Abstract
Context:
Understanding intersubject variability in glycemic control following exercise training will help individualize treatment.
Objective:
Our aim was to determine whether this variability is related to training-induced changes in insulin sensitivity or pancreatic β-cell function.
Design, Setting, and Participants:
We conducted an observational clinical study of 105 subjects with impaired glucose tolerance or type 2 diabetes.
Interventions and Main Outcome Measures:
Individual subject changes in fitness (VO2max), glycemia (glycosylated hemoglobin, fasting glucose, oral glucose tolerance test), insulin sensitivity (hyperinsulinemic-euglycemic clamp), oral glucose-stimulated insulin secretion (GSIS), and disposition index (DI) were measured following 12 to 16 weeks of aerobic exercise training. Regression analyses were used to identify relationships between variables.
Results:
After training, 86% of subjects increased VO2max and lost weight. Glycosylated hemoglobin, fasting glucose, and 2-hour oral glucose tolerance test were reduced in 69%, 62%, and 68% of subjects, respectively, while insulin sensitivity improved in 90% of the participants. Changes in glycemic control were congruent with changes in GSIS such that 66% of subjects had a reduction in first-phase GSIS, and 46% had reduced second-phase GSIS. Training increased first- and second-phase DI in 83% and 74% of subjects. Training-induced changes in glycemic control were related to changes in GSIS (P < .05), but not insulin sensitivity or DI, and training-induced improvements in glycemic control were largest in subjects with greater pretraining GSIS.
Conclusions:
Intersubject variability in restoring glycemic control following exercise is explained primarily by changes in insulin secretion. Thus, baseline and training-induced changes in β-cell function may be a key determinant of training-induced improvements in glycemic control.
Randomized controlled trials have shown that aerobic exercise training improves glycemic control in subjects with impaired glucose tolerance and type 2 diabetes (1–5). However, the HERITAGE Family Study found that the interindividual variability in the response to exercise is large (6). The natural progression of type 2 diabetes indicates that as insulin sensitivity deteriorates, pancreatic β cells initially compensate by secreting more insulin but, with time, compensatory hyperinsulinemia fails and hyperglycemia ensues (7). Although several exercise studies have focused attention on changes in insulin sensitivity, fewer have examined the role of insulin secretion. Traditionally, after exercise in nondiabetic subjects, insulin secretion is reduced relative to an increase in insulin sensitivity; but in type 2 diabetes, where β-cell function may be very poor, exercise training increases insulin secretion in the presence of increased insulin sensitivity (8–10). However, to evaluate the true clinical effect of exercise, glycemic control should be determined because measuring insulin sensitivity or insulin secretion alone does not indicate the state of glycemic control; such variables are simply mechanistic entities that govern glucose tolerance.
In our previous studies, comparisons of group means have shown statistically significant improvements in glycemia following exercise (5, 8, 11). However, like the HERITAGE study, we have consistently observed large intersubject variability. We recently reported that in nondiabetic subjects, impaired fasting glucose blunted the beneficial effect of exercise on glycemic control (12). We have also shown that among subjects with type 2 diabetes, high ambient hyperglycemia (hemoglobin A1c) predicts smaller improvements in glycosylated hemoglobin (HbA1c) following exercise (5, 13). Although data from the HERITAGE study show that exercise training–induced changes in fitness (14, 15) and glycemic control (16) in healthy subjects may be influenced by the skeletal muscle transcriptome, studies in subjects with dysregulated glycemic control indicate that pancreatic β-cell function may also play a role. For example, obese nondiabetic subjects exposed to diet-induced elevations in daytime glycemia exhibited a worsening of hyperinsulinemia following exercise training compared to those with lower daytime glycemia (17). Furthermore, Dela et al (9) demonstrated that subjects with type 2 diabetes who had poorer insulin secretory capacity and overt hyperglycemia exhibited a blunted exercise training–induced improvement in glycemic control and glucose-stimulated insulin secretion (GSIS). Accordingly, Krotkiewski et al (10) showed that lower baseline GSIS in type 2 diabetes predicted a smaller increment in GSIS following exercise training. These findings indicate that pancreatic β-cell function may influence exercise training–induced changes in glycemic control; however, a thorough examination of this concept has not been conducted.
The main purpose of this study was to determine the individual exercise-responsiveness (postminus pretraining change) of glycemic control and the underlying changes in insulin sensitivity and insulin secretion in a large cohort of subjects with dysregulated glucose tolerance, under the hypothesis that changes in β-cell function would be a major determinant of the training-induced improvement in glycemic control.
Materials and Methods
Subjects
This study includes individual subject data from those who had adhered to >90% of the prescribed exercise training, so as to remove the confounding effects of exercise volume. As such, this is a nonrandomized, single-arm intervention. It should also be noted that data from some of the subjects have been used previously to test hypotheses related to exercise training and insulin resistance (5, 11). Potential subjects from the local community underwent medical evaluation (medical history, physical examination, 75 g oral glucose tolerance test [OGTT], and blood chemistry) as part of eligibility screening. Volunteers engaged in regular exercise or weight loss programs, smokers, pregnant women, and those exhibiting symptoms of chronic pulmonary/hepatic/renal/cardiovascular/hematological disease were excluded from participation. All subjects had impaired glucose tolerance or type 2 diabetes according to American Diabetes Association criteria (18). Participants with type 2 diabetes were recently diagnosed (4.8 ± 0.9 y) and those who were previously diagnosed were being treated with antidiabetic medications (metformin [N = 14], sulfonylureas [N = 6], GLP-1-analogues [N = 2], and DPP-4-inhibitors [N = 1]). None of these subjects were receiving insulin therapy. Medications were withheld for 5 days prior to metabolic testing. All female subjects were postmenopausal. The study was approved by the Institutional Review Board at the Cleveland Clinic (Cleveland, Ohio) and by the Ethical Committee of the Capital Region of Denmark. All subjects provided informed consent before participation in the study.
Measurements
For 3 days prior to testing, subjects continued their typical dietary/activity habits and completed diet and exercise records. Clinical assessments were conducted at the Clinical Research Unit at the Cleveland Clinic and in the clinical research laboratory of the Centre of Inflammation and Metabolism (Copenhagen, Denmark). Body composition (height, weight, dual-energy x-ray absorptiometry to determine body fat) and aerobic fitness (VO2max measured during an incremental workload exercise test to exhaustion on a treadmill; details in Ref. 11) were measured. Glycemic control was determined by HbA1c (samples were collected in 54 subjects), fasting plasma glucose, the 2-hour glucose response to a standard 75 g OGTT, and incremental area under the glucose response curve during OGTT. Blood samples were collected at 0, 30, 60, 90, and 120 minutes during OGTT. Insulin sensitivity was measured using a 2-hour hyperinsulinemic (40 mU/m2/min) euglycemic (5 mM) clamp, using the method described by DeFronzo et al (19). Subjects with fasting glucose greater than 5 mM underwent a prolonged (<1 h) priming infusion of insulin to reduce glucose levels to 5 mM before exogenous glucose was administered. Oral GSIS was assessed by serum insulin and C-peptide responses during OGTT. All tests were conducted at baseline and repeated 2 to 4 days after the final exercise bout.
Intervention
All subjects participated in an aerobic exercise training intervention of 12 to 16 weeks in duration. Exercise consisted of treadmill walking or cycle ergometry performed for ∼60 minutes per day, on 4 to 5 days per week, at an intensity of ∼75% of VO2max. VO2max was measured every 2 to 4 weeks during the intervention to modify the absolute work load of exercise so as to maintain the appropriate relative intensity. Full details of our standard exercise intervention are published (5, 17).
Calculations
Insulin sensitivity was quantified as the mean steady-state rate of glucose disposal during the final 30 minutes of the glucose clamp divided by the mean steady-state plasma insulin (Si; μmol/kg/min/pM). Incremental area under the curve (AUC) (iAUC) for glucose (mM.min), insulin (pM.min), and C-peptide (pM.min) during OGTT was calculated using the trapezoid method. First-phase GSIS is the incremental insulin and C-peptide response during the first 30 minutes of OGTT (Δ0–30 min). Second-phase GSIS equals iAUC for insulin and C-peptide during 30 to 120 minutes of the OGTT. Insulin secretion rate sensitivity to changes in glucose (rate sensitivity) is defined as the average C-peptide response above basal over the average glucose stimulus above basal (20). Disposition index (DI), a measure of pancreatic β-cell insulin secretory compensation for deteriorating insulin sensitivity, and which reflects whole-body glucose disposition, was calculated as the product of (first- and second-phase) GSIS and Si, in accordance with prior work (21). Validation of the inverse relationship between GSIS and insulin sensitivity is provided in the Supplemental Methods and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Biochemical analyses
HbA1c was measured in whole blood by high-pressure liquid chromatography (Tosho G7). Samples for glucose analysis were collected into heparin-containing syringes and immediately analyzed (YSI Stat; ABL 700, Radiometer). Samples for C-peptide and insulin analyses were collected into plain blood tubes at room temperature and centrifuged immediately at 25°C for 15 minutes at 2000g. Sera were isolated and stored at −80°C until analysis by electrochemiluminescence immunoassay (E-modular, Roche).
Statistics
Exercise training-induced changes between pre- and postintervention means were examined using paired two-tailed t tests. In this study we define exercise responsiveness as the exercise-induced change (Δ; Δ = postminus pretraining mean). Relationships between intervention-induced changes in variables were determined by linear regression. Furthermore, relationships between the preintervention value and its corresponding intervention-induced change were also demonstrated using linear regression. Age, body mass index (BMI), sex, and time since diagnosis had no influence on the statistical outcomes from these regression analyses. Family history of diabetes was not recorded and thus not controlled for. Statistical analyses were conducted using Prism v6 (GraphPad) and SPSS v20 (IBM), and statistical significance was accepted when P < .05. All data represent mean ± SEM unless stated otherwise.
Results
Subjects
One hundred five older (61 ± 1 y) overweight/obese (BMI 33 ± 1 kg/m2) subjects were included in this observational study (Table 1). Fifty-six subjects had impaired glucose tolerance and 49 had type 2 diabetes. Mean adherence to the intervention was 94 ± 1% (= total exercise minutes performed divided exercise minutes prescribed).
Table 1.
Subject Characteristics and Glycemic Control
| Pre | Post | Δ | |
|---|---|---|---|
| Subject demographics | |||
| Sex, M/F | 46/59 | ||
| Age, y | 61 ± 1 | ||
| Weight, kg | 94.8 ± 1.7 | 89.7 ± 1.5b | −4.6 ± 0.5 |
| BMI, kg/m2 | 33.2 ± 0.5 | 31.5 ± 0.5b | −1.6 ± 0.2 |
| Fat, % | 40.7 ± 0.8 | 38.8 ± 0.9b | −1.9 ± 0.3 |
| VO2max, L/min | 2.15 ± 0.06 | 2.37 ± 0.07b | 0.23 ± 0.03 |
| VO2max, mL/kg/min | 22.7 ± 0.5 | 26.5 ± 0.6b | 3.8 ± 0.4 |
| Glycemic control | |||
| HbA1c (n = 54), % | 6.09 ± 0.11 | 6.06 ± 0.13 | −0.01 ± 0.06 |
| Fasting plasma glucose, mM | 6.42 ± 0.15 | 6.08 ± 0.16b | −0.35 ± 0.08 |
| 2-h OGTT glucose, mM | 11.0 ± 0.4 | 10.2 ± 0.4b | −0.8 ± 0.2 |
| ΔG 0–30 min, mM | 3.28 ± 0.14 | 3.26 ± 0.14 | −0.02 ± 0.15 |
| iAUC G 0–30 min, mM·min | 252 ± 7 | 236 ± 7b | −16.0 ± 3.3 |
| iAUC G 30–120 min, mM·min | 597 ± 36 | 580 ± 39a | −17.0 ± 14.3 |
Abbreviations: F, female; G, glucose; M, male; Δ0–30 min = increment between 0 and 30 minutes of OGTT. Δ values represent the exercise responsiveness of each variable.
Subjects (n = 105) with impaired glucose tolerance and recently diagnosed type 2 diabetes underwent 12 to 16 weeks of moderate-intensity aerobic exercise training, 5 days per week, 60 minutes per day. Body composition, aerobic fitness (VO2max), glucose tolerance (OGTT), insulin sensitivity (glucose clamp), and oral glucose-stimulated insulin secretion were measured.
Data indicate mean ± SEM paired t tests were used to compare pre- vs postexercise means. Statistical significance is represented by a P < .05 and b P < .001.
Effects of exercise training on physiological and metabolic outcomes (Table 1)
Body weight, BMI, and whole body adiposity were significantly decreased (−5 ± 1%) following exercise. Aerobic fitness was increased by +11 ± 1%. There was a significant reduction in several measures of glycemic control (fasting glucose, 2-h OGTT glucose, and AUC glucose); however, mean HbA1c was unchanged between pre- and postexercise. Serum insulin concentrations (fasting and during OGTT) decreased following exercise, whereas serum C-peptide levels during OGTT were unchanged. GSIS was reduced following exercise when calculated using serum insulin levels, but not when GSIS was calculated using C-peptide levels. There was a 60 ± 9% increase in insulin-sensitivity following exercise, and a 69 ± 15% and 82 ± 14% increase in first- and second-phase DI.
Intersubject exercise responsiveness
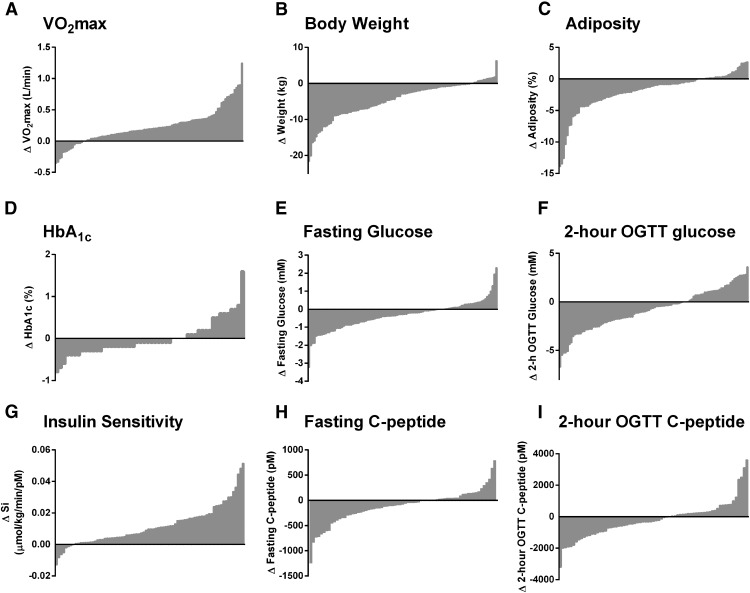
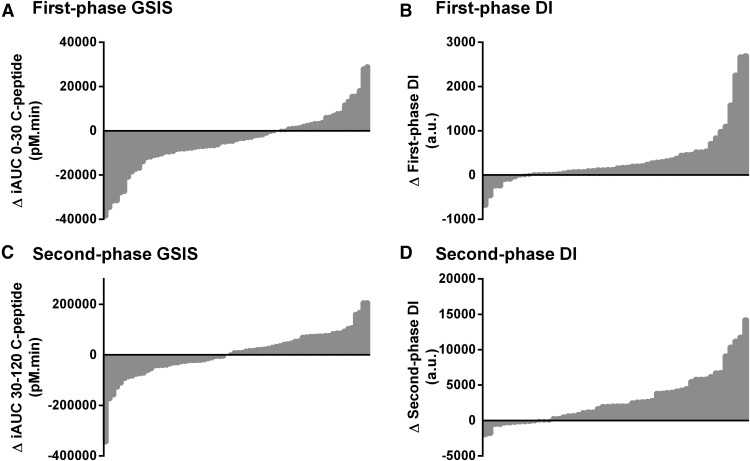
Following exercise, 86% of subjects increased VO2max (Figure 1A), 86% lost weight (Figure 1B), and 77% reduced whole-body adiposity (Figure 1C). Exercise reduced HbA1c (Figure 1D), fasting plasma glucose (Figure 1E), 2-hour plasma glucose during OGTT (Figure 1F), and AUC plasma glucose during OGTT in 69%, 62%, 68%, and 54% of subjects. Insulin-stimulated glucose disposal was increased following exercise in 90% of subjects (Figure 1G), but the training-induced changes in insulin secretion were less consistent: fasting C-peptide (Figure 1H) and 2-hour OGTT C-peptide (Figure 1I) were both decreased in 60% of the subjects, whereas 59% of subjects had a decrease in the incremental 30-minute C-peptide response to oral glucose, and insulin secretion rate sensitivity to changes in plasma glucose was reduced in 51% of subjects. First- and second-phase GSIS was decreased in 66% and 46% of subjects and first- and second-phase DI was increased in 83% and 74% of subjects (Figure 2). Additional exercise-responsiveness data, including trends in serum insulin, are provided in Supplemental Figure 1.
Figure 1.
Subjects with impaired glucose tolerance or type 2 diabetes (N = 105) underwent 12 to 16 weeks of moderate-intensity exercise training, 5 days per week, 60 minutes per day. Exercise responsiveness of (A) aerobic fitness, (B) body weight, (C) whole-body adiposity, (D) HbA1c (N = 54), (E) fasting glucose, (F) 2-hour OGTT glucose, (G) insulin sensitivity, (H) fasting C-peptide, and (I) 2-hour OGTT C-peptide were measured as the postminus preexercise (Δ) values. Individual subject data points are plotted on the x-axis. Y-axis data above the origin indicate an exercise training-induced increase in that variable; data below the origin represent a training-induced decrease.
Figure 2.
Subjects with impaired glucose tolerance or type 2 diabetes (N = 105) underwent 12 to 16 weeks of moderate-intensity exercise training, 5 days per week, 60 minutes per day. Exercise responsiveness of first-phase oral GSIS (A) and DI (B), and second-phase GSIS (C) and DI (D) were measured as the postminus preexercise (Δ) values. Individual subject data points are plotted on the x-axis. Y-axis data above the origin indicate an exercise training-induced increase in that variable; data below the origin represent a training-induced decrease. First-phase GSIS is reported as the area under the serum C-peptide response curve during the first 30 minutes following the ingestion of 75 g glucose. Second-phase GSIS is reported as the area under the C-peptide curve from 30 to 120 minutes after glucose ingestion. DI is calculated as the product of GSIS and insulin sensitivity.
Relationship between training-induced changes in glycemic control and training-induced changes in insulin sensitivity or β-cell function
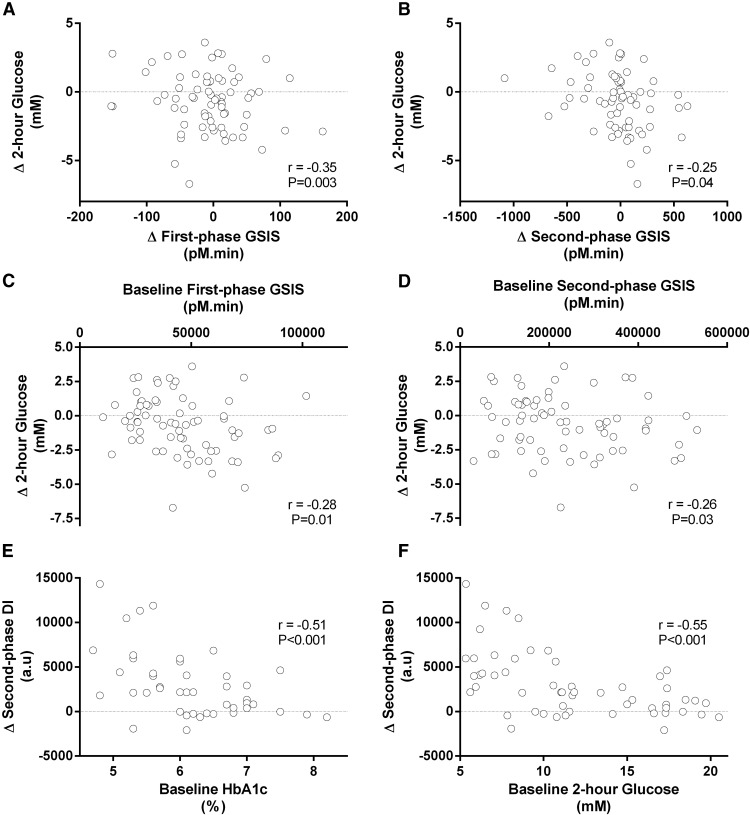
Table 2 indicates that exercise training–induced changes in ambient hyperglycemia (HbA1c and fasting plasma glucose) and measures of oral glucose tolerance (2-h glucose and AUC glucose during OGTT) were significantly correlated with training-induced changes in various markers of insulin secretion, but they were not related to training-induced changes in insulin sensitivity. For example, a larger increase in first- and second-phase GSIS was correlated with a better reduction in 2-hour OGTT glucose (Figure 3, A and B). Weight loss was related to training-induced changes in fasting and 2-hour OGTT glucose (both r = 0.22, P < .05), fasting C-peptide (r = 0.32, P < .01), insulin sensitivity (r = −0.61, P < .001), and DI (r = −0.57, P < .001); however, the correlations between training-induced changes in glycemic control and insulin secretion remained significant when controlling for weight loss.
Table 2.
Insulin Secretion, Insulin Sensitivity, and Disposition Index
| Pre | Post | Δ | |
|---|---|---|---|
| Serum insulin | |||
| Fasting, pM | 121 ± 8 | 90 ± 5b | −31 ± 7 |
| 2-h OGTT, pM | 754 ± 76 | 576 ± 91a | −180 ± 61 |
| ΔI 0–30 min, pM | 449 ± 48 | 345 ± 31a | −107 ± 33 |
| iAUC I 0–30 min, pM·min | 9872 ± 773 | 7391 ± 475b | −2480 ± 553 |
| iAUC I 30–120 min, pM·min | 71 554 ± 6537 | 52 368 ± 5660b | −19 670 ± 5021 |
| ΔI/G 0–30 min | 175 ± 22 | 124 ± 14a | −52 ± 17 |
| iAUC I/G 0–30 min | 42.5 ± 3.5 | 33.5 ± 2.3b | −9.0 ± 2.2 |
| iAUC I/G 30–120 min | 188 ± 21 | 142 ± 19a | −48 ± 14 |
| Serum C-peptide | |||
| Fasting, pM | 959 ± 54 | 841 ± 46a | −118 ± 38 |
| 2-h OGTT, pM | 3454 ± 187 | 3247 ± 195 | −207 ± 134 |
| ΔC 0–30 min, pM | 946 ± 95 | 931 ± 94 | −15 ± 63 |
| iAUC C 0–30 min, pM·min | 45 702 ± 2372 | 41 256 ± 2001 | −4446 ± 1553 |
| iAUC C 30–120 min, pM·min | 239 064 ± 15 131 | 244 987 ± 16 234 | 5923 ± 10592 |
| ΔC/G 0–30 min | 418 ± 60 | 359 ± 44 | −59 ± 53 |
| iAUC C/G 0–30 min | 187 ± 11 | 181 ± 11 | −6.6 ± 6.7 |
| iAUC C/G 30–120 min | 548 ± 51 | 578 ± 68 | 30 ± 52 |
| Rate sensitivity, pM/mM | 284 ± 171 | 207 ± 81 | −27 ± 205 |
| Clamp GDR, μmol/kg/min | 12.7 ± 0.7 | 18.7 ± 1.0b | 6.0 ± 0.7 |
| Clamp insulin, pM | 480 ± 27 | 460 ± 25 | −20.9 ± 16.4 |
| Insulin sensitivity, μmol/kg/min/pM | 0.0194 ± 0.0013 | 0.0309 ± 0.0022b | 0.012 ± 0.001 |
| First-phase DI, a.u. | 707 ± 64 | 1054 ± 113b | 351 ± 89 |
| Second-phase DI, a.u. | 3561 ± 317 | 6491 ± 672b | 2946 ± 489 |
Abbreviations: C, C-peptide; F, female; G, glucose; GDR, glucose disposal rate; I, insulin; M, male; Rate sensitivity, rate of change of serum C-peptide relative to the rate of change of plasma glucose; Δ0–30 min, increment between 0 and 30 min of OGTT. Δ values represent the exercise responsiveness of each variable.
Subjects (n = 105) with impaired glucose tolerance and recently diagnosed type 2 diabetes underwent 12- to 6 weeks of moderate-intensity aerobic exercise training, 5 days per week, 60 minutes per day. Body composition, aerobic fitness (VO2max), glucose tolerance (OGTT), insulin sensitivity (glucose clamp), and oral glucose-stimulated insulin secretion were measured.
Data indicate mean ± SEM. Paired t tests were used to compare pre- vs postexercise means. Statistical significance is represented by a P < .01 and b P < .001.
Figure 3.
Subjects with impaired glucose tolerance or type 2 diabetes (N = 105) underwent 12 to 16 weeks of moderate-intensity exercise training, 5 days per week, 60 minutes per day. Relationships between variables were analyzed by linear regression. (A) and (B) demonstrate that a greater training-induced increase in first- and second-phase GSIS (x-axis) was related to a greater training-induced decrease in 2-hour plasma glucose measured during OGTT (y-axis). (C) and (D) indicate that larger preintervention first- and second-phase GSIS (x-axis) were associated with larger training-induced improvements in oral glucose tolerance (y-axis). (E) and (F) show that poorer preintervention glycemic control, as indicated by high HbA1c (x-axis), was correlated with poorer training-induced improvements in the DI (y-axis). First-phase GSIS is measured as the area under the serum C-peptide response curve during the first 30 minutes following the ingestion of 75 g glucose. Second-phase GSIS is measured as the area under the C-peptide curve from 30 to 120 minutes after glucose ingestion. DI is calculated as the product of GSIS and insulin sensitivity.
Predicting training-induced changes in glycemic control, insulin sensitivity, or β-cell function from pretraining metabolic or endocrine variables
Pretraining measures of glycemic control were inversely correlated with training-induced increases in insulin sensitivity and first- and second-phase DI, such that high pretraining HbA1c (r = −0.50 and −0.51), fasting glucose (r = −0.40 and −0.44), 2-hour OGTT glucose (r = −0.49 and −0.55), and area under the OGTT glucose curve (r = −0.50 and −0.52) predicted smaller increases in these variables (all P < .001) (Figure 3, E and F). Longer type 2 diabetes disease duration (y) also predicted poorer training-induced decreases in 2-hour OGTT glucose (r = 0.40, P < .01) and area under the OGTT glucose curve (r = 0.53, P < .001), and poorer increases in insulin sensitivity (r = −0.36, P < .05), and second-phase DI (r = −0.41, P < .05). Furthermore, pretraining markers of β-cell insulin secretory function were inversely associated with training-induced decreases in glycemic control, such that high pretraining 2-hour OGTT C-peptide and first-phase GSIS predicted a greater training-induced decrease in 2-hour OGTT glucose (r = −0.31, P < .01; and r = −0.28, P < .05) and area under the OGTT glucose curve (r = −0.36, P < .01; and r = −0.27, P < .05) (Figure 3, C and D). Although pretraining insulin secretory function predicted changes in glycemic control, on the contrary, pretraining insulin sensitivity was not associated with training-induced changes in any variable (all comparisons, P > .05).
Discussion
We observed that regular aerobic exercise training improved aerobic fitness and body composition and increased insulin-mediated peripheral tissue glucose disposal in 86% to 90% of subjects. However, despite these beneficial effects in most subjects, glycemic control (HbA1c, fasting glucose, or oral glucose tolerance) improved in only two-thirds of subjects. This high intersubject variability in the exercise responsiveness of glycemic control was visually reflected by the intersubject variability in exercise training–induced changes in glucose-stimulated insulin secretion. We confirmed this objectively, demonstrating that training-induced changes in glycemic control were correlated with changes in insulin secretion; we found that high pretraining glucose-stimulated insulin secretory capacity predicted better training-induced improvements in glycemic control. In addition, although training-induced changes in DI were not related to changes in glycemic control, the DI was significantly increased following exercise and, interestingly, high ambient hyperglycemia before commencing training blunted training-induced improvements in the DI.
These data emphasize the large intersubject variability that exists in the exercise responsiveness of glycemic control. Previously, we documented that high ambient hyperglycemia in subjects with type 2 diabetes blunts the beneficial effect of aerobic exercise on glycemic control in that high HbA1c or 2-hour OGTT glucose predicts poor improvements in glycemic control following training (13). The Look-AHEAD study also recently showed that diabetes remission following exercise and diet intervention was more likely in persons with diabetes with a shorter disease history and lower initial HbA1c (22). Given that following a single exercise bout van Dijk et al (23) showed greater reductions in daytime glycemia in type 2 diabetes patients with higher HbA1c, these poorer outcomes following exercise training in more hyperglycemic diabetic subjects are perhaps unexpected. However, such observations are supported by prior evidence from us (12, 17) and others (9, 10), which have indicated that poor pretraining glycemic control may predict the effectiveness of exercise, and this may all depend on β-cell capacity at the time the intervention is initiated. Our new data complement prior evidence in that low pretraining GSIS (2-h OGTT C-peptide, and first- and second-phase C-peptide response to OGTT) predicted smaller training-induced decreases in glycemia (2-h and AUC OGTT glucose). Furthermore, high ambient hyperglycemia (HbA1c and fasting glucose) and poor glucose tolerance (OGTT) also predicted a poorer training-induced increase in the DI. The DI provides a measure of compensatory β-cell function (glucose-stimulated insulin secretion) relative to changes in the underlying degree of insulin sensitivity (insulin-stimulated glucose disposal) and in absolute terms indicates whole-body glucose disposition. It is therefore not just a measure of GSIS and is therefore not solely a measure of β-cell function. Also by nature of its calculation, changes in insulin sensitivity carry as much weight to the change in DI as do changes in insulin secretion. We found a significant increase in DI following training in a population of subjects representing both impaired glucose tolerant and type 2 diabetic phenotypes, where population means for insulin sensitivity were significantly increased (Table 2) and where population means for insulin secretory function were unchanged (Table 2). In this context, an increase in the DI value is primarily driven by the increment in insulin sensitivity. Accordingly, the variability in the training-induced change in DI is similar to the observed variability in insulin sensitivity (Figure 1). As such, DI is consistently increased following training in most subjects (83% and 74% of subjects for first- and second-phase DI, respectively); however, it was a poor predictor of the exercise-induced change in glycemic control (Table 3).
Table 3.
Relationships Between Exercise Training-induced Changes (Δ) in Measures of Glycemia and Training-induced Changes (Δ) in Insulin Sensitivity and Insulin Secretion
| R values | Δ HbA1c | Δ Fasting Glucose | Δ 2-h OGTT Glucose | Δ AUC OGTT Glucose |
|---|---|---|---|---|
| Δ Serum insulin | ||||
| Fasting | 0.27 | 0.47c | 0.31b | 0.14 |
| 2-h OGTT | 0.01 | 0.08 | 0.20 | 0.16 |
| ΔI 0–30 min | 0.00 | −0.06 | −0.11 | −0.10 |
| iAUC I 0–30 min | 0.10 | 0.15 | 0.06 | 0.02 |
| iAUC I 30–120 min | 0.02 | 0.04 | −0.02 | 0.04 |
| ΔI/G 0–30 min | −0.02 | −0.05 | −0.13 | −0.34c |
| iAUC I/G 0–30 min | 0.02 | 0.00 | −0.04 | 0.13 |
| iAUC I/G 30–120 min | 0.00 | 0.02 | −0.19a | −0.40c |
| Δ Serum C-peptide | ||||
| Fasting | 0.37a | 0.34b | 0.24a | 0.40c |
| 2-h OGTT | −0.19 | −0.01 | 0.28a | 0.42c |
| ΔC 0–30 min | −0.12 | −0.16 | 0.17 | 0.06 |
| iAUC C 0–30 min | 0.13 | 0.17 | 0.12 | 0.35b |
| iAUC C 30–120 min | −0.24 | −0.09 | 0.02 | 0.18 |
| ΔC/G 0–30 min | −0.14 | −0.09 | −0.11 | −0.13 |
| iAUC C/G 0–30 min | −0.06 | −0.18 | −0.14 | 0.18 |
| iAUC C/G 30–120 min | −0.17 | −0.02 | −0.25a | −0.42c |
| Rate sensitivity | −0.10 | 0.01 | −0.17 | −0.18 |
| Δ Insulin sensitivity | 0.04 | −0.04 | −0.15 | −0.13 |
| Δ First-phase DI | −0.04 | 0.02 | −0.23 | 0.08 |
| Δ Second-phase DI | −0.09 | −0.03 | −0.08 | 0.04 |
Abbreviations: C, C-peptide; G, glucose; I, insulin; Δ 0–30 min, increment between 0 and 30 minutes of OGTT.
Linear regression was used to compare training-induced changes in variables. Statistically significant correlations are represented by a P < .05, b P < .01, and c P < .001.
Despite showing significant improvements in insulin sensitivity, our observations that training-induced changes in glycemic control are related to changes in insulin secretion but not insulin sensitivity. Accordingly, we noted that higher pretraining GSIS predicts larger training-induced decreases in glucose tolerance, which adds to the evidence from Dela et al (9) that some residual β-cell function is required to maximize the beneficial effect of aerobic exercise on glycemic control. Decreased capacity to secrete insulin may be explained by the profound degree of glucotoxicity that exists in subjects with type 2 diabetes. Prolonged exposure to glucose can impair pancreatic β-cell function in vitro (reviewed in Refs. [24, 25]), and we (26) and others (27, 28) have demonstrated this experimentally in vivo. In β cells, it is likely that increased IL-1β signaling leads to insulin secretory dysfunction and cell death in type 2 diabetic models (29). Interestingly, Russell et al (30) provide in vitro evidence that IL-6 augments apoptosis in pancreatic β cells under IL-1β-mediated inflammatory attack. This is of relevance to the exercise setting because there is a well-documented acute and transient inflammatory response (increased muscle-derived plasma IL-6) to a single exercise bout (31). Thus, we speculate that accumulation of multiple exercise bouts exposes the endocrine pancreas to increased amounts of IL-6, which under conditions of glucolipotoxicity-induced inflammation (IL-1β) such as found in uncontrolled type 2 diabetes may augment β-cell death and subsequent insulin secretory dysfunction. Our unpublished in vitro data support this speculation, but further in vivo investigation is warranted.
The observation that ∼30% of subjects experienced a deterioration in glycemic control (when assessed by HbA1c, fasting glucose, or 2-h glucose) following exercise is concerning. This lack of a therapeutic benefit of exercise on glycemic control is also highlighted by the finding that mean values of HbA1c were not significantly changed between pre- and postintervention time points (Table 1), although this is possibly explained by the relatively short-term nature of the intervention (12–16 wk). This does however provide a good example that important information regarding individualized subject responses to treatments may be masked if only changes in group means are considered. This adverse effect of exercise on glycemic control was documented in the HERITAGE Study by Boulé and colleagues (6), whereby ∼40% of subjects decreased iv glucose tolerance. That study and ours are of relatively short duration (<20 wk) and outcomes of diabetic complications and diabetes-related mortality were not measured so it is difficult to comment on the long-term implications of these adverse effects on glycemic control. Promisingly, other data from the HERITAGE study showed that only 7% of participants exhibited deterioration in two or more components of the metabolic syndrome (32), suggesting that the impact of exercise responsiveness on the variability of cardiovascular disease risk may be small. Nevertheless, the clinical relevance of these findings is paramount, as they point toward the need for future research to gain insight mechanistically to the metabolic “nonresponder.” Ultimately, this understanding will potentially lead to the personalization of medical treatment and help maximize the benefit of physical activity.
We appreciate that correlations do not indicate causality. Future randomized controlled trials stratifying subjects by glycemia will determine whether our observations extrapolate to causal associations. Additional assessments of β-cell function are also warranted; for instance, insulin secretory responses to nonglucose secretagogues (incretins, arginine). However, other variables may be implicated. For example, the HERITAGE Study noted that the presence of a single (Pro12Ala) nucleotide polymorphism in peroxisome proliferator-activated receptor-γ increased training-induced improvements in glucose tolerance (16). Furthermore, metformin, a widely prescribed antihyperglycemic drug, may interact with exercise to impair outcomes regarding insulin sensitivity (33–35). In this study, genomic/transcriptomic analyses were not performed; however, antidiabetic medications (N = 14 metformin, N = 6 sulfonylureas, N = 2 GLP-1-analogues, N = 1 DPP-4-inhibitors) did not influence our outcomes; due to the small sample size, such analyses may be underpowered. Weight loss may also impact our outcome variables, and although training-induced weight loss was related to changes in fasting glucose (r = 0.23, P < .05) and insulin sensitivity (r = 0.61, P < .001) but no other measure related to glycemic control, baseline body weight did not predict exercise responsiveness of any variable. One final comment regarding our design is that changes in the groups' mean values for serum insulin but not C-peptide were found following exercise, indicating that hepatic insulin extraction was likely increased following training. This variable was not measured directly and is often neglected in exercise studies. Future work should consider hepatic insulin extraction as a variable of interest.
Future studies should incorporate a thorough clinical assessment of glycemic control to include HbA1c, fasting glucose, and an OGTT, so that appropriate expectations regarding the clinical effectiveness of exercise training can be drawn. Despite consistent improvements in fitness, body composition, and insulin sensitivity, there is high intersubject variability in the improvement of glycemic control following aerobic exercise training. This finding makes it clear that despite observing statistically significant improvements in insulin sensitivity, great caution should be taken when assuming that exercise training has a clinically meaningful outcome with regard to glycemic control. Herein we demonstrate that the intersubject variability in the exercise responsiveness of glycemic control is related to variability in exercise training–induced changes in insulin secretion, and that high pretraining ambient hyperglycemia and low insulin secretory function predict a poorer training-induced outcome. Hence, using aerobic exercise to treat patients with poorly controlled long-term type 2 diabetes may have limited chances of a successfully controlled glycemia. In such patients it seems sensible to improve β-cell function and achieve better glycemic control (possibly by pharmaceutical means) prior to incorporating aerobic exercise as an intervention. Future randomized controlled trials are crucial to determine an optimal approach. Overall, these findings are important in that they highlight the need for individualized treatment so as to maximize the benefit to treating obesity-related hyperglycemia.
Supplementary Material
Acknowledgments
We thank Lisbeth Andreasen (Rigshospitalet, Copenhagen, Denmark) for assistance with biochemical analyses and Julianne Filion (Cleveland Clinic, Cleveland, Ohio) for her assistance with subject recruitment and screening. We also thank Marc Cook (Cleveland Clinic) and Thomas Grøndahl and Kamilla Winding (Rigshospitalet) for their help with exercise training.
Trial registration: Clinical Trials NCT01234155.
Author contributions: T.P.J.S. conceptualized the idea for this study. T.P.J.S., S.K.M., K.K., J.M.H., S.R.K., and J.P.K. implemented the study. T.P.J.S., S.K.M., K.K., and J.M.H. analyzed the data. T.P.J.S., S.K.M., K.K., J.M.H., and J.P.K. discussed the data. T.P.J.S. wrote the manuscript. All authors edited the manuscript. T.P.J.S. had access to the data and can take responsibility for the integrity of the data and the accuracy of the analysis.
The study was funded by a Paul Langerhans program grant from the European Foundation for the Study of Diabetes (T.P.J.S.) and supported by the National Institutes of Health (NIH Grant RO1 AG12834 to J.P.K.). S.K.M. was supported by NIH Grant T32 DK007319. The Centre of Inflammation and Metabolism is supported by a center grant from the Danish National Research Foundation. The funding sources played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- DI
- disposition index
- GSIS
- glucose-stimulated insulin secretion
- HbA1c
- glycosylated hemoglobin
- iAUC
- incremental area under the glucose response curve
- OGTT
- oral glucose tolerance test.
References
- 1. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544 [DOI] [PubMed] [Google Scholar]
- 2. Balducci S, Zanuso S, Nicolucci A, et al. , Italian Diabetes and Exercise Study (IDES) Investigators Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170(20):1794–1803 [DOI] [PubMed] [Google Scholar]
- 3. Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304(20):2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369 [DOI] [PubMed] [Google Scholar]
- 5. Karstoft K, Winding K, Knudsen SH, et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36(2):228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulé NG, Weisnagel SJ, Lakka TA, et al. , and HERITAGE Family Study Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108–114 [DOI] [PubMed] [Google Scholar]
- 7. Jallut D, Golay A, Munger R, et al. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism. 1990;39(10):1068–1075 [DOI] [PubMed] [Google Scholar]
- 8. Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic β-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33(7):1561–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance β-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287(5):E1024–E1031 [DOI] [PubMed] [Google Scholar]
- 10. Krotkiewski M, Lönnroth P, Mandroukas K, et al. The effects of physical training on insulin secretion and effectiveness and on glucose metabolism in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1985;28(12):881–890 [DOI] [PubMed] [Google Scholar]
- 11. Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol. 2008;104(5):1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malin SK, Kirwan JP. Fasting hyperglycaemia blunts the reversal of impaired glucose tolerance after exercise training in obese older adults. Diabetes Obes Metab. 2012;14(9):835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomon TP, Malin SK, Karstoft K, Haus JM, Kirwan JP. The influence of hyperglycemia on the therapeutic effect of exercise on glycemic control in patients with type 2 diabetes mellitus [published online July 1, 2013]. JAMA Int Med. doi:10.1001/jamainternmed.2013.7783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keller P, Vollaard NB, Gustafsson T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol. 2011;110(1):46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timmons JA, Knudsen S, Rankinen T, et al. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol. 2010;108(6):1487–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruchat SM, Rankinen T, Weisnagel SJ, et al. Improvements in glucose homeostasis in response to regular exercise are influenced by the PPARG Pro12Ala variant: results from the HERITAGE Family Study. Diabetologia. 2010;53(4):679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solomon TP, Haus JM, Kelly KR, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92(6):1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223 [DOI] [PubMed] [Google Scholar]
- 20. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of β-cell function and insulin sensitivity. Diabetes. 2001;50(1):150–158 [DOI] [PubMed] [Google Scholar]
- 21. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672 [DOI] [PubMed] [Google Scholar]
- 22. Gregg EW, Chen H, Wagenknecht LE, et al. Association of an Intensive Lifestyle Intervention With Remission of Type 2 Diabetes. JAMA. 2012;308(23):2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Dijk JW, Manders RJ, Canfora EE, et al. Exercise and 24-h glycemic control: equal effects for all type 2 diabetic patients? Med Sci Sports Exerc. 2012. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. Prentki M, Corkey BE. Are the β-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45(3):273–283 [DOI] [PubMed] [Google Scholar]
- 25. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev. 2008;29(3):351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solomon TP, Knudsen SH, Karstoft K, Winding K, Holst JJ, Pedersen BK. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab. 2012;97(12):4682–4691 [DOI] [PubMed] [Google Scholar]
- 27. Ferner RE, Ashworth L, Tronier B, Alberti KG. Effects of short-term hyperglycemia on insulin secretion in normal humans. Am J Physiol. 1986;250(6 Pt 1):E655–E661 [DOI] [PubMed] [Google Scholar]
- 28. Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am J Physiol. 1996;270(2 Pt 1):E251–E258 [DOI] [PubMed] [Google Scholar]
- 29. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):314–321 [DOI] [PubMed] [Google Scholar]
- 30. Russell MA, Cooper AC, Dhayal S, Morgan NG. Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic β-cells. Islets. 5(2):95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(Pt 1):287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35(1):131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab. 2010;298(4):E815–E823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulé NG, Robert C, Bell GJ, et al. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care. 2011;34(7):1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.