Abstract
Background:
Smoking cessation for individuals with depressive disorders represents an important clinical issue. It often has been hypothesized that smoking cessation worsens negative affect as part of the withdrawal process in this population. However, studies examining the impact of smoking cessation on changes in affect in smokers with depression are limited and equivocal.
Methods:
This study examines affective processes in smokers with depression undergoing a 12-week smoking cessation intervention (N = 49). We used the Positive and Negative Affect Scale to measure participants’ positive affect (PA) and negative affect (NA) trajectories over the course of a quit attempt. We examined whether affective treatment response across the trial differed by prolonged smoking abstinence status and whether postquit affect differed by prequit affective treatment response, as well as the interaction of prequit affective response and abstinence status.
Results:
Prolonged abstainers showed significant increases in PA over the course of a quit attempt compared with nonabstainers. Prequit affective trajectories significantly predicted postquit affect for measures of both PA and NA. Lastly, the interaction of prequit affective trajectory and abstinence significantly predicted postquit levels of NA but not PA.
Conclusions:
This study adds to a burgeoning body of research demonstrating that significant improvements in psychological functioning can be observed among those who successfully quit smoking even in the most severe psychiatric group.
INTRODUCTION
Smoking cessation for individuals with depressive disorders represents an important clinical issue. Recent epidemiological data show that at least 30%–40% of individuals with depressive disorders are current smokers, which is about twice the rate of individuals who are not depressed (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Hall & Prochaska, 2009; Lasser et al., 2000; Lawrence, Mitrou, & Zubrick, 2009; Ziedonis et al., 2008). Thus, depressed smokers are an important group to target in population health–based tobacco control efforts.
Several models have been proposed to explain association of smoking with depression. The primary depression model, also known as the self-medication model, proposes an etiological link from depression to cigarette smoking. According to this model, depressed individuals are more likely to initiate smoking and progress to nicotine dependence in part because nicotine serves to manage negative affect or aid in coping with distress related to depressive symptom development (Glassman et al., 1990; Khantzian, 1997). Conversely, the primary smoking model proposes a causal relationship from smoking to depression. This model posits that smoking increases risk of developing depression due to alterations in neurotransmitter pathways following prolonged exposure to nicotine (Hughes, 1999; Markou & Kenny, 2002; Markou, Kosten, & Koob, 1998).
Traditionally, the primary depression or self-medication model has been accepted by researchers and clinicians alike, as depression is characterized by high negative affect and low positive affect (Watson, Clark, & Carey, 1988), and these affective disturbances may be exacerbated through attempting to quit smoking. This has been supported by findings that smoking cessation can provoke or exacerbate symptoms of depression (Covey, Glassman, & Stetner, 1997; Evins et al., 2008; Glassman, Covey, Stetner, & Rivelli, 2001) though it is possible that these effects are associated most strongly with unsuccessful quit attempts rather than successful abstinence (Berlin, Chen, & Covey, 2010; McClave et al., 2009). Contrary to the primary depression model, several recent studies have shown smoking abstinence to have no association with depressed mood (Kahler et al., 2002; Prochaska et al., 2008; Torres et al., 2010) or to predict improvements in psychological functioning (Berlin et al., 2010; Blalock, Robinson, Wetter, Schreindorfer, & Cinciripini, 2008; Kahler, Spillane, Busch, & Leventhal, 2011). However, studies examining the impact of smoking cessation on negative affect in depressed smokers are limited and equivocal.
There is also preliminary evidence to suggest that postquit affect may differ by prequit affective treatment response in this population. Although limited research has explored affective or other treatment processes in the context of smoking cessation, early gains have been shown to be predictive of positive treatment outcome in the depression literature (Kelly, Roberts, & Ciesla, 2005; Tang & DeRubeis, 1999). Two previous smoking cessation trials have shown that those with precessation improvement in affect are more likely to maintain these gains and become prolonged abstainers (Blalock et al., 2008; Kahler et al., 2011). Thus, in the current study, we expected that those who displayed early improvement in affect would be most likely to show further improvements in affective trajectories in the postquit period.
In addition to the impact of prolonged abstinence, abstinence may have concurrent effects on affect. Preliminary evidence suggests that being abstinent in a particular week is associated with lower levels of concurrent depressive symptoms (Dawkins, Powell, Pickering, Powell, & West, 2009; Kahler et al., 2011). Clinical observation would suggest that those who improve early in treatment and are abstinent following the quit date develop a sense of self-efficacy that helps to alleviate symptoms of depression, but this question has not yet been examined in the research literature.
Data for the current study were drawn from a randomized clinical trial comparing Cognitive Behavioral Analysis System of Psychotherapy (McCullough, 2000) in combination with standard smoking cessation treatment (CBASP/ST) to Health Education plus standard smoking cessation treatment (HE/ST) in smokers with current chronic depressive disorders. Primary abstinence outcomes for this clinical trial will be examined in a future paper. The current analyses focused on three primary aims related to affective functioning. First, we examined whether smoking abstainers had different patterns of pre- and postquit affective changes than relapsers. We hypothesized that those who were prolonged abstainers at the 3-month follow-up would report decreased negative affect and increased positive affect on the Positive and Negative Affect Scales (PANAS), both pre- and postquit, relative to nonabstainers. Second, we investigated whether prequit affective response to treatment in the first six prequit treatment sessions predicted postquit affective response. We hypothesized that those who showed significant improvement in their affective trajectories over the first six prequit treatment sessions would report decreased negative postquit affect and increased postquit positive affect compared with those who do not show improvement in affect during the prequit treatment sessions. Finally, we examined whether the interaction of early affective treatment response and postquit 7-day point prevalence abstinence predicted postquit affect. We hypothesized that those who showed the most improvement in prequit affect and who were abstinent following the quit date would show the greatest improvement in postquit affect.
METHODS
Participants
Participants were 49 smokers (19 women) seeking smoking cessation treatment recruited from the greater Houston metropolitan area. Study advertisement targeted those who were currently depressed and interested in quitting smoking. Participants were required to smoke 5 or more cigarettes per day at baseline and meet criteria for a chronic form of a depressive disorder (recurrent depression, major depressive episode with a duration of 2 years or more, or dysthymic disorder). Participants must have been experiencing or have been in partial remission of a major depressive episode at baseline. In addition, participants were required to score ≥ 8 on the Patient Health Questionnaire (PHQ), indicating at least moderate depressive symptoms, at the baseline session. Mood diagnoses were based on interview, at baseline, with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1994). Exclusion criteria included a history of psychotic or bipolar disorder or current principal Axis I disorder other than unipolar depression or nicotine dependence, contraindications for use of the nicotine patch, or presence of psychiatric symptoms in need of immediate treatment.
Procedures
A total of 642 individuals were assessed for basic eligibility using a brief telephone screen, with 190 of these individuals found eligible after this step and invited to attend an in-person screening. Of these, 96 did not attend the appointment, 15 did not meet study criteria, 18 declined, and 12 did not return for a randomization visit. Thus, 49 participants were randomized to receive either CBASP/ST or HE/ST. For both treatment groups, 12 treatment sessions were provided by clinical psychologists in the Department of Behavioral Science at the University of Texas MD Anderson Cancer Center. Participants were instructed to set a quit date following Week 6 of treatment. Nicotine replacement therapy (NRT) was also provided to participants in both groups. Participants were provided with a total of 8 weeks of NRT, beginning on the scheduled quit date and tapering from patches with 21mg nicotine dosages to 14 and 7mg patches. Data were collected from participants at 12 weekly treatment sessions and follow-up sessions at 3 and 6 months. As noted below, for purposes of the present analysis, treatment group was treated as a covariate.
Measures
Participants were assessed on a variety of interview, self-report, and biochemical measures at baseline and each treatment session and at 3 and 6 months after the targeted quit date. Relevant measures to the current study are discussed below.
PANAS
The PANAS (Watson, Clark, & Tellegen, 1988) was admin istered at each treatment session and at the 3- and 6-month follow-up visits. The PANAS is a widely used self-report measure of the experience of positive and negative affect within the past week. The measure consists of two 10-item mood scales, one for positive affect and one for negative affect.
Beck Depression Inventory II
Baseline severity of depressive symptoms was measured with the Beck Depression Inventory II (BDI-II; Beck, Steer, & Brown, 1996). The BDI-II is a widely used 21-item self-report measure developed to assess depressive symptoms in both normal and clinical populations.
Wisconsin Smoking Withdrawal Scale
The craving subscale of the Wisconsin Smoking Withdrawal Scale (WSWS) was used to assess subjective craving for cigarettes (Welsch et al., 1999). The WSWS is a well-validated measure of the major symptoms of nicotine withdrawal syndrome.
Fagerström Test for Nicotine Dependence
Baseline severity of nicotine dependence was measured with the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), a questionnaire that assesses various components of smoking behavior such as daily intake, difficulty in refraining from smoking, and other information related to patterns of intake.
Timeline Followback
Participant report of daily smoking behavior was collected with the timeline followback procedure (Brown, Burgess, Sales, Evans, & Miller, 1998). A computerized program was used to provide the interviewer with a calendar on which to record the amount of cigarettes smoked on each day since last contact, highlighting the days between contacts for easy reference.
Biochemical Verification of Smoking Status
Self-reported smoking status was verified with breath samples providing biochemical verification of abstinence. Participants’ expired CO was measured at each treatment session and follow-up visit using an EC50 Micro III Smokerlizer (Bedfont Scientific).
Abstinence Group Categorization
For Aims 1 and 2, prolonged abstinence status at the 3-month follow-up was used to assign participants as either prolonged abstainers or nonabstainers. Prolonged abstinence was defined as sustained abstinence from end of treatment (EOT; visit 12) to 3-month follow-up (Hughes et al., 2003), biochemically verified by expired CO (<10 ppm) at the 3-month follow-up assessment point. Participants were considered nonabstainers if they reported smoking on 7 or more consecutive days or smoking at least once each week over 2 consecutive weeks. Aim 3 examined the relationship between postquit abstinence and affect. For this aim, a 7-day point prevalence abstinence definition was used, in which abstinence was defined as self-report of no smoking, not even a puff, in the 7 days prior to the selected time point of interest (visits 8–12) plus a corresponding CO < 10 ppm.
Analytic Strategy
We used multilevel mixed-effects models (SAS Proc Mixed v9.2) to examine proposed study aims. This approach provides a generalization to the classic linear regression model using likelihood functions to estimate effects in place of least squares (McCullagh & Nelder, 1989). A mixed-model approach is well suited for analysis of repeated measures data as it allows for estimates of the correlation structure of the residuals and can efficiently handle unbalanced designs and missing data without excluding participants or imputing values (Gibbons, Hedeker, Waternaux, & Davis, 1988; Gibbons et al., 1993).
All analyses were run controlling for treatment group. Because no differences were found by including gender as a covariate, results are reported for the unadjusted models. Subjects were included as a random effect. Time was measured as days from quit date rather than session date to account for variability in time between each treatment session and distance from quit dates. Residual error variances over time were modeled as a heteroscedastic random effect, using an autoregressive function, because the homoscedastic model was not shown to improve model fit (Snijders & Berkhof, 2008). Slopes for average change over time in each of the affective measures are reported as point estimates (PE) and standard errors (SE). Missing abstinence data were addressed by using appropriate missing value imputation techniques for variables in the data analysis, including pattern mixture models. In the event of missing data, all individuals were treated as having smoked during that period (43% of the total observations—predominately at posttreatment), except when data were missing between two acquired datapoints that were either both coded as abstinent or when the first datapoint was coded as nonabstinent but the second datapoint was coded as abstinent.
RESULTS
Demographic, Smoking, and Depression-Related Characteristics
Demographic, smoking, and depression-related characteristics of the sample by abstinence groups at baseline are presented in Table 1. One-way analyses of variance were used to evaluate the abstinence group differences on continuous measures, whereas chi-square tests were used to evaluate abstinence group differences on categorical measures. In order to accommodate a zero cell in the marital status variable, Fisher’s exact test was performed in place of chi-square test, and no significant differences were found (p = .17). There was no main effect of abstinence group on any of the smoking and depression-related variables, but prolonged abstainers were more likely to be women than men, F(1,49) = 5.37, p = .021.
Table 1.
Baseline Demographic, Smoking and Depression-Related Characteristics of the Sample
| Characteristic | Abstinence group | ||
|---|---|---|---|
| Nonabstainers | Prolonged abstainers | Total | |
| N | 35 | 14 | 49 |
| Female (%) | 10 (28.57) | 9 (64.29) | 19 (38.78)* |
| Mean age, years (SD) | 41.46 (12.05) | 43.00 (10.43) | 41.90 (11.53) |
| Married (%) | 7 (20) | 0 (0) | 7 (14.29)* |
| White (%) | 26 (74.29) | 8 (57.14) | 34 (69.39) |
| Some college/bachelor’s degree (%) | 23 (65.71) | 11 (78.57) | 34 (69.39) |
| Mean expired carbon monoxide (SD) | 13.31 (8.23) | 14.14 (11.26) | 13.55 (9.09) |
| No. of years smoking (SD) | 21.14 (13.02) | 25.79 (10.47) | 22.47 (12.42) |
| Age started smoking (SD) | 17.91 (4.97) | 16.00 (4.96) | 17.37 (4.99) |
| Mean FTND score (SD) | 5.27 (1.82) | 5.38 (2.60) | 5.30 (2.04) |
| Mean BDI score (SD) | 25.54 (8.64) | 28.21 (7.53) | 26.31 (8.35) |
| Primary diagnosis: Depression (%) | 31 (88.57) | 14 (100) | 45 (91.84) |
| Primary diagnosis: Dysthymia (%) | 4 (11.43) | 0 (0) | 4 (8.16) |
Note. Depression = major depressive disorder; FTND = Fagerström Test for Nicotine Dependence; BDI = Beck Depression Inventory.
*p < .05.
At 3-month follow-up, prolonged abstinence rate was 28.6% (14/49). Prolonged abstinence status was verified by CO < 10 ppm, with mean CO value for prolonged abstainers of 2.10 (SD = 1.75), and mean CO for nonabstainers of 13.18 ppm (SD = 9.80). Attrition rate was 30.61% (34/49) at EOT and did not vary by treatment condition. As all participants were invited for follow-up assessment, attrition at 3-month follow-up was slightly lower (26.53%; 36/49).
Prolonged Abstinence and Affect
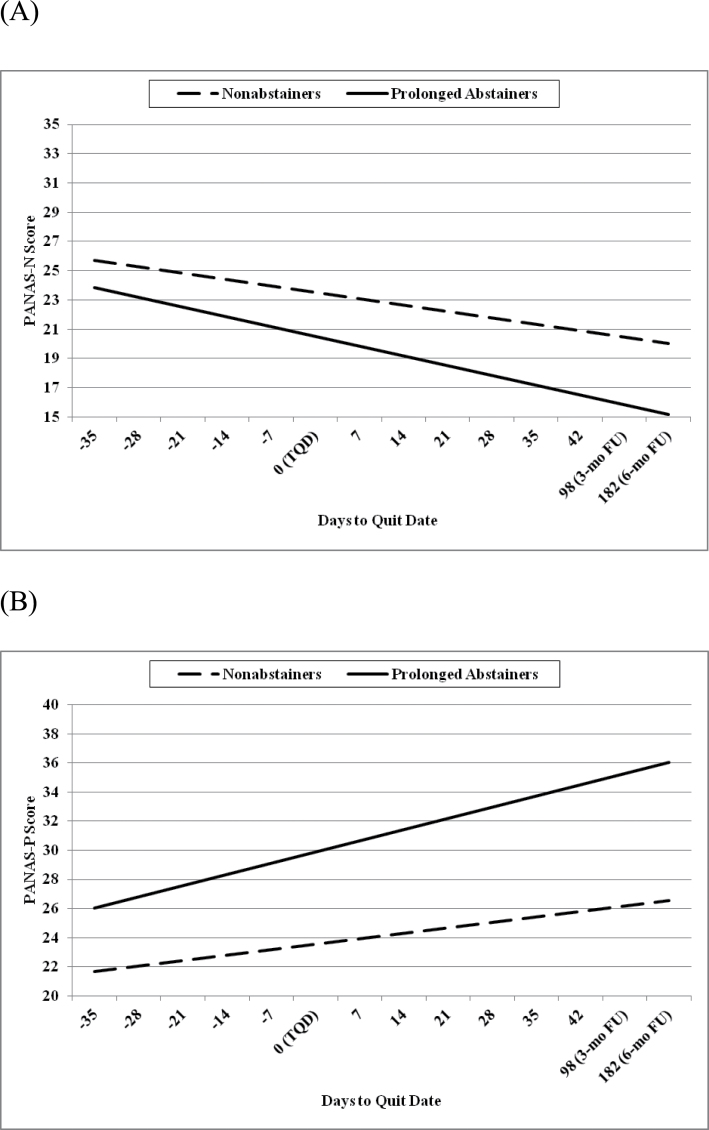
We conducted analyses to examine whether prolonged smoking abstinence was associated with affective changes over time. To evaluate Aim 1, models were run to examine the interaction of abstinence status (prolonged abstinence or nonabstinence at the 3-month follow-up) as a between-subjects factor and time (days from quit date) as a within-subjects factor on affect. For the measure of PA, the interaction of abstinence groups with days to quit date was significant, F(1,449) = 6.69, p = .010. As seen in Figure 1, slopes indicate that prolonged abstainers report increased PA over time (PE = 0.046, SE = 0.007), relative to nonabstainers (PE = 0.024, SE = 0.005). Effects remained significant after covarying for baseline PA and FTND score. For the measure of NA, the interaction of abstinence group with days to quit date was not significant, F(1,449) = 2.58, p = .11, indicating that prolonged abstainers and nonabstainers did not significantly differ in NA as a function of time.
Figure 1.
Slopes for (A) nonsignificant Positive and Negative Affect Scale–Negative Affect (PANAS-N) and (B) significant Positive Affect (PANAS-P) scores in prolonged abstainers versus nonabstainers. TQD = target quit date.
Related constructs of depressive symptoms and craving also were examined. Prolonged abstainers had significantly lower BDI-II scores at 3-month follow-up than nonabstainers, F(1,37) = 4.41, p = .04. However, the interaction of abstinence group with days to quit date was not significant in predicting BDI-II score, F(1,464) = 0.95, p = .33, indicating that prolonged abstainers and nonabstainers did not significantly differ in depressive symptoms as a function of time. For the measure of craving (WSWS-Craving), the interaction of abstinence groups with days to quit date was significant, F(1,425) = 15.81; p < .0001. Slopes indicate that prolonged abstainers report decreased craving over time (PE = −0.054; SE = 0.006), relative to nonabstainers (PE = −0.025; SE = 0.004).
Prequit Affect as a Predictor of Postquit Affect
To evaluate Aim 2, analyses were conducted to test whether the trajectory of prequit affective responses to treatment in the first six prequit treatment sessions predicted postquit affective response. Models were run using empirical Bayes estimates of prequit slope, time, and their interaction as predictors, covarying for treatment group and abstinence status, with postquit affective scores as dependent variables. There was a significant main effect for prequit PA slope, F(1,36) = 11.43, p = .002, indicating that prequit increases in PA slope were positively associated with postquit PA scores (PE = 36.09, SE = 10.67). There was also a significant main effect for prequit NA slope, F(1,36) = 25.77, p < .0001, indicating that prequit decreases in NA slope were positively associated with postquit NA scores (PE = 49.76, SE = 9.80).
For PA, a significant main effect was found for days from quit date, F = 11.43, p = .002, indicating that postquit PA scores were positively associated with days from quit date (PE = 0.010, SE = 0.005). For NA, no significant main effect for days from quit date was found, F(1,216) = 1.71, p = .19.
Interaction effects between prequit affect and time were examined, but the interactions were nonsignificant for PA, F(1,215) = 2.04, p = .16, and for NA, F(1,215) = 0.37, p = .54.
Interaction of Prequit Affect and Abstinence at Any Given Timepoint on Postquit Affect
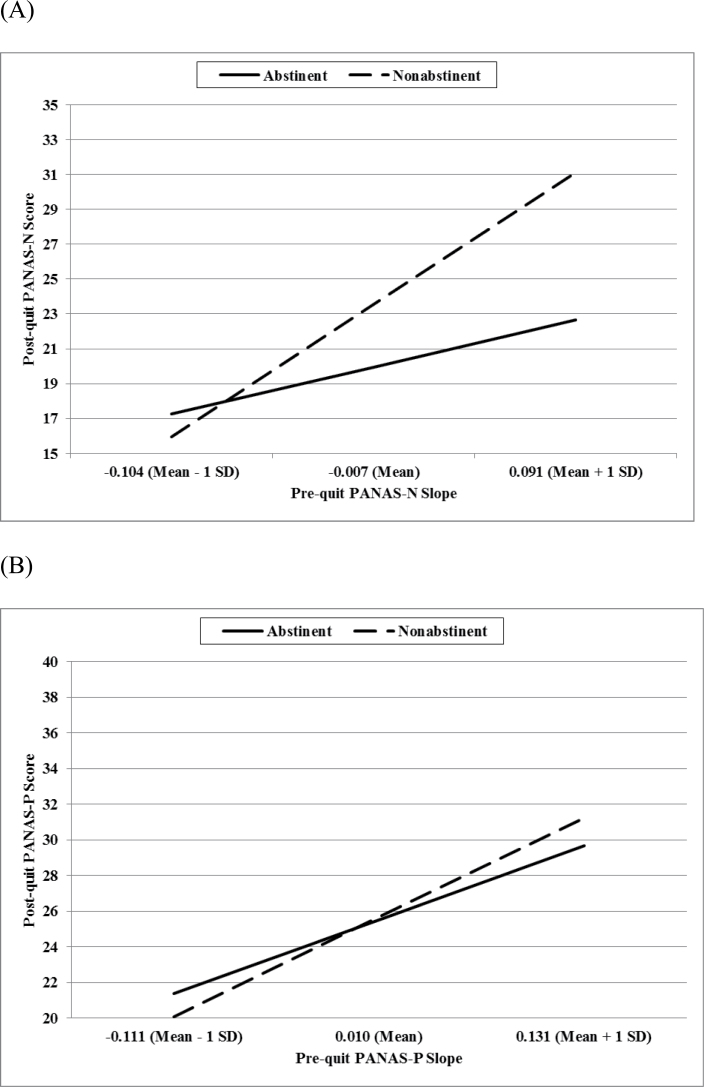
To evaluate Aim 3, analyses were conducted to examine the interaction of early affective treatment response and abstinence status on postquit affect. For Aim 3, abstinence status was defined as 7-day point prevalence at each postquit treatment session (Sessions 8–12) in order to identify dynamic, week-by-week effects of abstinence on affect. Models were run examining the interaction of prequit affective response to treatment as a between-subject factor and abstinence status as a within-subject factor in predicting postquit affect over time. As seen in Figure 2, the interaction of prequit affective response and abstinence status was not significant for PA, F(1,115) = 2.03, p = .16. For NA, the interaction of prequit affective response and abstinence status was significant, F(1,115) = 8.23, p = .005. Slopes indicate that among nonabstainers, increasing trajectories of prequit negative affect (i.e., poor response to treatment) was associated with a disproportionate rise postquit NA (PE = 50.05, SE = 17.45). Models were also run examining the three-way interaction of prequit affective response to treatment, abstinence status, and time in predicting postquit affect. Three-way interaction models were not significant for either PA, F(1,112) = 0.65, p = .42, or NA, F(1,112) = 0.59, p = .45.
Figure 2.
Interaction effects for (A) significant prequit PANAS-N slope by abstinence status (7-day point prevalence) on postquit PANAS-N score and (B) nonsignificant prequit PANAS-P slope by abstinence status (7-day point prevalence) on postquit PANAS-P score. SD = standard deviation.
DISCUSSION
Results of this study show that, among depressed smokers, those who were prolonged abstainers at the 3-month follow-up showed significant increases in PA over the course of a quit attempt compared with nonabstainers. No significant differences in NA were found between prolonged abstainers and nonabstainers although prolonged abstainers reported significantly lower levels of craving than nonabstainers. Prequit affective trajectories significantly predicted postquit affect for measures of both PA and NA. Lastly, the interaction of abstinence status and early affective response was significant in predicting affect over time for NA but not for PA. This suggests that, for those with increasing trajectories of prequit NA, nonabstainers show a disproportionate rise in NA following the quit date, relative to abstainers.
Results of this study highlight important affective differences between prolonged abstainers and nonabstainers. These affective differences may be related to individual differences in neurobiological or physical processes. Prolonged abstainers may have had a greater capacity to respond to non-cigarette rewards in the environment than nonabstainers (Buhler et al., 2010), thus increasing their levels of PA. In contrast, it is possible that nonabstainers represented a subset of smokers higher on trait anhedonia, which has been shown to predict persistence of nicotine dependence and risk of relapse following cessation (Cook, Spring, McChargue, & Doran, 2010), even after adjusting for other affective symptoms (Leventhal et al., 2008; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009). As smokers experience repeated bouts of stress and withdrawal symptoms during interruptions in smoking (Parrott, 1999), prolonged abstainers may also experience improvements in affect as a function of breaking this chronic withdrawal cycle.
The current study also highlights the important role of early affective response to treatment as a predictor of overall changes in affect. Replicating past research (Blalock et al., 2008), our results show that precessation changes in affect in smokers with current depression were important in distinguishing prolonged abstainers from nonabstainers. Those whose affective trajectories improve early in treatment may be better able to maintain these gains and reduce risk of relapse to smoking. Results underscore the importance of early affective changes that may serve as a useful target for smoking cessation intervention in this population.
An interesting interaction effect was detected between prequit changes in affect and abstinence on postquit NA. Among those at the same level of prequit NA slope, nonabstainers were shown to rise proportionally more than abstainers in postquit NA. In other words, prequit changes in NA were shown to have less of an impact on postquit NA for abstainers than for nonabstainers. This is consistent with other findings in establishing failure to quit smoking as a risk factor for exacerbation of NA (Torres et al., 2010). Although further validation is needed, it is possible that postquit abstinence may exert a protective effect, especially for those with worsening affective trajectories.
Although extant research has focused on negative affect and depressed mood as barriers to smoking cessation, the current study adds to emerging research supporting the critical role of low positive affect to smoking cessation efforts (Leventhal et al., 2008; McCarthy et al., 2008). Nonabstainers may represent a subset of depressed smokers with significant deficits in PA that call for tailored intervention. Accordingly, our findings provide additional rationale for interventions designed to enhance positive affect among smokers, such as behavioral activation and positive psychology. Interventions that address not only management of negative mood, but fostering of positive affect, may improve smoking cessation rates for depressed smokers.
Results of the current study provide partial support for the primary smoking model of smoking-depression cooccurrence, which posits that smoking increases risk of developing depression due to alterations in neurotransmitter pathways following prolonged exposure to nicotine (Hughes, 1999; Markou & Kenny, 2002; Markou et al., 1998). Although the hypothesis that prolonged abstainers would experience a decrease in NA was not supported, prolonged abstinence was not associated with a worsening of NA, as would be consistent with the primary depression or self-medication model. Thus, prolonged abstinence status did not predict an exacerbation of NA and, in fact, predicted improvement in levels of PA. This finding supports a key premise of the primary smoking model; namely, that abstinence from cigarettes supports improvements in psychological functioning over time. As abstinence was not associated with increased affective distress, our findings provide important rationale for targeting smoking cessation even in psychiatric populations of smokers.
The current study has several limitations. First, the study design does not allow definitive conclusions to be drawn regarding the directionality of relationships between abstinence status and affect. A causal role cannot be established when participants are not randomized to abstinence. Second, carbon monoxide (CO) was used for biochemical verification of smoking status. As the biochemical verification window of CO assessment is only 24hr, it is possible that this measure led to incorrectly classifying some lapsers as abstainers. Third, participants in the current study received time-intensive counseling and nicotine replacement therapy. To address questions of generalizability, it will be important for future research to examine the effect of prolonged abstinence on affect in different types of treatment, including brief interventions. Fourth, the relatively small sample size may have limited power to test some study aims. Sample size also prevented testing time-varying definitions of abstinence status following the quit attempt. However, balancing the limitation of small sample size, one strength of the current study was the use of an especially high-risk group of currently, chronically depressed smokers who have not often been included in research studies. Though the generalizability of these findings to less severe populations of smokers may be unclear, we are aware of one large clinical trial of nondepressed smokers that has also showed increasing PA as a function of abstinence status (Cinciripini et al., in press). Lastly, the scope of the current study included examining affective changes but not other components of the withdrawal process from nicotine. Although NA is thought to be an important component of the withdrawal process, it is also important to examine other aspects of withdrawal, such as craving.
Together with other recent findings (Berlin et al., 2010; Blalock et al., 2008; Kahler et al., 2011; Torres et al., 2010), results of the current study show that significant improvements in psychological functioning can be observed among those who successfully quit smoking even in a clinically depressed group when assessed in the context of intensive treatment. These results have implications for future smoking cessation efforts among high-risk psychiatric populations of smokers. Although smokers with current psychiatric disorders are not often encouraged to quit, cessation may actually contribute to improvements in psychiatric symptoms. This finding adds to burgeoning research that supports the role of abstinence in improving positive affect over time in depression-vulnerable smokers. This study contributes unique findings from a currently, chronically depressed sample of smokers.
FUNDING
This work was supported the National Institute of Mental Health (R01 MH076776-03 to JAB), National Cancer Institute (R25T CA057730 to ARM; S. Chang, Principal Investigator), and National Institutes of Health (P30 CA016672 to the University of Texas MD Anderson Cancer Center).
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We thank Tiffini Jones for coordinating data collection and Andrea Taylor for serving as a study clinician.
REFERENCES
- Beck A. T., Steer R. A., Brown G. (1996). Beck Depression Inventory (2nd ed.). San Antonio, TX: Harcourt Brace Educational Measurement; [Google Scholar]
- Berlin I., Chen H., Covey L. S. (2010). Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit day. Addiction (Abingdon, England), 105, 2209–2216.10.1111/j.1360-0443.2010.03109.x [DOI] [PubMed] [Google Scholar]
- Blalock J. A., Robinson J. D., Wetter D. W., Schreindorfer L. S., Cinciripini P. M. (2008). Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychology of Addictive Behaviors, 22, 122–128.10.1037/0893-164X.22.1.122 [DOI] [PubMed] [Google Scholar]
- Brown R. A., Burgess E. S., Sales S. D., Evans D. M., Miller I. W. (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12, 101–112.10.1037/0893-164X.12.2.101 [Google Scholar]
- Buhler M., Vollstadt-Klein S., Kobiella A., Budde H., Reed L. J., Braus D. F. … Smolka M. N. (2010). Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry, 67, 745–752.10.1016/j.biopsych.2009.10.029 [DOI] [PubMed] [Google Scholar]
- Cinciripini P. M., Robinson J. D., Karam-Hage M., Minnix J. A., Lam C., Versace F. … Wetter D. W. (in press). The effects of varenicline and bupropion-SR plus intensive counseling on prolonged abstinence from smoking, depression, negative affect and other symptoms of nicotine withdrawal. Archives of General Psychiatry, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J., Spring B., McChargue D., Doran N. (2010). Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research, 12, 978–982.10.1093/ntr/ntq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey L. S., Glassman A. H., Stetner F. (1997). Major depression following smoking cessation. American Journal of Psychiatry, 154, 263–265 [DOI] [PubMed] [Google Scholar]
- Dawkins L., Powell J. H., Pickering A., Powell J., West R. (2009). Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction (Abingdon, England), 104, 850–858.10.1111/j. 1360-0443.2009.02522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins A. E., Culhane M. A., Alpert J. E., Pava J., Liese B. S., Farabaugh A., Fava M. (2008). A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. Journal of Clinical Psychopharmacology, 28, 660–666.10.1097/JCP.0b013e31818ad7d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. (1994). Structured clinical interview for Axis I DSM-IV disorders, Patient Edition (SCID-II, version 2.0), New York: Biometrics Research Department, New York State Psychiatric Institute; [Google Scholar]
- Gibbons R. D., Hedeker D., Elkin I., Waternaux C., Kraemer H. C., Greenhouse J. B. … Watkins J. T. (1993). Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Archives of General Psychiatry, 50, 739–750.10.1001/archpsyc.1993.01820210073009 [DOI] [PubMed] [Google Scholar]
- Gibbons R. D., Hedeker D., Waternaux C., Davis J. M. (1988). Random regression models: A comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology Bulletin, 24, 438–443 [PubMed] [Google Scholar]
- Glassman A. H., Covey L. S., Stetner F., Rivelli S. (2001). Smoking cessation and the course of major depression: A follow-up study. Lancet, 357, 1929–1932.10.1016/S0140-6736(00)05064-9 [DOI] [PubMed] [Google Scholar]
- Glassman A. H., Helzer J. E., Covey L. S., Cottler L. B., Stetner F., Tipp J. E., Johnson J. (1990). Smoking, smoking cessation, and major depression. Journal of the American Medical Association, 264, 1546–1549.10.1001/jama.1990.03450120058029 [PubMed] [Google Scholar]
- Grant B. F., Hasin D. S., Chou S. P., Stinson F. S., Dawson D. A. (2004). Nicotine dependence and psychiatric disorders in the United States: Results from the National Epidemiologic Survey on alcohol and related conditions. Archives of General Psychiatry, 61, 1107–1115.10.1001/archpsyc.61.11.1107 [DOI] [PubMed] [Google Scholar]
- Hall S. M., Prochaska J. J. (2009). Treatment of smokers with co-occurring disorders: Emphasis on integration in mental health and addiction treatment settings. Annual Review of Clinical Psychology, 5, 409–431.10.1146/annurev.clinpsy.032408.153614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127.10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hughes J. R. (1999). Comorbidity and smoking. Nicotine & Tobacco Research, 1(Suppl. 2)S149–S152.10.1080/ 14622299050011981 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Keely J. P., Niaura R. S., Ossip-Klein D. J., Richmond R. L., Swan G. E. (2003). Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research, 5, 13–25.10.1080/1462220031000070552 [PubMed] [Google Scholar]
- Kahler C. W., Brown R. A., Ramsey S. E., Niaura R., Abrams D. B., Goldstein M. G. … Miller I. W. (2002). Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology, 111, 670–675.10.1037/0021-843X.111.4.670 [DOI] [PubMed] [Google Scholar]
- Kahler C. W., Spillane N. S., Busch A. M., Leventhal A. M. (2011). Time-varying smoking abstinence predicts lower depressive symptoms following smoking cessation treatment. Nicotine & Tobacco Research, 13, 146–150.10.1093/ntr/ntq213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. A., Roberts J. E., Ciesla J. A. (2005). Sudden gains in cognitive behavioral treatment for depression: When do they occur and do they matter? Behaviour Research and Therapy, 43, 703–714.10.1016/j.brat.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Khantzian E. J. (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4, 231–244.10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- Lasser K., Boyd J. W., Woolhandler S., Himmelstein D. U., McCormick D., Bor D. H. (2000). Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association, 284, 2606–2610.10.1001/jama.284.20.2606 [DOI] [PubMed] [Google Scholar]
- Lawrence D., Mitrou F., Zubrick S. R. (2009). Smoking and mental illness: Results from population surveys in Australia and the United States. BMC Public Health, 9, 285.10.1186/1471-2458-9-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. M., Kahler C. W., Ray L. A., Stone K., Young D., Chelminski I., Zimmerman M. (2008). Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. American Journal on Addictions, 17, 218–223.10.1080/10550490802019774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A., Kenny P. J. (2002). Neuroadaptations to chronic exposure to drugs of abuse: Relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotoxicity Research, 4, 297–313.10.1080/10298420290023963 [DOI] [PubMed] [Google Scholar]
- Markou A., Kosten T. R., Koob G. F. (1998). Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology, 18, 135–174.10.1016/S0893-133X(97)00113-9 [DOI] [PubMed] [Google Scholar]
- McCarthy D. E., Piasecki T. M., Lawrence D. L., Jorenby D. E., Shiffman S., Baker T. B. (2008). Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction (Abingdon, England), 103, 1521–1533.10.1111/j.1360-0443.2008.02275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave A. K., Dube S. R., Strine T. W., Kroenke K., Caraballo R. S., Mokdad A. H. (2009). Associations between smoking cessation and anxiety and depression among U.S. adults. Addictive Behaviors, 34, 491–497.10.1016/j.addbeh.2009.01.005 [DOI] [PubMed] [Google Scholar]
- McCullagh P., Nelder J. A. (1989). Generalized linear models, (2nd ed.). London: Chapman and Hall; [Google Scholar]
- McCullough J. P. (2000). Treatment for chronic depression: Cognitive behavioral analysis system of psychotherapy (CBASP). New York: Guilford Press; [DOI] [PubMed] [Google Scholar]
- Parrott A. C. (1999). Does cigarette smoking cause stress? The American Psychologist, 54, 817–820 [DOI] [PubMed] [Google Scholar]
- Prochaska J. J., Hall S. M., Tsoh J. Y., Eisendrath S., Rossi J. S., Redding C. A. … Gorecki J. A. (2008). Treating tobacco dependence in clinically depressed smokers: Effect of smoking cessation on mental health functioning. American Journal of Public Health, 98, 446–448.10.2105/AJPH.2006.101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T. A. B., Berkhof J. (2008). Diagnostic checks for multilevel models. In Goldstein H., Leeuw J. D., Meijer W. (Eds.), Handbook of multilevel analysis(pp. 141–176). New York: Springer; [Google Scholar]
- Tang T. Z., DeRubeis R. J. (1999). Sudden gains and critical sessions in cognitive-behavioral therapy for depression. Journal of Consulting and Clinical Psychology, 67, 894–904.10.1037/0022-006X.67.6.894 [DOI] [PubMed] [Google Scholar]
- Torres L. D., Barrera A. Z., Delucchi K., Penilla C., Pérez-Stable E. J., Muñoz R. F. (2010). Quitting smoking does not increase the risk of major depressive episodes among users of Internet smoking cessation interventions. Psychological Medicine, 40, 441–449.10.1017/S0033291709990560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Carey G. (1988). Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology, 97, 346–353 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070.10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Welsch S. K., Smith S. S., Wetter D. W., Jorenby D. E., Fiore M. C., Baker T. B. (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7, 354–361 [DOI] [PubMed] [Google Scholar]
- Ziedonis D., Hitsman B., Beckham J. C., Zvolensky M., Adler L. E., Audrain-McGovern J. … Riley W. T. (2008). Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research, 10, 1691–1715.10.1080/ 14622200802443569 [DOI] [PubMed] [Google Scholar]
- Zvolensky M. J., Stewart S. H., Vujanovic A. A., Gavric D., Steeves D. (2009). Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & Tobacco Research, 11, 323–331.10.1093/ntr/ntn037 [DOI] [PMC free article] [PubMed] [Google Scholar]