type 2 diabetes may be more pronounced than previously estimated.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute (Grants HL-47887, HL-47889, HL-47890, HL-47892, HL-47902, HL-55208, and R01-HL-58329) and the General Clinic Research Centers Program (Grants NCRR GCRC, M01 RR431, and M01 RR01346).
Some data were published previously in form of an abstract by Haffner SM, et al. Longitudinal progression of β-cell dysfunction: comparison of a direct method vs a fasting surrogate measure, in Diabetes. 2009;58(suppl 1):A265.
ClinicalTrials.gov Identifier: NCT00005135.
Disclosure Summary: A.F. is employed by Eli Lilly and Company. S.M.H., L.E.W., C.L., and A.J.G.H. have nothing to declare.
Footnotes
- AIR
- acute insulin response
- BCF
- β-cell function
- BMI
- body mass index
- GTS
- glucose tolerance status
- HOMA B
- homeostasis model assessment of β-cell function
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- IRAS
- Insulin Resistance Atherosclerosis Study
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- SI
- insulin sensitivity index.
References
- 1. Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 2. Hanley AJ, Wagenknecht LE, Norris JM, et al. Insulin resistance, β cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family study. Diabetologia. 2009;52:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UK prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258 [PubMed] [Google Scholar]
- 4. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker M, Mari A, Jayapaul MK, Bennett SM, Ferrannini E. Impaired β cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects. Diabetologia. 2005;48:2470–2476 [DOI] [PubMed] [Google Scholar]
- 6. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of β-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 7. Wagenknecht LE, Mayer EJ, Rewers M, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 8. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 9. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 10. Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 11. Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–256 [DOI] [PubMed] [Google Scholar]
- 12. Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 13. Zaccaro DJ, D'Agostino RB, Jr, Karter A, Bergman R, Wagenknecht LE. A comparison of the repeatability of insulin sensitivity with other cardiovascular disease risk factors. Can J Cardiol. 1997;13(suppl B):197B (Abstract) [Google Scholar]
- 14. Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes. 1994;43:1114–1121 [DOI] [PubMed] [Google Scholar]
- 15. Korytkowski MT, Berga SL, Horwitz MJ. Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism. 1995;44:1121–1125 [DOI] [PubMed] [Google Scholar]
- 16. Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–1384 [DOI] [PubMed] [Google Scholar]
- 17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 18. Marchetti P, Lupi R, Del Guerra S, et al. Goals of treatment for type 2 diabetes: β-cell preservation for glycemic control. Diabetes Care. 2009;32(suppl 2):S178–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA, San Antonio Metabolism Study Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 20. Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40:S21–S25 [DOI] [PubMed] [Google Scholar]
- 21. Lyssenko V, Almgren P, Anevski D, et al. ; Botnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 22. Kitabchi AE, Temprosa M, Knowler WC, et al. ; Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198 [DOI] [PubMed] [Google Scholar]
- 24. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 25. Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association, European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]